Introduction
In recent years, evidence has mounted in support of the importance of protein kinase CK2 (previously called casein kinase 2 or II) (EC 2.7.11.1) in diverse biological processes, and in particular in regard to its role in cell growth and proliferation in normal and disease states. In particular, emerging data have underscored a novel function of CK2 as a potent suppressor of apoptosis. Thus, it now appears that CK2 has a dual role in cell function, namely its involvement in growth and proliferation as well as in suppression of apoptosis. The latter function of CK2 is of particular significance with respect to its role in neoplasia since CK2 has been found to be consistently elevated in cancer cells which are known to demonstrate remarkable resistance to death. In this review, we have focused on the pertinent observations that implicate CK2 to have a global role as a suppressor of apoptosis and its relevance to the cancer phenotype.
General Characteristics of Protein Kinase CK2
CK2 (official acronym for the former name casein kinase 2 or II) (EC 2.7.11.1) is a ubiquitous protein serine/threonine kinase that is among the most highly conserved proteins in nature. A unique property of this kinase is that it can utilize both ATP and GTP as substrates. Its heterotetrameric structure consists of two catalytic subunits (42 kDa α and 38 kDa α′) and two regulatory subunits (28 kDa β) existing as α2β2, or αα′β2, or α′2β2 configurations. The two catalytic subunits are linked through the β subunits (Graham et al., 2000). The β subunits in turn may form a linkage with the nuclear matrix (Zhang et al., 1998) which is a key locus for CK2 signaling in the nucleus (Ahmed, 1999). The growth related functions of CK2 accord with CK2-mediated phosphorylation of a rather large number of substrates in the cell, many of which are nuclear-associated and are involved in gene expression and cell growth (for recent reviews see e.g., Guerra and Issinger, 1999; Ahmed et al., 2000; Tawfic et al., 2001; Ahmed et al., 2002; Pinna, 2002; Litchfield, 2003; Pyerin and Ackermann, 2003). The kinase is localized to both the nuclear and cytoplasmic compartments in the cell, and undergoes dynamic shuttling between various compartments which may relate to its functional activity in those compartments (Ahmed et al., 1993; Tawfic and Ahmed, 1994a, 1994b; Tawfic et al., 1996, 1997; Guo et al., 1998, 1999a, 1999b; Ahmed et al., 2000; Faust and Montenarh, 2000). A key feature of CK2 cell biology is that it is essential for cell survival (e.g., Padmanabha et al., 1990), and accordingly, attempts to produce CK2-knockout mice have been unsuccessful (Bouchou et al., 2003).
The nature of existence of CK2 subunits in the cell is a subject of current investigations which have demonstrated divergent results (e.g., Guerra et al., 1999; Martel et al., 2002; Salvi et al., 2006). Regulation of cellular CK2 appears to be complex and various hypotheses have been proposed on this subject (for reviews, see e.g., Allende and Allende, 1995; Ahmed, 1999; Montenarh and Faust, 2000; Pinna, 2002; Filhol et al., 2004; Olsten et al., 2005). To explain some of our data on CK2 responses to altered cell growth, we originally proposed that CK2 undergoes dynamic shuttling between cytoplasm and nucleus and further within the nucleus it undergoes differential distribution depending on the conditions to which the cell is exposed (Ahmed et al., 1993; Ahmed, 1999; Tawfic and Ahmed, 1994a, 1994b; Tawfic et al., 1996, 1997; Guo et al., 1998, 1999a, 1999b; Ahmed et al., 2000). Subsequently, it was demonstrated that CK2 translocation can take place to other parts of the cell further reinforcing the notion that dynamic intracellular shuttling of CK2 might represent a key mode of its regulation in the cell in response to diverse signals (Tawfic et al., 1997; Faust and Montenarh, 2000). Analogous to these considerations is the emerging view that spatiotemporal organization of individual CK2 subunits in living cells involves multimolecular assemblies (Filhol et al., 2004), and that association of discrete subpopulations of CK2 may be regulated independently (Olsten et al., 2005). In this context, it is noteworthy that molecular downregulation of CK2 (e.g., by treatment of cells with antisense CK2 ODN) in cell culture or in vivo demonstrates a distinct reduction in the signal in the nuclear fractions such as chromatin and nuclear matrix (Faust et al., 2000; Wang et al., 2001; Slaton et al., 2004).
CK2 and Cancer
Much evidence exists supporting that CK2 signal is dysregulated in all the cancers that have been examined (for reviews, see e.g., Guerra and Issinger, 1999; Tawfic et al., 2001; Wang et al., 2006b). Interestingly, it appears that CK2 by itself is not oncogenic but rather it co-operates with other molecules thereby altering the oncogenic potential in the cell. Experimental transgenic mouse models of neoplasia in which altered CK2 signal served as a double transgene have provided significant support to this notion (e.g., Kelliher et al., 1996; Xu et al., 1999). The presence of elevated CK2 in cancer appears to correlate with the pathological status of the cancer and may serve as a prognostic marker (Yenice et al., 1994; Gapany et al., 1995; Faust et al., 1996; Faust et al., 1999; Piazza et al., 2006; Laramas et al., 2007). Although CK2 is present ubiquitously in all cells, it is noteworthy that its distribution in the normal versus cancer cells is distinct. In normal cells CK2 is localized in a diffuse pattern in the nuclear and cytoplasmic compartments whereas in cancer cells its distribution is characterized by a higher level in the nuclear compartment (Faust et al., 1999; Laramas et al., 2007). As indicated above, this difference in the localization of CK2 depending on the nature of the cell may be significant in regard to its function in cancer. Originally, it was unclear as to how elevated CK2 was involved in cancer cell biology considering that its activity is high in normal rapidly proliferating cells. This raised the question if CK2 increase in cancer cells was simply a reflection of their high proliferation. However, a comparison of CK2 immuno-reactivity with that of Ki-67 in the same tumor section revealed that both Ki-67 and CK2 signals were apparent in the proliferating edge of the tumor but the CK2 signal was additionally widely distributed in various cells in the section. This observation suggested that CK2 signal is not simply a proliferation marker but rather reflects the pathological status of the cell (Faust et al., 1999), and is further supported by recent immunohistochemical analysis of prostate cancer specimens (Laramas et al., 2007). Additionally, as discussed subsequently, our demonstration that CK2 is a potent suppressor of apoptosis (Ahmed et al., 2002) provided a novel link of CK2 function in cancer cell biology since it is well known that cancer cells not only demonstrate dysregulated growth but also dysregulated cell death. Thus, consistently elevated CK2 signal would not only contribute to altered cell growth and proliferation in cancer cells but also to suppression of cell death related activities.
CK2 and Cell Death
Cell death is a complex phenomenon involving a variety of mechanisms. Of these, apoptosis (or programmed cell death) has gained considerable attention in regard to its potential significance in cancer cell death since it appears that dysregulation of apoptotic activity in cancer cells is an important feature of the cancer phenotype. Indeed, in certain cases, it may be even more significant than the dysregulation of cell growth (e.g., in the case of prostate cancer) (Kyprianou et al., 2000). Apoptosis in cells can be induced by a variety of means including, e.g., removal of growth and survival factors from the cells, treatment of cells with chemical agents, or treatment of cells with ligands for death receptors. We have investigated the involvement of CK2 in modulation of apoptosis induced by these various mechanisms.
One of the first studies on nuclear-associated protein kinase activity towards acidic nonhistone proteins employing the experimental model of rat ventral prostate subjected to altered androgenic status demonstrated that removal of the androgenic stimulus resulted in loss of phosphorylation of these proteins in the prostate which was very rapidly reversed in response to androgen administration in the animal (Ahmed and Ishida, 1971). Subsequently, we identified CK2 to play a significant role in phosphorylation of certain of these nuclear proteins (Goueli and Ahmed, 1991; Tawfic and Ahmed, 1994a, 1994b; Guo et al., 1998, 1999b); the variety of substrates of CK2 has expanded over time through investigations in various laboratories (for a review, see e.g., Meggio and Pinna, 2003). Our studies originally demonstrated that removal of the growth factor stimulus in the prostate gland (such as by androgen deprivation in the animal) resulted in rapid loss of nuclear CK2 such that it preceded the induction of apoptosis in prostate epithelial cells under these conditions. On the other hand, when growth stimulus was instituted by androgen administration in animals previously deprived of androgens there was a rapid shuttling of CK2 to the nuclear compartment of prostate epithelial cells prior to cell growth. Noteworthy was the observation that the translocated CK2 was differentially associated with the chromatin and nuclear matrix fractions in the nucleus (Ahmed et al., 1993; Tawfic and Ahmed, 1994b; Guo et al., 1998; Ahmed, 1999; Tawfic et al., 2001). Removal of growth factors from the culture media resulted in shuttling of CK2 from the nuclear to the cytoplasmic compartment and subsequent addition of the growth factors to the culture media demonstrated a reversal of this process with CK2 shuttling to the nucleus (Guo et al., 1999a). Further corroborative support for these considerations has been provided by recent documentation that nuclear accumulation of PTHrP (a key survival factor for cells subjected to apoptotic stimuli) effectively inhibits mitochondrial-mediated apoptosis through regulation of the expression, activity and sub-cellular trafficking of CK2 (Okoumassoun et al., 2007). Together, these observations point to a correlation of loss of nuclear CK2 to induction of apoptosis in response to removal of survival or growth factors whereas the reverse was the case upon administration of survival or growth factors. Thus, our observations provided the initial indication that dynamic changes in CK2 (especially in the nuclear compartment) play important role in cell growth and in cell death (Ahmed et al., 2000; Yu et al., 2001).
Further investigations of the involvement of CK2 in chemical mediated apoptosis demonstrated that treatment of cells with chemical agents such as etoposide and diethylstilbestrol which are well known chemical mediators of apoptosis caused an initial rapid shuttling of CK2 to the nuclear compartment presumably as a survival response of the cells. To provide a more direct confirmation of the role of CK2 as a suppressor of apoptosis we demonstrated that forced overexpression of CK2 prior to treatment of cells with etoposide or diethylstilbestrol strongly protected them against apoptosis (Guo et al., 2001). Analogous observations based on heat shock treatment of cells supported the role of CK2 in promoting cell survival (Ahmed et al., 2002; Davis et al., 2002), and subsequent studies employing radiation or UV treatment of cells have made similar observations (Kato et al., 2003; Yamane and Kinsella, 2005). It was previously suggested that CK2 may be involved in phosphorylation of ser-392 induced by UV damage of DNA (Keller et al., 2001). Recent documentation that CK2 mediates DNA repair following single strand damage (Loizou et al., 2004) may also be pertinent to regulation of cell death. Thus, it appears that CK2 impacts on apoptotic activity in cells mediated by chemical agents and also by physical stress.
An important means of induction of apoptosis in cells is via activation of the death receptor pathway. We and others have investigated the relationship between this mode of apoptosis and CK2, leading to the demonstration that CK2 impacts on apoptosis mediated by TNF-α, TRAIL, and FasL which bind to their cognate death receptors in prostate cancer cells (Wang et al., 2005a; Wang et al., 2006a) and other cancer cells (Ravi and Bedi, 2002; Izeradjene et al., 2005). To that end, we observed that when CK2 is moderately downregulated (by employing relatively low levels of chemical inhibitors of CK2 such as 4,5,6,7-tetrabromobenzotriazole (TBB) or apigenin or by molecular downregulation employing antisense CK2 or siRNA) there was a remarkable sensitization of cells towards death receptor ligands which under these conditions induced cell death at sub-optimal concentrations. Forced overexpression of CK2 impeded the effectiveness of the death receptor ligands in inducing apoptosis (Wang et al., 2005a; Wang et al., 2006a).
We originally demonstrated that treatment of cancer cells with antisense CK2 (or CK2 siRNA) resulted in potent induction of apoptosis (Faust et al., 2000; Wang et al., 2001; Ahmad et al., 2006). The induction of apoptosis was apparent when CK2 activity was reduced by 30–40% in the nuclear compartment (chromatin or nuclear matrix) (Faust et al., 2000; Wang et al., 2001). Recent studies by us (Wang et al., 2005b; Wang et al., 2006a; Ahmad et al., 2006; Ahmad et al., 2007) and by others (Ruzzene et al., 2002; Seeber et al., 2005; Pagano et al., 2007; Mishra et al., 2007; Hamacher et al., 2007) have also shown that chemical inhibitors of CK2 are effective in inducing cell death, and that induction of death by chemical agents is blocked by forced overexpression of CK2 (Wang et al., 2006a). Delivery of antisense CK2α into a xenograft prostate cancer in the mouse also demonstrated potent induction of apoptosis associated with significant reduction of the CK2 signal in the nuclear matrix (Slaton et al., 2004). Of note, analogous to results on the relation of nuclear CK2 to apoptotic signals are the observations that nuclear matrix-associated CK2 demonstrates discrete cell cycle related changes that are not apparent in the cytoplasmic compartment (Wang et al., 2003). Together, our studies indicate that a compartment of CK2 in the nucleus plays a key role in regulation of cell survival and death. The induction of apoptosis in vivo by intratumoral administration of antisense CK2 (Slaton et al., 2004), or a peptide that impairs phosphorylation by CK2 (Perea et al., 2004), has provided the proof of principle evidence for potential targeting of CK2 to produce cell death in vivo. Subsequently, we also originally reported that CK2 can be targeted by antisense CK2 delivered via the systemic route into the animal such that apoptotic response in tumor xenograft cells was observed in vivo (Ahmad et al., 2005). These observations have paved the way towards devising potential approaches to cancer therapy by targeting CK2 (Unger et al., 2004; Ahmad et al., 2005). It is noteworthy that chemopreventive dietary agents such as resveratrol and EGCG (epigallocatechin-3-gallate) which are known to have mild apoptotic activity in cancer cells appear to mediate this effect at least in part via targeting of CK2, raising the possibility of employing these polyphenolic compounds alongside sub-optimal levels of inhibitors of CK2 in combination chemotherapy (Ahmad et al., 2007). In the same context, it is noteworthy that multidrug resistance phenotype of CEM cells which are characterized by high CK2 level undergo reversion upon pharmacological inhibition of CK2 (Di Maira et al., 2007). Although concern persists regarding the potential of targeting CK2 for cancer therapy because of its ubiquitous nature, the aforementioned observations suggest an emerging interest in this signal as a therapeutic target. We are currently investigating an approach to circumvent the host toxicity issues by delivering the CK2 targeting agent encapsulated in a nanocapsule for delivery specifically to cancer cells in vivo (Unger et al., 2004; Ahmad et al., 2005; Wang et al., 2005b).
Downstream Targets of CK2 in the Apoptotic Machinery
Mounting evidence suggests that there may be multiple nuclear and cytoplasmic targets that impact on the apoptotic machinery in response to modulations in CK2. For example, caspases 2, 3, 8, and 9 respond to alteration in the CK2 signal in diverse manners (Shin et al., 2005; Wang et al., 2006a). Procaspase-2 was shown to be a target of CK2 such that its dephosphorylation results in its dimerization and activation (Shin et al., 2005). ARC, a protein that inhibits caspase-8 activity when phosphorylated, has also been identified as a CK2 target (Li et al., 2002). The hallmark of nuclear apoptotic activity indicated by lamin A cleavage is strongly elicited by downregulation of CK2 and prevented by its forced overexpression (Ahmad et al., 2006; Wang et al., 2006a). Among the Bcl-2 family, Bid has been shown to be phosphorylated by CK2 (and also CK1) at serine residues in the vicinity of caspase-8 recognition site thereby preventing its cleavage by activated caspase-8 (Desagher et al., 2001). In a preliminary observation, we noted that forced overexpression of CK2 in ALVA-41 prostate cancer cells caused an upregulation of Bid (Ahmed, et al., unpublished results). Subsequent studies have shown Bid to be an interaction partner of the catalytic subunit CK2α (Olsen et al., 2006). In our studies employing TRAIL as inducer of apoptosis in prostate cancer cells, we have found Bcl-xL and Bcl-2 proteins to be sensitive to CK2 status altered by treating cells with chemical inhibitors of CK2 (Wang et al., 2006a). Inhibition or downregulation of CK2 results in loss of Bcl-xL and Bcl-2 proteins with Bax being upregulated, whereas overexpression of CK2 results in prevention of such changes in these proteins (Wang et al., 2006a). The engagement of the mitochondrial pathway is clearly indicated by the alterations in cytochrome c release upon downregulation of CK2 while it is blocked by overexpression of CK2 (Wang et al., 2006a). These various studies suggest that mitochondrial pathway plays a role in CK2 regulation of apoptotic activity and that certain of the Bcl-2 family of proteins in the apoptotic machinery are among the targets of CK2.
Attempts to identify proximal effectors of CK2 mediated modulation of apoptosis have revealed that intracellular H2O2 production upon downregulation of CK2 in prostate cancer cells may be an important signal for induction of apoptosis under these conditions (Ahmad et al., 2006). These studies have shown that downregulation of CK2 by employing chemical inhibitors of CK2 or antisense CK2α or siRNA for CK2α to achieve downregulation of CK2 in prostate cancer cells (both androgen-sensitive ALVA-41 and –insensitive PC-3 cells) results in rapid increase in intracellular H2O2 which may be responsible for triggering the downstream pathways resulting in release of cytochrome c, activation of caspase-3, downregulation of IκB, translocation of NF-κB p65, and subsequent DNA fragmentation. These novel observations implicate a relationship between reactive oxygen species and CK2 such that inhibition of CK2 may result in elevation of intracellular H2O2 leading to activation of its downstream targets in the apoptotic machinery (Ahmad et al., 2006). In this context, it is of interest to note that SAG (sensitive to apoptosis gene) protein which is upregulated on hypoxia induction undergoes degradation on phosphorylation by CK2 at Thr-10 (He et al., 2007).
Another locus of CK2 mediated modulation of apoptotic activity appears to be the inhibitor of apoptosis proteins (IAPs). Among these, survivin has been shown to be influenced by the CK2 status in cells (Tapia et al., 2006). In accord with these observations, ongoing work in our laboratory has also shown that survivin expression cIAPs expression is reduced in prostate cancer cells upon downregulation of CK2; further, we have observed that cIAP1, cIAP2, and xIAP are also engaged downstream of the CK2 signal (Ahmed et al., unpublished data).
Certain other genes that play a role in apoptosis are also affected by CK2. For example, in studies on TRAIL mediated induction of apoptosis, we observed downregulation of cFLIPL which was prevented by overexpression of CK2 in PC-3 prostate cancer cells (Wang et al., 2006a); the significance of this observation may relate to the recent documentation that cFLIPL expression is necessary and sufficient to maintain resistance to TRAIL-induced apoptosis in prostate cancer cells (Zhang et al., 2004). Upon phosphorylation by CK2, Max is rendered insensitive to cleavage by caspases (Krippner-Heidenreich et al., 2001) while Myc is stabilized by CK2 mediated phosphorylation (Channavajhala and Seldin, 2002). In the NF-κB pathway, downregulation of CK2 by employing antisense CK2α resulted in nuclear translocation of NF-κB p65 (Ahmad et al., 2006). Promotion of aberrant activation of nuclear factor-κB by CK2 in transformed phenotype of breast cancer cells has been documented (Romieu-Mourez et al., 2002; Eddy et al., 2005). Aberrant NF-κB activation by serum factors involving CK2 mediated activation of IKK2 has been reported in head and neck squamous carcinoma cells (Yu et al., 2006). Likewise, CK2 has been found to phosphorylate the Fas-associated factor FAF1 in vivo and influence its translocation to the nucleus (Olsen et al., 2003). A recent study has demonstrated that IGFBP-3 which is known to promote apoptosis in cancer cells is a substrate for CK2 mediated phosphorylation at ser-167, and that phosphorylation of this site by CK2 limits the ability of IGFBP-3 to induce apoptosis in prostate cancer cells (Cobb et al., 2007). Analogous to these studies is the recent documentation that PML tumor suppressor which controls key pathways of growth suppression, induction of apoptosis and cellular senescence loses can undergo phosphorylation by CK2 at ser-517 which results in its ubiquitin-mediated degradation. These authors also found an inverse relation between CK2 and PML protein level in human lung cancer (Scaglioni et al., 2006). PTEN, another important signal related to cell death and survival, appears to be stabilized on phosphorylation by CK2 (Vazquez et al., 2001). In this context, it is interesting to note that AKT (a protein kinase with a role in cell survival) which requires phosphorylation at Ser-473 and Thr-308 by cognate kinases for its activation also has been found to harbor a CK2-specific phosphorylation site at Ser-129 that appears to contribute to its hyperactivation (Di Maira et al., 2005). In a preliminary study, we also noted that forced overexpression of CK2 influenced AKT activation in ALVA-41 prostate cancer cells (Ahmed et al., unpublished data). Further support for the interaction of CK2 and AKT has been provided in a recent report (Guerra, 2006).
Concluding remarks
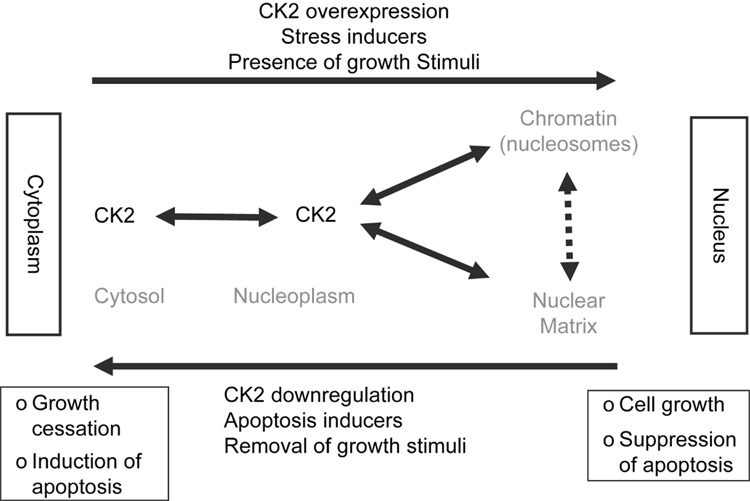
The brief overview of the functional activity of CK2 in the context of its ability to modulate cell death activity suggests that this signal has a broad ability to impact on diverse pathways engaged in the mediation of cell death. Fig. 1 depicts the dynamics of CK2 as involved in mediation of various functions in the cell under diverse conditions. As discussed above, a significance of these observations is that since CK2 downregulation by various means causes cell death, this approach has the potential of leading to novel strategies for cancer therapy. Studies in our laboratory are in progress along these line (Unger et al., 2004; Slaton et al., 2004; Ahmad et al., 2005; Wang et al., 2005b; Wang et al., 2006b).
Fig. 1.
Cell response in relation to spatio-temporal dynamics of CK2 as affected by various stimuli.
Summary
Protein kinase CK2 is a ubiquitous and highly conserved protein serine/threonine kinase that is indispensable for cell survival. CK2 has long been implicated in cell growth and proliferation, and studies from several laboratories have suggested that CK2 plays a global role in affecting cell growth related activities. Recently, we documented that CK2, besides its role in cell growth and proliferation, can potently suppress apoptosis. Considering that CK2 has been found to be elevated in all the cancers that have been examined, the ability of CK2 to suppress apoptosis is particularly important in the context of cancer cell pathobiology since these cells exhibit dysregulation of both cell proliferation and cell death. Thus, overexpression of CK2 in cancer cells may impart a survival advantage by its action as a suppressor of apoptotic activity in these cells while promoting cell growth. In experimental studies, we have shown that overexpression of CK2 in cells can potently inhibit apoptosis mediated by a variety of agents including removal of survival factors, chemical and physical agents, and death receptor ligands. On the other hand, inhibition of CK2 by chemical inhibitors or by its molecular downregulation by antisense CK2 ODN or siRNA leads to potent induction of apoptosis. Downregulation of CK2 is associated with apoptosis mediated via effects on several downstream targets, and it appears that CK2 may have a global impact on the apoptotic machinery. While CK2 is present in both the nuclear and cytoplasmic compartments, several of its cell growth and cell death related activities appear to be associated with its signalling to the nuclear structures such as chromatin and nuclear matrix. In general, shuttling of CK2 to these compartments correlates with its role in cell growth and suppression of apoptotic activity whereas loss of CK2 from the nuclear structures is associated with induction of apoptosis and cessation of cell growth. These various observations on the biology of CK2 have led to our original proposal that CK2 is a potentially important target for cancer chemopreventive and therapeutic approaches; this is now being substantiated by recent studies.
Acknowledgements
The original investigations by the authors are supported in part by a U.S.P.H.S. Research Grant CA-15062 awarded by the National Cancer Institute, Department of Health and Human Services, and by the Medical Research Funds of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad KA, Harris NH, Johnson AD, Lindvall HCN, Wang G, Ahmed K. Protein kinase CK2 modulates apoptosis induced by resveratrol and EGCG in prostate cancer cells. Mol Cancer Therap. 2007;6:1006–1012. doi: 10.1158/1535-7163.MCT-06-0491. [DOI] [PubMed] [Google Scholar]
- Ahmad KA, Wang G, Ahmed K. Intracellular Hydrogen Peroxide Production is an Upstream Event in Apoptosis Induced by Downregulation of CK2 in Prostate Cancer Cells. Mol Cancer Res. 2006;4:331–338. doi: 10.1158/1541-7786.MCR-06-0073. [DOI] [PubMed] [Google Scholar]
- Ahmad KA, Wang G, Slaton J, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anti-Cancer Drugs. 2005;16:1037–1044. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- Ahmed K. Nuclear matrix and protein kinase CK2 signaling. Crit Revs Eukaryotic Gene Expression. 1999;9:329–336. doi: 10.1615/critreveukargeneexpr.v9.i3-4.170. [DOI] [PubMed] [Google Scholar]
- Ahmed K, Davis AT, Wang H, Faust RA, Yu S, Tawfic S. Significance of protein kinase CK2 nuclear signaling in neoplasia. J Cell Biochem Supp. 2000;35:130–135. doi: 10.1002/1097-4644(2000)79:35+<130::aid-jcb1136>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: An emerging role for protein kinase CK2. Trends in Cell Biology. 2002;12:226–230. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- Ahmed K, Ishida H. Effects of testosterone on nuclear phosphoproteins of rat ventral prostate. Mol Pharmacol. 1971;7:323–327. [PubMed] [Google Scholar]
- Ahmed K, Yenice S, Davis A, Goueli SA. Association of casein kinase 2 (CK-2) with nuclear chromatin in relation to androgenic regulation of rat prostate. Proc Natl Acad Sci USA. 1993;90:4426–4430. doi: 10.1073/pnas.90.10.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende JE, Allende CC. Protein kinase CK2 : an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- Bouchou T, Vernet M, Blond O, Jensen HH, Pointou H, Olsen BB, Cochet C, Issinger O-G, Boldyreff B. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol. 2003;23:908–915. doi: 10.1128/MCB.23.3.908-915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 2002;21:5280–5288. doi: 10.1038/sj.onc.1205640. [DOI] [PubMed] [Google Scholar]
- Cobb L, Koyama S, Cohen P. Site-specific phosphorylation by intracellular kinases determines the apoptotic activity of IGFBP-3 in prostate cancer; Los Angeles, CA. AACR Annual Meeting Proceedings; 2007. Abstract #4393. [Google Scholar]
- Davis AT, Wang H, Zhang P, Ahmed K. Heat Shock Mediated Modulation of Protein Kinase CK2 in the Nuclear Matrix. J Cell Biochem. 2002;85:583–591. doi: 10.1002/jcb.10158. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- Di Maira G, Brustolon F, Bertacchini J, Tosoni K, Marmiroli S, Pinna LA, Ruzzene M. Pharmacological inhibition of protein kinase CK2 reverts the multidrug resistance phenotype of a CEM cell line characterized by high CK2 level. Oncogene. 2007 doi: 10.1038/sj.onc.1210495. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differentiation. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- Eddy SF, Guo S, Demicco EG, Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Inducible IkappaB kinase/IkappaB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-kappaB activation in breast cancer cells. Cancer Res. 2005;65:11375–11383. doi: 10.1158/0008-5472.CAN-05-1602. [DOI] [PubMed] [Google Scholar]
- Faust M, Montenarh M. Subcellular localization of protein kinase CK2 – A key to its function? Cell & Tissue Res. 2000;301:329–340. doi: 10.1007/s004410000256. [DOI] [PubMed] [Google Scholar]
- Faust RA, Gapany M, Tristani P, Davis A, Adams GL, Ahmed K. Elevated protein kinase CK2 activity in chromatin of head and neck tumors: Association with malignant transformation. Cancer Letts. 1996;101:31–35. doi: 10.1016/0304-3835(96)04110-9. [DOI] [PubMed] [Google Scholar]
- Faust RA, Niehans G, Gapany M, Knapp D, Cherwitz D, Davis A, Adams GL, Ahmed K. Subcellular immunolocalization of protein kinase CK2 in squamous cell carcinomas of the head and neck. Int J Biochem Cell Biol. 1999;31:941–949. doi: 10.1016/s1357-2725(99)00050-3. [DOI] [PubMed] [Google Scholar]
- Faust RA, Tawfic S, Davis AT, Bubash LA, Ahmed K. Antisense oligodeoxynucleotides against protein kinase CK2α inhibit growth of squamous cell carcinoma of head and neck in vitro. Head and Neck. 2000;22:341–346. doi: 10.1002/1097-0347(200007)22:4<341::aid-hed5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Filhol O, Martiel J-L, Cochet C. Protein kinase CK2: a new view of an old molecular complex. EMBO Rep. 2004;5:351–355. doi: 10.1038/sj.embor.7400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapany M, Faust RA, Tawfic S, Davis A, Adams G, Ahmed K. Association of elevated protein kinase CK2 activity with aggressive behavior of squamous cell carcinoma of the head and neck. Molec Med. 1995;1:659–666. [PMC free article] [PubMed] [Google Scholar]
- Goueli S, Ahmed K. Nature of intrinsic protein kinases involved in phosphorylation of non-histone proteins in intact prostatic nuclei: Further identification of androgen-sensitive protein kinase reaction. Mol Cell Biochem. 1991;101:145–155. doi: 10.1007/BF00229531. [DOI] [PubMed] [Google Scholar]
- Graham KC, Litchfield DW. The regulatory β subunit of protein kinase CK2 mediates formation of tetrameric CK2 complexes. J Biol Chem. 2000;275:5003–5010. doi: 10.1074/jbc.275.7.5003. [DOI] [PubMed] [Google Scholar]
- Guerra B. Protein kinase CK2 subunits are positive regulators of AKT kinase. Int J Oncol. 2006;28:685–693. [PubMed] [Google Scholar]
- Guerra B, Issinger O-G. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis. 1999;20:391–408. doi: 10.1002/(SICI)1522-2683(19990201)20:2<391::AID-ELPS391>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Guerra B, Siemer S, Boldyreff B, Issinger OG. Protein kinase CK2: evidence for a proteinase CK2β subunit fraction, devoid of the catalytic CK2α subunit in mouse brain and testicles. FEBS Lett. 1999;462:353–357. doi: 10.1016/s0014-5793(99)01553-7. [DOI] [PubMed] [Google Scholar]
- Guo C, Davis AT, Ahmed K. Dynamics of protein kinase CK2 association with nucleosomes in relation to transcriptional activity. J Biol Chem. 1998;273:13675–13680. doi: 10.1074/jbc.273.22.13675. [DOI] [PubMed] [Google Scholar]
- Guo C, Yu S, Davis AT, Ahmed K. Nuclear matrix targeting of the protein kinase CK2 signal as common downstream response to androgen or growth factor stimulation of prostate cancer cells. Cancer Res. 1999a;59:1146–1151. [PubMed] [Google Scholar]
- Guo C, Davis AT, Yu S, Tawfic S, Ahmed K. Role of protein kinase CK2 in phosphorylation of nucleosomal proteins in relation to transcriptional activity. Mol Cell Biochem. 1999b;191:135–142. [PubMed] [Google Scholar]
- Guo C, Yu S, Davis AT, Green JE, Ahmed K. A potential role of nuclear matrix-associated protein kinase CK2 in protection against drug-induced apoptosis in cancer cells. J Biol Chem. 2001;276:5992–5999. doi: 10.1074/jbc.M004862200. [DOI] [PubMed] [Google Scholar]
- Hamacher R, Saur D, Fritsch R, Reichert M, Schmid RM, Schneider G. Casein kinase II inhibition induces apoptosis in pancreatic cancer cells. Oncol Rep. 2007;18:695–701. [PubMed] [Google Scholar]
- He H, Tan M, Pamarthy D, Wang G, Ahmed K, Sun Y. CK2-mediated phosphorylation of SAG at Thr 10 regulates its stability but not its E3 ligase activity. Mol Cell Biochem. 2007;295:179–188. doi: 10.1007/s11010-006-9287-3. [DOI] [PubMed] [Google Scholar]
- Izeradjene K, Douglas L, Delaney A, Houghton JA. Casein kinase II (CK2) enhances death-inducing signaling complex (DISC) activity in TRAIL-induced apoptosis in human colon carcinoma cell lines. Oncogene. 2005;24:2050–2058. doi: 10.1038/sj.onc.1208397. [DOI] [PubMed] [Google Scholar]
- Kato T, Delhase M, Hoffmann A, Karin M. CK2 is a C-terminal IκB kinase responsible for NF-κB activation during the UV response. Mol Cell. 2003;12:829–839. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- Keller DM, Zeng X, Wang Y, Zhang QH, Kapoor M, Shu H, Goodman R, Lozano G, Zhao Y, Lu H. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol Cell. 2001;7:283–292. doi: 10.1016/s1097-2765(01)00176-9. [DOI] [PubMed] [Google Scholar]
- Kelliher MA, Seldin DC, Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIα. EMBO J. 1996;15:5160–5166. [PMC free article] [PubMed] [Google Scholar]
- Krippner-Heidenreich A, Talanian RV, Sekul R, Kraft R, Thole H, Ottleben H, Luscher B. Targeting of the transcription factor Max during apoptosis: phosphorylation-regulated cleavage by caspase-5 at an unusual glutamic acid residue in position P1. Biochem J. 2001;358:705–715. doi: 10.1042/0264-6021:3580705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N, Bruckheimer EM, Guo Y. Cell proliferation and apoptosis in prostate cancer: significance in disease progression and therapy. Histol Histopathol. 2000;15:1211–1223. doi: 10.14670/HH-15.1211. [DOI] [PubMed] [Google Scholar]
- Laramas M, Pasquier D, Filhol O, Ringeisen F, Desxotes J-L, Cochet C. Nuclear localization of protein kinase CK2 catalytic subunit (CK2α) is associated with poor prognostic factors in human prostate cancer. Eur J Cancer. 2007;43:928–934. doi: 10.1016/j.ejca.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Li PF, Li J, Muller EC, Otto A, Dietz R, von Harsdorf R. Phosphorylation by protein kinase CK2: a signaling switch for the caspase-inhibiting protein ARC. Mol Cell. 2002;10:247–258. doi: 10.1016/s1097-2765(02)00600-7. [DOI] [PubMed] [Google Scholar]
- Litchfield DW. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, Sarno S, Meggio F, Pinna LA, Caldecott KW. The protein kinase CK2 facilitates repair of chromosomal DNA single stranded breaks. Cell. 2004;117:17–28. doi: 10.1016/s0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- Martel V, Filhol O, Nueda A, Cochet C. Dynamic localization/association of protein kinase CK2 subunits in living cells: a role in its cellular regulation? Ann NY Acad Sci. 2002;973:272–277. doi: 10.1111/j.1749-6632.2002.tb04648.x. [DOI] [PubMed] [Google Scholar]
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- Mishra S, Pertz V, Zhang B, Kaur P, Shimada H, Groffen J, Kaziemierczuk Z, Pinna LA, Heisterkamp N. Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. Leukemia. 2007;21:178–180. doi: 10.1038/sj.leu.2404460. [DOI] [PubMed] [Google Scholar]
- Okoumassoun LE, Russo C, Denizeau F, Averill-Bates D, Henderson JE. Parathyroid hormone-related protein (PTHrP) inhibits mitochondrial-dependent apoptosis through CK2. J Cell Physiol. 2007;212:591–599. doi: 10.1002/jcp.21055. [DOI] [PubMed] [Google Scholar]
- Olsen BB, Jessen V, Højrup P, Issinger OG, Boldyreff B. Protein kinase CK2 phosphorylates the Fas-associated factor FAF1 in vivo and influences its transport into the nucleus. FEBS Lett. 2003;546:218–222. doi: 10.1016/s0014-5793(03)00575-1. [DOI] [PubMed] [Google Scholar]
- Olsen BB, Petersen J, Issinger O-G. BID, an interaction partner of protein kinase CK2α. Biol Chem. 2006;387:441–449. doi: 10.1515/BC.2006.059. [DOI] [PubMed] [Google Scholar]
- Olsten MEK, Weber JE, Litchfield DW. CK2 interacting proteins: emerging paradigms for CK2 regulation? Mol Cell Biochem. 2005;274:115–124. doi: 10.1007/s11010-005-3072-6. [DOI] [PubMed] [Google Scholar]
- Padmanabha R, Chen-Wu JLP, Hanna DE, Glover CVC. Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in S. cerevisiae. Mol Cell Biol. 1999;10:4089–4099. doi: 10.1128/mcb.10.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Poletto G, Di Maira G, Cozza G, Ruzzene M, Sarno S, Bain J, Elliott M, Moro S, Zagotto G, Meggio F, Pinna LA. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. ChemBioChem. 2007;8:129–139. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- Perea SE, Reyes O, Puchades Y, Mendoza O, Vispo NS, Torrens I, Santos A, Silva R, Acevedo B, López E, Falcón V, Alonso DF. Antitumor effect of a novel proapoptotic peptide that impairs the phosphorylation by the protein kinase 2 (casein kinase 2) Cancer Res. 2004;64:7127–7129. doi: 10.1158/0008-5472.CAN-04-2086. [DOI] [PubMed] [Google Scholar]
- Piazza FA, Ruzzene M, Currieri C, Montini B, Bonanni L, Chioetto G, Di Maira G, Barbon F, Cabrelle A, Zambello R, Adami F, Trentin L, Pinna LA. Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood. 2006;108:1698–1707. doi: 10.1182/blood-2005-11-013672. [DOI] [PubMed] [Google Scholar]
- Pinna LA. Protein kinase CK2: A challenge to canons. J Cell Sci. 2002;115:3873–3878. doi: 10.1242/jcs.00074. [DOI] [PubMed] [Google Scholar]
- Pyerin W, Ackermann K. The genes encoding human protein kinase CK2 and their functional links. Progr Nucl Acid Res Mol Biol. 2003;74:239–273. doi: 10.1016/s0079-6603(03)01015-8. [DOI] [PubMed] [Google Scholar]
- Ravi R, Bedi A. Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. Cancer Res. 2002;62:4180–4185. [PubMed] [Google Scholar]
- Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor-κB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002;62:6770–6778. [PubMed] [Google Scholar]
- Ruzzene M, Penzo D, Pinna LA. Protein kinase CK2 inhibitor 4,5,6,7- tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem J. 2002;364:41–47. doi: 10.1042/bj3640041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi M, Sarno S, Marin O, Meggio F, Itarte E, Pinna LA. Discrimination between the activity of protein kinase CK2 holoenzyme and its catalytic subunits. FEBS Lett. 2006;580:3948–3952. doi: 10.1016/j.febslet.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Scaglioni PP, Yung TM, Cai LF, Erdjument-Bromage H, Kaufman AJ, Singh B, Teruya-Feldstein J, Tempst P, Pandolfi PP. Cell. 2006;126:269–283. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Seeber S, Issinger O-G, Holm T, Kristensen LP, Guerra B. Validation of protein kinase CK2 as oncological target. Apoptosis. 2005;10:875–885. doi: 10.1007/s10495-005-0380-y. [DOI] [PubMed] [Google Scholar]
- Shin S, Lee Y, Kim W, Ko H, Choi H, Kim K. Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J. 2005;24:3532–3542. doi: 10.1038/sj.emboj.7600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaton JW, Sloper DT, Unger G, Davis A, Ahmed K. Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model. Mol Cancer Res. 2004;2:712–721. [PubMed] [Google Scholar]
- Tapia JC, Torres VA, Rodriguez DA, Leyton L, Quest AF. Casein kinase 2 (CK2) increases survivin expression via enhanced beta-catenin-T cell factor/lymphoid enhancer binding factor-dependent transcription. Proc Natl Acad Sci USA. 2006;103:15079–15084. doi: 10.1073/pnas.0606845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfic S, Ahmed K. Association of casein kinase 2 (CK-2) with nuclear matrix: Possible role in nuclear matrix protein phosphorylation. J Biol Chem. 1994a;269:7489–7493. [PubMed] [Google Scholar]
- Tawfic S, Ahmed K. Growth stimulus-mediated differential translocation of casein kinase 2 to the nuclear matrix: Evidence based on androgen action in the prostate. J Biol Chem. 1994b;269:24615–24620. [PubMed] [Google Scholar]
- Tawfic S, Davis AT, Faust RA, Gapany M, Ahmed K. Association of protein kinase CK2 with nuclear matrix: Influence of method of preparation of nuclear matrix. J Cell Biochem. 1997;64:499–504. [PubMed] [Google Scholar]
- Tawfic S, Faust RA, Gapany M, Ahmed K. Nuclear Matrix as an Anchor for Protein Kinase CK2 Nuclear Signalling. J Cell Biochem. 1996;62:165–171. doi: 10.1002/(sici)1097-4644(199608)62:2<165::aid-jcb4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol Histopathol. 2001;16:573–582. doi: 10.14670/HH-16.573. [DOI] [PubMed] [Google Scholar]
- Unger GM, Davis AT, Slaton JW, Ahmed K. Protein kinase CK2 as regulator of cell survival: Implications for cancer therapy. Curr Cancer Drug Targets. 2004;4:77–84. doi: 10.2174/1568009043481687. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Ahmed K. Modulation of death receptor-mediated apoptosis by CK2. Mol Cell Biochem. 2005a;274:201–205. doi: 10.1007/s11010-005-2952-0. [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Ahmed K. Role of CK2 in regulation of TRAIL induced apoptosis in prostate cancer cells. Cancer Res. 2006a;66:2242–2249. doi: 10.1158/0008-5472.CAN-05-2772. [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Unger G, Slaton JW, Ahmed K. CK2 signaling in androgen-dependent and –independent prostate cancer. J Cell Biochem. 2006b;99:382–391. doi: 10.1002/jcb.20847. [DOI] [PubMed] [Google Scholar]
- Wang G, Unger G, Ahmad KA, Slaton JW, Ahmed K. Downregulation of CK2 induces apoptosis in cancer cells – A potential approach to cancer therapy. Mol Cell Biochem. 2005b;274:77–84. doi: 10.1007/s11010-005-3077-1. [DOI] [PubMed] [Google Scholar]
- Wang H, Davis A, Yu S, Ahmed K. Response of cancer cells to molecular interruption of the CK2 signal. Mol Cell Biochem. 2001;227:167–174. [PubMed] [Google Scholar]
- Wang H, Yu S, Davis AT, Ahmed K. Cell cycle dependent regulation of protein kinase CK2 signaling to the nuclear matrix. J Cell Biochem. 2003;88:812–822. doi: 10.1002/jcb.10438. [DOI] [PubMed] [Google Scholar]
- Xu X, Landesman-Bollag E, Channavajhala PL, Seldin DC. Murine protein kinase CK2: gene and oncogene. Mol Cell Biochem. 1999;191:65–74. [PubMed] [Google Scholar]
- Yamane K, Kinsella TJ. CK2 inhibits apoptosis and changes its cellular localization following ionizing radiation. Cancer Res. 2005;65:4362–4367. doi: 10.1158/0008-5472.CAN-04-3941. [DOI] [PubMed] [Google Scholar]
- Yenice S, Davis AT, Goueli SA, Akdas A, Limas E, Ahmed K. Nuclear Casein kinase 2 (CK-2) activity in human normal, benign hyperplastic, and cancerous prostate. The Prostate. 1994;24:11–16. doi: 10.1002/pros.2990240105. [DOI] [PubMed] [Google Scholar]
- Yu M, Yeh J, Van Waes C. Protein kinase casein kinase 2 mediates inhibitor-κB kinase and aberrant nuclear factor-κB activation by serum factor(s) in head and neck squamous carcinoma cells. Cancer Res. 2006;66:6722–6731. doi: 10.1158/0008-5472.CAN-05-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Wang H, Davis A, Ahmed K. Consequences of CK2 signaling to the nuclear matrix. Mol Cell Biochem. 2001;227:67–71. [PubMed] [Google Scholar]
- Zhang P, Davis AT, Ahmed K. Mechanism of protein kinase CK2 association with nuclear matrix: Role of disulfide bond formation. J Cell Biochem. 1998;69:211–220. [PubMed] [Google Scholar]
- Zhang X, Jin T-G, Yang H, DeWolf WC, Khosravi-Far R, Olumi AF. Persistent c-FLIP(L) expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in prostate cancer. Cancer Res. 2004;64:7086–7091. doi: 10.1158/0008-5472.CAN-04-1498. [DOI] [PubMed] [Google Scholar]