Abstract
The mechanism(s) of the enhancement of the immune response by addition of aluminum salt adjuvants to parenterally administered protein-based vaccines is still the subject of debate. It has been hypothesized, however, that destabilization of the antigen structure on the surface of the adjuvant may be important for eliciting immune response. Also, it has been suggested that immune response to adjuvanted vaccines is reduced if the adjuvant particles become aggregated before administration because of processing steps such as freeze-drying. In this study, we tested these hypotheses and examined the immune response in a murine model to various liquid, freeze-dried and spray freeze-dried formulations of a model vaccine, bovine intestinal alkaline phosphatase adsorbed on aluminum hydroxide. Enzymatic activity of the alkaline phosphatase was used as a sensitive indicator of intact native antigen structure. By manipulating the secondary drying temperature during lyophilization, vaccines were produced with varying levels of alkaline phosphatase enzymatic activity and varying degrees of adjuvant aggregation, as assessed by particle size distribution. Anti-alkaline phosphatase titers observed in immunized mice were independent of both the antigen’s retained enzymatic activity and the vaccine formulation’s mean particle diameter.
Keywords: alkaline phosphatase, aluminum hydroxide, adjuvant, particle size distribution, immune response, protein conformation
INTRODUCTION
Recombinant protein antigens are only weakly immunogenic and must be formulated with an adjuvant in order to be used as a vaccine capable of provoking a protective immune response 1,2. Currently, the aluminum-salt adjuvants aluminum hydroxide and aluminum phosphate are the only adjuvants used in approved vaccines marketed in the U.S. Aluminum hydroxide, chemically aluminum oxyhydroxide or boehmite (AlOOH) 3, which was used in the current study, consists of needle-like primary particles with diameters of about 2 nm 4. In solution, these primary particles form stable porous aggregates that are 1–10 μm in diameter 4,5.
Several possible modes of action have been proposed for aluminum-salt adjuvants 6–9. One hypothesis is that these particles act as a depot at the site of injection, wherein the antigen is slowly released after administration 9,10. However this mechanism has been questioned, and more recently proposed mechanisms are that the adjuvant particles aid in delivery of the antigen to antigen-presenting cells, 5,6 that they serve as immunostimulators and elicit Th2 cytokines 11,12, and that they destabilize protein antigens on their surface, making the proteins more susceptible to proteolytic degradation 13,14.
The consideration of the role of protein conformation in immunogenicity is particularly relevant if the product formulation will be lyophilized. Lyophilization can perturb protein structure at any stage of the process including during freezing, drying, and reconstitution 15–17. It is typically desirable to have a final product which has a water content less than 1 % residual moisture 15. However, there are indications that extremely low levels of water may be detrimental to retention of native protein structure in lyophilized solids 18. Also, it has been shown that lyophilization may cause aggregation of aluminum adjuvant particles, which has been proposed to reduce the immune response to protein-based vaccines 19–21. In this study, we utilized differences in lyophilization process parameters such as freezing rates and secondary drying temperatures to manipulate the amount of retained native protein structure in a lyophilized vaccine formulation that used bovine intestinal alkaline phosphatase as a model antigen. The size distributions of the adjuvant particles were altered by the same variations in processing.
Based on previous studies, we hypothesized that the structure of the bound alkaline phosphatase on the adjuvant would have an effect on the immunogenicity of the vaccine. Specifically, we hypothesized that the more unfolded the protein became on the surface of the adjuvant 13,14, the stronger would be the immune response to the vaccine. We also hypothesized that shifts in the particle size distribution (PSD) to larger sizes due to aggregation of the adjuvant during lyophilization would lead to a reduction in immunogenicity. Vaccine formulations with varying degrees of antigen structural perturbation and various adjuvant PSD’s were then tested for their ability to provoke an immune response (anti-alkaline phosphatase IgG1 or IgE) in a murine model.
MATERIALS AND METHODS
Materials
Trehalose dihydrate (high purity, low endotoxin) was obtained from Ferro Pfanstiehl (Cleveland, OH). Tris (hydroxylmethyl aminomethane), bovine alkaline phosphatase (AP), and its enzymatic activity assay components (p-nitrophenyl phosphate, diethanolamine buffer and magnesium chloride) were purchased from Sigma Chemical Company (St. Louis, MO).Alhydrogel™ 2.0% (aluminum hydroxide adjuvant, AH), made by Brenntag Biosector, was purchased through E.M. Sergeant Pulp & Chemical Co, Inc (Clifton, NJ). 3-ml and 5-ml, 13-mm glass lyophilization vials (Product Numbers 68000316 and 68000344) and stoppers (Product Number 19500360) were obtained from West Pharmaceutical Services.
Sample Preparation
All formulations were prepared with sterile water for injection. AP was reconstituted and dialyzed into 25 mM Tris, pH 7.5. An aqueous solution was prepared containing 0.081 mg/ml AP (as estimated by an activity assay), 0.2 % w/v AH, 7.5 % w/v trehalose, and 25 mM Tris, pH 7.5. With the exception of the adjuvant suspension, which was maintained sterile, all aqueous solutions were passed through a 0.2 μm filter prior to formulation.
Enzymatic Activity
The enzymatic activity of AP in solution was measured by an assay based on the conversion of p-nitrophenyl phosphate (PNPP) to p-nitrophenol and inorganic phosphate, which was measured by the rate of increase in absorbance at 405 nm. Briefly, 50 μl of sample were added to a cuvette containing 1.5 ml of the PNPP substrate (assay concentration 14 mM) in diethanolamine buffer, pH 9.8, which was equilibrated at 37 °C. The slope of a plot of the absorbance at 405 nm vs. time, corrected for the slope measured using a blank sample, was used to determine the enzymatic activity of AP in each sample. Three runs were completed for each sample. Activity units were converted to AP concentration by using a conversion of 17.0 AU/mg AP as given by specifications from the manufacturer. Our analysis confirmed this value (data not shown). Lyophilized samples were first reconstituted with water prior to analysis.
Lyophilization
A FTS Systems Lyostar® lyophilizer was used to prepare freeze-dried samples. Samples were frozen by one of two processes. In the first, traditional freeze-drying (FD) was employed. After equilibrating for one hour at 5 °C, 1-ml samples in 3-ml vials were frozen by cooling them on a lyophilizer shelf at 0.5 °C/min to −40 °C. In the second method, spray freeze-drying (SFD) was used, wherein 1 ml of the formulation first was frozen by injecting 20 μl droplets into 3-ml vials containing liquid nitrogen. Then the nitrogen was allowed to boil off and sample vials were transferred to a lyophilizer shelf held at −40 °C. Sample vials were spaced in the lyophilizer so that they were not in contact with one another and the array of approximately 200 sample vials was encircled with a row of vials containing only water.
Primary drying of the samples was achieved by setting the shelf temperature to −20 °C and applying vacuum at 60 mTorr for 20 hours. Then secondary drying was commenced by increasing the shelf temperature. The final secondary drying temperatures were varied from 10 °C to 50 °C, with shelf warming rates of 0.2 °C/min to 0 °C and 0.5 °C/min to the final temperature in a given run. Samples were held at the final temperature under vacuum for 5 hours. Also, one group of samples was removed at the end of primary drying. Samples were sealed under vacuum and reconstituted to their original 1-ml volume with sterile water for injection prior to analysis.
Particle Size Distributions (PSD’s)
PSD’s were measured using a Beckman-Coulter LS230. Three one-ml samples were required for each run, and three replicates of each run were completed per formulation. Reported PSD’s are surface area weighted and are averages of three runs.
Water Content
A Karl Fischer assay was used to measure the amount of water remaining in samples processed by each lyophilization protocol. Moisture content was measured using a Mettler DL37 coulometric moisture analyzer (Highstown, NJ) using Photovolt (Indianapolis, Indiana) reagents. A small amount of each dried sample was compressed into a modified syringe and weighed, without exposure to air, prior to being added to the instrument for measurement. The average of three separate measurements is reported.
Mouse Care
All studies with animals were approved by the University of Colorado at Boulder Institutional Animal Care and Use Committee (Protocol # 06-05-RAN-02). All procedures were done at the MCDB Transgenic Facility at the University of Colorado, Boulder. Mice were housed 4 to a cage with food and water (acidified) available ad libitum.
Mouse Immunization and Serum Collection
Six female, 5-week old BALB/c mice (Jackson Laboratories) were used to assess the immunogenicity of each of the liquid control, FD and SFD samples. On the first day of the study, liquid formulations were prepared and lyophilized preparations were reconstituted with water for injection. Injections of 100 μl of the formulation containing 8.1 μg of AP and 0.2% AH were administered subcutaneously along the back of each mouse. Mice for controls received either a liquid vaccine containing 8.1 μg of AP and 0.2% AH (positive control), or an injection containing only buffer and 0.2% AH (negative control). For each sample type and control, a booster immunization was administered on day 14 in the same manner. Blood was collected via retro-orbital bleeding under anesthesia prior to each injection, 14 days following the initial injection and 14 days following the booster injection. Serum was separated by centrifugation at 15,000 rpm for 5 minutes, transferred to a clean centrifuge tube, and frozen at −20 ºC until analysis.
Enzyme-Linked Immunosorbent Assay (ELISA)
The antibody response to each vaccine was determined by ELISA. Each well of a 96-well plate (Nunc, Rochester, NY) was coated with 0.25 μg of AP in phosphate buffered saline (PBS), pH 7.4, overnight at 4 ºC. Plates were washed with PBS. Nonspecific sites were blocked using 1% bovine serum albumin (BSA, Fisher) in PBS. Plates were allowed to dry and were stored at 4 ºC until use. Serum samples were thawed at room temperature and serially diluted in 1% BSA in PBS from 1:20 to 1:1838, and 50 μl of each sample were added to the wells of the 96-well plate. Samples were incubated overnight at 4 ºC. After washing with PBS, sample wells were incubated with 50 μl horseradish peroxidase-conjugated goat-anti-mouse antibodies for IgG1 and IgE (Immunology Consultants Lab, Inc, Newberg, OR) at 1:10,000 dilution. Incubation was for 2 hours at room temperature with rotation (400 rpm). Plates were then washed and incubated with 100 μl of Ultra-TMB (Pierce, Rockfort, IL). After 15 minutes of development, the reaction was stopped with 100 μl of 1 N HCl. Absorbance of each well at 450 nm was determined using a FLUOstar OPTIMA microplate reader (BMG Labtech). Anti-AP titers were calculated as the dilution factor that gave an absorbance reading equal to two standard deviations greater than the average of the negative controls and were determined using a four-parameter fit analyzed using Softmax Pro software.
RESULTS
AP Activity in Liquid Solutions
To determine whether AP is bound to adjuvant and retains enzymatic activity when bound, AP was formulated with AH in 7.5 w/v% trehalose, 25 mM Tris buffer, pH 7.5 containing 0.2 % AH. The target AP concentration of 81.0 μg/ml has enzymatic activity of 1.38 AU/ml (with a conversion of 17.0 AU/mg AP). After centrifugation to pellet the AH and any associated, adsorbed AP, the supernatant was tested for AP enzymatic activity. No activity could be detected, suggesting that adsorption to AH was essentially complete. In contrast, when the pelleted AH was resuspended and tested for enzymatic activity, it exhibited 79 ± 4% of the enzymatic activity prior to adsorption. This minor loss of enzymatic activity due to binding to adjuvant (which likely reflects, at least in part, steric hindrances at the solid interface) suggests that the bound enzyme retains most of its native structure in the adsorbed state.
Water Content of Dried Solids
Figure 1 shows the water content of the dried powders after processing by FD or SFD. As the final temperature of the secondary drying step is increased, more water is desorbed from the dried sample. The FD samples retained more water than the SFD samples, with the exception of samples taken to a final secondary drying temperature of 50 °C. At this temperature samples processed by both methods retained the same water content.
Figure 1.
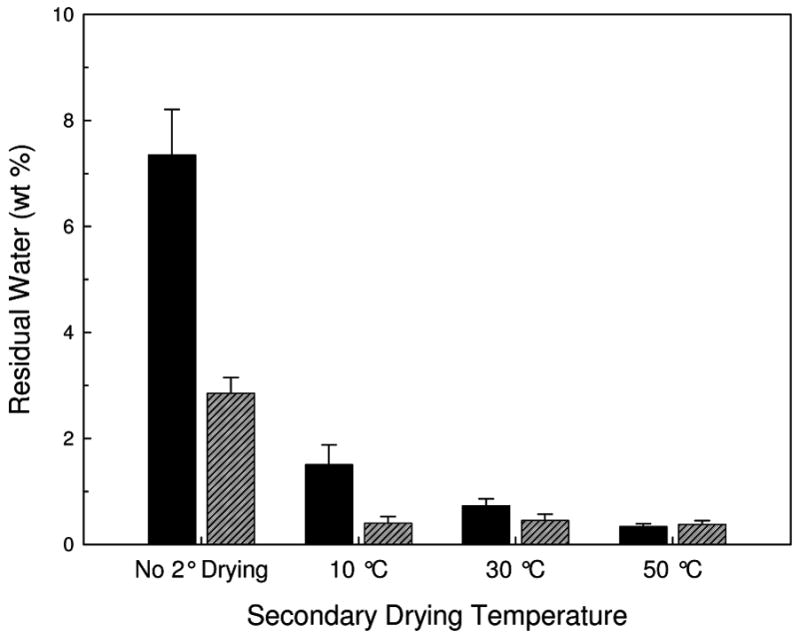
Residual water in FD (solid black) and SFD (diagonal-lined grey) vaccines as measured with Karl Fisher titration as a function of the secondary drying endpoint temperature. Error bars are the standard deviation of three samples.
Activity of AP in Reconstituted Solids
The fraction of AP activity retained after drying and reconstitution, compared to that in an identically-formulated liquid control, is presented in Figure 2. Samples that did not undergo secondary drying (and thus those with the greatest amount of residual water) retain almost 100% activity when compared to the liquid vaccine. In contrast, as the samples are dried further (higher secondary drying temperature), there is a drop in the protein activity for both the FD and SFD vaccines; however, the largest drop in activity occurs when the samples dry below 1 wt% water. The relationship between retained activity and residual water in the dried solid is shown in Figure 3.
Figure 2.
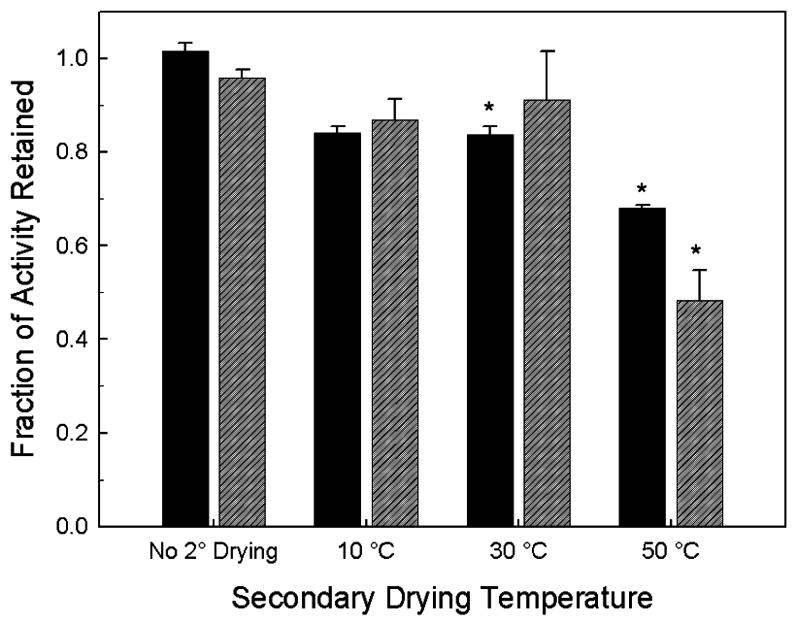
Retained enzymatic activity of AP in FD (solid black) and SFD (diagonal-lined grey) vaccines as a function of the secondary drying endpoint temperature as compared to the AP activity of liquid vaccine. Stars indicate statistical difference from liquid vaccine at a 95% confidence interval.
Figure 3.
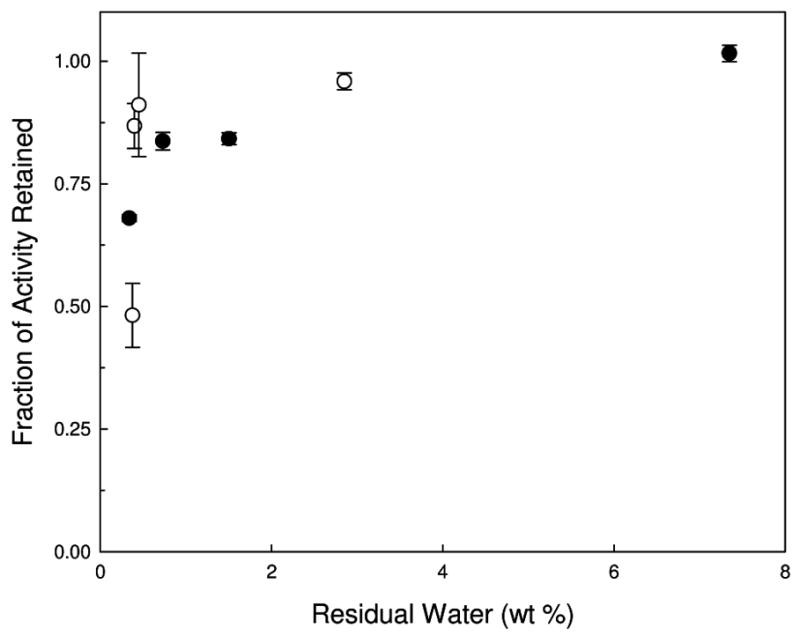
Fraction of AP enzymatic activity retained compared to AP activity in liquid vaccine as a function of residual water following lyophilization. Closed circles are FD samples while open circles are SFD samples.
PSD of AP Vaccines
The PSD and mean AH particle diameter measured following reconstitution of FD and SFD samples are shown in Figures 4 and 5. The mean particle diameter for the FD samples is much larger than that of the SFD samples, similar to the results of previous studies 20,22. The mean particle diameter for the FD samples does not depend on secondary drying temperature. However, for the SFD samples the particle diameter increases slightly with increasing secondary drying temperature (and decreasing water content).
Figure 4.
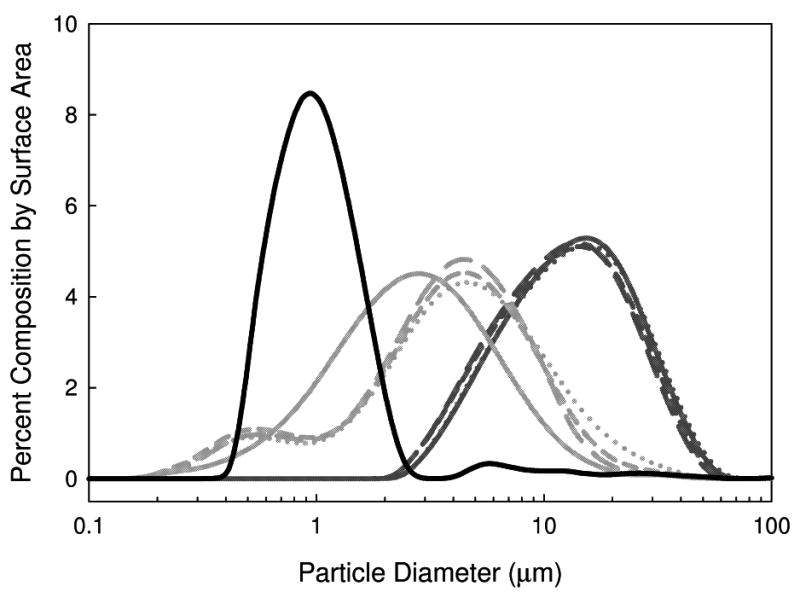
PSD of AH in vaccines formulated with AP in 7.5 w/v% trehalose. Lines are as follows: liquid vaccine: black solid line, SFD vaccines: light gray; FD vaccines: dark gray. Line types are indicative of samples processed with different secondary drying temperatures: long dash: 10 °C, short dash: 30 °C, and dotted: 50 °C. The solid light and dark gray lines are for the samples processed with no secondary drying.
Figure 5.
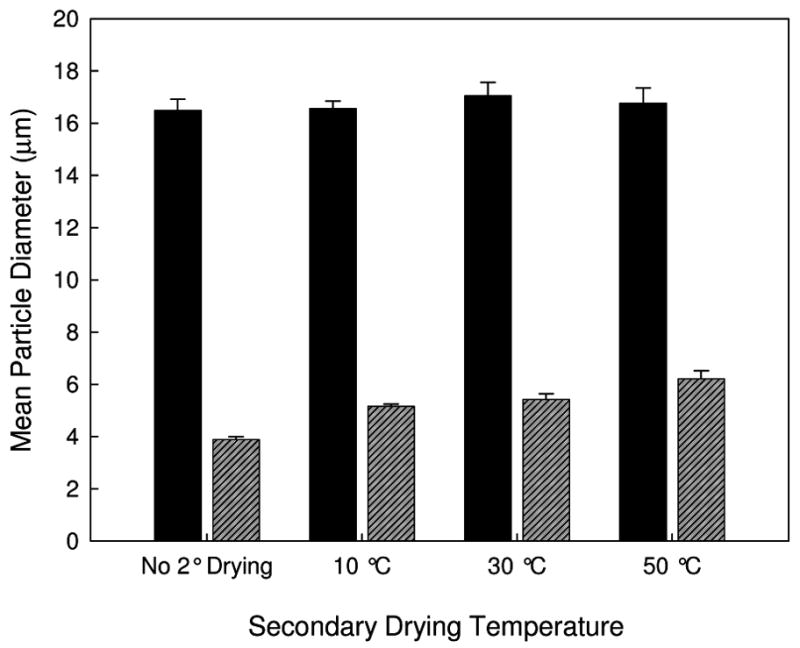
Mean AH particle diameter following FD and reconstitution (solid black) and SFD and reconstitution (diagonal-lined grey) for lyophilized vaccines containing AP.
Immunogenicity of AP Vaccines
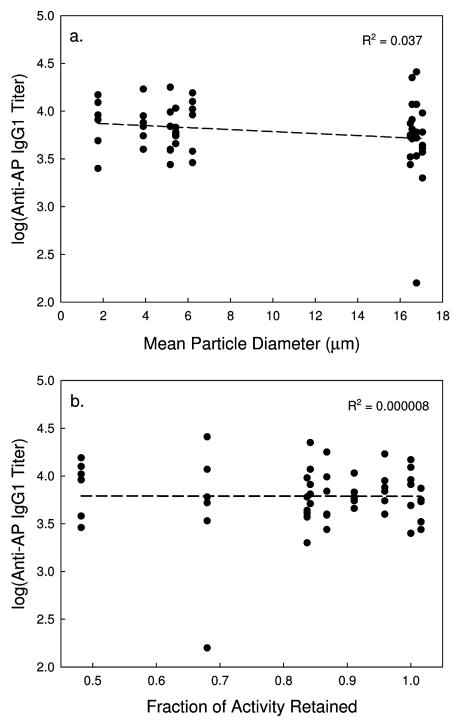
IgG1 titers following the first injection (primary response) and the booster injection (secondary response) are shown in Figure 6. As expected, IgG1 titers following the second injections are about two orders of magnitude greater than those observed following the first injection. More importantly, there is no difference in IgG1 titer between any of the different samples examined, after either the first or second injections. Even though there is almost an order of magnitude difference in the mean particle diameter between the different vaccine formulations, there is no correlation between IgG1 titers and the PSD of the adjuvant. Also, there is no correlation between the IgG1 response and the retained activity following lyophilization and reconstitution (Figure 7), even though the activity drops by about 50% in the driest samples. Presumably perturbation of protein structure accounts for the reduction in AP activity. No anti-AP IgE antibodies were observed in the samples tested.
Figure 6.
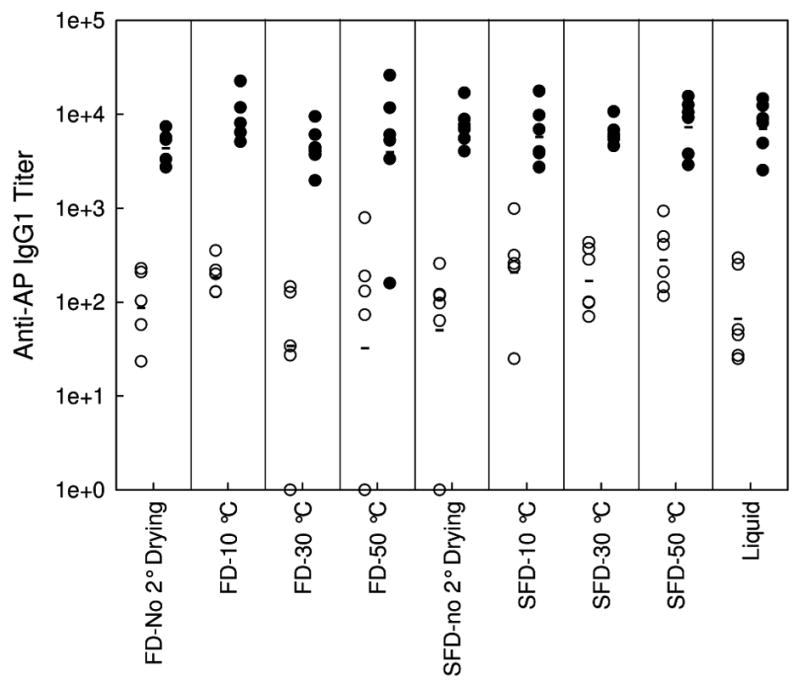
Anti-AP IgG1 titers of serum from Balb/c mice injected with 100 μl of AP vaccine following the first injection (open circles) and the booster injection (closed circles) as a function of processing and secondary drying endpoint temperatures. Mean titers of each vaccine is marked with a horizontal bar.
Figure 7.
Log(Anti-AP IgG1 titers) of serum from Balb/c mice injected with 100 μl of AP vaccine following after and two injections as a function of mean particle diameter (a.) and fraction of retained AP activity (b.) in the reconstituted vaccines. Dashed line is a linear regression of the data, with the R2 value shown.
DISCUSSION
AP vaccines were produced by varying the processing (FD and SFD) and secondary drying endpoint temperatures, giving rise to vaccines with varying water content, PSD’s, and AP enzymatic activity. As expected, samples processed at higher secondary drying temperatures had lower residual moisture content. At all secondary drying temperatures, with the exception of samples dried at 50 °C, SFD samples had lower residual water than corresponding FD samples, presumably as a result of better mass transfer of water vapor throughout the sample 17. Even though the same volume of both sample types were processed, the FD samples were formed a monolithic cake, while the SFD procedure produced small, ~20 μl spheres, shortening the diffusion distance required for water to escape from the sample. Typically, samples at the end of primary drying have been observed to have water contents around 20 % 23. However, in the current study, FD and SFD samples that were removed prior to the initiation of secondary drying had water contents of 7.3 and 2.9 wt%, respectively, indicating that some secondary drying occurred even at a shelf temperature of −20 ºC 24. Most importantly, by varying the processing method and the final temperature of secondary drying, ranges of PSD and AP catalytic activity were obtained, reflecting varying degrees of adjuvant aggregation and a range of perturbation of native AP conformation, respectively. Thus, we were able to test the effects of PSD and protein conformation on immunogenicity of the AP vaccine.
The first parameter of interest is the particle size for the adjuvant particles. Loss in immunogenicity following lyophilization of vaccines is often attributed to the aggregation of the aluminum salt adjuvants included in the formulation 20. It has been hypothesized that immunogenicity is a function of not only adjuvant surface area 20,21, but also adjuvant surface charge and morphology 25. In this study, the processing (liquid, FD, and SFD) and the secondary drying temperature were varied, resulting in vaccine samples with mean particle diameters between 1 and 17 μm. However, there was no correlation between mean particle diameter and the anti-AP IgG1 titer. This observation indicates that the particle diameter of the adjuvant may not be as important as previously hypothesized. This is the same result that we have recently reached for another vaccine formulation that used lysozyme as a model antigen. 26 There may be an upper limit to the particle size that would be effective at eliciting an immune response, but that upper particle size appears to be larger than the particle diameters obtained in the current with traditional lyophilization. Also, this particle size dependency may be more important for the elicitation of other antibody species, as Nygaard et. al. noticed a particle size dependency for the immune response to ovalbumin adsorbed on polystyrene particles. In that study, which monitored IgG2 and IgE antibodies, particle diameters >1 μm were ineffective at eliciting an immune response 21. We did not observe an IgE response to the AP vaccine.
The different processing and secondary drying temperatures also gave rise to a range of retained AP enzymatic activities. We assume that the activity of AP bound to adjuvant is a sensitive indication of the structure of the protein, with loss in activity indicating that there is a perturbation of the protein’s native conformation. Compared to control samples of AP adsorbed to AH in liquid formulations, almost 100% of initial AP activity is retained for samples that were lyophilized and removed prior to secondary drying or dried at the lower secondary drying temperatures, but there was a significant drop in enzymatic activity once the water content fell below 1 wt%. This loss of activity could be an effect of stresses caused by further dehydration of the formulation and/or stresses occurring during rehydration of the dried solids 17. The retained activity of the dried and reconstituted AP vaccines ranged from 50% to 100% of the enzymatic activity of the liquid control AP sample. However, no correlation was observed between this parameter and the IgG1 immune response. Contrary to previous studies that suggested that destabilization and conformational perturbation of the antigen protein on the surface of aluminum salt adjuvant is important for immunogenicity 13, we found significant IgG responses even in the absence of perturbation of AP conformation,, showing this property may be important for only certain antigens. We note that there is a possibility that the loss of AP activity (and the implied conformational perturbations associated with this loss) could be reversible upon injection into a physiological milieu. If this occurred, however, it would only strengthen the conclusion that, at least for AP, perturbation of native antigen structure is not needed to elicit an IgG response.
Acknowledgments
This work is supported by the NIH through Dynport Vaccine Co., LLC (A CSC Company) and HTD Biosystems under Grant No. NIAID 1 U 01 AI056514-01. ALC was supported through grants from the NIH Leadership Training in Biotechnology and the Graduate Assistantship in Areas of National Need Fellowship.
References
- 1.Singh M, O'Hagan D. Advances in vaccine adjuvants. Nat Biotechnol. 1999;17(11):1075–1081. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 2.O'Hagan DT, MacKichan ML, Singh M. Recent developments in adjuvants for vaccines against infectious diseases. Biomol Eng. 2001;18(3):69–85. doi: 10.1016/s1389-0344(01)00101-0. [DOI] [PubMed] [Google Scholar]
- 3.Shirodkar S, Hutchinson RL, Perry DL, White JL, Hem SL. Aluminum compounds used as adjuvants in vaccines. Pharm Res. 1990;7(12):1282–1288. doi: 10.1023/a:1015994006859. [DOI] [PubMed] [Google Scholar]
- 4.Johnston CT, Wang SL, Hem SL. Measuring the surface area of aluminum hydroxide adjuvant. J Pharm Sci. 2002;91(7):1702–1706. doi: 10.1002/jps.10166. [DOI] [PubMed] [Google Scholar]
- 5.Romero Mendez IZ, Shi Y, Hogenesch H, Hem SL. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine. 2006 doi: 10.1016/j.vaccine.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RK, Siber GR. Adjuvants for Human Vaccines - Current Status, Problems and Future-Prospects. Vaccine. 1995;13(14):1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RK, Rost BE. Aluminum Compounds as Vaccine Adjuvants. In: O'Hagan D, editor. Vaccine Adjuvants: Preparation Methods and Research Protocols. Totowa, NJ: Humana Press Inc; 2000. pp. 65–89. [Google Scholar]
- 9.Cox JC, Coulter AR. Adjuvants--a classification and review of their modes of action. Vaccine. 1997;15(3):248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 10.Callahan PM, Shorter AL, Hem SL. The importance of surface charge in the optimization of antigen-adjuvant interactions. Pharm Res. 1991;8(7):851–858. doi: 10.1023/a:1015843210358. [DOI] [PubMed] [Google Scholar]
- 11.Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121(1):134–145. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 12.HogenEsch H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine. 2002;20(Suppl 3):S34–39. doi: 10.1016/s0264-410x(02)00169-x. [DOI] [PubMed] [Google Scholar]
- 13.Jones LS, Peek LJ, Power J, Markham A, Yazzie B, Middaugh CR. Effects of adsorption to aluminum salt adjuvants on the structure and stability of model protein antigens. J Biol Chem. 2005;280(14):13406–13414. doi: 10.1074/jbc.M500687200. [DOI] [PubMed] [Google Scholar]
- 14.Thai R, Moine G, Desmadril M, Servent D, Tarride JL, Menez A, Leonetti M. Antigen stability controls antigen presentation. J Biol Chem. 2004;279(48):50257–50266. doi: 10.1074/jbc.M405738200. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997;14(8):969–975. doi: 10.1023/a:1012180707283. [DOI] [PubMed] [Google Scholar]
- 16.Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 2001;46(1–3):307–326. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 17.Webb SD, Golledge SL, Cleland JL, Carpenter JF, Randolph TW. Surface adsorption of recombinant human interferon-gamma in lyophilized and spray-lyophilized formulations. J Pharm Sci. 2002;91(6):1474–1487. doi: 10.1002/jps.10135. [DOI] [PubMed] [Google Scholar]
- 18.Breen ED, Curley JG, Overcashier DE, Hsu CC, Shire SJ. Effect of moisture on the stability of a lyophilized humanized monoclonal antibody formulation. Pharm Res. 2001;18(9):1345–1353. doi: 10.1023/a:1013054431517. [DOI] [PubMed] [Google Scholar]
- 19.Diminsky D, Moav N, Gorecki M, Barenholz Y. Physical, chemical and immunological stability of CHO-derived hepatitis B surface antigen (HBsAg) particles. Vaccine. 1999;18(1–2):3–17. doi: 10.1016/s0264-410x(99)00149-8. [DOI] [PubMed] [Google Scholar]
- 20.Maa YF, Zhao L, Payne LG, Chen D. Stabilization of alum-adjuvanted vaccine dry powder formulations: mechanism and application. J Pharm Sci. 2003;92(2):319–332. doi: 10.1002/jps.10294. [DOI] [PubMed] [Google Scholar]
- 21.Nygaard UC, Samuelsen M, Aase A, Lovik M. The capacity of particles to increase allergic sensitization is predicted by particle number and surface area, not by particle mass. Toxicol Sci. 2004;82(2):515–524. doi: 10.1093/toxsci/kfh287. [DOI] [PubMed] [Google Scholar]
- 22.Clausi AL, Merkley SA, Carpenter JF, Randolph TW. Accepted for Publication. Inhibition of Aggregation of Aluminum Hydroxide Adjuvant During Freezing and Drying. Journal of Pharmaceutical Sciences. doi: 10.1002/jps.21143. [DOI] [PubMed] [Google Scholar]
- 23.Miller DP, de Pablo JJ, Corti H. Thermophysical properties of trehalose and its concentrated aqueous solutions. Pharm Res. 1997;14(5):578–590. doi: 10.1023/a:1012192725996. [DOI] [PubMed] [Google Scholar]
- 24.Luthra S, Obert JP, Kalonia DS, Pikal MJ. Investigation of drying stresses on proteins during lyophilization: differentiation between primary and secondary-drying stresses on lactate dehydrogenase using a humidity controlled mini freeze-dryer. J Pharm Sci. 2007;96(1):61–70. doi: 10.1002/jps.20758. [DOI] [PubMed] [Google Scholar]
- 25.Hem SL, White JL. Characterization of aluminum hydroxide for use as an adjuvant in parenteral vaccines. J Parenter Sci Technol. 1984;38(1):2–10. [PubMed] [Google Scholar]
- 26.Clausi A, Cummiskey J, Merkley S, Carpenter J, Braun L, Randolph T. Influence of Particle Size and Antigen Binding on Effectiveness of Aluminum Salt Adjuvants in a Model Lysozyme Vaccine. J Pharm Sci. 2008 doi: 10.1002/jps.21390. in press. [DOI] [PubMed] [Google Scholar]