Abstract
A procedure for the purification of enzyme I (EI) and the protein HPr, the general components of the phosphoenolpyruvate:sugar phosphotransferase system, from Streptococcus mutans serotype c is presented. The method was also applied successfully to the purification of EI and HPr from Streptococcus salivarius, Streptococcus sobrinus, and Streptococcus sanguis. Using specific antibodies obtained against the proteins purified from S. mutans DR0001, we determined quantitatively by rocket electrophoresis the cellular levels of EI and HPr in a freshly isolated strain of S. mutans grown under various conditions in continuous culture. The activity of a few specific EIIs was also determined by an in vitro phosphorylation test. Results indicated that maximum EII activities for glucose, mannose, and 2-deoxyglucose were obtained under conditions of glucose limitation, at pH 7.0 and low dilution rate (D = 0.057/h). Increasing the amount of glucose or the dilution rate (D = 0.40/h) or decreasing the pH from 7.0 to 5.5 resulted in a 1.4- to 24-fold decrease in these activities. The EII activity for fructose was not influenced by the growth conditions in the same way as the other EIIs. The fructose EII was highest at pH 5.5 and at high dilution rate under conditions of glucose or nitrogen limitation and was always repressed at pH 7.0 and at low dilution rates. The intracellular levels of EI were also dependent on the growth conditions. The highest concentration (0.65 nmol/mg of protein) was observed in cells grown under glucose limitation at pH 7.0 and high dilution rate, and the lowest concentration (0.12 nmol/mg of protein) was found in cells grown under glucose excess at pH 7.0 and high dilution rate. The other general component of the phosphoenolpyruvate:sugar phosphotransferase system, the protein HPr, was not influenced significantly by varying growth conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Britton P., Lee L. G., Murfitt D., Boronat A., Jones-Mortimer M. C., Kornberg H. L. Location and direction of transcription of the ptsH and ptsI genes on the Escherichia coli K12 genome. J Gen Microbiol. 1984 Apr;130(4):861–868. doi: 10.1099/00221287-130-4-861. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L., Mayrand D., Vadeboncoeur C. Isolation of a novel protein involved in the transport of fructose by an inducible phosphoenolpyruvate fructose phosphotransferase system in Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):755–763. doi: 10.1128/jb.160.2.755-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami Y., Yamada T. Rate-limiting steps of the glycolytic pathway in the oral bacteria Streptococcus mutans and Streptococcus sanguis and the influence of acidic pH on the glucose metabolism. Arch Oral Biol. 1980;25(3):163–169. doi: 10.1016/0003-9969(80)90015-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Transport of glucose and mannose by a common phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus mutans GS5. Infect Immun. 1984 Mar;43(3):1106–1109. doi: 10.1128/iai.43.3.1106-1109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Hausman S. Xylitol-mediated transient inhibition of ribitol utilization by Lactobacillus casei. J Bacteriol. 1982 May;150(2):657–661. doi: 10.1128/jb.150.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo R. L., Waygood E. B. Determination of the levels of HPr and enzyme I of the phosphoenolpyruvate-sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Can J Biochem Cell Biol. 1983 Jan;61(1):29–37. doi: 10.1139/o83-005. [DOI] [PubMed] [Google Scholar]
- Orr M. D., Blakley R. L., Panagou D. Discontinuous buffer systems for analytical and preparative electrophoresis of enzymes on polyacrylamide gel. Anal Biochem. 1972 Jan;45(1):68–85. doi: 10.1016/0003-2697(72)90008-5. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault L., Vadeboncoeur C. Phosphoenolpyruvate-sugar phosphotransferase transport system of Streptococcus mutans: purification of HPr and enzyme I and determination of their intracellular concentrations by rocket immunoelectrophoresis. Infect Immun. 1985 Dec;50(3):817–825. doi: 10.1128/iai.50.3.817-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Regulation of glycolysis and sugar phosphotransferase activities in Streptococcus lactis: growth in the presence of 2-deoxy-D-glucose. J Bacteriol. 1983 May;154(2):819–830. doi: 10.1128/jb.154.2.819-830.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Gauthier L. The phosphoenolpyruvate: sugar phosphotransferase system of Streptococcus salivarius. Identification of a IIIman protein. Can J Microbiol. 1987 Feb;33(2):118–122. doi: 10.1139/m87-020. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Proulx M., Trahan L. Purification of proteins similar to HPr and enzyme I from the oral bacterium Streptococcus salivarius. Biochemical and immunochemical properties. Can J Microbiol. 1983 Dec;29(12):1694–1705. doi: 10.1139/m83-260. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C. Structure and properties of the phosphoenolpyruvate: glucose phosphotransferase system of oral streptococci. Can J Microbiol. 1984 Apr;30(4):495–502. doi: 10.1139/m84-073. [DOI] [PubMed] [Google Scholar]
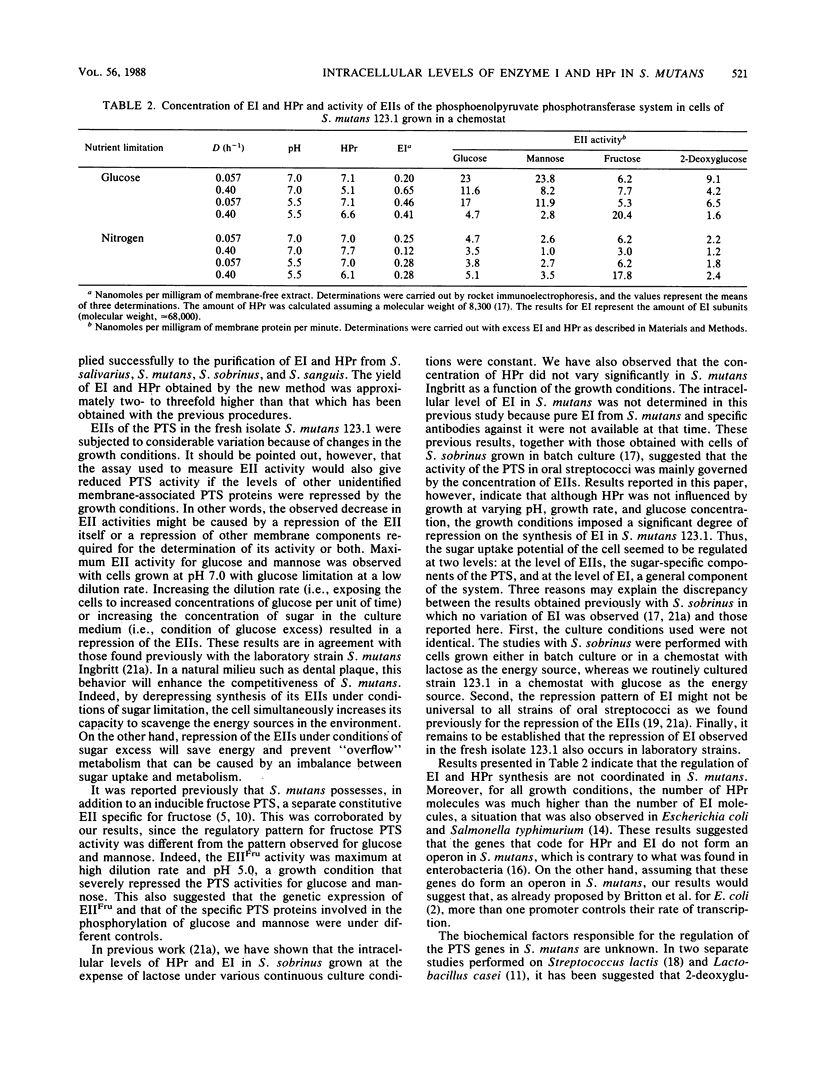
- Vadeboncoeur C., Thibault L., Neron S., Halvorson H., Hamilton I. R. Effect of growth conditions on levels of components of the phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans and Streptococcus sobrinus grown in continuous culture. J Bacteriol. 1987 Dec;169(12):5686–5691. doi: 10.1128/jb.169.12.5686-5691.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Trahan L. Comparative study of Streptococcus mutans laboratory strains and fresh isolates from carious and caries-free tooth surfaces and from subjects with hereditary fructose intolerance. Infect Immun. 1983 Apr;40(1):81–90. doi: 10.1128/iai.40.1.81-90.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]