Abstract
OBJECTIVES
To use static and dynamic magnetic resonance imaging (MRI) to compare dimensions of the bony pelvis and soft tissue structures in a sample of African-American and white women.
METHODS
This study used data from 234 participants in the Childbirth and Pelvic Symptoms Imaging Study, a cohort study of 104 primiparous women with an obstetric anal sphincter tear, 94 who delivered vaginally without a recognized anal sphincter tear and 36 who underwent by cesarean delivery without labor. Race was self-reported. At 6–12 months postpartum, rapid acquisition T2-weighted pelvic MRIs were obtained. Bony and soft tissue dimensions were measured and compared between white and African-American participants using analysis of variance, while controlling for delivery type and age.
RESULTS
The pelvic inlet was wider among 178 white women than 56 African-American women (10.7±0.7 cm compared with 10.0.±0.7 cm, P<.001). The outlet was also wider (mean intertuberous diameter 12.3±1.0 cm compared with 11.8±0.9 cm, P<.001). There were no significant differences between racial groups in interspinous diameter, angle of the subpubic arch, anteroposterior conjugate, levator thickness, or levator hiatus. In addition, among women who delivered vaginally without a sphincter tear, African-American women had more pelvic floor mobility than white women. This difference was not observed among women who had sustained an obstetric sphincter tear.
CONCLUSION
White women have a wider pelvic inlet, wider outlet, and shallower anteroposterior outlet than African-American women. In addition, after vaginal delivery, white women demonstrate less pelvic floor mobility. These differences may contribute to observed racial differences in obstetric outcomes and to the development of pelvic floor disorders.
Magnetic resonance imaging (MRI) has been in use in the characterization of the female pelvis since the mid 1980s.1 Advantages of MRI include multiplanar images as well as dynamic visualization of the soft tissues of the female pelvic floor. As a result, MRI has become an important adjunct to physical examination and fluoroscopy for the evaluation of pelvic anatomy.2
Before MRI, conventional radiography suggested that the architecture of the bony pelvis differs between white and African-American women. More recently, differences in the dimensions of the posterior pelvis3 have been observed with MRI. The potential clinical implications of racial differences in anatomy include a possible association with variations in obstetric outcomes4–6 and in the incidence of pelvic floor disorders.7–11 In fact, stress urinary incontinence and pelvic organ prolapse appear to be less common among African-American than white women.7,9–11 These observations have led us to hypothesize that racial differences in pelvic anatomy might be responsible for observed differences in racial patterns of pelvic floor disorders.
The objective of this study was to compare MRI dimensions of the bony pelvis in African-American and white women. In addition, the study evaluated MRI measurements based upon soft tissues within the pelvis, including static images at rest and dynamic measurements at rest and during Valsalva. Our goal is to confirm earlier data12 suggesting racial differences in pelvic anatomy between African-American and white women.
MATERIALS AND METHODS
The Childbirth and Pelvic Symptoms study (CAPS)13 was performed by the Pelvic Floor Disorders Network, a multicenter network supported by the National Institute of Child Health and Human Development. The CAPS study was a prospective cohort study of primiparous women designed to study the relationship between vaginal delivery with a sphincter laceration and subsequent incontinence. Women in this study were recruited from the 921 participants in CAPS. Methods of the CAPS study have been reported in detail13 and are briefly summarized here. Enrollment into this study was conducted from September 2003 to February 2005. Three cohorts of primiparous women were recruited while the women were hospitalized after a singleton delivery. The primary cohort of interest consisted of women with an anal sphincter tear (n=104). Two comparison groups were recruited: women who delivered vaginally without a clinically recognized anal sphincter tear (n=94) and women who underwent cesarean delivery without labor (n=36). We attempted to include all women who delivered with a sphincter laceration. For each woman with an anal sphincter tear recruited for this study, we recruited the next consecutive woman who delivered vaginally without a clinically recognized sphincter tear. We attempted to include all women who delivered by cesarean without labor.
At the time of (or shortly after) their 6-month telephone interviews for the CAPS study, CAPS participants were approached to join the CAPS Imaging Study,14 which correlated standardized imaging (MRI and endoanal ultrasonography), physical examination findings, and symptom assessment. The MRIs obtained for the CAPS Imaging Study provided the data for this secondary analysis.
This research protocol was approved at the institutional review boards at all clinical sites and the central data coordinating center. All women provided informed consent for participation. Data for this investigation were obtained 6–12 months after delivery. Weight and height were measured, and body mass index was calculated for each subject. Race was self-reported. Subjects were allowed to report more than one race but were asked to select a primary racial category if more than one race was indicated.
The MRI protocol was standardized at a 1-day training session, led by the expert consulting radiologist at the central site before study initiation. After centralized training, images were acquired using a 1.5T magnet with the patient in a supine position and a surface array coil wrapped around the pelvis. Ultrasound gel (60 mL) was placed in the rectum. After localizer images, we obtained sagittal ultra-fast T2-weighted images (rest and strain), and transverse and coronal T2-weighted images (rest). For straining images, participants were coached to strain without elevating the lumbosacral spine or thighs. Each dynamic image required 2 seconds for acquisition.
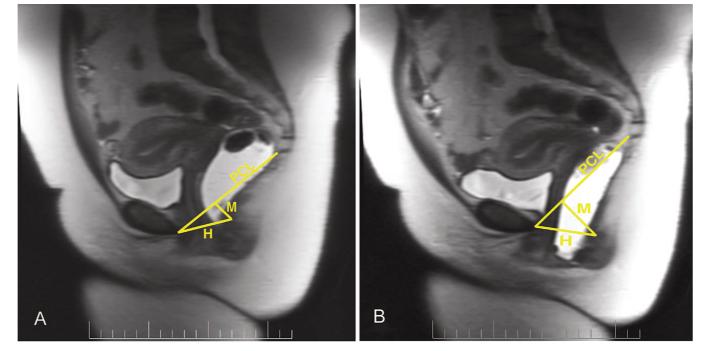
On sagittal images, the pubococcygeal line was used to represent the normal location of the pelvic floor. Rest and maximal strain midsagittal images were obtained to evaluate the descent of the bladder neck and anorectal junction, anteroposterior length of the hiatus, and angle of the levator plate with the pubococcygeal line. The angle of the posterior rectal wall relative to the pubococcygeal line was measured at rest and during Valsalva. The H line, the distance from the inferior posterior aspect of the symphysis to the posterior rectal wall, was calculated. This represents the anteroposterior width of the genital hiatus. The distance from the posterior end of the H line, measured perpendicular to the pubococcygeal line, represented the M line. On the midsagittal image, we also obtained the following bony measures15: sacral length and depth, the obstetric conjugate (from the sacral promontory to the superior symphysis), and the anteroposterior outlet (from the last vertical joint of the coccyx to the inferior symphysis) (Fig. 1).
Fig. 1.
Representative T2-weighted midsagittal magnetic resonance images are shown, both at rest (A) and with maximal strain (B). The pubococcygeal line (PCL), connecting the inferior border of the symphysis and the last vertical joint of the coccyx, represents the location of the normal pelvic floor. The H line is the distance from the inferior symphysis to the posterior rectal wall, at the level of the anorectal junction. The M line is the distance perpendicular from the pubococcygeal line to the same point on the posterior rectal wall.
Axial measurements of levator muscle thickness were obtained at the level of the constrictor urethrae muscle. The width of the genital hiatus was obtained at the cranial-most image that included the symphysis. Bony measurements obtained on axial images included the angle of the pubic arch (in degrees, with the symphysis as the apex), the intertuberous diameter (measured from the posterior and medial cortex of the ischial tuberosities), and the interspinous diameter (measured from the posterior ischial spines).
Using the coronal image that included the femoral heads and fovea, we measured the transverse inlet (from the inner aspect of the ischial cortex at the level of the fovea on each side). The transverse diameter of the pelvic inlet was measured at the level of the fovea. On oblique coronal images obtained in the plane of the sacrum, the maximum transverse inlet diameter was measured again.
Standardized images were obtained at six clinical sites. Images were reviewed by the site radiologist and a central radiologist. Image interpretation was standardized through a full day of in-person training for research radiologists. The radiology investigators were masked to the subjects’ obstetric characteristics and race. Our prior research (personal communication: Mark E. Lockhart, Julia R. Fielding, Holly E. Richter, Linda Brubaker, Caryl G. Salomon, Wen Ye, et al. Reproducibility of Dynamic MRI pelvic measures: a multi-site study. Submitted to Radiology, 2007) suggested high variability among readers of pelvic MRI measurements, particularly with respect to soft-tissue parameters. As a result, this research used the measures obtained by the central reader in all cases.
The mean and standard deviations for each dimension were calculated for African-American and white women. There were too few women of other races for meaningful comparisons. The initial analysis compared the two racial groups, adjusting only for cohort. Since the African-American participants were younger than the white participants (5% and 95% percentiles were 16.6–32.7 years of age for African-American women and 19.9–38.8 years of age for white women, P<.001), we then examined the potential confounding effect of age by adding it as a covariate to each analysis. When adjustment for age significantly changed the result or age was significant (in either the African-American or white population), we performed a second analysis restricted to the subpopulation of women under the age of 30, adjusting for cohort; the limit of age 30 was chosen because there were too few African-Americans above the limit to provide a reliable estimate of the age effect. Otherwise, we report results from the initial analyses. For all measures, the interaction effect between cohort and race was also examined using only subjects under the age of 30 in the two larger cohorts; there were insufficient observations in the cesarean delivery cohort for inclusion in this analysis.
Normal support was defined as the bladder neck above the pubococcygeal line with strain. In cases of normal support, descent of the bladder neck was not quantified. If the bladder neck descended below the pubococcygeal line, the descent was measured in centimeters. A similar strategy was used for the angle of levator plate with rest and with straining. Again, descent was measured only if the levator plate was below the pubococcygeal line. When the angle of the levator plate extended below the pubococcygeal line, the angle was measured in degrees. To compare these measures between African-American and white women, we first used the ϰ2 test to compare the proportions of women with abnormal descent across races. When no significant difference was detected, analysis of variance was used to test for a difference in the severity of descentbetween races (eg, among women with abnormal support). In all analyses, we adjusted for cohort effect. We did not adjust for height, body mass index, or site because they had no effect on the inferences.
RESULTS
Two hundred forty-six women were enrolled in the CAPS Imaging Study, including 56 (22.8%) African-American and 178 (72.3%) white women. Ten (4.1%) women reported other races, and two (0.8%) were of unknown race. These 12 women were excluded because there were too few for meaningful comparisons. Two subjects reported more than one race and were classified according to the “primary” race indicated by the participant. The demographic and obstetric characteristics of the two groups are shown in Table 1.
Table 1.
Characteristics of African-American and White Participants
| African American (n=56) | White (n=178) | P | |
|---|---|---|---|
| Age (y, mean±SD) | 22.0±5.1 | 28.8±5.6 | <.001 |
| Cohort [n (%)] | .80 | ||
| Anal sphincter tear | 25 (44.6) | 79 (44.4) | |
| Cesarean delivery | 7(12.5) | 29 (16.3) | |
| Vaginal delivery control | 24 (42.9) | 70 (39.2) | |
| BMI at 6 months postpartum (kg/m2, mean±SD) | 28.7±7.3 | 26.6±6.3 | .048 |
| Height (m, mean±SD) | 1.64±0.07 | 1.66±0.07 | .023 |
SD, standard deviation; BMI, body mass index.
Magnetic resonance imaging pelvimetry measures are shown in Table 2. Among white women, the pelvic outlet was significantly wider (mean intertuberous diameter 12.3±1.0 cm compared with 11.8±0.9 cm, P<.001), and the pelvic inlet was also significantly wider (10.7±0.7 cm compared with 10.0.±0.7 cm, P<.001) than for African-American women. The length of the sacrum was longer for white women (12.1±1.3 cm compared with 10.7±1.5 cm, P<.001). The two groups did not differ with respect to the angle of the subpubic arch, the anteroposterior conjugate or the depth of sacral hollow. With respect to the interspinous diameter and the anteroposterior outlet, differences between race varied by delivery cohort (P values for interaction=.004 and .016, respectively). Among women who delivered vaginally without a sphincter tear, the anteroposterior outlet was significantly shallower among white women than African-American women (mean anteroposterior outlet 11.0±1.1 cm compared with 12.2±1.2 cm, P<.001). Although the relationship between race and interspinous diameter varied significantly by cohort, there were no significant racial differences in interspinous diameter in either cohort.
Table 2.
Bony Pelvic Dimensions of African-American and White Participants
| African American (n=56) | White (n=178) | P* | |
|---|---|---|---|
| Transverse inlet diameter (coronal view) | 10.0±0.7 | 10.7±0.7 | <.001 |
| Transverse inlet diameter (oblique view) | 11.8±0.7 | 12.6±0.7 | <.001 |
| Interspinous distance | 10.3±0.9 | 10.5±0.8 | .15† |
| Intertuberous distance | 11.8±0.9 | 12.3±1.0 | .001 |
| Angle of subpubic arch (degrees) | 82.8±6.7 | 83.7±7.0 | .32 |
| Anteroposterior conjugate | 12.1±1.0 | 12.3±1.1 | .25 |
| Length of sacrum and coccyx | 10.7±1.5 | 12.1±1.3 | <.001 |
| Depth of sacral hollow | 3.8±0.7 | 4.0±0.8 | .46‡ |
| Anteroposterior outlet | 11.7±1.2 | 11.1±1.0 | <.001† |
All dimensions are in centimeters unless otherwise noted. Values are expressed as mean±standard deviation.
P values are adjusted for delivery cohort.
Adjusted for cohort and for significant interaction of cohort and race. P values for interaction of cohort and race: interspinous distance=0.004; anteroposterior outlet=0.016.
P values adjusted for cohort and age and restraining analysis to a subpopulation younger than 30 years of age.
Magnetic resonance imaging soft tissue results for African-American and white women are shown in Table 3. The levator hiatus width was similar between African-American and white women. The left levator sling was narrower among white women (P<.001), but there was no difference between racial groups in the right sling (P=.61). For five soft-tissue measures (Table 3), the differences between race varied by delivery cohort. Among women who delivered vaginally without a sphincter tear, the H line with straining was shorter among white than African-American women (4.8±1.9 cm compared with 5.9±1.2 cm, P<.001), and the H line difference was similarly smaller among white women (0.7±0.9 cm compared with 1.4±1.1 cm, P=.012). Also, in the vaginal delivery cohort, the M line with straining was shorter among white women (2.2±1.1 cm compared with 3.2±1.5 cm, P=.008), and the M line difference was also smaller among white women (0.9±1.0 cm compared with 1.9±1.4 cm, P=.003). No significant differences in these measures were observed between races in the cohort of women with an anal sphincter tear.
Table 3.
Soft Tissue Pelvic Dimensions of African-American and White Participants
| African American (n=56) |
White (n=178) |
P* | P (Restricted)† | |
|---|---|---|---|---|
| Width of levator hiatus | 3.8±0.6 | 3.8±0.5 | .52 | |
| Width of right levator sling muscle | 0.4±0.2 | 0.4±0.2 | .61 | |
| Width of left levator sling muscle | 0.6±0.2 | 0.5±0.2 | <.001 | |
| H line (rest) | 4.5±0.8 | 4.4±0.7 | .35 | |
| H line (strain) | 5.6±1.1 | 5.2±1.2 | .009‡ | <.001 |
| H line difference (not seen at site) | 1.1±0.9 | 0.8±1.0 | .044§ | .012 |
| M line (rest) | 1.1±0.5 | 1.4±0.6 | .49 | |
| M line (strain) | 2.6±1.4 | 2.5±1.2 | .52‡ | .008 |
| M line difference | 1.5±1.3 | 1.1±1.1 | .40§ | .003 |
| Distance from bladder neck to pubococcygeal line (rest) | 2.3±0.6 | 2.1±0.5 | .89§ | .072 |
All dimensions are in centimeters unless otherwise noted. Values are expressed as mean±standard deviation
P values are adjusted for delivery cohort.
P value for difference between African-American and white women, restricted to women who delivered by vaginal birth without sphincter tear. P values are listed only if there is a significant interaction between delivery group and race.
Adjusted for cohort and for significant interaction of cohort and race.
P values are adjusted for cohort and age and restraining analysis to a subpopulation younger than 30 years of age.
Magnetic resonance imaging assessment of bladder neck support (Table 4) was similar between racial groups. Also, there was no racial difference in the angle of the levator plate. For those with abnormal descent, the mean descent was not significantly different between white and African-American women.
Table 4.
Soft Tissue Descent of Pelvic Structures With Straining, Comparing African-American and White Participants
| Race |
||||||
|---|---|---|---|---|---|---|
| African American (n=56) |
White (n=178) |
P |
||||
| Abnormal Descent [n (%)] |
Mean±SD Descent† |
Abnormal Descent [n (%)] |
Mean±SD Descent† |
Abnormal Descent* |
Mean Descent† |
|
| Distance from bladder neck to pubococcygeal line |
24 (44.4) | 1.1±0.6 | 74 (41.8) | 0.9±0.6 | .76 | .17‡ |
| Angle of levator plate with pubococcygeal line |
35 (64.8) | 2.0±1.2 | 95 (53.7) | 2.1±1.0 | .15 | .47 |
All dimensions are in centimeters unless otherwise noted.
P value for ϰ2 test (controlling for cohort).
Mean descent was quantified only for those with abnormal descent. P value for analysis of variance (controlling for cohort).
Adjusted for significant interaction for cohort and race (P value for interaction is .020).
DISCUSSION
We identified significant racial differences in bony pelvic parameters between African-American and white women. Our results confirm and extend findings from the 1940s and 1950s, when conventional radiographic pelvimetry suggested racial differences in pelvic type. We found that African-American women have narrower transverse diameters of the bony pelvis than white women (pelvic inlet and intertuberous distance). Also, among women delivering vaginally without a sphincter tear, African-American women had a deeper pelvic anteroposterior diameter (outlet). Radiographic pelvimetry data previously described these characteristics as the anthropoid pelvic type and suggested that this pelvic type was seen in 44.5% of African-American women but only 27.6% of white women.12,16 We did not use the previously described categories to classify pelvic type in our research because those categories are founded on qualitative comparisons rather than quantitative measures.
The conventional teaching regarding the anthropoid pelvis is that it is characterized by a long sacrum of “average curvature.”17 However, we unexpectedly found that African-American women had a significantly shorter sacrum than the white subjects. Baragi et al3 analyzed bony pelvic measurements from a collection of anthropological specimens in which the specimen collectors assigned race. Their findings demonstrated a smaller posterior and total pelvic area in the African-American as compared to white women. This finding has clinical implications for obstetric practice. It is possible that this difference in sacral shape could impact the course of labor for white compared with African-American women. Historically, the anthropoid pelvis has been associated with an increase in “serious arrest” of labor.17 This association should be reassessed in the light of current MRI data.
Although the bony pelvis is one of the factors that influence the course of labor, pelvimetry does not play a major role in modern obstetrics.18 Since the mid-1900s clinicians have sought a reliable and clinically useful assessment of the maternal pelvis. The use of X-ray pelvimetry declined substantially as clinicians recognized the limited clinical utility of this investigation.19–21 Other imaging modalities have been explored, including computed tomographic pelvimetry for the management of breech pregnancy.22 Magnetic resonance imaging pelvimetry was described in the mid-1980s,1 but still does not have a clear clinical role in obstetrics. Zaretsky et al18 described a clinical series of 107 women who underwent predelivery MRI and suggested that MRI data were more clearly associated with labor dystocia than other imaging modalities.
Magnetic resonance imaging is a valuable tool in the investigation of pelvic anatomy and has been used to define soft tissue structures, especially in women with pelvic floor disorders. In this cohort of new mothers, we did not detect consistent differences in the levator muscle width, although the left levator was slightly wider in African-American women. However. the observed difference was small and not clinically meaningful (approximately, 1 mm).
In the vaginal delivery cohort, H line lengthened more with straining among African-American women than white women, suggesting a greater widening of the levator hiatus among African-American women. Similarly, the M line lengthened more with straining in African-American women, suggesting greater levator descent. Given that the majority of vaginal deliveries occur without a recognized anal sphincter tear, the vaginal delivery cohort is most generalizable to a typical obstetric population. Therefore, our results suggest significant racial differences in pelvic floor laxity after vaginal birth, with greater laxity in African-American women. It is unclear whether this difference might impact the later development of pelvic organ prolapse.
Greater lengthening of the H line and M line in African-American women could reflect increased tissue elasticity. Investigators have previously reported that African-American primiparas were less likely to deliver with second-degree or greater lacerations and more likely to deliver with their perineum intact.23,24 This might be due to inherent differences in connective tissue composition. Investigators using a mouse model have shown that a failure to maintain elastin fiber homeostasis caused significant pelvic floor disorders and proposed this as a potential mechanism in human females as well.25 However, we are unaware of any characterization of racial differences in the elastin content of the pelvic connective tissue. We suggest this as an area of further investigation. Another possibility is that the increased H Line and M Line in African-American women are a soft tissue consequence of the bony pelvis differences by race, namely a longer anteroposterior diameter of the outlet in African-American women.
Our analysis has several limitations. First, our population was recruited specifically to compare the incidence of postpartum fecal incontinence between women with an anal sphincter laceration and two comparison groups and, therefore, is not representative of a general obstetric population. Second, our findings are limited to racial comparisons between the two major races in the United States, black and white. In addition, we treated race as a categorical variable, but we recognize that race cannot be accurately classified into simple categories. This may obscure some racial differences. Finally, some of the observed differences were statistically significant but small in absolute magnitude. For example, the mean difference between the length of the sacrum in African-American and white women was 0.4 cm.
Despite these limitations, these data describe MRI bony and soft tissue anatomy using a standardized image acquisition technique in a relatively large group of women. These participants, from different geographic regions of the United States, were not selected by race or traditional categories of pelvic type. This greatly increases the generalizability of our findings and provides a sound base for further evaluation of racial differences in the female pelvis.
Acknowledgments
Supported by grants from the National Institute of Child Health and Human Development (U01 HD41249, U10 HD41268, U10 HD41248, U10 HD41250, U10 HD41261, U10 HD41263, U10 HD41269, and U10 HD41267).
Footnotes
For members of the Pelvic Floor Disorders Network, see the Appendix online at www.greenjournal.org/cgi/content/full/111/4/914/DC1.
Presented at the annual meeting of the Radiological Society of North America, Chicago, Illinois, November 29, 2007.
Financial Disclosure
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Stark DD, McCarthy SM, Filly RA, Parer JT, Hricak H, Callen PW. Pelvimetry by magnetic resonance imaging. AJR Am J Roentgenol. 1985;144:947–50. doi: 10.2214/ajr.144.5.947. [DOI] [PubMed] [Google Scholar]
- 2.Macura KJ. Magnetic resonance imaging of pelvic floor defects in women. Top Magn Reson Imaging. 2006;17:417–26. doi: 10.1097/RMR.0b013e3180417dc8. [DOI] [PubMed] [Google Scholar]
- 3.Baragi RV, Delancey JO, Caspari R, Howard DH, Ashton-Miller JA. Differences in pelvic floor area between African American and European American women. Am J Obstet Gynecol. 2002;187:111–5. doi: 10.1067/mob.2002.125703. [DOI] [PubMed] [Google Scholar]
- 4.Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563–8. doi: 10.1080/14767050600682487. [DOI] [PubMed] [Google Scholar]
- 5.Chung JH, Garite TJ, Kirk AM, Hollard AL, Wing DA, Lagrew DC. Intrinsic racial differences in the risk of cesarean delivery are not explained by differences in caregivers or hospital site of delivery. Am J Obstet Gynecol. 2006;194:1323–8. doi: 10.1016/j.ajog.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 6.Hollard AL, Wing DA, Chung JH, Rumney PJ, Saul L, Nageotte MP, et al. Ethnic disparity in the success of vaginal birth after cesarean delivery. J Matern Fetal Neonatal Med. 2006;19:483–7. doi: 10.1080/14767050600847809. [DOI] [PubMed] [Google Scholar]
- 7.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–6. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Harvey MA, Johnston S. A review of the epidemiology and pathophysiology of pelvic floor dysfunction: do racial differences matter? J Obstet Gynaecol Can. 2005;27:251–9. doi: 10.1016/s1701-2163(16)30518-7. [DOI] [PubMed] [Google Scholar]
- 9.Bump RC. Racial comparisons and contrasts in urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1993;81:421–5. [PubMed] [Google Scholar]
- 10.Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol. 2007;109:1396–403. doi: 10.1097/01.AOG.0000263469.68106.90. [DOI] [PubMed] [Google Scholar]
- 11.Thom DH, van den Eeden SK, Ragins AI, Wassel-Fyr C, Vittinghof E, Subak LL, et al. Differences in prevalence of urinary incontinence by race/ethnicity. J Urol. 2006;175:259–64. doi: 10.1016/S0022-5347(05)00039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steer CM. 2nd Saunders; Philadelphia (PA): 1959. Moloy’s evaluation of the pelvis in obstetrics. [Google Scholar]
- 13.Borello-France D, Burgio KL, Richter HE, Zyczynski H, Fitzgerald MP, Whitehead W, et al. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863–72. doi: 10.1097/01.AOG.0000232504.32589.3b. [DOI] [PubMed] [Google Scholar]
- 14.Richter HE, Fielding JR, Bradley CS, Handa VL, Fine P, FitzGerald MP, et al. Endoanal ultrasound findings and fecal incontinence symptoms in women with and without recognized anal sphincter tears. Obstet Gynecol. 2006;108:1394–401. doi: 10.1097/01.AOG.0000246799.53458.bc. [DOI] [PubMed] [Google Scholar]
- 15.Handa VL, Pannu HK, Siddique S, Gutman R, VanRooyen J, Cundiff G. Architectural differences in the bony pelvis of women with and without pelvic floor disorders. Obstet Gynecol. 2003;102:1283–90. doi: 10.1016/j.obstetgynecol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham FG, Williams JW. Williams obstetrics. 19th Appleton & Lange; Norwalk (CT): 1993. [Google Scholar]
- 17.Moloy HC, Steer CM. Moloy’s evaluation of the pelvis in obstetrics. 3d Plenum Medical Books Co.; New York (NY): 1975. [Google Scholar]
- 18.Zaretsky MV, Alexander JM, McIntire DD, Hatab MR, Twickler DM, Leveno KJ. Magnetic resonance imaging pelvimetry and the prediction of labor dystocia. Obstet Gynecol. 2005;106:919–26. doi: 10.1097/01.AOG.0000182575.81843.e7. [DOI] [PubMed] [Google Scholar]
- 19.Gordon A, Pinchen C, Walker E, Tudor J. The changing place of radiology in obstetrics. Br J Radiol. 1984;57:891–3. doi: 10.1259/0007-1285-57-682-891. [DOI] [PubMed] [Google Scholar]
- 20.Jagani N, Schulman H, Chandra P, Gonzalez R, Fleischer A. The predictability of labor outcome from a comparison of birth weight and x-ray pelvimetry. Am J Obstet Gynecol. 1981;139:507–11. doi: 10.1016/0002-9378(81)90508-1. [DOI] [PubMed] [Google Scholar]
- 21.Varner MW, Cruikshank DP, Laube DW. X-ray pelvimetry in clinical obstetrics. Obstet Gynecol. 1980;56:296–300. [PubMed] [Google Scholar]
- 22.van Loon AJ, Mantingh A, Serlier EK, Kroon G, Mooyaart EL, Huisjes HJ. Randomised controlled trial of magnetic-resonance pelvimetry in breech presentation at term. Lancet. 1997;350:1799–804. doi: 10.1016/S0140-6736(97)05431-7. [DOI] [PubMed] [Google Scholar]
- 23.Howard D, Davies PS, DeLancey JO, Small Y. Differences in perineal lacerations in black and white primiparas. Obstet Gynecol. 2000;96:622–4. doi: 10.1016/s0029-7844(00)00956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handa VL, Danielsen BH, Gilbert WM. Obstetric anal sphincter lacerations. Obstet Gynecol. 2001;98:225–30. doi: 10.1016/s0029-7844(01)01445-4. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Zhao Y, Pawlyk B, Damaser M, Li T. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol. 2006;168:519–28. doi: 10.2353/ajpath.2006.050399. [DOI] [PMC free article] [PubMed] [Google Scholar]