Abstract
Glioblastoma multiforme (GBM) is an invasive and aggressive primary brain tumor which is associated with a dismal prognosis. We have earlier developed a macroscopic, intracranial, syngeneic GBM model, in which treatment with adenoviral vectors (Ads) expressing herpes simplex virus type 1 thymidine kinase (HSV1-TK) plus ganciclovir (GCV) resulted in survival of ~20% of the animals. In this model, treatment with Ads expressing Fms-like tyrosine kinase 3 ligand (Flt3L), in combination with Ad-HSV1-TK improves the survival rate to ~70% and induces systemic antitumor immunity. We hypothesized that the growth of a large intracranial tumor mass would cause behavioral abnormalities that can be reversed by the combined gene therapy. We assessed the behavior and neuropathology of tumor-bearing animals treated with the combined gene therapy, 3 days after treatment, in long-term survivors, and in a recurrent model of glioma. We demonstrate that the intracranial GBM induces behavioral deficits that are resolved after treatment with Ad-Flt3L/Ad-TK (+GCV). Neuropathological analysis of long-term survivors revealed an overall recovery of normal brain architecture. The lack of long-term behavioral deficits and limited neuropathological abnormalities demonstrate the efficacy and safety of the combined Ad-Flt3L/Ad-TK gene therapy for GBM. These findings can serve to underpin further developments of this therapeutic modality, leading toward implementation of a Phase I clinical trial.
IntroductIon
Glioblastoma multiforme (GBM) is a highly invasive and aggressive primary brain tumor. The median survival (~12 months after diagnosis) has remained unchanged for the past several decades despite advances in surgery, chemotherapy, and radiotherapy.1–6 Surgical success is often limited by a poorly defined tumor border because of the highly infiltrative nature of GBM and its close proximity to vital brain structures. Chemotherapy plus radiation are unable to eliminate all residual GBM cells. Residual glioma cells, often become resistant to chemotherapeutic agents, and give rise to recurrent GBM tumors, a hallmark of the disease.6 Gene therapy has been proposed as a new therapeutic approach to treat this devastating disease.2–4,6–8
Therapeutic approaches include the use of oncolytic, conditionally replicating viral vectors,7,9,10 replication-defective viral vectors to transfer conditionally cytotoxic genes such as herpes simplex virus type 1 thymidine kinase (HSV-1-TK),8,11,12 death receptor ligand interactions using TRAIL or FasL,13–15 and immunostimulatory genes such as interleukin-2 (IL-2), IL-12, interferon-β, tumor necrosis factor-α, and granulocyte–macrophage colony-stimulating factor.6,16–18 Several gene therapy strategies have been implemented in clinical trials to treat GBM, using adenoviral, adeno-associated viral (AAV), HSV-1, and retroviral vectors.6 Most of the clinical trials using gene therapy approaches to treat GBM have been Phase I safety trials, and therefore efficacy data cannot be extracted from these trials. One large Phase III trial using retroviral vectors expressing HSV1-TK failed to extend patients’ survival.19 To date, two gene therapy clinical trials have shown statistically significant prolongation of life expectancy (~2 month increase), by using adenoviral vectors to deliver HSV1-TK.8,12 In a Phase IIa trial Sandmair et al. compared delivery of TK into the tumor cavity with either replication-defective adenoviral vectors or retroviral vector-producing cells.12 Patients treated with an adenoviral vector expressing TK showed prolonged survival. In a Phase IIb clinical trial conducted by Immonen et al., 36 patients were randomized and treated with either current standard of care consisting of radical tumor resection followed by radiotherapy, or with standard of care plus intra-operative injections of an adenoviral vector expressing HSV1-TK into the margins of the tumor cavity following tumor resection. The mean survival time for patients treated with adenoviral vectors was 70.6 weeks as compared to 39 weeks in those who received standard of care alone.8 This approach has been recently tested in a large multicenter Phase III trial.20
We have shown earlier that treatment of microscopic tumors with Ad-TK plus ganciclovir (GCV) effectively inhibits tumor progression of syngeneic intracranial GBM in 100% of the animals.21 In order to model the characteristics of human GBM more closely, we developed a large, macroscopic, syngeneic rat model of GBM. In this stringent GBM animal model Ad-TK plus GCV resulted in the survival of only ~20% of the animals, while other single therapies tested in this model failed.22 We therefore used this model to test the efficacy of novel, combination gene therapies, and demonstrated that the efficacy of Ad-TK is greatly enhanced when combined with gene transfer of Fms-like tyrosine kinase 3 ligand (Flt3L), rescuing ~70% of the treated, tumor-bearing animals.22,23 We have also shown that treatment with Ad-Flt3L and Ad-TK is effective in a multifocal model of intracranial GBM, in which only one GBM lesion was treated.24
Striatal lesions induced by using the neurotoxin 6-hydroxydopamine are used to generate a model of Parkinson’s disease in rats, resulting in rapid reduction in the tyrosine hydroxylase (TH) immunoreactive cells in the striatum25 and elicits behavioral deficits such as asymmetry in limb use and abnormalities in amphetamine-induced turning behavior and sensorimotor reactivity.26–29 These behavioral abnormalities can be quantitated and used as response variables for testing the efficacy of Parkinson’s disease therapies.26–29 Because the growth of the GBM in the striatum induces the displacement of normal striatal tissue, seen as a displacement of TH-immunoreactive axons in the striatum, we sought to use the same tests to assess the behavioral impact of the combined Ad-Flt3L/Ad-TK-mediated tumor progression/regression in this model.
Because the GBM tumor cells were implanted into the striatum we hypothesized that tumor growth would cause behavioral abnormalities in the experimental animals. Further, we also hypothesized that because the combined gene therapy approach using Flt3L with TK plus GCV was effective at inducing tumor regression and long-term survival of the treated animals, the treatment would also correct the behavioral abnormalities. We therefore assessed the behavior and neuropathology of tumor-bearing animals treated with our combined gene therapy at three time points: 3 days after treatment, in long-term survivors (60 days after tumor implantation), and in long-term survivors that had been rechallenged with a second GBM tumor in the contralateral brain hemisphere (200 days after the initial tumor implantation).
Our results demonstrate that the large, syngeneic, intracranial GBM displaces the normal striatal tissue and induces behavioral deficits. We show a complete resolution of tumor-induced behavioral deficits as a result of gene therapy-mediated tumor regression in long-term survivors. In addition, behavioral abnormalities were not observed in long-term survivors that had been rechallenged with a second GBM tumor. Neuropathological analysis of long-term survivors after tumor rechallenge revealed an overall recovery of normal architecture of the injected striatum. The lack of long-term behavioral deficits and limited neuropathological abnormalities demonstrate the efficacy and good safety profile of the combined Flt3L and TK adenoviral vector-mediated gene therapy for GBM. These results may form the basis for further developments, leading toward the implementation of a Phase I clinical trial for GBM using this combined gene therapy strategy.
Results
Tumor growth induces behavioral deficits
We evaluated the behavioral consequences resulting from the growth of an intracranial tumor mass implanted in the striata of Lewis rats (Figure 1a). Three days post-treatment with Ad-Flt3L and Ad-TK (+GCV) or saline (control group) (i.e., 13 days after tumor implantation) the animals were analyzed for amphetamine-induced rotational behavior. The day after the behavioral tests were completed, three animals from each group were killed and the brains were processed for neuropathology. The rest continued to be monitored for tumor progression and were later used in further behavioral tests.
Figure 1. Glioblastoma multiforme tumor growth induces behavioral abnormalities.
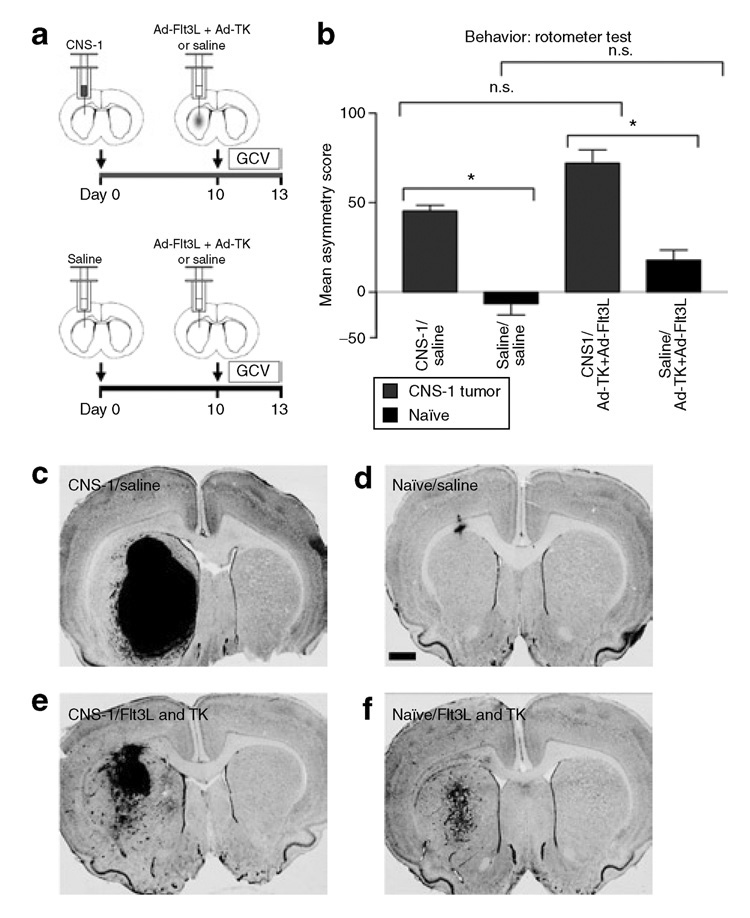
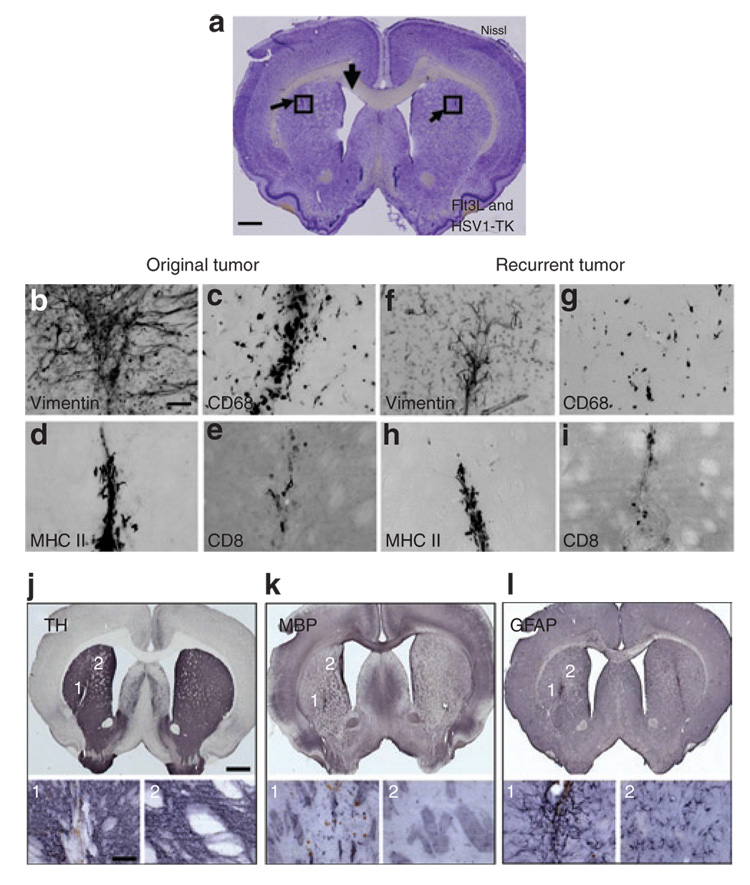
(a) Schematic representation of the experimental paradigm. 5,000 CNS-1 cells were injected unilaterally into the striata of Lewis rats. Naïve rats were injected with an equal volume of saline. Ten days later 5 × 107 infection units (i.u.) of Ad-Flt3L and 5 × 107 i.u. of Ad-TK were injected intratumorally (n = 12) or into the striata of naïve Lewis rats (n = 11). As controls, an equal volume of saline was injected into tumor bearing (n = 13) or naïve animals (n = 11). Twenty-four hours after viral vector injection, animals received twice-daily injections of ganciclovir (GCV) (25 mg/kg, intraperitoneally twice daily for 7 days). (b) Amphetamine-induced rotational behavior was assessed 3 days after treatment (i.e., 13 days after tumor implantation). All tumor-bearing animals (red bars) exhibited asymmetric rotational movement toward the tumor-bearing striatum, when compared with naïve control animals (black bars) (*P < 0.05, two-way analysis of variance). Behavioral abnormalities were absent in nontumor-bearing animals treated with Ad-Flt3L and Ad-TK when compared with animals receiving saline alone. Individual bars represent mean asymmetry scores from 11 to 13 animals per group. Error bars represent mean values ± SEM. (c–f) The animals were killed the day after behavioral testing, and NissI staining was used for elucidating the gross morphology of the brain. Nissl staining indicates the presence of CNS-1 tumors in all animals treated with either (e) Ad-Flt3L and Ad-TK or (c) saline. (e) Tumor regression was evident in tumor-bearing animals treated with Ad-Flt3L and Ad-TK. Nissl staining of naïve animals treated with (f) Ad-Flt3L and Ad-TK or (d) saline reveals minimal anatomical abnormalities localized to the area surrounding the needle tract. (f) Naïve animals treated with Ad-Flt3L and Ad-TK (+GCV) displayed more intense Nissl staining around the needle tract when compared with (d) naïve animals treated with saline alone. Ad, adenovirus; Flt3L, Fms-like tyrosine kinase 3 ligand; n.s., no significance.
In animals with unilateral 6-hydroxydopamine striatal lesions, amphetamine induces stereotypical rotational behavior ipsilateral to the lesion.26–29 As expected, an analysis of amphetamine-induced rotational behavior revealed that all the tumor-bearing animals (red bars) exhibited asymmetric rotation toward the tumor-bearing striatum when compared to animals without tumors (black bars) [Figure 1b; P < 0.05, two-way analysis of variance (ANOVA)]. These data demonstrate that significant behavioral abnormalities result from large intracranial tumors growing within the striatum, thereby showing that the tumors disrupt normal striatal function. Also, these data suggest that rotational behavior can be used as a surrogate end point to monitor both tumor progression and treatment efficacy in vivo. There were no differences in rotational behavior between tumor-bearing animals treated with Ad-Flt3L/Ad-TK and those treated with saline (Figure 1b; P > 0.05, two-way ANOVA), thereby indicating that gene therapy treatment did not produce behavioral abnormalities over and above those produced by tumor growth. No behavioral abnormalities were observed in naïve animals (not implanted with CNS-1 cells) treated with either Ad-Flt3L/Ad-TK or saline (Figure 1b; P > 0.05, two-way ANOVA). The lack of short-term behavioral abnormalities in control animals (without tumor) treated with Ad-Flt3L and Ad-TK is indicative of high safety profile of these vectors for intracranial administration at the dose tested (5 × 107 infection units of each vector).
Tumor growth displaces nerve terminals and axon bundles in the rat striatum
Nissl staining reveals the presence of CNS-1 tumors in all the tumor-implanted animals that had been treated with either Ad- Flt3L in combination with Ad-TK (Figure 1e) or with saline (Figure 1c) at 3 days after treatment. Notably, substantial tumor regression was already evident in tumor-bearing animals treated with Ad-Flt3L and Ad-TK (+GCV) (Figure 1e), as compared to saline-treated animals. In naïve animals (not implanted with CNS-1 cells) treated with Ad-Flt3L and Ad-TK or with saline (Figure 1d and f), Nissl staining reveals the absence of gross anatomical abnormalities within the brain beyond the scarring surrounding the needle tract (Figure 1e).
Staining for TH and for myelin basic protein (MBP) demonstrate that CNS-1 tumor growth displaces and compresses nerve terminals (TH+) and axon bundles (MBP+) coursing throughout the striatum (Figure 2a). Tumor-induced alterations to striatal structure are most likely to account for the behavioral abnormalities detected. Treatment with Ad-Flt3L and Ad-TK (+GCV) was able to induce tumor regression even at this early time point; as a result, there are smaller areas of disrupted striatal structure visualized through the staining of dopaminergic fibers (TH), or axon bundles traversing the striatum (MBP) (Figure 2b). We observed enlargement of the ventricles in the hemisphere where the tumor had been implanted (Figure 2b, black arrow), indicating some residual reduction in striatal mass. High-magnification images of immunoreactivity for markers of macrophages/microglial cells (CD68), CD8 + T cells (CD8), and cells expressing major histocompatibility class II reveal that tumors are heavily infiltrated with immune cells, regardless of treatment type. CNS-1 cells within the tumor mass exhibit vimentin expression, as expected of activated astrocyte-derived glioma cells (Figure 2a and b).
Figure 2. Neuropathological correlates of behavioral phenotype in tumor-bearing lewis rats 5 days after treatment with Ad-Flt3L and Ad-tK, or with saline.
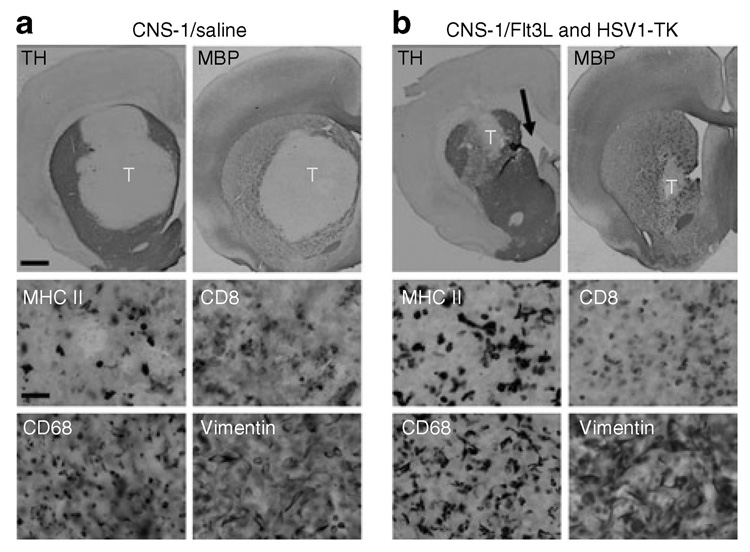
(a) Representative brain sections from animals depicted in Figure 1a stained for tyrosine hydroxylase (TH) and myelin basic protein (MBP) reveals that CNS-1 tumor growth displaces nerve terminals (TH+) and axon bundles (MBP+) within the striata of tumor-bearing animals that had been treated with an intratumoral injection of saline 3 days earlier. The lack of TH and MBP immunoreactivity of CNS-1 tumor cells results in a well-delineated tumor mass (labeled “T”). High-magnification images taken within the tumor mass reveal a high level of immune cell infiltration including cells expressing major histocompatibility class II (MHC II), CD8 + T cells (CD8), and macrophages/microglia (CD68). CNS-1 cells were identified using vimentin staining. (b) Treatment with Ad-Flt3L- and Ad-TK-induced tumor regression and, as a result, such animals show a smaller area of striatal disruption of TH+ axons and myelin (MBP+) 3 days after treatment. Enlargement of the ventricles ipsilateral to the tumor-injected hemisphere (black arrow) is observed as early as 5 days after treatment. High-magnification images taken within the tumor mass reveal a high level of immune cell infiltration including cells expressing MHC II+, CD8 + T cells (CD8), and macrophages/ microglia (CD68). CNS-1 cells were identified using vimentin staining. Scale bars represent 1,000 and 50 µm, respectively. Ad, adenovirus; Flt3L, Fms-like tyrosine kinase 3 ligand; HSV1-TK, herpes simplex virus type 1 thymidine kinase.
In naïve animals (not implanted with CNS-1 cells) treated with Ad-Flt3L and Ad-TK (Figure 3b, TH and MBP) or with saline (Figure 3a, TH and MBP), TH and MBP immunoreactivity demonstrate minimal disruption to the striatum beyond scarring at the needle tract. While vimentin immunoreactive cells are detected in the Ad-Flt3L/Ad-TK animals and in the salinetreated animals, an analysis of immunoreactive cell morphology indicates that in these animals vimentin labels the reactive astrocytes (Figure 3a and b). Tissue disruption and inflammation at the needle tract was restricted to an area of tissue surrounding the injection site (Figure 3).
Figure 3. Neuropathological correlate of behavioral phenotype 5 days after treatment with Ad-Flt3l and Ad-tK, or with saline, into brains of naive lewis rats.
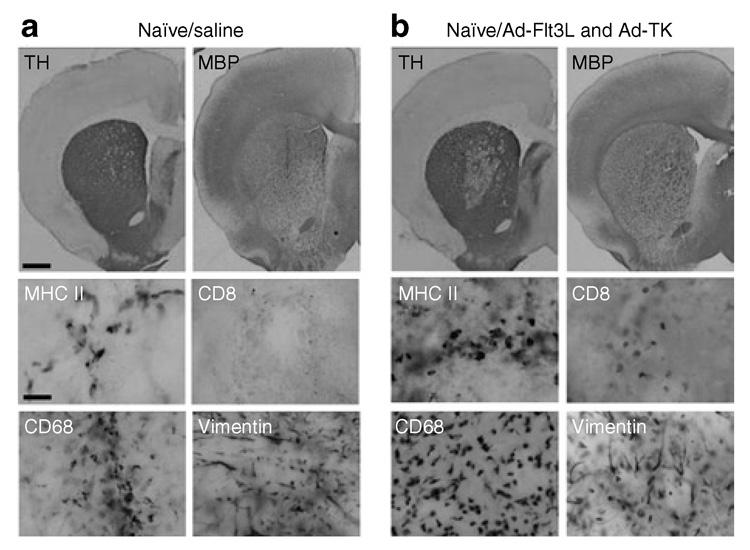
(a) Representative brain sections from animals depicted in Figure 1a. Staining for tyrosine hydroxylase (TH) and myelin basic protein (MBP) of brain sections from naïve animals treated with saline reveals tissue damage to the striatum localized to the area surrounding the injection site 3 days after intratumoral saline injection. High-magnification images taken at the injection site indicate the presence of macrophages/microglia (CD68) and cells expressing major histocompatibility class II (MHC II), but not CD8 + T cells (CD8). Vimentin immunoreactive cells are detected, with morphology indicative of reactive astrocytes. (b) Staining for TH and MBP of brain sections from naïve animals treated with Ad-Flt3L and Ad-TK reveals an area of decreased TH staining around the needle tract 3 days after intratumoral adenovirus (Ad) delivery. High-magnification images taken at the injection site indicate the presence of macrophages/microglia (CD68) and cells expressing MHC II as well as CD8 + T cells. Morphological analysis of vimentin immunoreactive cells suggests that they are reactive astrocytes. Scale bars represent 1,000 and 50 µm, respectively. Flt3L, Fms-like tyrosine kinase 3 ligand; TK, thymidine kinase.
Recovery from behavioral deficits in Ad-Flt3l and Ad-tK treated long-term GBM survivors
Next we tested for any potential behavioral abnormalities in long-term GBM survivors treated with Ad-Flt3L and Ad-TK (Figure 4a). One-way ANOVA analysis of amphetamine-induced rotational behavior in long-term survivors and naïve, age-matched control animals treated with an intracranial injection of saline or Ad-Flt3L and Ad-TK showed no significant differences in rotational asymmetry (Figure 4b). Tumor-bearing animals treated with saline did not survive to day 60 for evaluation. Log-rank test of Kaplan–Meier survival curves showed that treatment with Ad- Flt3L and Ad-TK (n = 11) improved the survival of tumor-bearing rats (P < 0.001). Approximately 80% of the rats treated with Ad- Flt3L and Ad-TK survived to day 60 while all the tumor-bearing animals treated with saline succumbed to tumor growth by day ~15 post-GBM implantation (n = 8) (Figure 4c).
Figure 4. Reversal of behavioral deficits caused by tumor burden within 60 days after Ad-Flt3l/Ad-tK treatment.
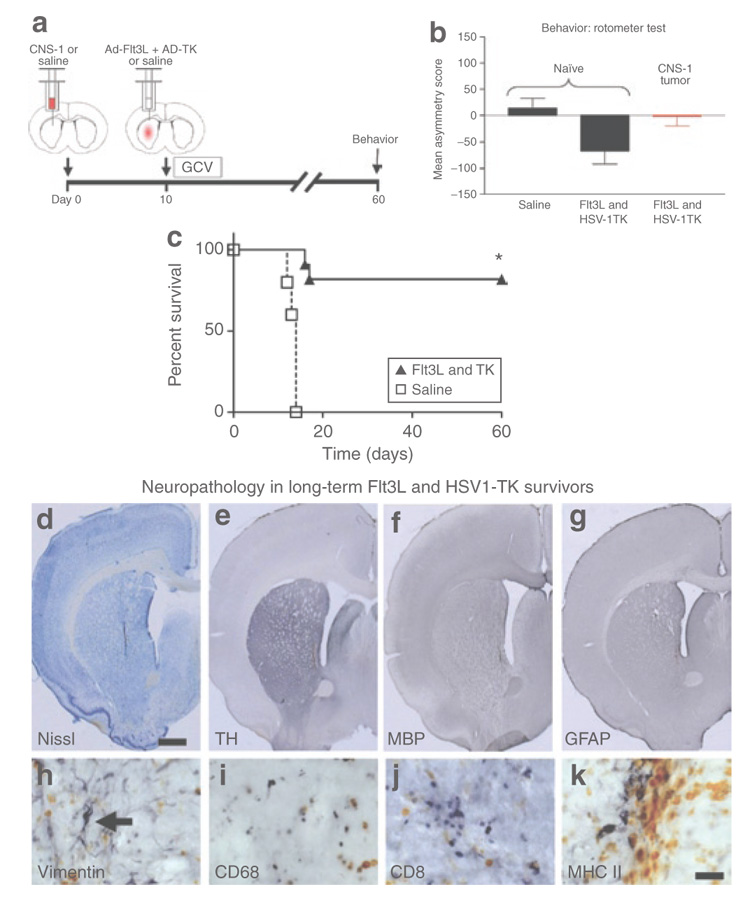
(a) Schematic representation of experimental paradigm. 5,000 CNS-1 cells were injected unilaterally into the striata of naïve Lewis rats. Another group of naïve rats was injected with an equal volume of saline. Ten days later 5 × 107 infection units (i.u.) of Ad-Flt3L and 5 × 107 i.u. of Ad-TK were injected intratumorally or into the striata. Saline was injected into tumor-bearing or naïve control animals. Twenty-four hours after the viral vector injection, the animals received twice-daily injections of ganciclovir (GCV). Long-term survivors were assessed for abnormalities in amphetamine-induced rotational behavior. (b) Analysis of amphetamine-induced rotational behavior in long-term survivors (n = 9) and in naïve, age-matched control animals treated with an intracranial injection of saline (n = 6) or with Ad-Flt3L and Ad-TK (n = 7) showed no significant difference in rotational asymmetry (one-way analysis of variance). Tumor-bearing animals treated with saline did not survive to day 60 for evaluation. Individual bars represent mean asymmetry scores from 6 to 9 animals/group. Error bars represent mean values ± SEM. (c) Log-rank test of Kaplan–Meier survival curves showed that treatment with Ad-Flt3L and Ad-TK (+GCV) (n = 11) improved the survival of tumor bearing rats (P < 0.001). Approximately 80% of the rats treated with Ad-Flt3L and Ad-TK (+GCV) survived to day 60, whereas all the tumor-bearing animals treated with saline succumbed to tumor growth by approximately day 15 after glioblastoma multiforme implantation (n = 8). Representative brains of long-term survivors were evaluated for structural integrity using (d) Nissl staining and (e) immunoreactivity for tyrosine hydroxylase (TH), (f) for myelin basic protein (MBP), and (g) for glial fibrillary acidic protein (GFAP). Tissue damage to the striatum was limited to the area immediately adjacent to the injection site. Ventricles enlarged to varying degrees were observed in the ipsilateral hemisphere of tumor implantation in long-term survivors. (h) High-magnification images taken at the needle tract show vimentin positive immunoreactivity with morphology suggestive of reactive astrocytes (black arrow). Immunoreactivity indicates the presence of (i) macrophages/microglia, (j) CD8 + T cells, and (k) cells expressing major histocompatibility class II (MHC II), which were limited to the area immediately adjacent to the injection site in long-term survivors. Scale bars represent 1,000 and 50 µm, respectively. Orange color represents hemosiderin within macrophages. Ad, adenovirus; Flt3L, Fms-like tyrosine kinase 3 ligand; TK, thymidine kinase.
The brains of the long-term survivors were evaluated for structural integrity using Nissl staining (Figure 4d) and immunoreactivity for TH (Figure 4e), MBP (Figure 4f), and glial fibrillary acidic protein (Figure 4g). Damage to the striatum and inflammatory cell influx was limited to the tissue immediately adjacent to the needle tract. Varying degrees of enlargement of the ventricles were observed in the hemisphere ipsilateral to the tumor implantation in long-term survivors. These data demonstrate that the initial influx of inflammatory cells into the tumor mass was mostly resolved by day 60 (Figure 4i–k).
Treatment with Ad-Flt3l and Ad-tK does not affect behavior after rechallenge with a second tumor in the contralateral hemisphere
In order to mimic the recurrent features of GBM, long-term survivors (60 days) were rechallenged with 5,000 CNS-1 cells injected into the contralateral hemisphere. No further treatments were administered. Behavior was assessed 140 days after rechallenge, and the animals were killed 180 days after rechallenge and their brains analyzed for neuropathology and immune cellular infiltrates (Figure 5a).
Figure 5. Behavior remains unaffected after rechallenge, in a recurrent model of glioma.
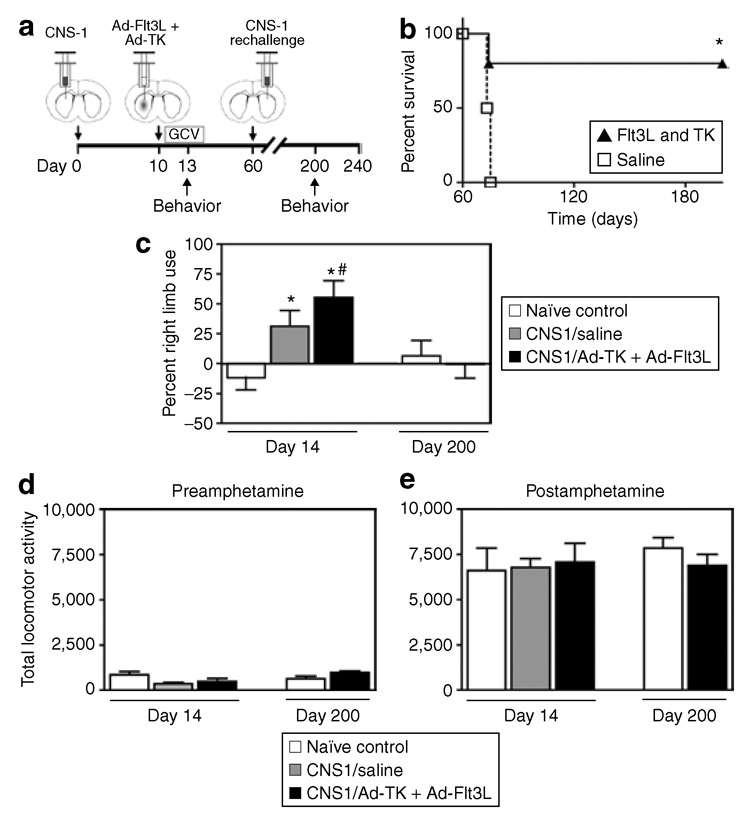
(a) Schematic representation of experimental paradigm. Five thousand CNS-1 cells were injected unilaterally into the striata of Lewis rats. Ten days later 5 × 107 infection units (i.u.) of Ad-Flt3L and 5 × 107 i.u. of Ad-TK, or saline as a control, were injected intratumorally. Twenty-four hours after viral vector injection, the animals received twicedaily injections of ganciclovir (GCV). Long-term survivors were implanted with 5,000 CNS-1 in the contralateral hemisphere 60 days after the initial tumor implantation. No further treatment was administered. Tumor-bearing long-term survivors, tumor-bearing saline-treated animals, and naïve, age-matched animals were tested at 13 and 200 days post-tumor implantation. Tumor-bearing saline-treated animals were tested at the 13-day time point only, because they succumb to tumor burden at approximately day 15 post-tumor implantation. Animals were killed 240 days after initial tumor implantation for neuropathology evaluation. (b) Log-rank test of Kaplan–Meier survival curves showed that 80% of long-term survivors lived to 180 days after the tumor cell rechallenge (240 days after the initial tumor implantation, n = 5). As expected, naïve control animals implanted with tumor cells on the day of rechallenge died within ~15 days (*P < 0.001; n = 5). (c) The cylinder test revealed forelimb use asymmetry in tumor-bearing animals treated with saline or with Ad-TK + Ad-Flt3L at day 13 after tumor implantation, when compared with naïve control animals (*P < 0.05 versus naïve, Student’s t-test, n = 8–10). When tested at day 200, there were no differences observed in the forelimb use asymmetry in longterm survivors after rechallenge, when compared with naïve age-matched controls. Statistically significant differences in forelimb use asymmetry were observed in the Ad-TK + Ad-Flt3L-treated tumor-bearing animals between day 13 and day 200 (#P < 0.05 versus naïve, Student’s t-test). Individual bars represent the percentage of right limb use from 8 to 10 animals/group. Error bars represent mean values ± SEM. Assessment of (d) baseline locomotor activity or (e) amphetamine treatment–induced locomotor activity demonstrated no significant differences among the treatment groups. Individual bars represent average locomotor activity scores from 8 to 10 animals/group. Error bars represent mean values ± SEM. Ad, adenovirus; Flt3L, Fms-like tyrosine kinase 3 ligand; TK, thymidine kinase.
After tumor rechallenge, 80% of the animals survived long term; 6 months after the tumor rechallenge they were perfused fixed for neuropathological analysis. In order to verify the viability of the CNS-1 cells and their capacity to form tumors, these cells were implanted in control animals on the same day as the rechallenge. All these control animals died within ~15 days (*P < 0.001) (Figure 5b), thereby confirming the tumor-forming capacity and viability of the CNS-1 cells used in the rechallenge experiment.
As amphetamine-induced rotational abnormalities resolved by day 60, additional tests were implemented to test for forelimb use asymmetry (the cylinder test) and gross locomotor (baseline and amphetamine-induced locomotor activity) deficits at day 200. Naïve, age-matched control animals were injected with saline intracranially and used as controls for behavioral assessment of long-term survivors after rechallenge. First, behavioral testing was performed on the animals 3 days after intratumoral injection of either Ad-TK + Ad-Flt3L or saline (i.e., 13 days after tumor implantation) to demonstrate the behavioral consequences of tumor burden and short-term delivery of the combined gene therapy. Significant differences were observed in forelimb use asymmetry in tumor-bearing rats treated with either saline or gene therapy, when compared with naïve rats (Figure 5c, *P < 0.05 versus naïve, Student’s t-test, n = 8–10). Further, statistically significant differences in forelimb use asymmetry were also observed between Ad-TK + Ad-Flt3L-treated tumor-bearing animals tested at day 13 versus day 200 (Figure 5c, #P < 0.05, Student’s t-test, n = 8–10). These behavioral abnormalities resolved by day 200 in long-term survivors treated with gene therapy and implanted with a secondary GBM in the contralateral hemisphere. There were no significant differences in baseline locomotor activity (Figure 5d) or amphetamine-induced locomotor activity (Figure 5e) in any of the treatment groups, indicating that the behavioral abnormalities (e.g., amphetamine-induced rotational behavior and forelimb use asymmetry) found were not a consequence of nonspecific effects on locomotor activity.
Ad-Flt3L and Ad-TK-treated animals that survived primary and recurrent GBM were killed 240 days after the initial tumor implantation and were evaluated for neuropathology and the presence of immune cellular infiltrates using immunohistochemistry. Nissl staining and immunocytochemistry reveal scarring and inflammation limited to the injection site in both hemispheres and an enlarged ventricle ipsilateral to the site of the original tumor implantation (Figure 6). Immunoreactivity with markers for TH and MBP reveals limited tissue disruption in close proximity to the injection site, but otherwise normal expression patterns throughout the striatum, thereby indicating that damage to nerve terminals and axon bundles is minimal in long-term survivors after GBM rechallenge (Figure 6).
Figure 6. Neuropathological correlates in long-term survivors after glioblastoma multiforme rechallenge; minimal long-term side-effects.
The animals described in Figure 5a, treated with Ad-Flt3L and Ad-TK and surviving the primary and recurrent tumors, were killed 240 days after the initial tumor implantation and were subjected to immunohistochemical evaluation for neuropathology and the presence of immune cellular infiltrates in both hemispheres of the brain. Representative brain sections are shown. (a) Nissl staining reveals scar tissue adjacent to the injection site in both hemispheres and an enlarged ventricle ipsilateral to the site of the original tumor implantation. (b,f) Vimentin staining and morphological analysis of immunoreactive cells suggests the presence of activated astrocytes at both injection sites. Immunoreactivity for (c,g) macrophages, (d,h) major histocompatibility class II (MHC II) + immunoreactive cells, and (e,i) CD8 + T cells reveals low levels of infiltration of immune cells both at the original and recurrent tumor injection sites. (j,k) Immunoreactivity with markers for tyrosine hydroxylase (TH) and myelin basic protein (MBP) reveals slight tissue disruption in close proximity to the injection site, but otherwise normal expression patterns throughout the striatum. Images showing disruptions to (j1) nerve terminals and (k1) axon bundles are limited to the injection sites. Images from an area of the striatum distant from the injection site show (j2) normal TH distribution and (k2) MBP-coated axons. (l1) Increased glial fibrillary acidic protein (GFAP) immunoreactivity is localized to areas close to both injection sites, indicating the presence of activated astrocytes. (l2) Normal GFAP immunoreactivity is observed at an area of the striatum distant from the injection site. Ad, adenovirus; Flt3L, Fms-like tyrosine kinase 3 ligand; TK, thymidine kinase.
Discussion
Attempts at brain tumor therapy through induction of antibrain tumor immune responses have been challenged by our poor understanding of the brain’s immune responses, the disseminated nature of the disease, the high rate of glioma recurrences, the high rate of mutations occurring in glioma cells, and concerns that induction of an immune response within the brain could lead to autoimmune disease.
Importantly, so far it has been difficult to stimulate an immune response directly from within the brain parenchyma itself. In order to overcome some of these limitations we have developed a therapeutic approach based on the recruitment to the brain of antigenpresenting cells, dendritic cells. These cells, which are essential for stimulating an antitumor immune response, are usually missing from the normal brain parenchyma.30–33 In our approach, recruitment of dendritic cells to the brain is induced by Flt3L expression, 30 while the killing of tumor cells is induced by HSV1-TK and GCV.21–23,34 This therapeutic combination not only induces tumor regression and long-term survival, but also inhibits the growth of secondary tumor implanted in the contralateral hemisphere in long-term survivors, thereby suggesting the successful induction of anti-GBM systemic immunity.
In this study we examined the behavioral consequences of glioma growth within the striatum, and the effects of Ad-Flt3L/ Ad-TK gene therapy on tumor progression and behavioral patterns. Herein we report the development of behavioral abnormalities caused by the growth of a large intracranial GBM tumor implanted into the striatum. Following intratumoral treatment with Ad-Flt3L and Ad-TK (+GCV), we demonstrated complete resolution of tumor-induced behavioral deficits in long-term survivors of GBM. Moreover, the long-term neuropathological consequences observed were minimal.
Unilateral lesions of the striatum, or of its dopaminergic innervation, induce abnormal rotational behavior. Abnormal rotation, and the reversal of the abnormality after therapy, are a standard method of evaluating novel treatments for Parkinson’s disease, a disease that affects the structure and function of the basal ganglia.26–29 We tested the hypothesis that the growth of a large intracranial tumor would affect behavior as the tumors infiltrate, displace and compress striatal tissue.
The development of a large intracranial GBM induced asymmetric rotational movements toward the tumor-bearing striatum in response to amphetamine, and forelimb asymmetry in the cylinder test, thereby demonstrating that significant behavioral abnormalities result from tumor-induced disruption of striatal structure and function. In contrast, neither baseline locomotor activity nor amphetamine-stimulated locomotor activity was different across the treatment groups. This finding demonstrates that, at the time of behavioral testing, there was no generalized, nonspecific reduction in locomotor activity produced by tumor implantation or gene therapy. In addition, the interpretation of the amphetamine-induced rotational behavior was not confounded by differential sensitivity to amphetamine. We have earlier established that the syngeneic CNS-1 tumor model reproduces several histopathological characteristics of human GBM.1 The behavioral deficits encountered in this rat GBM model demonstrate its utility for testing the safety and efficacy of novel therapeutic strategies for GBM. Also, the data reported suggest that rotational behavior can be used as a surrogate end point of both tumor progression and treatment efficacy. Importantly, tumor-induced behavioral deficits were reversed by successful elimination of the tumors by gene therapy. This indicates that the behavioral deficits detected are functional, and normal behavior is restored after successful elimination of the tumor.
Enlarged ventricles were consistently observed ipsilateral to the striatum implanted with GBM. Enlargement of the ventricular space could be the result of loss of striatal cells or increased pressure in the ventricular system during the tumor elimination process. Most likely it reflects loss of striatal mass. However, in this model, such loss did not preclude behavioral recovery. This recovery is supported by the fact that large lesions into the striatum or the nigro-striatal pathway are necessary for behavioral deficits to become evident, and the plasticity of the nigro-striatal system. Therefore, in our model, the loss, if any, of striatal mass is below the threshold needed to cause permanent behavioral deficits. Short-term, transient immune infiltrates were observed in naïve animals injected with adenoviral vectors expressing Flt3L and TK (Figure 1f), and these resolved within 60 days after Ad administration (Figure 3d). This is caused by a transient inflammatory response to the adenoviral vectors.35,36
One of the clinical hallmarks of human GBM is the high rate of tumor recurrence. The majority of patients succumb to a recurrent tumor that develops usually 6–12 months following radical surgical resection and subsequent chemo- and/or radiotherapies. Recurrent tumors are often difficult to treat, because the recurrent GBM cells are resistant to previously utilized chemo- and radiotherapies. 6 When tumor-bearing Ad-Flt3L and Ad-TK-treated animals were rechallenged with tumor cells in the contralateral hemisphere, 80% survived long term despite receiving no further treatment. These animals showed neither gross locomotor abnormalities nor limb use asymmetry, and there was an absence of neuropathology in the contralateral hemisphere. These data suggest that the second GBM implantation in the contralateral hemisphere of long-term survivors 2 months after gene therapy did not result in the development of a second GBM because of the presence of an anti-GBM immune response in these animals.
In summary, our data demonstrate that tumors growing within the striata of rats cause behavioral abnormalities that are reversible upon successful tumor elimination with combined cytotoxic and immune-stimulatory gene therapy. Flt3L in combination with TK constitutes an effective and safe gene therapy strategy for GBM. These results further support the need to proceed toward implementation of Phase I clinical testing of Ad-Flt3L and Ad-TK for primary and recurrent GBM in human patients.
Materials and Methods
Adenoviral vectors and cell lines
The adenoviral vectors used in this study are first generation, replication-deficient, recombinant adenovirus type 5 vectors (Ad) encoding transgenes driven by the human cytomegalovirus intermediate early promoter situated within the E1 region: Ad-TK expressing HSV-1 thymidine kinase21–23 and Ad-Flt3L expressing human soluble Flt3L.22,30 They were generated and characterized by us as described earlier37 –39 All viral preparations were tested to ensure absence of replicationcompetent adenovirus and lipopolysaccharide contamination using methodologies described earlier.37–39
CNS-1 cells were maintained in culture in Dulbecco’s modified Eagle’s medium (CellGro, San Diego, CA) supplemented with 10% fetal bovine serum (Omega Scientific, Tarzana, CA) and 1% penicillin/streptomycin (CellGro, San Diego, CA). On the day of injection, the cells were trypsinized, counted using trypan blue to exclude dead cells, and resuspended in phosphate-buffered saline at a final concentration of 5,000 cells in 3 µl. The cells were stored on ice until use in the injection.
Syngeneic intracranial rat tumor models and controls
Male Lewis rats (220–250 g, Harlan, Indianapolis, IN) were unilaterally injected in the striatum (from bregma: +1 mm anterior, +3 mm lateral, and from dura: −5 mm with either 3 µl of phosphate-buffered saline containing 5,000 CNS-1 cells or phosphate-buffered saline alone as a control.22 Ten days later, 5 × 107 infection units each of Ad-Flt3L and Ad-TK were mixed together and resuspended in a final volume of 3 µl of saline. Utilizing the same drill hole, the vectors were delivered in three locations (−5.5, 5.0, and −4.5 mm from dura, 1 µl/injection site) within the tumor mass or striatum. Control animals received an intracranial injection of saline alone. On the day after vector injection 25 mg/kg GCV (Roche Laboratories, Nutley, NJ) was injected intraperitoneally twice daily for 7 days.
Sixty days after the initial tumor implantation, long-term survivors were rechallenged with an intracranial injection of 5,000 CNS-1 cells into the contralateral striatum (from bregma: +1 mm anterior, −3 mm from lateral, −5 mm from dura). No further treatment was administered. Animals were monitored daily and killed by terminal perfusion at the first signs of moribund behavior. In order to control for cell viability and tumor progression in vivo, CNS-1 cells were also intracranially injected into naïve rats.
The animals were monitored daily and killed at the first signs of moribund behavior, or at the end of the experiment, by terminal perfusion-fixation with oxygenated, heparinized tyrode solution followed by 4% paraformaldehyde in phosphate-buffered saline. The brains were removed for histopathological analysis. Animals were housed in a humidity- and temperature-controlled vivarium on a 12:12 hour light/dark cycle (lights on 07:00) with free access to food and water. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by Cedars- Sinai Medical Center Institutional Animal Care and Use Committee.
Behavioral analysis
Rotational asymmetry
Amphetamine-induced rotational behavior was assessed using the RotoMax apparatus and software (AccuScan Instruments, Columbus, OH). Rotational behavior was monitored for 90 minutes after subcutaneous injection of 1.5 mg/kg d-amphetamine sulfate (Sigma, St. Louis, MO). Tumor-bearing animals or naïve, age-matched animals were tested 13 and 59 days after the initial tumor implantation. Asymmetry values were calculated as the number of clockwise rotations minus the number of counterclockwise rotations. The statistical significance of rotational asymmetry was determined using two-way ANOVA at day 13 and one-way ANOVA at day 60.
Cylinder test
The cylinder test was used for detecting asymmetry and abnormalities in forelimb use.29,40,41 Tumor-bearing long-term survivors, tumor-bearing saline-treated animals, and naïve, age-matched animals were tested ~13 and 200 days after tumor implantation. Tumor-bearing saline-treated animals were tested only at the 13-day time point because all the animals in this group succumb to tumor burden at approximately day 15 after tumor implantation. The cylinder test was performed with dim, indirect room lighting (09:00 to 14:00 hours). The rats were not habituated to the room or the test apparatus before testing. Contacts made by each forepaw with the wall of a 20.3 cm wide clear cylinder were scored from videotape over a 10-minute period in slow motion by two independent observers, blinded as to the experimental grouping. Forelimb contact with the walls of the cylinder was recorded, measuring only the initial contact. The data were converted to the number of placements of the right or the left forelimb divided by the total number of placements, or the number of simultaneous placements of both forelimbs divided by the total number of placements. Rats that made <20 placements were not included in the final statistical analysis (one subject per group was eliminated). The statistical significance of forelimb use asymmetry values was determined using Student’s t-test, corrected for multiple comparisons.
Open field test
Spontaneous motor behavior was assessed using the open field apparatus (San Diego Instruments, San Diego, CA). Tumor-bearing long-term survivors, tumor-bearing saline-treated animals, and naïve, age-matched animals were tested ~13 and 200 days after tumor implantation. Tumor-bearing saline-treated animals were tested at the 13-day time point only, because all the animals in this group succumb to tumor burden at approximately day 15 after tumor implantation. In order to ensure consistency of the data, testing was performed during the light cycle (09:00 and 14:00 hours) in a well-lit room 30 minutes after being transported in their home cages. Baseline spontaneous locomotor activity was recorded for 30 minutes in a 40.6 × 40.6 cm3 × 38.1 cm3 enclosed box, using photobeam breaks and optical sensors. Locomotor activity was then monitored for 120 minutes after subcutaneous injection of 1.5 mg/kg d-amphetamine sulfate (Sigma, St. Louis, MO). The data were recorded as the total number of photobeam breaks over the observation period. The statistical significance of total locomotor activity was assessed using Student’s t-test and, when failing the normality criteria, they were analyzed by Mann–Whitney test.
Immunocytochemistry
Sections of the striatum (60 µm), were used for immunohistochemical examination of the structural integrity of the brain and to look for immune cells markers using methodologies described by us earlier.1,22,42 The primary antibodies used in the immunohistochemical analyses were: (i) ED1/CD68 (mouse anti-ED1, 1:1,000; Serotec, Raleigh, NC; cat. no. MCA341R) which labels macrophages/activated microglia, (ii) CD8 (mouse anti-CD8, 1:1,000; Serotec, Raleigh, NC; cat no. MCA48G) which labels CD8 + T cells, (iii) major histocompatibility class II (mouse anti-I-A, 1:1,000; Serotec, Raleigh, NC; cat no. MCA46G) which labels B lymphocytes, dendritic cells, and macrophages expressing I-A, (iv) vimentin (mouse monoclonal antivimentin, 1:1,000; Sigma, St. Louis, MO; cat no. V6603) which labels CNS-1 tumor cells and activated astrocytes, (v) TH (rabbit polyclonal anti-TH, 1:1,000; Calbiochem, La Jolla, CA; cat no. 657012) which labels nerve terminals, (vi) MBP (mouse monoclonal anti- MBP, 1:1,000; Chemicon, Temecula, CA; cat no. MAB1580) which labels axon bundles, and (vii) glial fibrillary acidic protein (mouse monoclonal antiglial fibrillary acidic protein 1:1,000; Chemicon, Temecula, CA; cat no. MAB3402) which labels activated astrocytes. Biotinylated secondary antibodies (anti-mouse or anti-rabbit, 1:1,000; DAKO, Glostrup, Denmark) were visualized using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) and developed with diaminobenzidine and glucose oxidase. Nissl staining was performed on mounted, air-dried brain sections. The sections were mounted and incubated in cresyl violet (0.1%; Sigma, St. Louis, MO) for 15 minutes. They were washed in destain solution (70% ethanol, 10% acetic acid) for 1 minute and then dehydrated (100% ethanol and xylene) and mounted. The tissues were analyzed and photographed using a Zeiss Axioplan 2 microscope (Thornwood, NY).
Statistical analysis
Statistical analysis was performed using log-rank test of Kaplan–Meier survival curves, two-way or one-way ANOVA, and Student’s t-tests or, when failing the normality criteria, the Mann–Whitney test corrected for multiple comparisons. The cutoff point for significance was considered as P < 0.05.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) Grant 1R01 NS44556.01, Minority Supplement NS445561.01; 1R21-NSO54143.01; 1UO1 NS052465.01, 1 RO3 TW006273-01 to M.G.C.; NIH/NINDS Grants 1 RO1 NS 054193.01; RO1 NS 42893.01, U54 NS045309-01, and 1R21 NS047298-01 to P.R.L. The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics to P.R.L. and M.G.C., respectively, The Linda Tallen and David Paul Kane Foundation Annual Fellowship and the Board of Governors at Cedars-Sinai Medical Center. G.D.K. is supported by NIH/NINDS 1F32 N50503034-01. M.C. is supported by NIH/NINDS 1F32 NS058156.01. R.N.P. is supported by the Levine Family Fund Research Endowment. We thank Shlomo Melmed and Leon Fine for their support and academic leadership. The authors have no conflicting financial interests.
REFERENCES
- 1.Candolfi M, Curtin JF, Nichols WS, Muhammad AG, King GD, Pluhar GE, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro MG, Cowen R, Williamson IK, David A, Jimenez-Dalmaroni MJ, Yuan X, et al. Current and future strategies for the treatment of malignant brain tumors. Pharmacol Ther. 2003;98:71–108. doi: 10.1016/s0163-7258(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 3.Curtin JF, King GD, Candolfi M, Greeno RB, Kroeger KM, Lowenstein PR, et al. Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr Top Med Chem. 2005;5:1151–1170. doi: 10.2174/156802605774370856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King GD, Curtin JF, Candolfi M, Kroeger KM, Lowenstein PR, Castro MG. Gene therapy and targeted toxins for glioma. Curr Gene Ther. 2005;5:535–537. doi: 10.2174/156652305774964631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prados MD, McDermott M, Chang SM, Wilson CB, Fick J, Culver KW, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J Neurooncol. 2003;65:269–278. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 6.Pulkkanen KJ, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12:585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 7.Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:625–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 10.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 11.Klatzmann D, Valery CA, Bensimon G, Marro B, Boyer O, Mokhtari K, et al. A phase I/II study of herpes simplex virus type 1 thymidine kinase “suicide” gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum Gene Ther. 1998;9:2595–2604. doi: 10.1089/hum.1998.9.17-2595. [DOI] [PubMed] [Google Scholar]
- 12.Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 13.Rubinchik S, Yu H, Woraratanadharm J, Voelkel-Johnson C, Norris JS, Dong JY. Enhanced apoptosis of glioma cell lines is achieved by co-delivering FasL-GFP and TRAIL with a complex Ad5 vector. Cancer Gene Ther. 2003;10:814–822. doi: 10.1038/sj.cgt.7700651. [DOI] [PubMed] [Google Scholar]
- 14.Tsurushima H, Yuan X, Dillehay LE, Leong KW. Radioresponsive tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene therapy for malignant brain tumors. Cancer Gene Ther. 2007;14:706–716. doi: 10.1038/sj.cgt.7701065. [DOI] [PubMed] [Google Scholar]
- 15.Uzzaman M, Keller G, Germano IM. Enhanced proapoptotic effects of tumor necrosis factor-related apoptosis-inducing ligand on temozolomide-resistant glioma cells. J Neurosurg. 2007;106:646–651. doi: 10.3171/jns.2007.106.4.646. [DOI] [PubMed] [Google Scholar]
- 16.Palu G, Cavaggioni A, Calvi P, Franchin E, Pizzato M, Boschetto R, et al. Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: a pilot study in humans. Gene Ther. 1999;6:330–337. doi: 10.1038/sj.gt.3300805. [DOI] [PubMed] [Google Scholar]
- 17.Ren H, Boulikas T, Lundstrom K, Soling A, Warnke PC, Rainov NG. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene--a phase I/II clinical protocol. J Neurooncol. 2003;64:147–154. doi: 10.1007/BF02700029. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida J, Mizuno M, Nakahara N, Colosi P. Antitumor effect of an adeno-associated virus vector containing the human interferon-β gene on experimental intracranial human glioma. Jpn J Cancer Res. 2002;93:223–228. doi: 10.1111/j.1349-7006.2002.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 20.ArkTherapeutics. Cerepro™ (sitimagene ceradenovec)—treatment for brain cancer (malignant glioma) 2007 < http://www.arktherapeutics.com/main/products.php?content=products_cerepro>.
- 21.Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 22.Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King GD, Muhammad AKM, Curtin JF, Barcia C, Puntel M, Liu C, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2007 doi: 10.1215/15228517-2007-045. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;9:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- 26.Hurtado-Lorenzo A, Millan E, Gonzalez-Nicolini V, Suwelack D, Castro MG, Lowenstein PR. Differentiation and transcription factor gene therapy in experimental parkinson’s disease: sonic hedgehog and Gli-1, but not Nurr-1, protect nigrostriatal cell bodies from 6-OHDA-induced neurodegeneration. Mol Ther. 2004;10:507–524. doi: 10.1016/j.ymthe.2004.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 28.Torres EM, Dunnett SB. Amphetamine induced rotation in the assessment of lesions and grafts in the unilateral rat model of Parkinson’s disease. Eur Neuropsychopharmacol. 2007;17:206–214. doi: 10.1016/j.euroneuro.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Vercammen L, Van der Perren A, Vaudano E, Gijsbers R, Debyser Z, Van den Haute C, et al. Parkin protects against neurotoxicity in the 6-hydroxydopamine rat model for Parkinson’s disease. Mol Ther. 2006;14:716–723. doi: 10.1016/j.ymthe.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- 32.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- 33.Pashenkov M, Teleshova N, Link H. Inflammation in the central nervous system: the role for dendritic cells. Brain Pathol. 2003;13:23–33. doi: 10.1111/j.1750-3639.2003.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zermansky AJ, Bolognani F, Stone D, Cowsill CM, Morrissey G, Castro MG, et al. Towards global and long-term neurological gene therapy: unexpected transgene dependent, high-level, and widespread distribution of HSV-1 thymidine kinase throughout the CNS. Mol Ther. 2001;4:490–498. doi: 10.1006/mthe.2001.0479. [DOI] [PubMed] [Google Scholar]
- 35.Barcia C, Gerdes C, Xiong W, Thomas CE, Liu C, Kroeger KM, et al. Immunological thresholds in neurological gene therapy: highly efficient elimination of transduced cells may be related to the specific formation of immunological synapses between T cells and virus-infected brain cells. Neuron Glia Biol. 2007;2:309–322. doi: 10.1017/S1740925X07000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther. 2001;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- 37.Cotten M, Baker A, Saltik M, Wagner E, Buschle M. Lipopolysaccharide is a frequent contaminant of plasmid DNA preparations and can be toxic to primary human cells in the presence of adenovirus. Gene Ther. 1994;1:239–246. [PubMed] [Google Scholar]
- 38.Dion LD, Fang J, Garver RI., Jr Supernatant rescue assay vs. polymerase chain reaction for detection of wild type adenovirus-contaminating recombinant adenovirus stocks. J Virol Methods. 1996;56:99–107. doi: 10.1016/0166-0934(95)01973-1. [DOI] [PubMed] [Google Scholar]
- 39.Southgate T, Kingston P, Castro MG. Gene transfer into neural cells in vivo using adenoviral vectors. In: Gerfen JN, Mc Kay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. New York: Wiley; 2000. pp. 4.23.21–24.23.40. [Google Scholar]
- 40.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp Neurol. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- 41.Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J Neurosci. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas CE, Abordo-Adesida E, Maleniak TC, Stone D, Gerdes G, Lowenstein PR. Gene transfer into rat brain using adenoviral vectors. In: Gerfen JN, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. New York: Wiley; 2000. p. 4.23.1.p. 4.23.40. [DOI] [PubMed] [Google Scholar]