Abstract
Quantitative analyses of malaria parasite development are necessary to assess the efficacy of control measures. Such analyses in the mammalian host have been difficult to implement, lagging behind the use of antiparasitic drugs, vaccine development and transmission-blocking strategies. Even less is known about the genetic, environmental and other factors that impact sporogony in the mosquito host. Here, we summarize current knowledge and review a first attempt to model sporogonic development quantitatively.
Sporogonic bottlenecks and transmission control
Strategies aimed at blocking the survival and development of Plasmodium falciparum, the causative agent of severe human malaria, hold promise for transmission control. One prevailing hypothesis known as the ‘bottleneck hypothesis’ states that malaria parasites experience high levels of attrition in some sporogonic stages, with a later recovery. The implications of this hypothesis for interventions to control malaria transmission by mosquitoes are considerable.
Sinden et al. [1] recently presented a series of data-driven models of parasite survivorship across sporogonic developmental stages in an attempt to test for density dependence of parasite development within the context of the bottleneck hypothesis. The authors utilized accumulated data from their own laboratory, which were collected by a variety of researchers and generated using a single clone of the murine parasite Plasmodium berghei ANKA (clone 234, with one exception) and a single inbred line of Anopheles stephensi. The models they describe are based on the same basic formula but predict different parasite growth kinetics depending on particular parameters. In general terms, Sinden et al. [1] concluded that the efficiency of P. berghei development is linked closely to its population density. Very low infection densities result in transmissible parasites at every point except at the ookinete–oocyst transition.
Despite these efforts, stumbling blocks remain in the way of our understanding of this system. In particular, parasite viability – or, more appropriately, the capacity of one stage to transform into a subsequent stage – has not been sufficiently considered in estimates of survivorship. Furthermore, the use of models such as P. berghei has come under increased scrutiny, given recent data that have revealed crucial biological differences in sporogony between non-human parasites and P. falciparum [2] and the differential responses of Anopheles gambiae to these parasites [3]. Nevertheless, most researchers in the field agree that targeting sporogony to break the cycle of transmission requires the kind of detailed understanding presented by Sinden et al. [1].
Gametocytogenesis and the ookinete transition
Although observations of variable gametocyte quality are well documented, tools to quantify gametocyte quality as a crucial factor in the equation of sporogony are frustratingly absent. Gametocyte quality is a product of the effects of factors that are intrinsic to the parasite and host-derived or environmental factors. As commonly referred to in the literature, intrinsic factors in the mammalian host include gametocyte maturity, the sum of molecular [4] and morphological developmental changes [5], and senescence or a decline in infectivity to the mosquito vector that can be defined as gametocyte ‘half-life’ [5]. Gametocyte maturation and senescence might be regulated by host environmental factors, including immunity to infection [6], in addition to anemia [7] and antimalarial drug treatment, both of which can increase gametocyte numbers [8].
In the mosquito host, abiotic factors, including decreases in temperature and pH, stimulate transition to the invasive ookinete, while mosquito- and blood-derived factors determine survival. These latter factors induce a fundamental developmental switch in Plasmodium that is characterized by a massive upregulation of gene transcription [9]. During mosquito feeding, parasite-infected erythrocytes are concentrated by diuresis, a process whereby water and dissolved solutes – including glucose – are removed from the immediate environment of the blood mass. This decrease in glucose concentration might stimulate rapid gametogenesis, based on observations that glucose starvation triggers changes in the expression of rRNAs [10], as well as other stage-specific genes [9]. Indeed, Fang et al. [10] suggest that upregulation of sexual-stage specific rRNAs in the parasite during glucose starvation is consistent with a shift away from glucose utilization in the mosquito.
Malaria parasites rely on glycolysis for energy generation in the blood [11]. In the blood-filled mosquito midgut, glucose availability is rapidly reduced and, thus, parasites upregulate the expression of mitochondrial genes that are related to oxidative phosphorylation [9]. The initial drop in glucose might trigger competition for limited carbohydrate resources in the mosquito and could greatly impact the timing and success of ookinete maturation. Clearly, gametocyte infectivity and the transition to the ookinete stage are not constants. Drakeley et al. [12] commented, ‘There is a general consensus that successful transmission is more about “quality” than quantity of parasites, reflected in the low gametocyte densities and the low prevalence of naturally acquired transmission-blocking antibodies in individuals in endemic areas’.
The ookinete to oocyst transition
Considerable osmotic and structural damage to the midgut epithelium occurs during Plasmodium invasion. Defenses against this damage involve physical barriers, pathogen recognition and antiparasite responses. Within 24 h of the blood meal, a thick, chitinous peritrophic membrane (PM) can form around the blood bolus and provide a physical barrier to pathogen invasion. Ookinetes of some species secrete chitinases that locally degrade the PM and express membrane-attack-complex proteins [9,13]. Apoptotic elimination of damaged midgut epithelial cells might also function in pathogen defense [13]. Finally, midgut anti-pathogen responses limit parasite development. In most cases, these factors include reactive oxygen and/or nitrogen species and recognition and/or effector proteins and peptides that opsonize parasites, interfere with invasion or otherwise result in parasite death [14].
Sporozoite development
The effects of parasite burden, even at the low infections typical in field-collected mosquitoes, impose a physiological cost to reproduction [15]. Such selection pressure would be consistent with the hypothesis of Riehle et al. [16] that P. falciparum infection in Anopheles gambiae in Mali, for example, results from a failure of immune mechanisms that limit parasite development. As such, only a small proportion of the mosquito population suffers from parasite-induced physiological damage.
Antiparasite responses are active when sporozoites enter the mosquito hemocoel [17], and only ∼20% of sporozoites successfully invade the salivary glands [18]. Anti-parasite resistance is affected by relatively few genetic loci [19]. Nonetheless, a single oocyst can produce sufficient numbers of sporozoites to yield an inoculum – as few as ten sporozoites – that is infective [20]. Intriguingly, the number of transmitted sporozoites correlates weakly to sporozoite load in salivary glands at low, but not high, infection levels [21]. The fact that infection can occur with fewer than a dozen parasites has important implications for intervention: oocyst prevalence in the mosquito must be reduced to zero to have an impact on transmission – a goal that will be difficult to achieve.
Data-driven modeling of Plasmodium infection: the implications for sporogony
The work of Sinden et al. [1] represents the first attempt at invertebrate ‘in-host’ modeling of the malaria parasite. However, the dynamics of gametocytes and infectivity in the human host – which directly impact sporogony and some aspects of the bottleneck hypothesis – have been the subject of several different models. This work has been driven primarily by the lack of a clear correlation between the presence or absence of gametocytes in the blood and the ability of a host to infect mosquitoes. That is, visible peripheral gametocytemia might not translate into infected mosquitoes, whereas submicroscopic gametocytemia (as detected by PCR, for example) can contribute substantially to mosquito infection [22]. In response to these obstacles, Ross et al. [23] fitted a statistical model to describe the relationship between the density of P. falciparum asexual parasites and infectivity. Ross et al. indicate that their ‘estimate for the percentage of gametocytes that are functional is low, in agreement with estimates from life tables’ [23]. Parasite loss, therefore, at the macrogametocyte–ookinete transition described by Sinden et al. [1] might be impacted by factors that have nothing to do with processes in the mosquito host. As such, the intersection of these two fields – statistical and/or mathematical modeling of human host infectivity and modeling of sporogony – must be strengthened to better define this host transition.
Model systems and the need to face reality
Sinden et al. [1] rightly recognize that future studies should focus on laboratory An. gambiae–P. falciparum associations, with key studies being ‘those on the parasites and vectors in their numerous and different endemic areas’. Vaughan [24] recently reviewed some key biological differences between the murine parasites and P. falciparum, and among laboratory and field strains of P. falciparum. Despite these differences, comparative functional genomics of laboratory models representing a range of Plasmodium species can be used broadly to define the genetic basis for gametocyte infectivity. Furthermore, studies with non-human parasites can reveal whether gametocyte infectivity has a common evolutionary history in Plasmodium or whether it is a complex trait that can be defined in a species-specific manner.
Many interesting questions remain regarding the impact of parasite biology on the quantitative dynamics of sporogony. For example, parasite metabolic strategies reflect the presence and absence of nutrients in their specific environments and the evolutionary history of the parasites. The malaria parasite lacks key glycolytic enzymes, cannot synthesize certain fatty acid precursors and has limited lipid metabolism [11]. Furthermore, the effects of habitat quality on mosquito nutrition, which directly impacts parasite nutrition, cannot be excluded in the refinement of models of sporogony. As such, it will be important to mimic in the laboratory those environmental conditions that account for most of the observed variation to generate sufficient numbers of infected mosquitoes for statistical analyses.
Mechanistic computational simulations of sporogony, similar to those carried out in the setting of human acute inflammation [25], might help to discern patterns within the complexity of sporogony. Mechanistic simulations depict biological interactions (e.g. among cells, their products and the outcomes that result under a given set of conditions). These simulations are often used to settle controversies that might be based on different starting conditions or other experimental differences among groups studying any given complex biological system [26]. Unlike statistical models, mechanistic models offer the possibility of prediction outside of the data on which they were trained. Depending on the level of detail with which biological processes are simulated, such models can indicate mechanisms that underlie the observed outcomes and, for malaria, offer the promise of identifying novel parasite control strategies (Figure 1).
Figure 1.
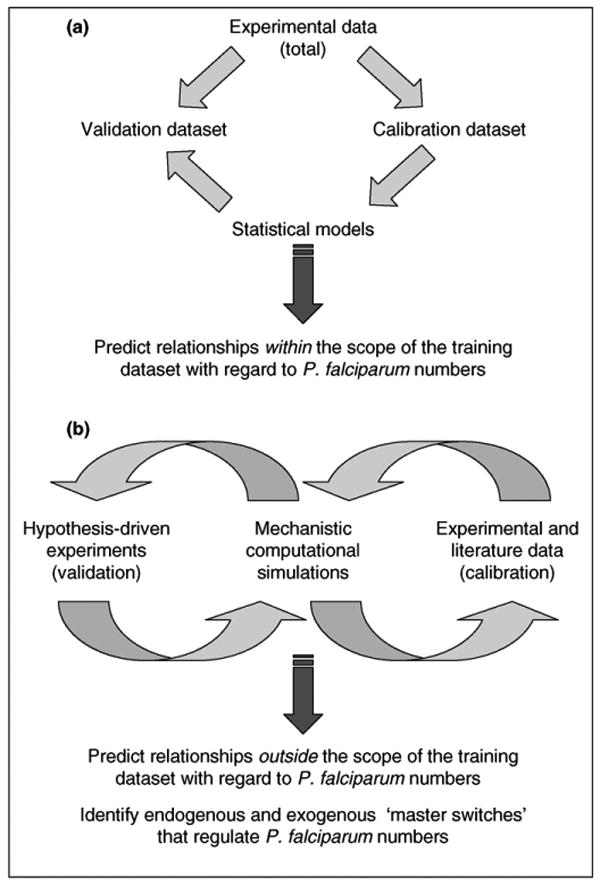
Statistical and mechanistic modeling. Statistical modeling (a) attempts to define associations among variables in a given dataset, with no imputation of mechanistic interactions. This type of modeling typically requires that experimental data be split into separate calibration and validation datasets for modeling purposes and, thus, statistical models can only be used with confidence when predicting data within the experimental conditions of the original dataset. By contrast, mechanistic models (b) attempt to recreate a given set of biological interactions. This type of modeling is best carried out iteratively with experimental work, in repeated cycles of literature mining and experimentation. Thus, mechanistic modeling is carried out iteratively, as follows: model building (largely using literature data) → model calibration (using new experiments) → further experimentation (for validation of model predictions) → model building (i.e. model revision). This iterative cycle can be used to gain insights outside of the original dataset used for model building.
Finally, recent work by Raberg et al. [27] highlights another form of control in the host–parasite relationship that might be relevant to sporogony. Mammalian hosts employ two different strategies to defend against Plasmodium infection: resistance (i.e. the ability to limit parasite burden) and tolerance (i.e. the ability to limit damage by a given parasite burden) [27]. In a manner similar to selection for enhanced antiparasite resistance, animals can evolve tolerance to Plasmodium infection [27]. Given that the pathogenesis of parasite infection in the mosquito is less than that observed in the mammalian host, tolerance might have contributed considerably to the co-evolution of the mosquito and the parasite. Here, too, mathematical modeling of tolerance in the setting of the mammalian inflammatory response [28] might inform experimental studies of sporogony.
Acknowledgments
S.L. and A.L.D. are supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID) (AI50663, AI60664) and the National Center for Research Resources (Research Facilities Improvement Grant C06 RR-12088–01). Y.V. is supported by NIAID grant AI50663 and the National Institute of General Medical Sciences grant P50-GM-53789.
References
- 1.Sinden RE, et al. Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS Pathog. 2007;3:e195. doi: 10.1371/journal.ppat.0030195. www.plospathogens.org. [DOI] [PMC free article] [PubMed]
- 2.Cohuet A, et al. Anopheles and Plasmodium: from laboratory models to natural systems in the field. EMBO Rep. 2006;7:1285–1289. doi: 10.1038/sj.embor.7400831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tahar R, et al. Immune response of Anopheles gambiae to the early sporogonic stages of the human malaria parasite Plasmodium falciparum. EMBO J. 2002;21:6673–6680. doi: 10.1093/emboj/cdf664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young JA, et al. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Talman AM, et al. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J. 2004;3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter R, Miller LH. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Organ. 1979;57(Suppl 1):37–52. [PMC free article] [PubMed] [Google Scholar]
- 7.Nacher M, et al. Decreased hemoglobin concentrations, hyperparasitemia, and severe malaria are associated with increased Plasmodium falciparum gametocyte carriage. J Parasitol. 2002;88:97–101. doi: 10.1645/0022-3395(2002)088[0097:DHCHAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Price R, et al. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60:1019–1023. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- 9.Hall N, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 10.Fang J, et al. The effects of glucose concentration on the reciprocal regulation of rRNA promoters in Plasmodium falciparum. J Biol Chem. 2004;279:720–725. doi: 10.1074/jbc.M308284200. [DOI] [PubMed] [Google Scholar]
- 11.Ginger ML. Niche metabolism in parasitic protozoa. Philos Trans R Soc Lond B Biol Sci. 2006;361:101–118. doi: 10.1098/rstb.2005.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drakeley C, et al. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 2006;22:424–430. doi: 10.1016/j.pt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol. 2005;21:573–580. doi: 10.1016/j.pt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Akman-Anderson L, et al. Bloodfeeding as an interface of mammalian and arthropod immunity. In: Beckage N, editor. Insect Immunology. Academic Press; 2008. pp. 151–179. [Google Scholar]
- 15.Hogg JC, Hurd H. The effects of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae s. l. in north east Tanzania. Parasitology. 1997;114:325–331. doi: 10.1017/s0031182096008542. [DOI] [PubMed] [Google Scholar]
- 16.Riehle MM, et al. Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science. 2006;312:577–579. [Google Scholar]
- 17.Hillyer JF, et al. Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. Int J Parasitol. 2007;37:673–681. doi: 10.1016/j.ijpara.2006.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg R, Rungsiwongse J. The number of sporozoites produced by individual malaria oocysts. Am J Trop Med Hyg. 1991;45:574–577. doi: 10.4269/ajtmh.1991.45.574. [DOI] [PubMed] [Google Scholar]
- 19.Niare O, et al. Genetic loci affecting resistance to human malaria parasites in a West African mosquito vector population. Science. 2002;298:213–216. doi: 10.1126/science.1073420. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg R, et al. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg. 1990;84:209–212. doi: 10.1016/0035-9203(90)90258-g. [DOI] [PubMed] [Google Scholar]
- 21.Medica DL, Sinnis P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect Immun. 2005;73:4363–4369. doi: 10.1128/IAI.73.7.4363-4369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shekalaghe SA, et al. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop Med Int Health. 2007;12:547–553. doi: 10.1111/j.1365-3156.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 23.Ross A, et al. Relationships between host infectivity to mosquitoes and asexual parasite density in Plasmodium falciparum. Am J Trop Med Hyg. 2006;75:32–37. doi: 10.4269/ajtmh.2006.75.32. [DOI] [PubMed] [Google Scholar]
- 24.Vaughan JA. Population dynamics of Plasmodium sporogony. Trends Parasitol. 2007;23:63–70. doi: 10.1016/j.pt.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Vodovotz Y, et al. Translational systems biology of inflammation. PLoS Comput Biol. 2008;4:1–6. doi: 10.1371/journal.pcbi.1000014. www.ploscompbiol.org. [DOI] [PMC free article] [PubMed]
- 26.Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- 27.Raberg L, et al. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- 28.Day J, et al. A reduced mathematical model of the acute inflammatory response II. Capturing scenarios of repeated endotoxin administration. J Theor Biol. 2006;242:237–256. doi: 10.1016/j.jtbi.2006.02.015. [DOI] [PubMed] [Google Scholar]


