Abstract
Prions are the infectious agents responsible for prion diseases, which appear to be composed exclusively by the misfolded prion protein (PrPSc). The mechanism of prion transmission is unknown. In this study we attempted to detect prions in urine of experimentally infected animals. PrPSc was detected in ~ 80% of the animals studied, whereas no false positives were observed among the control animals. Semi-quantitative calculations suggest that PrPSc concentration in urine is around 10-fold lower than in blood. Interestingly, PrPSc present in urine maintains its infectious properties. Our data indicate that low quantities of infectious prions are excreted in the urine. These findings suggest that urine is a possible source of prion transmission.
Keywords: Prion, transmissible spongiform encephalopathy, PMCA, diagnosis, scrapie
1. Introduction
Prions are the infectious agents responsible for a group of fatal neurodegenerative diseases, collectively called Transmissible Spongiform Encephalopathies (TSEs) that affect humans and several species of mammals [1–3]. Creutzfeldt-Jakob disease (CJD) is the most common TSE in humans, and scrapie in sheep, Bovine spongiform encephalopathy (BSE) in cattle and chronic wasting disease (CWD) in cervids are the most prevalent prion diseases in animals [2]. Although prion diseases are relatively rare in humans, the recent appearance of variant CJD (vCJD), which is linked to consumption of BSE contaminated food, have raised concern about a possible epidemic outbreak in the human population [4]. BSE is still a significant health and economical problem, and other animal diseases have become a permanent source of concern. Scrapie, for example, is endemic in various countries and CWD is spreading dramatically fast among wild and captive cervids in North America. Finally, it is now clear that vCJD can be iatrogenically transmitted from human to human by blood transfusion raising fears of a second wave of infection [5,6].
Unlike conventional infectious micro-organisms, the TSE agent appears to be devoid of genetic material and instead composed exclusively by a misfolded form of the prion protein (PrPSc) [3]. PrPSc has the intriguing ability to replicate in the body of infected individuals by propagating its misfolding to the normal prion protein (PrPC) [3]. PrPSc is not only the main component of the infectious agent and the most likely triggering factor in brain damage, but it is also the only validated surrogate biomarker for the disease and its sensitive detection is critical for disease diagnosis and to prevent further spreading of TSEs [7]. Currently, there is no validated method to detect PrPSc. Hampering the efforts to develop a reliable biochemical diagnosis for TSEs is the fact that PrPSc quantity in peripheral tissues or biological fluids is extremely low and under the limit of detection of standard techniques [7].
With the aim of facilitating PrPSc biochemical detection, we have developed a novel technique that enables PrPSc amplification in the test tube. This method, termed protein misfolding cyclic amplification (PMCA), is based on converting large amounts of PrPC triggered by undetectable quantities of PrPSc [8]. In a cyclic manner, conceptually analogous to polymerase chain-reaction (PCR), PrPSc is incubated with excess PrPC to enlarge the PrPSc aggregates, which are then sonicated to generate multiple smaller units for the continued formation of new PrPSc [8]. The newly generated protein exhibits the same biochemical and structural properties as brain-derived PrPSc and strikingly it is infectious to wild-type animals, producing a disease with similar characteristics to the illness produced by brain-isolated prions [9]. PMCA is highly specific for detection of PrPSc and leads to several million folds increase on sensitivity as compared to standard western blot assays [10,11]. The technology has been applied to replicate the misfolded protein from diverse species [12] and has enabled detection of prions in the blood of infected animals, both at the symptomatic and pre-symptomatic phases of the disease [10,13].
Although the highest concentration of PrPSc is present in the nervous system, its presence has been reported with a variable degree of success in peripheral tissues, such as lymphoid organs, peripheral nerves, skeletal muscle, kidney, mammary glands, olfactory mucosa and CSF (for reviews, see [7,14,15]). Blood and urine represent the ideal biological fluids for routine non-invasive diagnosis. Various reports of experimental and natural transmission have shown that blood carries infectivity (for reviews, see [6,15,16]) and as described above, we have been able to detect successfully PrPSc in animal blood. Although, experiments with urine have been for the most part negative, recent studies reported very small infectivity titers in urine of a tiny proportion of scrapie sick rodents [17,18]. However, PrPSc was not detected in these studies, presumably because the quantity secreted in the urine is below the limit of detection of the technology employed. The main goal of the current study is to attempt detection of PrPSc in urine of experimentally infected animals using the highly sensitive PMCA technology.
2. Materials and Methods
Sample collection and Preparation
Urine from healthy and sick animals was collected using metabolic cages. Sick and healthy animals were of similar age. Urine was processed as schematically described in Figure 1A. Brain tissue from healthy hamsters was used in PMCA reactions as a substrate for amplification. To prepare brain homogenate, animals were first perfused with phosphate-buffered saline (PBS) plus 5 mM EDTA prior to harvesting the tissue. Ten percent brain homogenates (w/v) were prepared in conversion buffer (PBS containing NaCl 150mM, 1.0% Triton X-100, 4mM EDTA and the Complete Protease Inhibitor Cocktail from Roche, Switzerland). The samples were clarified by a brief, low-speed centrifugation (1500 rpm for 30s) using an Eppendorf centrifuge (Hamburg, Germany), model 5414. Samples were stored frozen at −80°C.
Figure 1. Identification of the experimental conditions to process urine samples for PrPSc detection by PMCA.
Urine from several hamsters at the symptomatic stage of the disease (and uninfected controls) produced by i.p. inoculation of 263K prions was collected with metabolic cages. Twelve ml of urine was pooled and processed as schematically illustrated in panel A. First, urine was centrifuged at a low speed (5,000 × g for 20 min) to remove debris. The supernatant was collected and divided in 2 groups: one was dialyzed overnight against PBS at 4°C using a membrane with 30,000 Da cutoff; the other sample was left untreated. Both samples were divided into two identical aliquots, one of which was supplemented with 1 volume of 20% sarkosyl and the other one was left untreated. After 30 min incubation at 4°C, all the samples were centrifuged at 100,000 × g for 1h at 4°C. Pellet of each sample was resuspended directly in 80 ul of 10% normal hamster brain homogenate and subjected to 96 cycles of PMCA (30 min incubation at 37°C followed by a pulse of 30 s sonication, as described in Methods). Then, 8 µl of this sample were diluted into 72µl of normal brain homogenate and a new round of 96 PMCA cycles was performed. This process was repeated several times. After each round of PMCA, 20 µl of the sample was used for detection of PrPSc by western blot after PK digestion (50 ug/ml for 1h at 37°C), using 3F4 antibody. Panel B shows the results of serial rounds 5 and 6 of PMCA. No signal was observed before the fifth round. Sx: samples from sick animals; Cx: samples from control animals. All samples were treated with PK before electrophoresis, except the normal brain homogenate (NBH) in which –PK is indicated.
PMCA Procedure
Urine samples after the processing described in figure 1Awere resuspended into 10% healthy brain homogenate. Samples were loaded onto 0.2-ml PCR tubes and positioned on an adaptor placed on the plate holder of a microsonicator (Misonix Model 3000, Farmingdale, NY). Each PMCA cycle consisted of 30 min incubation at 37°C followed by a 20 sec pulse of sonication set at potency of 7. Samples were incubated without shaking immersed in the water of the sonicator bath. The microplate horn was kept in an incubator set at 37° C during the whole process. After a round of 96 cycles, an aliquot of the amplified material was diluted 10-folds into normal brain homogenate and a new round of 96 PMCA cycles was performed. This procedure was repeated several times as indicated in the text. The detailed protocol for PMCA, including reagents, methods and troubleshooting, has been published elsewhere [19,20].
PrPSc detection
Samples were incubated with 50 µg/ml of PK for 60 min at 45°C with shaking. The digestion was stopped by adding electrophoresis sample buffer. Proteins were fractionated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, electroblotted into nitrocellulose membrane, and probed with 3F4 antibody (Signet, Dedham, MA) diluted 1:5,000 in PBS, 0.05% Tween-20. The immunoreactive bands were visualized by enhanced chemoluminesence assay (Amersham, Piscataway, NJ). Western blots signals were analyzed by densitometry, using a UVP Bioimaging system EC3 apparatus (Upland, CA).
In Vivo Infectivity Studies
Syrian Golden hamsters were used as an experimental model of scrapie. Animals were 4- to 6-weeks old at the time of inoculation. For urine collection animals were injected i.p. with 100 ul of 10% brain homogenate. To assess infectivity, anesthesized animals were injected stereotaxically into the right hippocampus with 2 µl of the sample. The onset of clinical disease was measured by scoring the animals twice a week using the following scale: 1, Normal animal; 2, Mild behavioral abnormalities including hyperactivity and hypersensitivity to noise; 3, Moderate behavioral problems including tremor of the head, ataxia, wobbling gait, head bobbing, irritability and aggressiveness; 4, Severe behavioral abnormalities including all of the above plus jerks of the head and body and spontaneous backrolls; 5, Terminal stage of the disease in which the animal lies in the cage and is no longer able to stand up. Animals scoring level 4 during two consecutive weeks were considered sick and were sacrificed to avoid excessive pain using exposition to carbonic dioxide. Brains were extracted and one hemisphere was frozen for biochemical studies and the other was used for histological analysis. The scrapie infectious material used in these studies was titrated and 1 LD50 was obtained in a brain dilution of approximately 1 × 109.
Histopathological studies
Brain tissue was fixed in 10% formaldehyde solution, cut in sections and embedded in paraffin. Serial sections (6µm thick) from each block were stained with hematoxylin-eosin, or incubated with monoclonal antibodies recognizing PrP or the glial fibrillary acidic protein, using our previously described protocols [9]. Immunoreactions were developed using the peroxidase-antiperoxidase method, following manufacturer’s specifications. Antibody specificity was verified by absorption. Samples were visualized with a Zeiss microscope.
3. Results
In order to attempt PrPSc detection using PMCA, we collected urine from several hamsters at the symptomatic phase of the disease produced by intraperitoneal (i.p.) inoculation of 263K prions. First, we tested several experimental conditions to process urine samples for PrPSc detection by PMCA. Urine from either sick or control animals was collected using metabolic cages. Samples were pooled and processed as described in Figure 1A. Four different conditions were tested including combinations between dialysis and sarkosyl precipitation. After processing, the samples were subjected to several rounds of 96 cycles of PMCA as described in Methods. After each round of PMCA, 20 µl of the sample was used for detection of PrPSc by western blot after PK digestion. As shown in Fig. 1B, after 6 serial rounds of PMCA, PrPSc was detectable in 3 of the 4 conditions tested. The conclusion of these results is that a simple high speed centrifugation, after removing large debris, is sufficient to obtain an adequate sample. Neither the dialysis nor the sarkosyl precipitation steps gave better results in terms of PMCA amplification.
To evaluate the presence of PrPSc in individual samples of urine, we collected urine from 5 scrapie sick animals i.p. infected by the hamster strain Hyper (HY) as well as control un-infected animals of similar age. As before, several serial rounds of PMCA were unsuccessful to detect PrPSc in any of the urine samples tested. However, after 6, 7 or 8 rounds of 96 PMCA cycles each, we were able to detect a PrPSc signal by western blot in the urine of 3 or 4 of the 5 animals studied, respectively (Fig 2). Conversely, PrPSc was not detected in the urine of any of the 5 control uninfected hamsters tested, indicating that the procedure is specific for detection of prion infected samples. These results indicate that PMCA enable detection of PrPSc in urine of scrapie sick hamsters with 80% sensitivity and 100% specificity. Larger number of samples would be needed to have a more accurate estimation of sensitivity and specificity. It should be noted that although we have been able recently to generate infectious PrPSc “de novo” (without the addition of brain PrPSc), this requires modification of some PMCA parameters (unpublished observations). Under standard PMCA conditions, as those used in this study, spontaneous generation of infectious material does not occur within the number of cycles used in this study.
Figure 2. PrPSc detection in urine of sick hamsters by PMCA.
Three ml of urine from 5 clinically sick hamsters (infected i.p. with HY prions) and 5 control animals was collected using metabolic cages. The samples were processed as described in figure A1. Briefly, urine was centrifuged at 5,000 × g for 20 min to remove debris. The supernatant was collected and subjected to a high speed centrifugation at 100,000 × g for 1h at 4°C to precipitate PrPSc. Pellet was resuspended directly in 80 ul of 10% normal hamster brain homogenate. Samples were subjected to serial rounds of 96 cycles of PMCA, as described [10,11]. 20 µl of the sample was used for detection of PrPSc by western blot after PK digestion. The figure shows only the rounds 5, 6, 7 and 8, since the first four rounds of PMCA did not show signal in any of the samples. More than 7 rounds of PMCA do not show any more positive signals. Sx: samples from sick animals; Cx: samples from control animals. All samples were treated with PK before electrophoresis, except the normal brain homogenate (NBH) in which –PK is indicated.
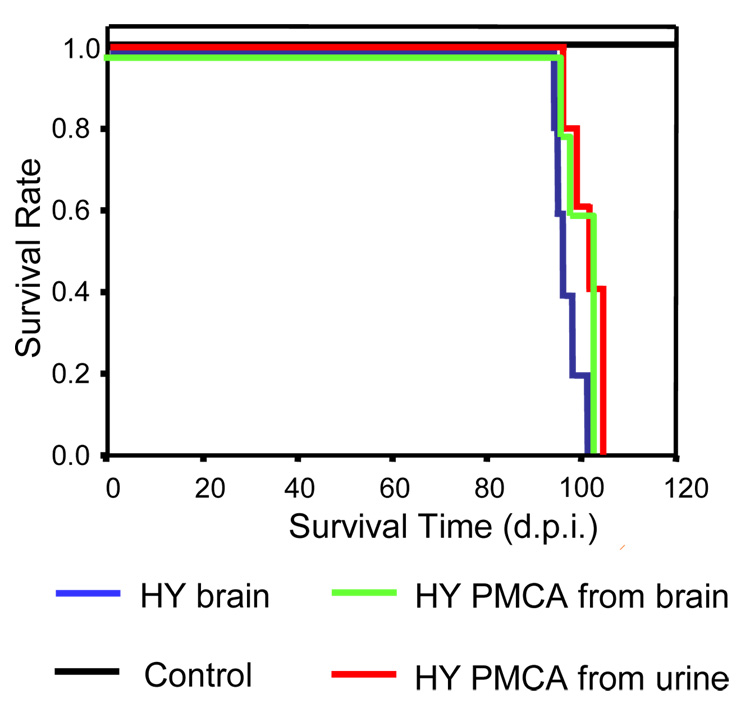
To assess whether PrPSc present in urine has the conformational properties required to produce infectivity, in vitro generated PrPSc by PMCA starting from urine was injected into wild type hamsters. All inoculated animals developed clear signs of HY prion disease at an average of 101.6 days after inoculation (Fig. 3). The incubation period was not significantly different to that obtained with a similar quantity of PrPSc obtained from the brain of sick animals (97.8 days) or PMCA-generated starting with HY brain inoculum (100.1 days) (Fig. 3). Conversely, none of the animals inoculated with the equivalent material, but starting from urine of normal animals, developed disease. The disease exhibits the clinical characteristics typical of HY hamster scrapie, including hyperactivity, motor impairment, head wobbling, muscle weakness and aggressiveness. Histopathological and biochemical studies confirmed that the disease produced from urinary prions exhibited the typical characteristics of HY scrapie, including spongiform degeneration (Fig. 4A), PrPSc accumulation (Fig. 4B) and astroglyosis (Fig. 4C). The quantity and biochemical characteristics of PrPSc were also indistinguishable from HY prions (Fig. 4D). This data indicates that the infectious properties and strain characteristics of prions are maintained in the urinary excreted PrPSc.
Figure 3. Urinary PrPSc is infectious.
To assess whether PrPSc amplified from urine maintain the infectious properties, we inoculated intra-cerebrally 5 wild type hamsters with the sample S4 after 7 serial rounds of PMCA. As controls, groups of hamsters were inoculated with equivalent quantities of brainderived HY PrPSc and PMCA-generated PrPSc starting from HY brain. The later was generated as previously described (9). Briefly a 104 dilution of HY brain was diluted into healthy hamster brain homogenate and subjected to 48 PMCA cycles. Thereafter, an aliquot of the amplified material was diluted 10-fold into healthy hamster brain homogenate and amplified again. This procedure was repeated to reach a 1020 dilution of brain inoculum in order to eliminate any brain derived PrPSc. A negative control group was included consisting of normal urine samples subjected to the same procedure of serial PMCA amplification. The onset of clinical signs was monitored as described in Methods and animals were considered sick when they reach clinical level 4, characterized by extensive behavioral problems including tremor of the head, ataxia, wobbling gait, head bobbing, irritability, aggressiveness, jerks of the head and body and spontaneous backrolls. At this time, animals were sacrificed to avoid further pain and this is the number provided in the graph.
Figure 4. Pathological and biochemical features of the disease produced by inoculation of PrPSc amplified from urine.
As positive control we used animals inoculated with brain-derived HY prions and as negative control we analyzed brains of uninfected hamsters. A: spongiform degeneration was assessed by hematoxilin-eosin staining in diverse areas of the brain, including medulla, hippocampus and superior colliculus. B: PrPSc accumulation was studied by histological staining with anti-PrP 3F4 antibody as described in methods. C: Reactive astrocytes were visualized by immunological staining with antibodies against glial fibrillar acidic protein (GFAP). D: PrPSc accumulation was evaluated by western blot after PK digestion. Different quantities of brain homogenate (dilutions 1:25, 1:50 and 1:100 with respect to the brain) were loaded into the gel. All samples were treated with PK, except for the normal brain homogenate (NBH) were –PK is indicated. Results shown in panels A–D are representative of several animals analyzed.
4. Discussion
In this study we show that PrPSc with the capability to convert PrPC into the misfolded form and produce disease is excreted in urine by most of scrapie sick hamsters. Detection of PrPSc in urine was possible only after extensive amplification by PMCA, suggesting that the quantity of prions in urine is very small. As reported previously, 7 serial rounds of PMCA enable detection of 1.3 ag of PrPSc in 20 ul of sample, which is equivalent to a 10−12 dilution from brain [11]. Considering that in this study we detected the majority of the samples after 6 serial rounds of PMCA, our estimation is that the quantity of PrPSc in urine is between 1010–1011 fold lower than in brain. Since the PrPSc concentration in the brain of a sick hamster is around 50 ug/ml [11], we estimate that urine contains approximately 0.5–5 fg/ml of PrPSc, expressed as monomer concentration. Considering recent reports of the minimum size of the most infectious prion particle corresponds to a PrPSc oligomer containing around 20 molecules of PrP monomer [21], we estimate that there are around 500–5000 PrPSc oligomeric molecules in each ml of hamster urine. Comparison with the quantity of PrPSc present in blood of sick hamsters, which is around 109–1010 lower than in brain [10], we estimate that the concentration of PrPSc in urine is in average 10-fold lower than in blood.
PrPSc in urine retains the infectious properties, since injection of the agent amplified from this fluid produced a disease indistinguishable from the one induced by in vivo isolated material. Interestingly, animals inoculated with PrPSc amplified from the HY strain (both from brain and urine) showed a similar incubation time as those injected with the same quantity of PrPSc from sick brain. This is different to what we obtained previously with the 263K hamster prion strain in which the in vitro generated protein had consistently lower infectivity per unit of PrPSc, compared to the in vivo produced protein [9]. This is surprising considering that 263K and HY strains are fairly similar in most of the properties. So far, among the many prions from different species and strains we have amplified and shown to be infectious (data not shown), 263K is the only one that has a lower specific infectivity when amplified in vitro. Currently, we do not have an explanation for this phenomenon, but a recent publication from Kretschmar’s group reported that immobilization of PMCA generated 263K PrPSc resulted in the same infectivity than brain derived PrPSc [22]. This result suggests that the in vitro produced protein was the same strain and the lower infectivity was due to a different size distribution which results in a more rapid biological clearance.
Our findings suggest that urine is a possible source of prion transmission. Since urine produced by animals potentially infected with prions is permanently released and likely concentrated in environmental samples, such as soil and grass, this route may prove very relevant for spreading of TSEs in wild and captive animals such as cervids, sheep and cattle. It is known that PrPSc is highly resistant to degradation and infectivity can survive in the environment for a long time [23]. Recent studies have shown that PrPSc adsorbs efficiently into soil where it remains infectious and that both infectivity and PrPSc can stay intact in soil for long periods of time [24–26]. Contamination of soil with urinary prions may contribute to spreading prion disease among animals, which are known to ingest large amounts of soil, including cattle, sheep and cervids [24,26,27]. Worrisomely, the continuous excretion of urine and the extremely high resistance of prions may lead to a progressive accumulation of infectious material in the environment, with potentially catastrophic consequences in the future.
One of the top priorities in the prion field is to minimize further spreading of TSEs to humans or animals by limiting the exposure to contaminated material [7,14]. This is a difficult problem, because prion diseases have a long clinically-silent incubation period in which infected individuals may unknowingly transmit the disease. In addition, it is possible that many individuals may remain as sub-clinical carriers during their entire life, constituting a permanent source of prions [28]. Therefore, the development and validation of procedures to detect even the tiniest quantities of infectious material is of paramount importance [7,15]. Implementation of a large scale program to screen animals at risk of infection and diagnosis of the human population requires detection of prions in easily accessible samples, such as blood or urine. Our results showing that PrPSc can be detected in urine of a large proportion of infected animals provide a promising avenue for a sensitive and non-invasive biochemical diagnosis of prion diseases. Adaptation of PMCA for detection of prions in urine of naturally infected animals and humans may offer a great possibility for routine testing of prion infections.
Acknowledgments
This research was supported by NIH grants NS049173, AI77774 and AG14359 to CS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguzzi A, Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–327. doi: 10.1016/s0092-8674(03)01031-6. [DOI] [PubMed] [Google Scholar]
- 2.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. Prions. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadsworth JD, Collinge J. Update on human prion disease. Biochim. Biophys. Acta. 2007;1772:598–609. doi: 10.1016/j.bbadis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Llewellyn CA, Hewitt P, Knight RS, Amar K, Cousens S, Mackenzie J, Will RG. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 6.Ironside JW. Variant Creutzfeldt-Jakob disease: risk of transmission by blood transfusion and blood therapies. Haemophilia. 2006;12(Suppl 1):8–15. doi: 10.1111/j.1365-2516.2006.01195.x. [DOI] [PubMed] [Google Scholar]
- 7.Soto C. Diagnosing prion diseases: needs, challenges and hopes. Nat. Rev. Microbiol. 2004;2:809–819. doi: 10.1038/nrmicro1003. [DOI] [PubMed] [Google Scholar]
- 8.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 9.Castilla J, Saá P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Castilla J, Saa P, Soto C. Detection of prions in blood. Nat. Med. 2005;11:982–985. doi: 10.1038/nm1286. [DOI] [PubMed] [Google Scholar]
- 11.Saa P, Castilla J, Soto C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem. 2006;281:35245–35252. doi: 10.1074/jbc.M603964200. [DOI] [PubMed] [Google Scholar]
- 12.Soto C, Anderes L, Suardi S, Cardone F, Castilla J, Frossard MJ, Peano S, Saa P, Limido L, Carbonatto M, Ironside J, Torress JM, Pocchiari M, Tagliavini F. Pre-symptomatic detection of prions by cyclic amplification of protein misfolding. FEBS Lett. 2005;579:638–642. doi: 10.1016/j.febslet.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Saa P, Castilla J, Soto C. Presymptomatic detection of prions in blood. Science. 2006;313:92–94. doi: 10.1126/science.1129051. [DOI] [PubMed] [Google Scholar]
- 14.Ingrosso L, Vetrugno V, Cardone F, Pocchiari M. Molecular diagnostics of transmissible spongiform encephalopathies. Trends Mol. Med. 2002;8:273–280. doi: 10.1016/s1471-4914(02)02358-4. [DOI] [PubMed] [Google Scholar]
- 15.Aguzzi A, Glatzel M. Prion infections, blood and transfusions. Nat. Clin. Pract. Neurol. 2006;2:321–329. doi: 10.1038/ncpneuro0214. [DOI] [PubMed] [Google Scholar]
- 16.Brown P, Cervenakova L, Diringer H. Blood infectivity and the prospects for a diagnostic screening test in Creutzfeldt-Jakob disease. J. Lab Clin. Med. 2001;137:5–13. doi: 10.1067/mlc.2001.111951. [DOI] [PubMed] [Google Scholar]
- 17.Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, Gaspert A, Seifert B, Miele G, Aguzzi A. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310:324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 18.Kariv-Inbal Z, Ben Hur T, Grigoriadis NC, Engelstein R, Gabizon R. Urine from scrapie-infected hamsters comprises low levels of prion infectivity. Neurodegener. Dis. 2006;3:123–128. doi: 10.1159/000094770. [DOI] [PubMed] [Google Scholar]
- 19.Saa P, Castilla J, Soto C. Cyclic amplification of protein misfolding and aggregation. Methods Mol. Biol. 2005;299:53–65. doi: 10.1385/1-59259-874-9:053. [DOI] [PubMed] [Google Scholar]
- 20.Castilla J, Saa P, Morales R, Abid K, Maundrell K, Soto C. Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol. 2006;412:3–21. doi: 10.1016/S0076-6879(06)12001-7. [DOI] [PubMed] [Google Scholar]
- 21.Silveira J, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiber P, Giese A, Piening N, Mitteregger G, Thomzig A, Beekes M, Kretzschmar HA. Generation of genuine prion infectivity by serial PMCA. Vet. Microbiol. 2007;123:346–357. doi: 10.1016/j.vetmic.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Brown P, Gajdusek DC. Survival of scrapie virus after 3 years' interment. Lancet. 1991;337:269–270. doi: 10.1016/0140-6736(91)90873-n. [DOI] [PubMed] [Google Scholar]
- 24.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions Adhere to Soil Minerals and Remain Infectious. PLoS. Pathog. 2006;2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. Scrapie Agent (Strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS. ONE. 2007;2:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. Transmissibility of Prion Disease Is Enhanced by Binding to Soil Particles. PLoS. Pathog. 2007;3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton I, Abrahams P. Soil ingestion--a major pathway of heavy metals into livestock grazing contaminated land. Sci. Total Environ. 1983;28:287–294. doi: 10.1016/s0048-9697(83)80026-6. [DOI] [PubMed] [Google Scholar]
- 28.Bishop MT, Hart P, Aitchison L, Baybutt HN, Plinston C, Thomson V, Tuzi NL, Head MW, Ironside JW, Will RG, Manson JC. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 2006;5:393–398. doi: 10.1016/S1474-4422(06)70413-6. [DOI] [PubMed] [Google Scholar]