Abstract
Pulmonary hypertension is an uncommon complication of sarcoidosis, but in severe pulmonary disease it occurs frequently. It is an important cause of cryptogenic dyspnea in sarcoidosis patients, and can occur despite the absence of pulmonary fibrosis. The true prevalence is unknown. With the advent of specific therapies for pulmonary hypertension, there has been a resurgence of interest in the pathophysiology, diagnosis, and treatment of sarcoidosis-associated pulmonary hypertension. This article reviews the status of the current epidemiologic, pathophysiologic and therapeutic knowledge regarding this entity.
Keywords: sarcoidosis, pulmonary hypertension, epoprostenol, endothelin
“.....the lungs, heart, and circulation should be thought of as a single apparatus for the transfer of oxygen and carbon dioxide between the atmosphere and the working tissues.” [1]
Pulmonary hypertension (PH) is defined as a mean pulmonary artery pressure (mPAP) higher than 25 mm Hg at rest or 30 mm Hg with exercise [2,3] . The disease can be idiopathic (familial or sporadic) or associated with several disorders that ultimately induce remodeling of the pulmonary circulation and result in a permanent elevation of the pulmonary vascular resistance. Idiopathic pulmonary arterial hypertension is a rare disease affecting 6–15 individuals per million [4,5]; non-idiopathic forms are considered to be much more common, particularly in patients with chronic respiratory disorders.
Sarcoidosis is a multisystem granulomatous disorder that affects the lungs in 90–95% of patients [6]. The disease has an estimated incidence of 10–35 cases per 100,000 people in the United States, with an overall mortality of 1–5% [7,8]. Initially considered a rare manifestation of long-standing sarcoidosis, sarcoidosis-associated pulmonary hypertension (SAPH) is now being recognized as an important complication that can result in significant morbidity and mortality. Although SAPH occurs more frequently in patients with evidence of advanced parenchymal fibrosis, it can also be present despite no apparently significant interstitial lung disease. Severe untreated pulmonary arterial hypertension carries an extremely poor prognosis and is associated with higher mortality in patients with interstitial lung diseases and sarcoidosis [9,10]. Early diagnosis and consideration of treatment options may be keys to improve patient outcomes.
History
The modern history of sarcoidosis probably began in 1898, when Jonathan Hutchinson, a London dermatologist, described a case of a patient with “Mortimer’s malady”, allegedly a patient with evidence of cutaneous disease [11]. In the initial description, the disease was thought to be a form of cutaneous lupus. Sarcoidosis was recognized as a distinct pathological entity a year later, when Cesar Boeck described the histology of the “sarkoid” granulomas [12]. By 1929, Mitchell Bernstein had described cardiopulmonary system involvement by sarcoidosis [13], the same year that Werner Forssmman revolutionized the study of cardiac and pulmonary hemodynamics by demonstrating that cardiac catheterization was possible in humans [14].
Between 1930 and 1950, several clinical, radiological and pathological studies established that the involvement of the lung was common in patients with sarcoidosis [15–17]. Furthermore, right ventricular failure was recognized as an explanation for dyspnea, cyanosis and death in patients with advanced lung disease. In 1948, for example, Mallory described six patients with granulomatous pneumonitis consistent with sarcoidosis, and parenchymal fibrosis [18]. In this report, the author presented evidence of invasion and thrombosis of the pulmonary vasculature. Two years later, the clinical relevance of these findings was suggested by Austrian, who noted elevated pulmonary artery pressures in two patients with sarcoidosis [19]. In the following decades, several small cohorts of patients with SAPH began to elucidate the frequency, pathophysiology and prognosis of pulmonary hypertension in sarcoidosis patients. In the past decade, with the advent of specific medications for treatment of PAH, interest in SAPH has burgeoned dramatically.
Epidemiology
The actual prevalence of SAPH has not been established. In patients with significant parenchymal lung disease, the disease was historically thought to be rare, with involvement of the pulmonary vasculature in <5% of the cases. Unfortunately most of the early cohorts were identified retrospectively based on autopsies or case series of patients with clinically-diagnosed right heart failure. For example, Mayock et al performed a review of 145 patients with sarcoidosis from nine published series, and found only six (4.1%) patients with evidence of pulmonary hypertension and cor pulmonale [20]. Similarly, a series of 320 autopsies in Japanese patients with sarcoidosis revealed 18 cases of cor pulmonale (5.6%) [21]. No hemodynamic measurements were made in any of these reports.
It is clear that the measurement technique and the entry criteria for performing screening influence the ascertained prevalence of SAPH. It is also probable that ethnicity affects development of the disease. Thus, estimates of prevalence have been widely variable (Table 1). Separate reviews of right heart catheterization (RHC) results among 104 subjects in Italy and Poland suggested that the prevalence of resting PH is 6–23% [22–24]. Higher chest radiograph (CXR) stage was associated with increased risk in all three series, with approximately one-half of those with Stage III CXR demonstrating at least mild elevations of mean PA pressure. When the screening is limited to patients with more advanced parenchymal disease, the prevalence is substantially higher. Emirgil et al reported 15 patients with sarcoid-related pulmonary fibrosis who had RHC, of whom 10 (67%) had evidence of PH at rest [25]. In a retrospective evaluation of 106 patients with sarcoidosis, Sulica et al. found that 51% of the patients with a pre-existing clinical indication for performing echocardiography had evidence of PH by transthoracic echocardiography (TTE) [26]. In this report, the majority of patients also had radiographic evidence of advanced disease. Finally, in a cohort of 363 sarcoidosis patients listed for lung transplantation between 1995 and 2002, 74% of the group who had RHC had some form of PH; approximately one third of the patients had severe PH (mPAP ≥ 40 mmHg) [27].
Table 1.
Ascertained prevalence of sarcoidosis-associated pulmonary hypertension in several populations
| Author/year | Location | Population | Number | Method | Prevalence (%) |
|---|---|---|---|---|---|
| Handa [28] 2006 | Single center, Japan | Prospective study of unselected clinic patients | 212† | TTE | 5.7 |
| Rizzato [22] 1983 | Single center, Italy | Unspecified patients who had RHC | 50 | RHC | 6* |
| Gluskowski [24] 1990 | Single center, Poland | Prospective study of CXR Stage II and III | 24 | RHC | 12.5* |
| Gluskowski [23] 1984 | Single center, Poland | Unspecified patients who had RHC | 30 | RHC | 23 |
| Baughman [29] 2006 | Single center, U.S. | Subjects with dyspnea disproportionate to PFTs | 53 | RHC | 47 |
| Sulica [26] 2005 | Single center, U.S. | Retrospective review of patients who had TTE for clinical indications | 106 | TTE | 51 |
| Emergil [25] 1971 | Single center, U.S. | Series of severe sarcoidosis patients | 15 | RHC | 67 |
| Shorr [27] 2005 | UNOS database, U.S. | All lung transplant candidates who also had RHC | 363 | RHC | 74** |
| Milman [30] 2008 | Single center, Denmark | All lung transplant candidates who also had RHC | 24 | RHC | 79 |
RHC: right heart catheterization; TTE: transthoracic echocardiography; UNOS: United Network for Organ Sharing; PFT: pulmonary function test
34/246 subjects had no evaluable TR jet
Additional 20–33% of subjects had exercise-induced PH (mean PAP ≥ 30 mmHg)
36% had severe PH (mPAP > 40 mm Hg)
In a prospective observational study using TTE, 246 Japanese patients with predominantly chronic sarcoidosis but good overall lung function were evaluated at a referral center [28]. Of the 212 patients with adequate echocardiograms, only 12 (5.7%) had PH (defined as systolic PAP ≥ 40 mmHg). Restrictive pulmonary physiology was the only independent risk factor for SAPH. In contrast, when only patients with unexplained dyspnea are studied, the prevalence appears to be higher. For example a recent single-center retrospective review of 53 patients with sarcoidosis found that 25/53 (47%) of the patients with persistent dyspnea had a mean PAP ≥ 25 mmHg [29]. Importantly, only 69% of the patients in this report had evidence of CXR Stage 3–4 sarcoidosis. A recent review of Danish patients prospectively characterized with right heart catheterization at the time of referral for lung transplantation reported that 79% (19/24 subjects) had mPAP >25 mm Hg [30]. These subjects had severe pulmonary sarcoidosis, as evidenced by chroncity (median duration 11 years), CXR stage (75% with Stage IV) and FVC (median 41% predicted).
A subgroup of patients with sarcoidosis appears to have excessive elevations of pulmonary arterial pressures during exercise. In an Italian cohort of 62 sarcoidosis patients published more than two decades ago, ten patients with normal resting hemodynamics exhibited exercise-induced PH [22]. Two subsequent reports from the same group in Poland demonstrated similar findings. In the first report, 3 of 10 patients with stage II CXR developed PA pressure elevation of 20 mmHg above baseline and all 10 patients with stage III CXR had increases > 10 mmHg [23]. Some of the patients demonstrated very dramatic (50–100 mm Hg) elevations of mean PAP with exercise, despite normal resting PA pressures. A prospective follow-up study of unselected patients with Stage 2 or 3 CXR, persistence of disease for at least 6 months and mild average restriction (mean FVC 82 ± 17% predicted) revealed an even higher prevalence [24]. Based on a threshold of 10 mmHg increase in mean PAP with exercise, 18 of 24 patients demonstrated exercise-induced SAPH. Finally, using multiple uptake gated acquisition (MUGA) scans in subjects with unremarkable echocardiograms, Baughman et al. noted 12 of 14 had a significant drop in the right ventricular ejection fraction associated with exercise and attributed to the development of PH [31].
Most of the available studies have shown an average age at the time of diagnosis of 40–60 years. In the U.S., SAPH appears to be more common in females and African-Americans, but this finding reflects only the overall demographic trends for sarcoidosis, and neither gender nor race have been independently associated with the disease . In the Japanese experience, male gender was associated with SAPH in univariate analysis, although the total number was small [28]. Finally, although some reports have suggested that the incidence of SAPH may be even higher when including hemodynamic evaluations with exercise, the prevalence of exercise-induced SAPH in larger cohorts of unselected sarcoidosis patients is currently unknown.
In conclusion, currently, ascertainment bias has confounded efforts to accurately assess the true prevalence of PH in patients with sarcoidosis. Almost all the published data are derived from referral centers, and most of the data are from selected patient populations within these institutions. Translating the findings in the Japanese and European centers to the U.S. experience is also problematic, since ethnicity substantially influences sarcoidosis phenotype. For example, in Japan, cardiac involvement is extremely common and could contribute to SAPH. In general, the lack of prospective surveys, reliance on echocardiography, and selection bias have led to poorly generalizable estimation of the overall prevalence.
Pathophysiology
The World Health Organization (WHO) currently classifies pulmonary hypertension into five groups, with sarcoidosis included as Class V (miscellaneous) [32]. Compared with idiopathic pulmonary fibrosis, where pulmonary hypertension is probably caused by fibrotic destruction of the distal capillary bed, several mechanisms may be relevant in SAPH. Thus, it is not incorrect to state that the mechanisms for SAPH could be grouped into all five WHO categories [33].
The granulomatous inflammation in sarcoidosis involves the lung in a lymphatic distribution. This distribution places the granulomas adjacent to the pulmonary artery in the bronchovascular area and to the veins that run within the interlobular septae (Figure 1). Because of this proximity, the pulmonary arteries are commonly involved by granulomas (Figure 2a) or giant cells (Figure 2b) in pulmonary sarcoidosis. This vascular involvement may involve the adventitia, media or intima of the pulmonary arteries. Pathological studies have shown that 69–100% of subjects have invasion of the vessel walls by granulomas that result in occlusive changes, perivascular fibrosis, or granulomatous pulmonary angiitis [34,35]. Similarly, scarring and occlusion by granulomas and giant cells may occur in the pulmonary veins (Figure 3), mimicking pulmonary veno-occlusive disease from a hemodynamic standpoint [34–37]. Obliteration of these vessels by either active granulomatous inflammation and by fibrosis affords one mechanism for development of pulmonary hypertension in patients with advanced radiographic stages. Furthermore, this preferential location of the granulomas adjacent to these vessels may explain the present of SAPH even in patients with little apparent radiologic involvement of the lung parenchyma.
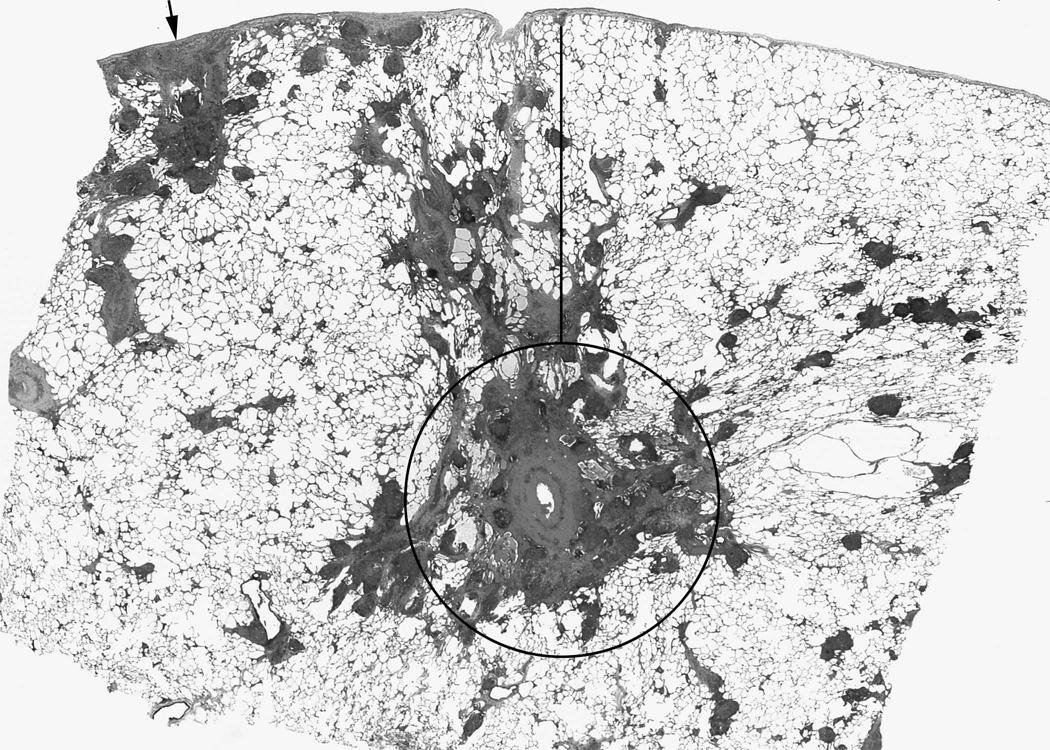
Figure 1. Distribution of granulomas in pulmonary sarcoidosis.
Sarcoidosis granulomas are found in a lymphatic distribution, including surrounding the bronchovascular bundle (circled area); the interlobular septae which also contain the pulmonary veins (adjacent to the line); and the subpleural area (arrow). Because of this anatomic orientation, granulomatous inflammation frequently encroaches on the vascular structures, even in the absence of overt fibrosis or vascular wall inflammation (Hematoxylin and eosin).
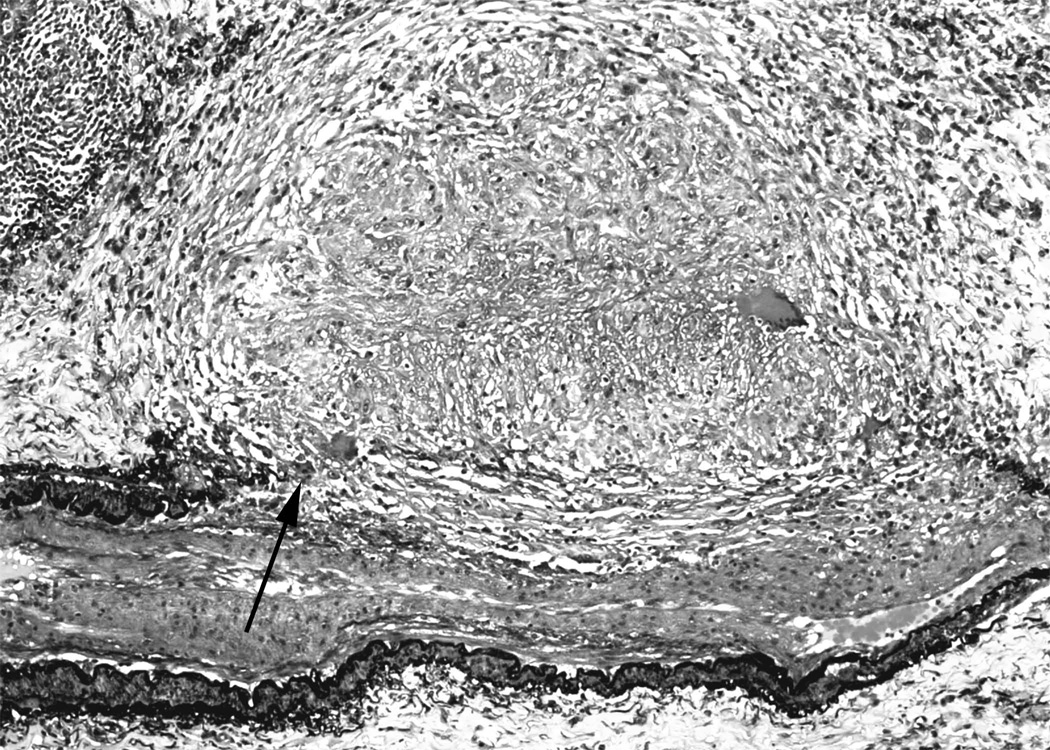
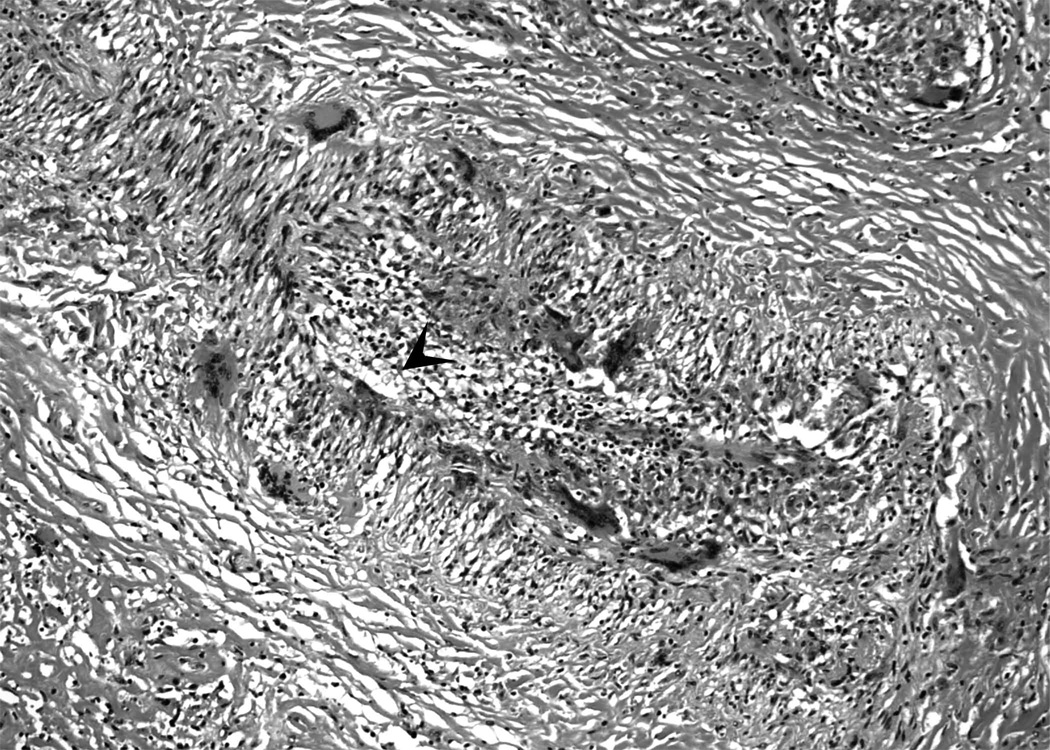
Figure 2. Granulomatous arteritis in sarcoidosis.
2a. Movat pentachrome stain showing granulomas obliterating the internal and external elastic laminae and smooth muscle (arrow) of medium size artery. There is secondary intimal fibroplasia seen in the center of the artery. (Movat pentachrome).
2b. In some cases, only giant cells and mononuclear cell infiltrates may be seen in sarcoidosis-associated arteritis. Arrowhead indicates the vascular lumen. Numerous giant cells may be seen in the vessel wall (Hematoxylin and eosin).
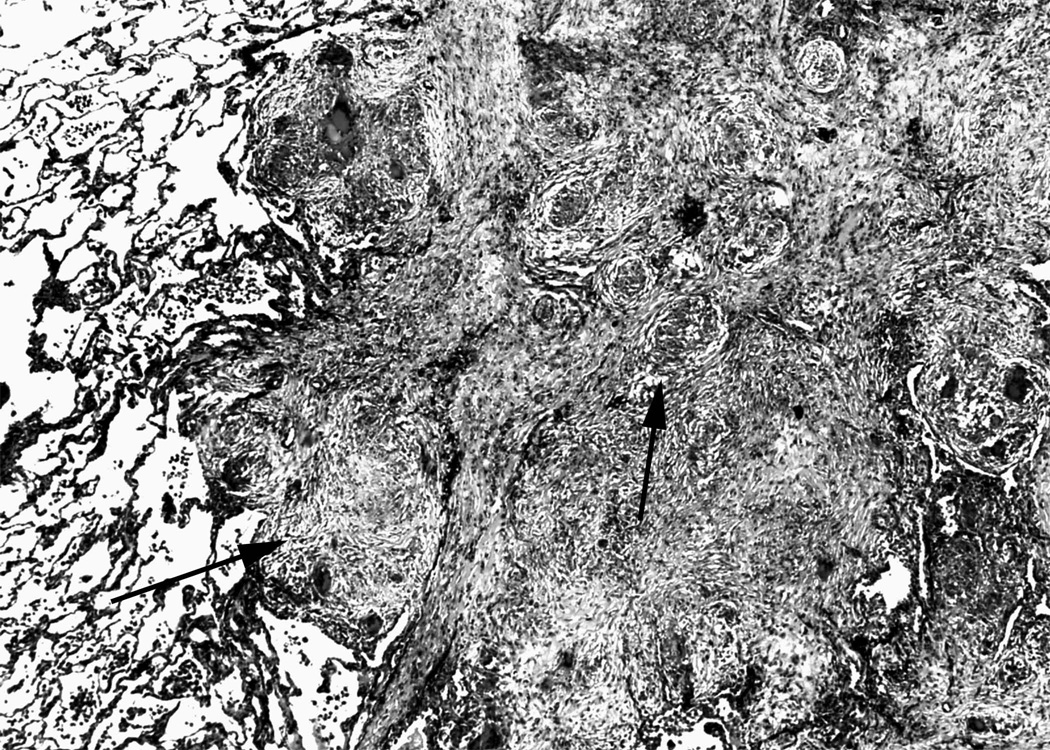
Figure 3. Obliteration of pulmonary venous drainage by exuberant granuloma formation.
Movat pentachrome stain of an interlobular septum showing extensive fibrosis and obliteration of the venous structures by granulomas. Perivenular distribution of granulomas is typical in sarcoidosis. Arrows indicate compressive granulomas. (Movat pentachrome).
Although the hypothesis that SAPH results from fibrotic or granulomatous obliteration of the pulmonary microvasculature in advanced disease fits most patients, this may not fully explain why some individuals with no radiographic evidence of interstitial lung disease develop PH [38,39]. In a recent retrospective review of twenty-two patients with SAPH, 32% of the patients developed pulmonary hypertension in the absence of overt parenchymal fibrosis [40]. Fifty percent of the patients had an elevation in the mPAP that was considered out of proportion to the changes in lung mechanics [40]. An isolated case study described the presence of plexiform lesions in a patients with SAPH, but the significance of this observation is unclear, since these lesions have not been observed in larger series of SAPH [40,41]. Several reports suggest other possible mechanisms, including extrinsic compression of pulmonary arteries by enlarged hilar lymph nodes (Figure 4) [40,42,43], diastolic or systolic dysfunction from myocardial sarcoidosis [44,45] and hypoxic vasoconstriction. Obstructive sleep apnea, which is common in sarcoidosis patients [46], may also contribute to the development of elevated pulmonary resistance. Finally, although the possibility of isolated vasoreactivity has been deemed to be a sufficient explanation for some cases of SAPH [47], this concept continues to be mainly hypothetical in the absence of corresponding pathological data demonstrating that there is not granulomatous inflammation in these subjects.
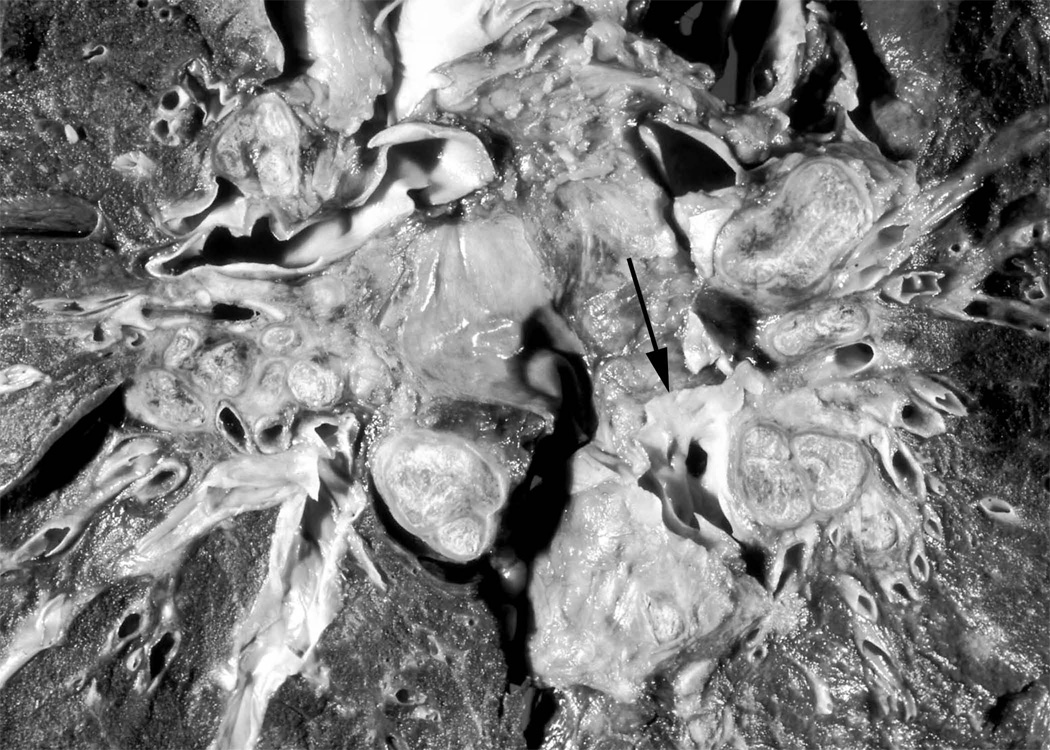
Figure 4. Compression of central pulmonary vascular structures by bulky lymph nodes.
Coronal section of thorax of post-mortem specimen from a patient who died from end-stage SAPH. There is extensive hilar compression of the main pulmonary arteries. Arrow denotes the left lower lobe pulmonary artery, which is subtotally occluded by extrinsic compression of calcified lymph nodes that are present throughout the mediastinum. Image reproduced from Farver CF. Chapter 31: Sarcoidosis. In Dail and Hammar’s Pulmonary Pathology, 3rd edition. Editors, Tomashefski, Cagle, Farver, Fraire. Figure 18B. With kind permission of Springer Science and Business Media.
Endothelin has been implicated in the development of idiopathic pulmonary hypertension. Endothelin-1 (ET-1) is found in abundance in the lung, where it is produced by smooth muscle, endothelial and airway epithelial cells [48]. Binding of ET-1 with its receptor, ETA, induces significant pulmonary vasoconstriction; in addition, ET-1 promotes proliferation of smooth muscle cells and fibroblasts, and in animal models has been linked to development of pulmonary fibrosis [49,50]. Urinary and plasma levels of ET-1 are elevated in patients with sarcoidosis, and in some instances, treatment with corticosteroids is associated with a normalization of the plasma ET-1 levels [51]. Similarly, ET-1 levels appear to be elevated in bronchoalveolar lavage (BAL) fluid from patients with sarcoidosis, systemic sclerosis and idiopathic pulmonary fibrosis [52]. A study that limited BAL analysis to 22 patients with sarcoidosis found approximately 25% had elevated levels of ET-1 [53]. Immunostaining in these patients suggested that alveolar macrophages may be an important source for ET-1. However, it is currently unknown whether ET-1 is only a biomarker for the severity of inflammation or an important mediator of vascular remodeling in sarcoidosis.
Clinical presentation
Pulmonary hypertension should be suspected in any sarcoidosis patient with dyspnea, hypoxemia, or clinical evidence suggesting right heart failure, particularly if symptoms appear to be out of proportion to the degree of parenchymal lung disease. Unfortunately these symptoms are often present in patients without PH. Other considerations when patients present with unexplained dyspnea or exercise limitation include myopathy (skeletal or respiratory muscle) large airways obstruction, occult cardiac disease, depression, and anemia [54–56].
The most common symptom in patients with SAPH is progressive dyspnea on exertion. Other common complaints include cough, chest pain, palpitations and symptoms suggesting right heart failure such as lower extremity edema and syncope. Only the signs of right heart failure are independent predictors of elevated right-sided pressures, but their sensitivity is low. In a retrospective analysis of 106 patients who had echocardiography for clinical reasons, only 21% of the patients with SAPH had pre-test evidence of right heart failure (elevated jugular venous pressure, lower extremity edema and/or right ventricular heave) compared to none of the patients without pulmonary hypertension [26].
Other rare reported presentations of SAPH include sudden death due to compression of large pulmonary arteries [57], main pulmonary vein occlusion caused by intravascular sarcoidosis [58] and combined SAPH and portal hypertension [59]. Although there are case reports of SAPH simulating pulmonary veno-occlusive disease (PVOD) [36,37,40,60], we believe that this simply represents the usual distribution of granulomatous inflammation, since the pulmonary venous drainage is closely approximated with the lymphatics. The histopathology of the reported sarcoid-induced PVOD more closely resembles burned-out sarcoidosis than actual PVOD (Figure 3). The term PVOD associated with sarcoidosis therefore represents a misnomer and should be avoided.
Imaging Studies
Pulmonary hypertension may be present despite the absence of overt fibrotic changes on chest imaging, but the great majority of patients with SAPH appear to have Stage 3 or 4 chest radiographs. In an initial report of patients with chronic cor pulmonale and sarcoidosis, 30% were found to have only perihilar infiltrates [61]. Subsequent studies have confirmed that fibrosis is present in approximately 2/3 of subjects, and the CXR may be normal in 9–10% [26,40]. High resolution computed tomography (HRCT) of the chest may suggest additional mechanisms. For example, 3 of 14 (21%) of a series of SAPH patients with pulmonary fibrosis also had extrinsic compression of large pulmonary arteries [40]. Overall, however, specific high resolution chest CT findings are not generally helpful for predicting the presence versus absence of SAPH [28].
A common clinical strategy is to measure the main pulmonary artery diameter (MPAD), the ratio of the pulmonary artery (PA) to ascending aorta (AA) or the ratio of segmental artery to bronchus to detect the presence of PH in patients with parenchymal lung disease. Unfortunately, this strategy appears to be unreliable in pulmonary fibrosis [62] and sarcoidosis [29].
Pulmonary function tests and oxygenation
Depending on the degree of parenchymal involvement, patients with SAPH typically exhibit hypoxemia, decreased carbon monoxide diffusing capacity (DLCO) and restrictive physiology. Most patients with SAPH require at least some supplemental oxygen; among lung transplant candidates, a requirement for 3 liters or more of supplemental O2 more than doubled the risk of PH [27]. In the same cohort, 93.4% of the subjects with mean PAP >40 mmHg required at least some supplemental oxygen. In the experience at Mt. Sinai hospital in New York, all patients identified with SAPH by TTE exhibited exercise-induced desaturation [26]. Based on these reports, it can be concluded that the absence of desaturation below 90% with exercise suggests a low likelihood of significant SAPH.
In radiographic stages 0–3, SAPH patients classically exhibit a mild restrictive defect with a disproportionate decrease in DLCO. In the Mt. Sinai series, of the 46 patients without pulmonary fibrosis the ratio of percent predicted FVC to percent predicted DLCO was 1.9 ± 1.1 in SAPH versus 1.3 ± 0.6 in subjects without PH [26]. The scleroderma experience suggests that serial decline in DLCO despite FVC >70% predicted portends the development of pulmonary hypertension [63]; the utility of serial PFT data has not been reported in SAPH. In patients with pulmonary fibrosis, there is characteristically a marked reduction in FVC, FEV1, DLCO and PaO2. However, these abnormalities may be more pronounced in SAPH compared to patients without pulmonary hypertension [26,40]. It is important to recognize that pulmonary function tests, while more severely impaired in patients with SAPH [28], may not correlate well with measured pulmonary artery pressures [29,40].
Finally, the six minute walk test is useful only when it is normal, since there are multiple reasons for abnormal tests in sarcoidosis patients. It is probably most helpful for serial measurements of cardiopulmonary function. A prospective study of 142 consecutive patients seen a tertiary center evaluated the utility of the 6 minute walk [64]. The fourteen patients with proven SAPH had a shorter six-minute walk distance (median, 280 meters; range, 61 to 404) than those without proven SAPH (median, 411 m; range, 46 to 747; p < 0.0001). Although the lowest oxygen saturation was correlated with six-minute walk distance, the presence of PH was not an independent risk factor in this series.
Hemodynamics
The gold standard for the diagnosis of SAPH is direct measurement of the pulmonary artery pressures with RHC. Pulmonary hypertension is present when the mPAP exceeds 25 mmHg at rest or 30 mmHg with exercise. Measurement of the transpulmonary gradient [the difference between the mPAP and the pulmonary capillary wedge pressure (PCWP)] is useful to exclude left ventricular disease associated with cardiac sarcoidosis or other causes. However, since LV end-diastolic volume may be impaired by septal flattening in severe pulmonary hypertension it is mandatory to correlate PCWP measurements with echocardiographic assessment of interventricular dependence.
The importance of RHC was emphasized by a recent series of 53 patients who had both echocardiography and RHC [29]. In these patients, TTE was unable to establish the diagnosis of elevated RVSP in 16/53 (30%) of the patients with increased PA pressures. In addition, 24% of the patients with elevated pulmonary pressures had a PCWP > 20 mmHg. These findings suggest that intrinsic cardiac disease (e.g. diastolic left ventricular dysfunction) should be excluded prior to diagnosing SAPH. Despite the presence of increased PAP with exercise in a subset of sarcoidosis patients, the role of routine exercise testing during RHC is unknown.
Echocardiography
Doppler echocardiography is frequently used to evaluate patients with pulmonary hypertension. Although studies have reported that Doppler analysis of the tricuspid regurgitant velocity by echocardiography may be used to estimate pulmonary pressures [65], many patients with PH have no evidence of tricuspid regurgitation. When useful data are obtained, the sensitivity and specificity of TTE for diagnosing any degree of pulmonary hypertension has varied from 0.69–1.0 and 0.68–0.98 respectively in idiopathic PH; correlation coefficients between echocardiography and RHC are generally robust for PAP between 50 and 100 mmHg [66–69]. These data have generated enthusiasm among clinicians for use of TTE as a screening tool when PH is suspected.
However, patients with parenchymal lung disease pose a challenge since the presence of lung disease may obscure echocardiographic windows and render accurate estimation of PAP difficult [70]. In these patients, the correlation between RVSP and PAP is highly variable and TTE may underestimate peak right ventricular pressures, particularly in patients with severe PH [71,72]. In a large study that included 374 transplant candidates, estimation of the RVSP was possible in only 44% [73]. Moreover, although the overall correlation between echocardiogram-estimated systolic PAP and RHC was moderately good (r=0.69), approximately half of the pressure estimations were inaccurate by more than 10 mm Hg. Echocardiographic estimation of PAP thus appears to have inadequate positive and negative predictive value in patients with advanced lung disease. A more recent single-center review of patients with sarcoidosis who had RHC when persistent dyspnea was deemed disproportionate to PFT abnormalities showed a similar correlation between TTE and RHC (r=0.79) [29]. Nevertheless, the authors reported that 7/9 patients for whom RVSP could not be assessed by TTE had SAPH confirmed by pulmonary artery catheterization.
In patients with SAPH, echocardiography can establish the presence of other cardiac causes of dyspnea such as left ventricular systolic or diastolic dysfunction, valvular abnormalities, pericardial effusion or the presence of right to left shunts. In the absence of tricuspid regurgitation, the diagnosis of SAPH should be suspected when there are echocardiographic findings of right ventricular pressure overload, including right ventricular hypertrophy, systolic dysfunction, flattening of the interventricular septum or an abnormal ratio of the interventricular septum to posterior left ventricular wall thickness [74]. The absence of these findings, nevertheless, does not exclude the possibility of SAPH [22] and therefore RHC is currently still considered necessary for definitive diagnosis.
Management
The optimal management strategy for pulmonary hypertension associated with sarcoidosis is unknown. Treatment has typically involved use of systemic anti-inflammatory medications, anticoagulants, pulmonary vasodilators, endothelin receptor antagonists and supplemental oxygen. The available literature regarding these therapeutic strategies is limited to small cohort studies, retrospective analyses and case reports.
Immunomodulating medications
Theoretically, anti-inflammatory medications could have a major impact on some patients with SAPH [75,76]. The results of the available trials, however, have not demonstrated consistent benefits from use of immunosuppressants in SAPH. In a small study of 24 patients, the effects of corticosteroid treatment on pulmonary hemodynamics were evaluated by RHC [24]. The patients were treated with an initial dose of 60 mg/d of prednisolone tapered to 25 mg at six months and continued for one year. Only two of four subjects with PH at rest had significant improvement of pulmonary pressures after one year of therapy. Interestingly, although almost all patients had improvement in pulmonary function, elevated exercise-induced pulmonary pressures decreased in only half of the group. Other investigators have noted similar results, with hemodynamic improvements noted in 0–30% after corticosteroid treatment [29,40].
Although these results suggest that corticosteroids may be helpful in a subset of patients with SAPH, it appears that the benefits are difficult to predict. Based on the available data, corticosteroids or other immunomodulators may be most useful for patients with evidence of active inflammation or compression of central vascular structures by bulky lymph nodes. The role of steroids in patients with established parenchymal fibrosis is less clear. To our knowledge, there are no available studies that have systematically addressed the use of other immune modulating agents in SAPH.
Pulmonary vasodilators
Currently available vasodilators include prostacyclin analogues, calcium channel blockers and phosphodiesterase inhibitors. Use of these agents in patients with pulmonary fibrosis has been controversial given concerns for severe hypoxemia or pulmonary edema caused by intrapulmonary shunting [77–79]. Since SAPH is often caused by fibrosis with end-stage pulmonary microcirculatory “fixed” abnormalities, it has also been unclear whether these agents have any beneficial impact on the pulmonary vascular resistance.
To address this issue, Preston et al. treated eight patients with SAPH using intravenous epoprostenol (EPO), inhaled nitric oxide (iNO) or calcium channel blockers [44] (Table 2). All patients had advanced sarcoidosis (CXR stages 3–4) and severe pulmonary hypertension (average mPAP 55 mmHg). The acute response in mPAP was greater for those receiving iNO (decrease of 18±4%) compared to patients that received EPO at doses of 2–8 ng/kg/min (6±2% decrease). No patients had an acute response with nifedipine. Five patients continued treatment with iNO at 100–200 ppm and one received iNO plus EPO; follow-up showed that patients were able to maintain stable functional class, although both mPAP and PVR tended to increase. Other reports that included patients with sarcoidosis and parenchymal fibrosis who received low dose epoprostenol therapy reported favorable hemodynamic responses without worsening hypoxemia [80]. A more recent study that included two patients with severe SAPH reported an excellent initial hemodynamic response (40% decrease in mPAP) in one patient [29].
Table 2.
Reports of vasodilator therapy in sarcoidosis
| No. treated | FVC%* | mPAP * (mmHg) | Treatment (number) | Follow up (mo) | Outcome | Observations | |
|---|---|---|---|---|---|---|---|
| Preston, 2001 [44] | 8 | 48 | 55 | iNO (5) CCB (2) |
6–24 | 5/8 subjects died in 0–18 months | Acute vasodilator response in 7/8 subjects |
| Culver, 2005 [84] | 11 | 57 | 76† | EPO (4) EPO + Bos (4) Bos (3) |
2–30 | 8/11 subjects died or required lung transplant Short-term hemodynamic or functional benefit observed in all subjects | Hemodynamic response most evident in patients with FVC ≥70% |
| Foley, 2005 [85] | 1 | 64 | 55 | Bosentan | 24 | mPAP decreased to 23 mmHg at six months | Improved functional class (NYHA IV to II) |
| Sharma, 2005 [86] | 1 | 40 | 78+ | Bosentan | 12 | Improved six minute walk distance | Improved functional class (NYHA IV to II) |
| Nunes, 2006 [40] | 1 | N/A | N/A | Iloprost (1) | 4 | Died awaiting lung transplant | |
| Baughman, 2006 [29] | 7 | 64 | 83 | EPO (1) EPO + Bos (1) Bos (4) CCB (1) |
6 | Significant decrease in mPAP after 6 months in 5/7 patients | Immunosupressive therapy was increased |
| Fisher, 2006 [83] | 7 | 59 | 57 | EPO (7) | 0–49 | The majority of patients responded to EPO therapy; 4/7 patients alive & without transplant | One episode of pulmonary edema; One sudden death 4 hours after EPO initiation |
| Milman, 2008 [30] | 12 | 41 | 36 | Sildenafil | 1–12 | mPAP decreased >20% in 50% of subjects; cardiac output improved in 86% | No benefit on 6 minute walk test |
CXR= chest x-ray; FVC=mean forced vital capacity; mPAP= average mean pulmonary artery pressure; iNO=inhaled nitric oxide; CCB=calcium channel blocker; EPO= epoprostenol; Bos=bosentan; NYHA=New York Heart Association
Mean or median value for the entire reported population, including those treated with vasodilators
Right ventricular systolic pressure estimate by echocardiogram
Systolic pulmonary artery pressure
Fatal pulmonary edema has been reported after initiation of intravenous prostacyclin agents in patients with pulmonary hypertension associated with PVOD or other fibrotic lung diseases such as scleroderma [81,82]. Fisher et al. reported one patient out of seven who developed non-cardiogenic pulmonary edema after treatment with epoprostenol [83]. Given that patients with SAPH may have significant impairment of the pulmonary venous system, therapy with pulmonary vasodilators should be started with caution. However, the available literature does not indicate that patients with SAPH have an excessively elevated risk of developing this complication.
Little is known about the use of other inhaled agents in SAPH. In the report by Nunes et al, one patient with SAPH and pulmonary fibrosis was treated with inhaled iloprost without any clinical benefit [40].
In our center, eight patients received treatment with prostanoid agents alone or in combination with endothelin antagonists, in addition to standard therapy with immunosuppressants [84]. Despite a high proportion of patients with evidence of severe parenchymal scarring (63%), none experienced episodes of vasodilator-induced shunting. Significant short-term benefits of vasoactive therapy were observed among most of the patients, especially those with preserved vital capacity (FVC ≥ 70% of predicted). However, progression to lung transplant or death over 12–18 months was extremely common.
The largest published long-term experience with prostanoids, however, suggests that circumspection is warranted prior to use of these agents [83]. In this report, which included mainly patients with severe restrictive physiology, 6 of 7 subjects demonstrated an acute vasodilator response to low doses of EPO (75% had ≥25% drop in PVR). Six patients were subsequently treated with EPO and one patient received treatment with subcutaneous treprostinil. However, one patient developed pulmonary edema and another patient died hours after administration of EPO. After a mean follow-up of 29 months, only four patients enjoyed transplant-free survival, with continued EPO dose titration (mean dose 55 ng/kg/min).
In summary, most of the available literature regarding the use of pulmonary vasodilators includes patients with advanced stage sarcoidosis. The experience is greatest with the use of epoprostenol. In many cases, it has been used as a palliative measure or a “bridge” to transplantation. The limited evidence suggests that some patients with SAPH may respond well to prostanoid therapy, but that a few individuals do develop pulmonary edema. For the remainder, the therapy appears to be well-tolerated despite use of high doses, and long term outcomes may be improved. It is still unknown if the potential benefits from prostanoids differ between patients with or without advanced parenchymal disease.
Endothelin antagonists
As described earlier, there is some evidence that endothelin may play a role in the pathogenesis of SAPH. Bosentan, a specific endothelin-1 antagonist, has been approved by the FDA for the treatment idiopathic PAH and scleroderma-related PAH. The experience with bosentan in SAPH is limited to scattered case reports, often in combination with other agents [29,85,86].
At our institution, we identified seven patients with SAPH who were treated with bosentan [84]. Three patients received bosentan and four received bosentan in addition to prostanoid therapy. Four of the subjects demonstrated objective improvement after short-term (6–18 months) follow-up, but the other three died from their disease. A similar report that included 4 patients treated with bosentan alone showed a significant drop in the pulmonary pressures after six months follow-up [29]. These reports are too few to provide guidance for clinicians, but they generally demonstrate the possibility of efficacy for some SAPH subjects. The role of endothelin antagonists in combination with other pulmonary vasodilators such as epoprostenol or sildenafil remains unclear. As for other causes of PH, it seems reasonable to limit the first-line use of endothelin antagonists to subjects with mild to moderate (New York Heart Association functional class 2–3) dyspnea.
Phosphodiesterase inhibitors
Sildenafil, a potent phosphodiesterase type 5 (PDE-5) inhibitor, has theoretical benefits in pulmonary hypertension due to parenchymal lung disease, since it may preserve ventilation-perfusion matching better than prostanoids [79]. Small case series have suggested benefits in IPF [79], scleroderma [87], and cystic fibrosis [88]. Moreover, in patients with end-stage COPD and IPF-associated pulmonary hypertension, the beneficial hemodynamic effects of sildenafil may also impact exercise tolerance and 6 minute walk distance [89,90]. A recent series described the use of sildenafil in Danish patients listed for lung transplant due to end-stage sarcoidosis [30]. The subjects had severe restrictive lung disease (mean FVC 41% predicted). Twelve patients with mPAP >25 mm Hg were treated with oral sildenafil (median dose 150 mg daily) for 1–12 months. Although the 6 MWT did not improve, there were substantial reductions in mPAP (48 ± 15 vs 39 ± 13 mm Hg), PVR (10.7 ± 4.8 vs 5.6 ± 4.0 Wood units) and improved cardiac index (2.3 ± 0.5 vs 2.9 ± 1.0 L/min/m2). Although these findings are encouraging, the role of sildenafil compared with prostanoids or endothelin antagonists for SAPH is currently unknown.
Prognosis
Sarcoidosis is generally considered a disease with a favorable long term prognosis. Although the disease remits spontaneously in nearly two-thirds of patients, about 1–5% patients die from to progressive respiratory failure, central nervous system disease or myocardial involvement [8,91,92]. Right ventricular failure has been described in up to 30% of sarcoidosis-related deaths [20,93]. Patients with substantial pulmonary fibrosis have a particularly elevated risk. A single-center retrospective cohort study of 41 patients with stage III and IV disease listed for OLT suggested that right atrial pressure > 15 mm Hg was the strongest independent predictor of mortality in this population [94]. Kaplan-Meier analysis estimated survival of 51% at one year and 25% at two years when mPAP ≥ 35 mmHg. RAP ≥ 15 mmHg increased the risk of death by 5.2 fold. A follow-up analysis of the United Network Organ System (UNOS) database confirmed that higher pulmonary artery pressures portend lower survival among patients awaiting lung transplant [10,95]. These reports have led the International Society for Heart and Lung Transplantation to recommend early assessment for lung transplantation in patients with SAPH [96]. However, these recommendations are limited by the ascertainment bias of the reports from which they are derived.
Compared to patients with radiographic evidence of advanced stage, little is known about the natural history and prognosis of SAPH in the absence of significant pulmonary fibrosis. A recent report proposed that the subset of patients with SAPH and no evidence of pulmonary fibrosis may have a shorter time between diagnosis of sarcoidosis and diagnosis of PH, as well as higher pulmonary vascular resistance compared to patients with parenchymal fibrosis [40]. However, ascertainment bias again is likely influencing this observation. It is currently not known if these patients represent a subgroup in early stages of the disease or if they have a different natural history compared to patients with severe interstitial lung disease. The profound heterogeneity in this group of patients suggests that clinical caution and close follow-up for evidence of progression are warranted. Whether patients with SAPH without pulmonary fibrosis who are treated with pulmonary vasodilators or endothelin antagonists have better outcomes compared to patients with parenchymal fibrosis is also currently not known.
In conclusion, the presence of pulmonary hypertension appears to confer poor prognosis in patients with sarcoidosis. Particularly at risk are patients with evidence of severe parenchymal disease. Clinicians must be vigilant for the development of SAPH since this complication appears to be associated with worse outcomes. Prompt recognition and referral to an experienced center for consideration of initiation of specific therapy and/or transplant evaluation are important considerations.
Conclusions
We conclude that SAPH may be present irrespective of the degree of parenchymal involvement. It may account for a substantial proportion of dyspnea among all sarcoidosis patients, and appears to be common in those with severe parenchymal disease. A major gap in current clinical practice is identification of a cost-effective, reliable, non-invasive method to screen for the presence of SAPH. Further studies are needed to further characterize the natural history more closely, to establish whether there is a role for routine trials of augmented immunosuppression, and to define the effects of the therapeutic options. Early identification of patients at risk for developing this complication might facilitate preventive efforts.
Variable response rates to different agents or combination of drugs reflect the fact the fact that this particular complication of sarcoidosis may be the consequence of quite complex underlying mechanisms including granulomatous fibrosis, sarcoid-induced occlusive venopathy and granulomatous inflammation of pulmonary arteries. Although the presence of pulmonary fibrosis may indicate the possibility of irreversible derangement and portend a worse outcome, its presence should not preclude a cautious therapeutic trial. If the patient is a candidate, concomitant evaluation for lung transplantation should be considered as well.
Acknowledgments
This work was supported by grant HL081538 from the NHLBI (to DAC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: DAC and JP are site investigators on an investigator-sponsored trial (Actelion)
Bibliography
- 1.Henderson LJ. Blood: a study in general physiology. London: Oxford University Press; 1928. [Google Scholar]
- 2.Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Rubin LJ. Diagnosis and management of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:7S. doi: 10.1378/chest.126.1_suppl.7S. [DOI] [PubMed] [Google Scholar]
- 4.Jing ZC, Xu XQ, Han ZY, et al. Registry and survival study in chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest. 2007;132:373. doi: 10.1378/chest.06-2913. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 6.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 7.Rybicki BA, Major M, Popovich J, Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 8.Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149. [PubMed] [Google Scholar]
- 9.Ryu JH, Krowka MJ, Pellikka PA, et al. Pulmonary hypertension in patients with interstitial lung diseases. Mayo Clin Proc. 2007;82:342. doi: 10.4065/82.3.342. [DOI] [PubMed] [Google Scholar]
- 10.Shorr AF, Davies DB, Nathan SD. Outcomes for patients with sarcoidosis awaiting lung transplantation. Chest. 2002;122:233. doi: 10.1378/chest.122.1.233. [DOI] [PubMed] [Google Scholar]
- 11.Hutchison J. Cases of Mortimer's malady (lupus vulgaris multiplex non ulcerans et non serpiginosis) Arch Surg. 1898;9:307. [Google Scholar]
- 12.Boeck C. Multiple benign sarkoid of the skin. J Cutan Genitourin Dis. 1899;17:543. [Google Scholar]
- 13.Bernstein M, Konzlemann FW, Sidlick DM. Boeck's sarcoid: report of a case with visceral involvement. Arch Intern Med. 1929;44:721. [Google Scholar]
- 14.Forssmann W. Die soniderung des rechten herzens. Klin Wschr. 1929;8:2085. [Google Scholar]
- 15.Ricker W, Clark M. Sarcoidosis: a clinicopathologic review of three hundered cases, including twenty-two autopsies. Am J Clin Path. 1949;19:725. doi: 10.1093/ajcp/19.8.725. [DOI] [PubMed] [Google Scholar]
- 16.Reisner D. Boeck's sarcoid and systemic sarcoidosis: a study of thirty-five cases. Am Rev Tuberc. 1944;49:289. [Google Scholar]
- 17.Freiman DG. Medical progress: sarcoidosis. N Engl J Med. 1948;239:664. doi: 10.1056/NEJM194810282391804. [DOI] [PubMed] [Google Scholar]
- 18.Mallory TB. Pathology of pulmonary fibrosis, including chronic pulmonary sarcoidosis. Radiology. 1948;51:468. doi: 10.1148/51.4.468. [DOI] [PubMed] [Google Scholar]
- 19.Austrian R, McClement JH, Renzetti AD, Jr, et al. Clinical and physiologic features of some types of pulmonary diseases with impariment of alveolar-capillary diffusion: the syndrome of "alveolar-capillary block". Am J Med. 1951;11:667. doi: 10.1016/0002-9343(51)90019-8. [DOI] [PubMed] [Google Scholar]
- 20.Mayock RL, Bertrand P, Morrison CE, et al. Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med. 1963;35:67. doi: 10.1016/0002-9343(63)90165-7. [DOI] [PubMed] [Google Scholar]
- 21.Iwai K, Tachibana T, Takemura T, et al. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol Jpn. 1993;43:372. doi: 10.1111/j.1440-1827.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 22.Rizzato G, Pezzano A, Sala G, et al. Right heart impairment in sarcoidosis: haemodynamic and echocardiographic study. Eur J Respir Dis. 1983;64:121. [PubMed] [Google Scholar]
- 23.Gluskowski J, Hawrylkiewicz I, Zych D, et al. Pulmonary haemodynamics at rest and during exercise in patients with sarcoidosis. Respiration. 1984;46:26. doi: 10.1159/000194667. [DOI] [PubMed] [Google Scholar]
- 24.Gluskowski J, Hawrylkiewicz I, Zych D, et al. Effects of corticosteroid treatment on pulmonary haemodynamics in patients with sarcoidosis. Eur Respir J. 1990;3:403. [PubMed] [Google Scholar]
- 25.Emirgil C, Sobol BJ, Herbert WH, et al. The lesser circulation in pulmonary fibrosis secondary to sarcoidosis and its relationship to respiratory function. Chest. 1971;60:371. doi: 10.1378/chest.60.4.371. [DOI] [PubMed] [Google Scholar]
- 26.Sulica R, Teirstein AS, Kakarla S, et al. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest. 2005;128:1483. doi: 10.1378/chest.128.3.1483. [DOI] [PubMed] [Google Scholar]
- 27.Shorr AF, Helman DL, Davies DB, et al. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur Respir J. 2005;25:783. doi: 10.1183/09031936.05.00083404. [DOI] [PubMed] [Google Scholar]
- 28.Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129:1246. doi: 10.1378/chest.129.5.1246. [DOI] [PubMed] [Google Scholar]
- 29.Baughman RP, Engel PJ, Meyer CA, et al. Pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:108. [PubMed] [Google Scholar]
- 30.Milman N, Burton CM, Iversen M, et al. Pulmonary hypertension in end-stage pulmonary sarcoidosis: therapeutic effect of sildenafil? J Heart Lung Transplant. 2008;27:329. doi: 10.1016/j.healun.2007.11.576. [DOI] [PubMed] [Google Scholar]
- 31.Baughman RP, Gerson M, Bosken CH. Right and left ventricular function at rest and with exercise in patients with sarcoidosis. Chest. 1984;85:301. doi: 10.1378/chest.85.3.301. [DOI] [PubMed] [Google Scholar]
- 32.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 33.Baughman RP. Pulmonary hypertension associated with sarcoidosis. Arthritis Res Ther. 2007;9 Suppl 2:S8. doi: 10.1186/ar2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen Y, Moon S, Huang CT, et al. Granulomatous pulmonary angiitis in sarcoidosis. Arch Pathol Lab Med. 1977;101:170. [PubMed] [Google Scholar]
- 35.Takemura T, Matsui Y, Oritsu M, et al. Pulmonary vascular involvement in sarcoidosis: granulomatous angiitis and microangiopathy in transbronchial lung biopsies. Virchows Arch A Pathol Anat Histopathol. 1991;418:361. doi: 10.1007/BF01600167. [DOI] [PubMed] [Google Scholar]
- 36.Hoffstein V, Ranganathan N, Mullen JB. Sarcoidosis simulating pulmonary veno-occlusive disease. Am Rev Respir Dis. 1986;134:809. doi: 10.1164/arrd.1986.134.4.809. [DOI] [PubMed] [Google Scholar]
- 37.Schachter EN, Smith GJ, Cohen GS, et al. Pulmonary granulomas in a patient with pulmonary veno-occlusive disease. Chest. 1975;67:487. [PubMed] [Google Scholar]
- 38.Levine BW, Saldana M, Hutter AM. Pulmonary hypertension in sarcoidosis. A case report of a rare but potentially treatable cause. Am Rev Respir Dis. 1971;103:413. doi: 10.1164/arrd.1971.103.3.413. [DOI] [PubMed] [Google Scholar]
- 39.Smith LJ, Lawrence JB, Katzenstein AA. Vascular sarcoidosis: a rare cause of pulmonary hypertension. Am J Med Sci. 1983;285:38. doi: 10.1097/00000441-198301000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61:68. doi: 10.1136/thx.2005.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tayal S, Voelkel NF, Rai PR, et al. Sarcoidois and pulmonary hypertension--a case report. Eur J Med Res. 2006;11:194. [PubMed] [Google Scholar]
- 42.Westcott JL, DeGraff AC., Jr Sarcoidosis, hilar adenopathy, and pulmonary artery narrowing. Radiology. 1973;108:585. doi: 10.1148/108.3.585. [DOI] [PubMed] [Google Scholar]
- 43.Damuth TE, Bower JS, Cho K, et al. Major pulmonary artery stenosis causing pulmonary hypertension in sarcoidosis. Chest. 1980;78:888. doi: 10.1378/chest.78.6.888. [DOI] [PubMed] [Google Scholar]
- 44.Preston IR, Klinger JR, Landzberg MJ, et al. Vasoresponsiveness of sarcoidosis-associated pulmonary hypertension. Chest. 2001;120:866. doi: 10.1378/chest.120.3.866. [DOI] [PubMed] [Google Scholar]
- 45.Bargout R, Kelly RF. Sarcoid heart disease: clinical course and treatment. Int J Cardiol. 2004;97:173. doi: 10.1016/j.ijcard.2003.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Turner GA, Lower EE, Corser BC, et al. Sleep apnea in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:61. [PubMed] [Google Scholar]
- 47.Barst RJ, Ratner SJ. Sarcoidosis and reactive pulmonary hypertension. Arch Intern Med. 1985;145:2112. [PubMed] [Google Scholar]
- 48.Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res. 2001;2:90. doi: 10.1186/rr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z, Krasnici N, Luscher TF. Endothelin-1 potentiates human smooth muscle cell growth to PDGF: effects of ETA and ETB receptor blockade. Circulation. 1999;100:5. doi: 10.1161/01.cir.100.1.5. [DOI] [PubMed] [Google Scholar]
- 50.Hocher B, Schwarz A, Fagan KA, et al. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am J Respir Cell Mol Biol. 2000;23:19. doi: 10.1165/ajrcmb.23.1.4030. [DOI] [PubMed] [Google Scholar]
- 51.Letizia C, Danese A, Reale MG, et al. Plasma levels of endothelin-1 increase in patients with sarcoidosis and fall after disease remission. Panminerva Med. 2001;43:257. [PubMed] [Google Scholar]
- 52.Reichenberger F, Schauer J, Kellner K, et al. Different expression of endothelin in the bronchoalveolar lavage in patients with pulmonary diseases. Lung. 2001;179:163. doi: 10.1007/s004080000058. [DOI] [PubMed] [Google Scholar]
- 53.Terashita K, Kato S, Sata M, et al. Increased endothelin-1 levels of BAL fluid in patients with pulmonary sarcoidosis. Respirology. 2006;11:145. doi: 10.1111/j.1440-1843.2006.00826.x. [DOI] [PubMed] [Google Scholar]
- 54.Spruit MA, Thomeer MJ, Gosselink R, et al. Skeletal muscle weakness in patients with sarcoidosis and its relationship with exercise intolerance and reduced health status. Thorax. 2005;60:32. doi: 10.1136/thx.2004.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kabitz HJ, Lang F, Walterspacher S, et al. Impact of impaired inspiratory muscle strength on dyspnea and walking capacity in sarcoidosis. Chest. 2006;130:1496. doi: 10.1378/chest.130.5.1496. [DOI] [PubMed] [Google Scholar]
- 56.Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472. doi: 10.1378/chest.127.2.472. [DOI] [PubMed] [Google Scholar]
- 57.Martin JM, Dowling GP. Sudden death associated with compression of pulmonary arteries in sarcoidosis. Cmaj. 1985;133:423. [PMC free article] [PubMed] [Google Scholar]
- 58.Padia SA, Budev M, Farver CF, et al. Intravascular sarcoidosis presenting as pulmonary vein occlusion: CT and pathologic findings. J Thorac Imaging. 2007;22:268. doi: 10.1097/RTI.0b013e3180437e3f. [DOI] [PubMed] [Google Scholar]
- 59.Salazar A, Mana J, Sala J, et al. Combined portal and pulmonary hypertension in sarcoidosis. Respiration. 1994;61:117. doi: 10.1159/000196320. [DOI] [PubMed] [Google Scholar]
- 60.Portier F, Lerebours-Pigeonniere G, Thiberville L, et al. [Sarcoidosis simulating a pulmonary veno-occlusive disease] Rev Mal Respir. 1991;8:101. [PubMed] [Google Scholar]
- 61.Battesti JP, Georges R, Basset F, et al. Chronic cor pulmonale in pulmonary sarcoidosis. Thorax. 1978;33:76. doi: 10.1136/thx.33.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zisman DA, Karlamangla AS, Ross DJ, et al. High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2007;132:773. doi: 10.1378/chest.07-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48:516. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
- 64.Baughman RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest. 2007;132:207. doi: 10.1378/chest.06-2822. [DOI] [PubMed] [Google Scholar]
- 65.Berger M, Haimowitz A, Van Tosh A, et al. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 66.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 67.Chan KL, Currie PJ, Seward JB, et al. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol. 1987;9:549. doi: 10.1016/s0735-1097(87)80047-5. [DOI] [PubMed] [Google Scholar]
- 68.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 69.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 70.Homma A, Anzueto A, Peters JI, et al. Pulmonary artery systolic pressures estimated by echocardiogram vs cardiac catheterization in patients awaiting lung transplantation. J Heart Lung Transplant. 2001;20:833. doi: 10.1016/s1053-2498(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 71.Bossone E, Duong-Wagner TH, Paciocco G, et al. Echocardiographic features of primary pulmonary hypertension. J Am Soc Echocardiogr. 1999;12:655. doi: 10.1053/je.1999.v12.a99069. [DOI] [PubMed] [Google Scholar]
- 72.Brecker SJ, Gibbs JS, Fox KM, et al. Comparison of Doppler derived haemodynamic variables and simultaneous high fidelity pressure measurements in severe pulmonary hypertension. Br Heart J. 1994;72:384. doi: 10.1136/hrt.72.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 74.Bossone E, Bodini BD, Mazza A, et al. Pulmonary arterial hypertension: the key role of echocardiography. Chest. 2005;127:1836. doi: 10.1378/chest.127.5.1836. [DOI] [PubMed] [Google Scholar]
- 75.Rodman DM, Lindenfeld J. Successful treatment of sarcoidosis-associated pulmonary hypertension with corticosteroids. Chest. 1990;97:500. doi: 10.1378/chest.97.2.500. [DOI] [PubMed] [Google Scholar]
- 76.Davies J, Nellen M, Goodwin JF. Reversible pulmonary hypertension in sarcoidosis. Postgrad Med J. 1982;58:282. doi: 10.1136/pgmj.58.679.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castro PF, Bourge RC, McGiffin DC, et al. Intrapulmonary shunting in primary pulmonary hypertension: an observation in two patients treated with epoprostenol sodium. Chest. 1998;114:334. doi: 10.1378/chest.114.1.334. [DOI] [PubMed] [Google Scholar]
- 78.Olschewski H, Ghofrani HA, Walmrath D, et al. Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. Am J Respir Crit Care Med. 1999;160:600. doi: 10.1164/ajrccm.160.2.9810008. [DOI] [PubMed] [Google Scholar]
- 79.Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 80.Jones K, Higenbottam T, Wallwork J. Pulmonary vasodilation with prostacyclin in primary and secondary pulmonary hypertension. Chest. 1989;96:784. doi: 10.1378/chest.96.4.784. [DOI] [PubMed] [Google Scholar]
- 81.Palmer SM, Robinson LJ, Wang A, et al. Massive pulmonary edema and death after prostacyclin infusion in a patient with pulmonary veno-occlusive disease. Chest. 1998;113:237. doi: 10.1378/chest.113.1.237. [DOI] [PubMed] [Google Scholar]
- 82.Farber HW, Graven KK, Kokolski G, et al. Pulmonary edema during acute infusion of epoprostenol in a patient with pulmonary hypertension and limited scleroderma. J Rheumatol. 1999;26:1195. [PubMed] [Google Scholar]
- 83.Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130:1481. doi: 10.1378/chest.130.5.1481. [DOI] [PubMed] [Google Scholar]
- 84.Culver DA, Minai OA, Chapman JT, et al. Treatment of pulmonary hypertension in sarcoidosis. Proc Am Thoracic Soc. 2005;2:A862. [Google Scholar]
- 85.Foley RJ, Metersky ML. Successful Treatment of Sarcoidosis-Associated Pulmonary Hypertension with Bosentan. Respiration. 2005 doi: 10.1159/000089815. [DOI] [PubMed] [Google Scholar]
- 86.Sharma S, Kashour T, Philipp R. Secondary pulmonary arterial hypertension: treated with endothelin receptor blockade. Tex Heart Inst J. 2005;32:405. [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenkranz S, Diet F, Karasch T, et al. Sildenafil improved pulmonary hypertension and peripheral blood flow in a patient with scleroderma-associated lung fibrosis and the raynaud phenomenon. Ann Intern Med. 2003;139:871. doi: 10.7326/0003-4819-139-10-200311180-00030. [DOI] [PubMed] [Google Scholar]
- 88.Montgomery GS, Sagel SD, Taylor AL, et al. Effects of sildenafil on pulmonary hypertension and exercise tolerance in severe cystic fibrosis-related lung disease. Pediatr Pulmonol. 2006;41:383. doi: 10.1002/ppul.20393. [DOI] [PubMed] [Google Scholar]
- 89.Madden BP, Allenby M, Loke TK, et al. A potential role for sildenafil in the management of pulmonary hypertension in patients with parenchymal lung disease. Vascul Pharmacol. 2006;44:372. doi: 10.1016/j.vph.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 90.Collard HR, Anstrom KJ, Schwarz MI, et al. Sildenafil improves walk distance in idiopathic pulmonary fibrosis. Chest. 2007;131:897. doi: 10.1378/chest.06-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med. 1996;100:423. doi: 10.1016/S0002-9343(97)89518-6. [DOI] [PubMed] [Google Scholar]
- 92.Takada K, Ina Y, Noda M, et al. The clinical course and prognosis of patients with severe, moderate or mild sarcoidosis. J Clin Epidemiol. 1993;46:359. doi: 10.1016/0895-4356(93)90150-y. [DOI] [PubMed] [Google Scholar]
- 93.Sones M, Israel HL. Course and prognosis of sarcoidosis. Am J Med. 1960;29:84. doi: 10.1016/0002-9343(60)90009-7. [DOI] [PubMed] [Google Scholar]
- 94.Arcasoy SM, Christie JD, Pochettino A, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest. 2001;120:873. doi: 10.1378/chest.120.3.873. [DOI] [PubMed] [Google Scholar]
- 95.Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients withv sarcoidosis awaiting lung transplantation. Chest. 2003;124:922. [PubMed] [Google Scholar]
- 96.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]