Abstract
The potencies and selectivity of peptide CRF antagonists is increased through structural constraints suggesting that the resulting ligands assume distinct conformations when interacting with CRF1 and CRF2 receptors. To develop selective CRF receptor agonists, we have scanned the sequence -Gln-Ala-His-Ser-Asn-Arg- (residues 30–35 of [DPhe12,Nle21,38]Ac-hCRF4-41) with an i-(i + 3) bridge consisting of the Glui-Xaa-Xbb-Lysi+3 scaffold where residues i = 30, 31 and 32. When i = 31 stressin1-A, a potent CRF1 receptor-selective agonist was generated. In vitro, stressin1-A was equipotent to h/rCRF to release ACTH. Astressin1-A showed a low nanomolar affinity for CRF1 receptor (Ki = 1.7 nM) and greater than 100-fold selectivity versus CRF2 receptor (Ki = 222 nM). Stressin1-A released slightly less ACTH than oCRF in adult adrenal-intact male rats, with increased duration of action. Stressin1-A, injected intra-peritoneally, induced pellet output (a CRF1 receptor-mediated response) and did not influence gastric emptying and blood pressure (CRF2 receptor-mediated responses).
Introduction
In previous reports describing the rationale used in the discovery of astressin (a potent non-selective antagonist at both CRF1 and CRF2 receptors) and astressin2-B (a CRF2 receptor-selective antagonist), we emphasized the importance of subtle structural constraints that led to the desired compounds. Whereas a Glu30-Lys33 side chain to side chain covalent lactam constraint increased affinity of linear CRF antagonists (astressin) for CRF1 and CRF2 receptors, 2 we found that a Glu32-Lys35 side chain to side chain bridge in h/rCRF fragments and the corresponding Glu31-Lys34 bridge in sauvagine fragments yielded potent ligands (astressin2-B) that are highly selective for the CRF2 receptor.3 This selectivity (>100-fold) was demonstrated using radioreceptor assays with cloned receptor cell lines and autoradiographic studies on rat brain slices. Additionally, we identified unique substitutions (CαMeLeu) that conferred on these antagonists long duration of action in vivo.4
Interestingly, extension of these sequences to full length CRF analogs yielded CRF1/CRF2 receptor (astressin-derived) agonists that were only 2- to 5-fold more potent than the 1 parent analogues as well as agonists that had lost some of their CRF2 receptor-selectivity. However, the discovery of urocortin 2 (Ucn 2)5 and urocortin 3 (Ucn 3)6 which are potent CRF2 receptor-selective agonists provided the needed tools to study the physiological role of the CRF2 receptor. Whereas a large number of studies used oCRF as a preferential CRF1 receptor-selective ligand, we disclosed the structure of stressin1-A1 which was equipotent to oCRF at CRF1 receptor and about 4 times less potent at CRF2 receptor than oCRF, resulting in a 4-fold increase in selectivity. Independently, Tezval et al. described cortagine with about 200-fold selectivity for the CRF1 receptor.7 Although any of these molecules may be used for the study of the pharmacological or physiological CRF1-mediated activities, we hypothesized that fragments of the most potent and selective analogue might yield a potent and CRF1 receptor-selective antagonist. It is noteworthy that nonpeptide CRF1 receptor-selective antagonists were available at the time of the initiation of this research, yet they are not necessarily the best tools for unraveling the physiological role of the CRF1 receptor because they do not limit their action to the peripheral or central compartments. Although one would expect that peptide CRF1 receptor agonists administered in the periphery would not cross the blood brain barrier, it is known that centrally administered peptides may leak to the periphery.
We disclosed the structure of stressin1-A (cyclo(31-34)[DPhe12,Nle21,38,Glu31,Lys34]Ac-hCRF(4-41)), a CRF1 receptor-selective agonist, at the 31st Annual Meeting of the Society of Neuroscience held in San Diego, CA.1 When it comes to nomenclature, we proposed to call CRF antagonists, astressins3 and use stressins for agonists with no subscript or subscript 1 or 2 (as for the antagonists) to indicate no selectivity or selectivity for either the CRF1 receptor or the CRF2 receptor, respectively. The essential role of the CRF1 receptor in mediating ACTH release from the pituitary in response to stress or exogenous administration of CRF or Ucn 1 is well established using CRF1 receptor knockout mouse model or selective nonpeptide CRF1 receptor subtype antagonists.8,9 In addition, convergent in vivo and in vitro studies have emphasized the role for the CRF1 receptor in stress- and peripheral administration of CRF-induced stimulation of colonic motor function (motility, transit, defecation and diarrhea).10,11 In contrast, in the upper gastrointestinal tract, CRF, Ucn 1, or Ucn 2 injected peripherally, exerts an inhibitory effect on 3,10,12–14 gastric motility and transit that is mediated by CRF2 receptors in rats and mice.
Peripheral CRF2 receptors also play an important role in the modulation of cardiovascular function.15 Peripheral administration of Ucn 2 induced hypotension that was blocked by the selective CRF2 receptor antagonists, astressin2-B or anti-sauvagine-30 in rats.16 Conversely, CRF2 receptor knockout mice displayed elevated blood pressure (BP), and did not respond to peripheral injection of Ucn 1 by change in BP.17 The expression of CRF2, but not CRF1 receptors in the endothelial cells of arteries, further supported a direct action of urocortins within the vascular system.15,18
While selective CRF2 receptor endogenous ligands, namely Ucn 2 and Ucn 3, have been characterized recently,5,6,19 there is no evidence for the existence of endogenous CRF1 receptor-selective ligands. Ovine CRF displays features of a preferential but not selective CRF1 receptor agonist and Ucn 1 has equal affinity for both CRF receptors.19 Because there is increased evidence for a modulation of CRF1 receptor-mediated actions by CRF2 receptors,20,21 it is important to probe the CRF signaling pathways using selective CRF1 receptor agonists. Here we describe some of the SAR studies that led to the discovery of stressin1-A. Additionally, we report its biological characterization in in vitro and in vivo CRF1 receptor mediated bioassays such as the release of ACTH, and the stimulation of colonic motor function along with the absence of CRF2 receptor-mediated biological responses on gastric emptying and BP in rats.
Results and Discussions
Synthesis and physicochemical characterization
All analogues shown in Table 1 were synthesized using the solid phase method of Merrifield on a methylbenzhydrylamine resin (MBHAR) using the Boc-strategy with orthogonal protection of the side-chains of the lysine (Fmoc) and glutamic acid (OFm) residues to be cyclized.22–24 Main-chain assembly was mediated in most cases by diisopropylcarbodiimide (DIC). The best results were obtained when the peptide chain was assembled in its entirety prior to cleavage of the Fmoc and OFm protecting groups and when the lactam formation was mediated by TBTU or BOP. The peptides were cleaved and deprotected in HF and purified with reversed phase high performance liquid chromatography (RPHPLC). Peptides were characterized using RPHPLC, CZE and MS (Table 1).
Table 1.
Physical and biological properties of CRF analogues.
| Cpd Name | HPLCa | CZEb | MSc calc | MSc found | CRF1d (nM) | CRF2d (nM) | Relative Potencye | |
|---|---|---|---|---|---|---|---|---|
| 1 | r/hCRF | >98 | 96 | 4755.5 | 4755.5 | 1.0 (0.2–4.6) | 6.2 (2.0–19) | 1.0 |
| 2 | oCRF | 99 | 98 | 4668.50 | 4668.5 | 1.2 (0.9–1.6) | 52 (21–128) | 1.0 |
| 3 | Cortagine | 96 | >98 | 4439.35 | 4440.3 | 3.4 (2.5–4.8 | 102 (46–225) | 0.63 (0.37–1.1) |
| 4 | cyclo(30-33)[DPhe12,Nle21,38,Glu30,Lys33]Ac-hCRF(4-41) | 97 | 95 | 4440.52 | 4440.4 | 0.7 (0.2–2.0) | 1.3 (0.5–3.6) | 4.3 (2.5–7.8) |
| 5 | linear[DPhe12,Nle21,38,Glu30,Lys33]Ac-hCRF(4-41) | 96 | 95 | 4458.54 | 4458.6 | 0.5 (0.2–1.3) | 1.6 (1.5–1.8) | 4.5 (2.7–7.6) |
| 6 | linear[DPhe12,Nle21,38,Glu31,Lys34]Ac-hCRF(4-41) | 98 | >98 | 4488.54 | 4488.5 | 2.7 (2.2–3.3) | 252 (114–560) | 1.0 (0.47–2.3) |
| 7 | cyclo(31-34)[DPhe12,Nle21,38,Glu31,Lys34]Ac-hCRF(4-41) (stressin1-A) | >98 | 98 | 4470.53 | 4470.3 | 1.7 (1.1–2.6) | 222 (137–361) | 1.1 (0.53–2.2) |
| 8 | linear[DPhe12,Nle21,38,MeLeu27,40,Glu31,Lys34]Ac-hCRF(4-41) | >98 | 98 | 4516.57 | 4516.5 | 2.7 (0.7–10) | 261 (105–654) | 1.1 (0.3–5.5) |
| 9 | cyclo(31-34)[DPhe12,Nle21,38,MeLeu27,40,Glu31,Lys34]Ac- hCRF(4-41) | 97 | 94 | 4498.56 | 4498.3 | 0.8 (0.5–1.1) | 47 (21–102) | 2.2 (0.8–6.2) NP |
| 10 | cyclo(31-34)[DPhe12,Nle21,Glu31,Lys34]oCRF | 98 | 94 | 4704.57 | 4704.55 | 66 (28.5–154) | >1000 | |
| 11 | cyclo(31-34)[DPhe12,Nle21,MeLeu27,Glu31,Lys34]oCRF | 95 | 94 | 4718.6 | 4718.29 | 50 (18–139) | >1000 | |
| 12 | cyclo(31-34)[DPhe12,Nle21,Glu31,Lys34]Ac-oCRF(9-41) | 95 | 91 | 3895.15 | 3895.13 | >500 | >500 | |
| 13 | cyclo(32-35)[DPhe12,Nle21,38,Glu32,Lys35]Ac-hCRF(4-41) | 96 | 95 | 4362.45 | 4362.5 | 1.8 (0.6–5.0) | 1.8 (1.2–2.7) | 2.7 (1.4–5.3) |
| 14 | cyclo(32-35)[DPhe12,Nle21,38,MeLeu27,40,Glu32,Lys35]Ac- hCRF(4-41) | 95 | 90* | 4390.48 | 4390.6 | 1.3 (0.6–2.9) | 1.5 (1.0–2.2) | 2.8 (1.4–5.8) |
| 15 | cyclo(32-35)[DPhe12,Nle21,38,MeLeu27,40,Glu32,Lys35]Ac-hCRF(6-41) | >98 | 95* | 4196.37 | 4196.4 | 3.9 (2.2–7.0) | 3.0 (1.0–9.2) | 6.1 (3.8–9.7) |
| 16 | cyclo(32-35)[DPhe12,Nle21,38,MeLeu27,40,Glu32,Lys35]Ac-hCRF(7-41) | >98 | 90* | 4083.29 | 4083.3 | 11 (8.0–15.0) | 1.6 (1.1–2.4) | 0.32 (0.21–0.48) |
| 17 | cyclo(32-35)[DPhe12,Nle21,38,MeLeu27,40,Glu32,Lys35]Ac-hCRF(8-41) | >98 | 95* | 3996.26 | 3996.2 | 15.6 (9.0–26.0) | 2.0 (1.6–2.4) | 0.025 (0.013–0.043) |
Percent purity determined by HPLC using buffer system: A = TEAP (pH 2.5) and B = 60% CH3CN/40% A with a gradient slope of 1% B/min, at flow rate of 0.2 mL/min on a Vydac C18 column (0.21 × 15 cm, 5 μm particle size, 300 Å pore size). Detection at 214 nm.
Capillary zone electrophoresis (CZE) was done using a Beckman P/ACE System 2050 or 5500. Field strength of 15 kV at 30 °C, mobile phase: 100 mM sodium phosphate (85:15, H2O:CH3CN) pH 2.50, on a Supelco P175 capillary (75 μm ID × 50 cm length).
CZE basic; Field strength of 13 kV at 30 °C; mobile phase: 100 mM sodium borate (85:15, H2O:CH3CN) pH 8.50, on an Agilent μSIL-FS capillary (75 μm ID × 50 cm length). Detection at 214 nm for both systems.
The observed m/z of the monoisotope compared with the calculated [M + H]+ monoisotopic mass.
The numbers given in Table 1 reflect the inhibitory binding constants, Ki, for the analogues’ binding to the cloned hCRF1 and mCRF2β receptors derived from competitive radioligand displacement assays using the non-selective 125I-labeled agonist [Tyr0,Glu1,Nle17]sauvagine as the radioligand. Ki values were calculated by PRISM software. Values in parentheses are 95% confidence limits.
Potencies are relative to ovine CRF in the in vitro rat pituitary cell culture assay, with 95% confidence limits in parentheses.
Biological characterization in vitro
Selected peptides were tested in the rat dispersed anterior pituitary cell culture assay25 to determine their relative potency and in cloned receptor-based assays to determine their binding affinities for two receptors (hCRF1 receptor and mCRF2β receptor)26 in order to determine their selectivity (Table 1). In earlier publications, we showed that deletion of the N-terminal residues (1–3)27 up to (1–7)28 yielded agonists with somewhat reduced relative potencies as the size of the peptide decreased. For example, whereas oCRF, Ac-oCRF and oCRF(4-41) were equipotent in the rat dispersed anterior pituitary cell culture assay, oCRF(6-41) and oCRF(7-41) had 11% and 0.5% of oCRF’s potency, respectively.29
SAR studies based on in vitro assays
The assay measuring the inhibition of CRF-stimulated release of ACTH from rat anterior pituitary cells in culture was instrumental in identifying astressin as a new lead for potent CRF antagonists because it was 32 times more potent than any of its predecessors [α-hel-CRF(9-41) or [DPhe12,Nle21,38]hCRF(12-41)].30 In the same series, cyclo(26-29), cyclo(28-31) and cyclo(29-32)[DPhe12,Glui,Lysi+3,Nle21,38]hCRF(12-41) were ten times less potent while cyclo(24-27), cyclo(25-28), cyclo(27-30), cyclo(31-34) and cyclo(32-35)[DPhe12,Glui,Lysi+3,Nle21,38]hCRF(12-41) were less than 2% as potent. With the availability of receptor assays for the CRF1 receptor and the CRF2 receptor26 and as rationalized in the introduction, the Glui, Lysi+3 cyclic lactams spanning the [DPhe12,Nle21,38]Ac-hCRF(4-41) sequence (i = 30, 31 and 32) were tested for their affinities for the two cloned CRF receptors (Table 1). A good correlation was found between the relative in vitro potencies derived from the measure of ACTH stimulation in rat anterior pituitary cells in culture by increasing doses of the agonists and Ki at CRF1 receptor for analogues 4–9 (Table 1). Binding affinities for the CRF2 receptor, however, diverged significantly from binding affinities for CRF1 in the case of both linear [DPhe12,Glu31,Lys34,Nle21,38]Ac-hCRF(4-41) (6) and cyclo(31-34)[DPhe12,Glu31,Lys34,Nle21,38]Ac-hCRF(4-41) (7) in that they had very high affinity for the CRF1 receptor and low affinity for the CRF2 receptor. This was the first indication that CRF1 receptor-selectivity measured by the ratio of the CRF2 receptor Ki/CRF1 receptor Ki = 93 and 130, respectively, could be modulated by the introduction of a structural constraint similar to that used to achieve CRF2 receptor selectivity in astressin2-B3 and consistent with that used for the potent antagonists astressin and astressin B.2,31
Although a cyclic constraint in 7 and 9 helped identify a region of the CRF molecule that could be altered to modulate selectivity towards CRF1 receptor-selectivity, the fact that the corresponding linear 6 and 8 also showed similar selectivity suggested that intramolecular (ionic) forces could also induce preferential secondary structures. Introduction of CαMe-leucine (CαMeLeu) at positions 27 and 40 was particularly beneficial (conferred long duration of action) in the cases of astressin B and astressin2-B and was well tolerated in 8 and its corresponding cyclic 9 when compared to 6 and 7 with respect to CRF1 receptor-selectivity.
Because oCRF (2) was by itself CRF1 receptor-selective, we hypothesized that the introduction of the Glu31-Xaa-Xaa-Lys34 scaffold in [DPhe12,Nle21]oCRF would result in an even more CRF1 receptor-selective analogue (10) than an analogue with the same substitution in Ac-hCRF4-41 (4). Unexpectedly, this modification resulted in significant loss of binding affinity at CRF1 receptor (Ki = 66 nM) and the CRF2 receptor (Ki > 1000 nM). The additional CαMeLeu substitution of 10 to yield 11 resulted in a slight increase in CRF1 receptor binding affinity in a manner similar to what was observed after CαMethylation of 7 to yield 9.
Analogue 12, resulting from the deletion of 8 residues at the N-terminus of 10, was hypothesized to be a potential CRF1 receptor-selective antagonist yet was essentially inactive in both CRF1 receptor and CRF2 receptor binding assays.
Shifting the i-(i+3) bridge consisting of the Glui-Xaa-Xbb-Lysi+3 scaffold in [DPhe12,Nle21,38]Ac-hCRF4-41 from positions 31–34 to 32–35 restored high binding affinity at both the CRF1 receptor and the CRF2 receptor (13) and CαMethylation at positions 27 and 40 was well tolerated (14). Agonists 13 and 14 were equipotent (Ki = ca 1.5 nM) at both receptors. In view of these results, we wondered whether the favorable constraints brought about by the 32–35 bridge would also favor a bioactive conformation despite further deletions at the N-terminus as we had shown for the 30–33 bridge.22 Indeed, analogues derived from 14 (missing residues 1–3 from the original CRF sequence) and missing residues 1–5 (15), 1–6 (16) and 1–7 (17) retained considerable binding affinity, yet with decreasing relative potencies (Table 1).
While this work was in progress, and after our disclosure of the structure of stressin1-A, Tezval et al.7 reported the synthesis and biological characterization of cortagine, [Glu21,Ala40][Svg1-12]x[human CRF14-30]x[Svg30-40], a chimera derived from 2 fragments of sauvagine (residues 1–12 and 30–40) and hCRF(residues 14–30) with two substitutions: Glu21 to reduce affinity to the CRF binding protein and Ala40 to increase CRF1 receptor selectivity. It is interesting that using two different, yet structurally based approaches, analogs of CRF were identified that showed CRF1 receptor selectivity. In our assays however, we found that cortagine was equipotent (relative potency = 0.63) to oCRF (relative potency = 1.0) in the in vitro pituitary cell culture assay and somewhat less selective than had been reported (ratio of CRF2 receptor Ki/CRF1 receptor Ki = 200 compared to 30 shown in Table 1)7 which is not statistically different from the ratio of CRF2 receptor Ki/CRF1 receptor Ki = 43 for oCRF. This relatively minor difference may be attributed to the fact that both compounds have very low affinity for the CRF2 receptor and thus, their Kis are not well defined.
In vivo biological characterization
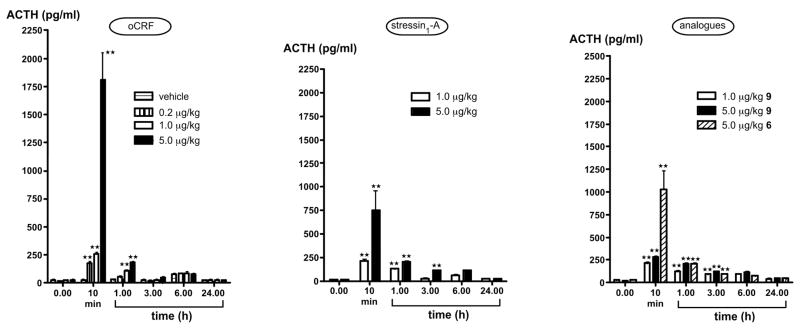
ACTH release: As shown in Figure 2, plasma ACTH levels of vehicle-injected rats remained below 80 pg/mL at all times and showed the expected circadian variations. Both CRF and stressin1-A induced significant (P<0.01) and dose-related increases in ACTH levels at the 10 min time point, but the pituitary response to 5.0 μg oCRF/kg (1,810 ± 239 pg ACTH/mL) was significantly larger than that measured after 5.0 μg stressin1-A/kg (752 ± 210 pg ACTH/mL, P<0.01). Animals administered 1.0 or 5.0, but not 0.2 μg oCRF/kg still had elevated (P<0.01) ACTH values 1 h after injection, and these values had returned to control levels by 3 h. Stressin1-A, and 9 at 1.0 and 5.0 μg/kg and 6 at 5.0 μg/kg also increased (P<0.01) ACTH concentrations 1 h after treatment. The observation that the higher dose of the three peptides maintained up-regulated (P<0.01) ACTH release at the 3 h time points suggests that their duration of action is slightly extended, compared to that of oCRF.
Figure 2.
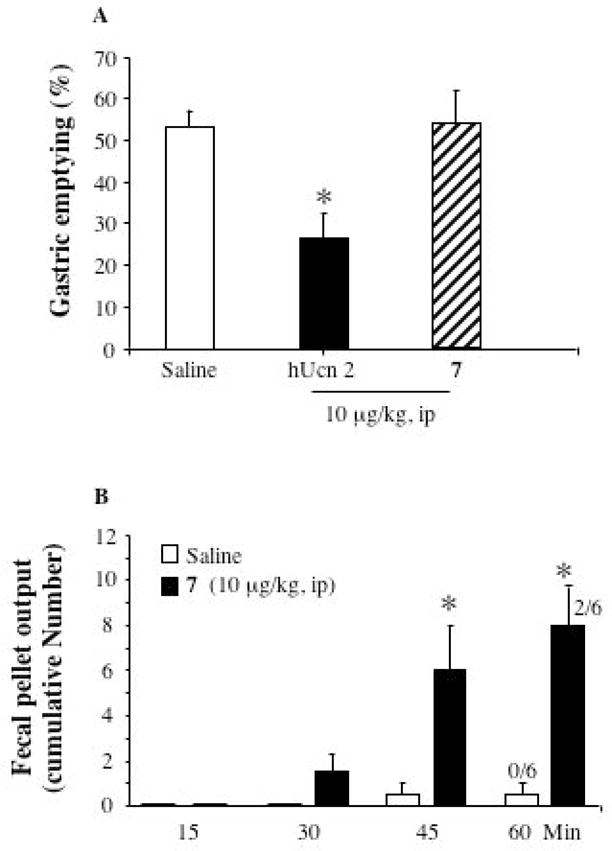
A = Ucn 2 (hUcn 2) but not 7 (stressin1-A) injected ip decreased gastric emptying of a viscous noncaloric meal in conscious rats. Overnight fasted rats were injected ip with saline, Ucn 2 or 7 (stressin1-A), and 10 min later gavaged with the phenol red methylcellulose solution and 20 min later gastric emptying was monitored. Each column is the mean ± SE of 5–6 rats/group. *p < 0.05 compared with both vehicle and stressin1-A groups. B = 7 injected ip stimulated colonic motor function in conscious rats. Non fasted rats were injected ip with saline or 7 (stressin1-A, 10 μg/kg) and fecal pellet output and diarrhea in conscious rats was monitored. Each column represents the mean ± SE of 6 rat/group. The numbers on the bars are the number of rats with diarrhea over the total in a group. #P<0.05 vs. saline and stressin1-A; * P<0.05 vs. saline.
Gut motor function and blood pressure
Peptides were tested in three in vivo assays that are known to be mediated by either the CRF1 receptor or the CRF2 receptor. These are a colonic propulsive motor function test in non fasted rat, gastric emptying of non nutrient meal in an overnight fasted rat model and arterial blood pressure measure in an overnight fasted and urethane anesthetized rat model. CRF1 receptor activation is well established to increase colonic transit and motility11,14 while peripheral CRF2 activation by CRF and related family members decreases gastric emptying10,12–14 and blood pressure16 in rodents. Because these activities are mediated by either one or the other CRF receptor (as shown), these measurements were critical in our evaluation of the peptides’ selectivity. We found that Ucn 2, but not stressin1-A, injected ip, delayed gastric emptying in conscious rats. Stressin1-A injected ip at 10 μg/kg had no significant effect on gastric emptying of a viscous noncaloric solution in conscious rats (51.0 ± 8.8% compared with 50.0 ± 2.5% in the vehicle group: p = 0.92 ; Figure 2A). By contrast, ip hUcn 2 given at the same dose significantly reduced gastric emptying to 26.4 ± 6.9% compared to both vehicle and stressin1-A groups (Figure 2A). We also found that stressin1-A injected ip stimulated colonic motor response in conscious rats. In non fasted conscious rats, stressin1-A injected ip at 10 μg/kg significantly increased the 60 min fecal pellet output when compared to saline injected rats (8.0 ± 1.6 vs. 0.5 ± 0.5/h; Figure 2B). Time course of the cumulative effects of stressin1-A on fecal pellet showed an increased response at 30 min that became significant at 45 and 60 min. In addition, stressin1-A induced diarrhea in 33% of the rats whereas none of the saline injected rats had diarrhea as monitored 60 min post injection (Figure 2B). Finally, stressin1-A, injected iv, did not modify arterial blood pressure. In urethane anesthetized rats, mean basal blood pressure was 75.9 ± 3.5 mm Hg and was not significantly modified after vehicle iv injection (77.4 ± 1.3 mm Hg; p = 0.14 vs. basal). Neither consecutive iv injections of stressin1-A at 3, 10 or 30 μg/kg nor of cortagine 1 at 3, 10 or 30 μg/kg produced significant changes in blood pressure compared to vehicle (+2.3 ± 1.4%, p > 0.05 ; −0.1 ± 0.7%, p > 0.05; − 1.6 ± 2%, p > 0.05 for stressin1-A and 1.4 ± 0.8%, p > 0.05; 0.1 ± 0.3%, p > 0.05; −3.2 ± 0.7%, p > 0.05 for cortagine) (data not shown).
Using one in vivo bioassay (colonic fecal expulsion and diarrhea) in which the role of CRF1 receptors has been well established to mediate the effects of peripheral injection of CRF and Ucn 1,11 and two assays (decrease in gastric emptying and blood pressure changes induced by CRF or urocortins) mediated by CRF2 receptor, we showed that peripheral administration of stressin1-A resulted solely in a CRF1 receptor-mediated biological response. We previously reported that the CRF1 receptor/CRF2 receptor agonists, Ucn 1 or CRF,19 injected ip at 10 μg/kg stimulated colonic transit while inhibiting gastric emptying through CRF1 and CRF2 receptors, respectively, in rats.14 Therefore the lack of change in gastric emptying together with potent stimulation of colonic motor function induced by stressin1-A injected ip provided in vivo evidence for the CRF1 receptor selectivity of stressin1-A. This is further corroborated by the lack of change in blood pressure when stressin1-A was injected iv and by several literature reports that emphasize the specific role of CRF2 receptors in the CRF or Ucn 1-induced drop in blood pressure.3,16,17
To conclude, potent peptidic CRF2 receptor agonists (urocortins 2 and 3) and antagonists (astressin2-B, anti-sauvagine-30) have been described previously. Here we report the development of stressin1-A and present in vitro and in vivo data providing convergent evidence that stressin1-A is a novel potent and selective CRF1 receptor agonist that will be very useful for characterizing biological actions mediated by the CRF1 receptor.32 Structural studies (NMR) are presently being carried out in order to determine the conformational differences that contribute to receptor selectivity. Still missing is a potent and long acting peptide CRF1 receptor antagonist to complete the panoply of peptide reagents that are critical for unraveling the physiological roles of the CRF1 receptor and the CRF2 receptor, their possible inhibitory or potentiating interactions both centrally and peripherally and ultimately, their pharmacological clinical potential.
Experimental Section
Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA) weighing 250–300 g were housed in group cages under controlled illumination (12:12-h light-dark cycle starting at 6 AM), humidity, and temperature (21–23°C) and had free access to tap water and Purina rat chow. Protocols were approved by the respective IACUCs of the University of California Los Angeles and Veteran Affairs Greater Los Angeles Healthcare System Animal Research Committees and the Salk Institute. When required by the experiments, rats were deprived of food but had free access to tap water for ~16 h before the experiment.
Administration of peptides
Immediately before injection, stressin1-A was dissolved in vehicle (water or saline). Human Ucn 2 and cortagine were dissolved in water. Ip and iv injections were done respectively in 0.3 mL and in 0.1 mL.
Rat dispersed pituitary cell culture assay
CRF analogues were tested for agonist activity in an in vitro assay measuring release of ACTH by collagenase dispersed rat anterior pituitary cells in culture.27,29 For each in vitro experiment, approximately 50 anterior pituitary glands from male Sprague-Dawley rats were dissociated by collagenase treatment and plated (0.16 × 106 cells/well in 48-well plates) in medium containing 2% fetal bovine serum (FBS). Three days after plating, the cells were washed three times with fresh medium containing 0.1% bovine serum albumin (BSA) and incubated for 1 h. Following the 1 h pre-incubation, the cells were washed once more and the test peptides were applied in 1 mL final volume of media. At the end of a 3 h incubation period, the media were collected and 10 μL tested for their level of ACTH using a radioimmunoassay (Nichols Institute Diagnostic).
Cloned receptor-based binding assays
The Ki values given in Table 1 reflect the affinities of the analogues for the cloned type 1 human and type 2β mouse CRF receptors. The values were derived from competitive radioligand displacement assays using the non-selective 125I-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine as the radioligand and crude membrane fractions from CHO cells stably expressing the respective receptors.26 Briefly, 200,000 cpm (ca. 0.5 nM) 125I-[Tyr0,Glu1,Nle17]-sauvagine were combined with increasing concentrations of peptide (0.1 nM - 1,000 nM) in 0.2 mL assay buffer (50 mM Na Hepes, pH 7.5; 10 mM MgCl2; 2 mM EGTA; 0.1% BSA) and incubated for 90 min at 18 ºC.
Measure of gastric emptying of non nutrient meal
Gastric emptying of a non nutrient viscous meal was determined by the phenol red method as described previously in conscious over night fasted rats.33 The non-caloric meal consisted of a viscous suspension of continuously stirred 1.5% methylcellulose (wt/vol) containing phenol red (50 mg/100 mL) given orogastrically through a stainless steel gavage tube (in 1.5-mL volume) to conscious rats. At 20 min after the administration of the solution, rats were euthanized by CO2 inhalation. The abdominal cavity was opened, the gastroesophageal junction and the pylorus were clamped, and the stomach was isolated and rinsed in 0.9% saline. The stomach was placed into 100 mL 0.1 N NaOH and homogenized (Polytron; Brinkman Instruments, Westbury, NY). The suspension was allowed to settle for 60 min at room temperature, then 5 mL supernatant was added to 0.5 mL of 20% trichloroacetic acid (wt/vol) and centrifuged at 3,000 rpm at 4°C for 20 min. After the supernatant was mixed with 4 mL 0.5 N NaOH, the absorbance of the sample was read at 560 nm (Shimazu 260 Spectrophotometer). The absorbance of the phenol red recovered from animals euthanized immediately after gavage of the liquid meal was taken as a standard 0% emptying. The percentage of emptying during the 20-min period was calculated with the following formula: percent emptying = (1 – absorbance of test sample/absorbance of standard) × 100.
Measure of colonic propulsive motor function
In non fasted conscious rats, defecation was monitored as described previously34 by counting the number of fecal pellets excreted every 15 min for 1 h. The incidence of diarrhea was assessed as percent of rats that developed watery stool from the total number of treated rats.
Measure of arterial blood pressure
Over night fasted rats were anesthetized with urethane (1.5 g/kg). Each rat was then fitted with a tracheal cannula for ventilation, a jugular vein catheter for hydration through continuous perfusion of sterile saline (0.4 mL/h) and for peptide injection, and a femoral artery catheter for BP measurement. The femoral catheter was connected to a preamplifier (model 600; Millar Instruments, Houston, TX), the output of which was subsequently amplified via a single-ended connection to the transducer amplifier. BP was acquired online at a sampling rate of 300 Hz via a Micro1401 A/D interface (Cambridge Electronic Design, Cambridge, UK) connected to a Pentium II class computer running Spike 2 (Cambridge Electronic Design) version 5 data acquisition software.
Effect of iv oCRF and stressin1-A on ACTH release in vivo
Adrenal-intact adult (ca. 60-day-old) male Sprague-Dawley rats were implanted with iv cannulae as described earlier.20 The test was conducted under minimum-stress conditions in fully awake and freely-moving animals that were not handled for either peptide injection or blood sampling. A first blood sample was obtained, immediately followed by the iv injection of the vehicle, oCRF (0.2, 1.0 or 5.0 μg/kg) or stressin1-A (1.0 or 5.0 μg/kg). Subsequently, blood samples were obtained 10 min, as well as 1, 3, 6 and 24 h later. ACTH levels were measured in plasma with an immunoradiometric assay from DiaSorin Inc. (Stillwater, MN).
Effect of intraperitoneal hUcn 2 and stressin1-A on gastric emptying in conscious rats
Rats were deprived of food overnight but had free access to tap water for ~16 h before the experiment. Fasted rats were injected ip (0.3 mL) with saline, Ucn 2 (10 μg/kg) or stressin1-A (10 μg/kg). At 10 min after the ip injection, the phenol red methylcellulose solution was delivered through orogastric gavage (1.5 mL), and gastric emptying was determined 20 min later. Doses of peptides were based on previous dose-response studies showing a maximal response of peripheral injection of Ucn 2 on gastric emptying in rats and mice.12,14
Effect of ip stressin1-A on colonic motor response in conscious rats
Rats were injected ip with saline or stressin1-A (10 μg/kg) and then immediately placed in individual cages to monitor the fecal pellet output and diarrhea response. These colonic responses were monitored every 15 min for 60 min after the ip injection. The dose of stressin1-A was selected on a previous dose-response study showing maximal colonic response to CRF injected ip at 10 μg/kg.14,34
Effect of iv stressin1-A and cortagine on arterial blood pressure in anesthetized rats
Basal BP was monitored for 150 to 200 min using an acute catheter positioned into the femoral artery in urethane anesthetized rats (n = 3). Each animal received consecutive intrajugular injections of vehicle (distilled water), stressin1-A at 3, 10 and 30 μg/kg, and cortagine at 3, 10 and 30 μg/kg at 15–20 min intervals. BP was recorded throughout the studies. For each treatment, mean basal BP was recorded for 5 min before and for 2 min 30 sec after the injection. Normalized values were obtained by subtracting mean basal BP from the mean BP after injection to obtain the net BP response and dividing it by the mean basal BP, yielding the percent change from the basal state.
Statistical analysis
All results are expressed as mean ± SEM. Comparison between multiple groups was performed by one-way ANOVA followed by Student Newman-Keuls multiple comparisons.
Figure 1.
Plasma ACTH levels in rats injected with the vehicle, 2 (oCRF), 6, 7 (stressin1-A) and 9. Each point represents the mean ± SEM of 6–7 animals. **, P<0.01 vs. vehicle.
Acknowledgments
This work was supported by NIH grant DK 057238 (YT), DK 026741 (JR), DK 041301 (Animal core, YT), DK 068155 (MM), VA Merit Award, and a VA Senior Career Scientist award. Dr. Gourcerol is the recipient of a French Gastroenterology Society (SNFGE) fellowship. We thank Ron Kaiser, Charleen Miller, William Low, Dr. Wolfgang Fischer, Cristin Roach, Brian Baridon and Yaira Haas for technical assistance and Mass spectrometric analysis. We thank Debbie Doan for manuscript preparation. J.R. is The Dr. Frederik Paulsen Chair in Neurosciences Professor.
Additional abbreviations
- BP
blood pressure
- BOP
(benzotriazol-1-yloxy)-tris(dimethylamino)phosphonium-hexafluorophosphate
- BSA
bovine serum albumin
- h/rCRF
human/rat corticotropin releasing factor
- oCRF
ovine CRF
- CRF1
CRF receptor 1
- CRF2
CRF receptor 2
- mCRF2
mouse CRF2
- DIC
diisopropylcarbodiimide
- CZE
capillary zone electrophoresis
- DCM
dichloromethane
- DMF
dimethylformamide
- Fmoc
9-fluorenylmethoxycarbonyl
- HBTU
O-(benzotriazol-l-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- HPLC
high performance liquid chromatography
- ip
intraperitoneal
- iv
intravenously
- LSIMS
liquid secondary ion mass spectrometry
- MeOH
methanol
- MS
mass spectrometry
- NMP
N-methylpyrrolidone
- Ofm
O-fluorenylmethyl
- RPHPLC
reversed phase high performance liquid chromatography
- SAR
structure activity relationships
- sc
subcutaneous
- TBTU
O-(benzotriazol-l-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate
- TEAP
triethylammonium phosphate
- TEA
triethylamine
- TFA
trifluoroacetic acid
- TFE
2,2,2-trifluoroethanol
- Xaa
any amino acid
Footnotes
Note: The structure of stressin1-A was first disclosed in a Neuroscience meeting poster.1 Rivier, J. E.; Gulyas, J.; Kirby, D.; Kunitake, K.; Donaldson, C. et al. Receptor-selective corticotropin releasing factor analogs. In 31st Annual Meeting of the Society for Neuroscience: San Diego, CA, 2001; Session number 413.417.
The abbreviations for amino acids are in accord with the recommendations of the IUPAC-IUB Joint Commission on Biochemical Nomenclature (European J. Biochem, 1984, 138, 9–37).
References cited
- 1.Rivier JE, Gulyas J, Kirby D, Kunitake K, Donaldson C, Vaughan J, Perrin M, Koerber S, Martinez V, Taché Y, Rivier C, Vale W. Receptor-selective corticotropin releasing factor analogs. 31st Annual Meeting of the Society for Neuroscience; San Diego, CA. 2001. Session number 413.417. [Google Scholar]
- 2.Gulyas J, Rivier C, Perrin M, Koerber SC, Sutton S, Corrigan A, Lahrichi SL, Craig AG, Vale WW, Rivier J. Potent, structurally constrained agonists and competitive antagonists of corticotropin releasing factor (CRF) Proc Natl Acad Sci USA. 1995;92:10575–10579. doi: 10.1073/pnas.92.23.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martínez V, Wang LX, Taché Y, Vale W. Potent and long acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 4.Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL. Constrained corticotropin releasing factor (CRF) antagonists (Astressin analogues) with long duration of action in the rat. J Med Chem. 1999;42:3175–3182. doi: 10.1021/jm9902133. [DOI] [PubMed] [Google Scholar]
- 5.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of Urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci USA. 2004;101:9468–9473. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein and related peptides. Proc Soc Exper Biol Med. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- 9.Bale T, Vale W. Annual Review of Pharmacology and Toxicology: Annual Reviews. 2004. CRF and CRF receptors: Role in stress responsivity and other behaviors; pp. 525–557. [DOI] [PubMed] [Google Scholar]
- 10.Taché Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Mot. 2004;16(Suppl 1):1–6. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 11.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: Role of CRF receptor subtypes 1 and 2. J Pharmacol Exper Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 13.Wang LX, Martínez V, Rivier JE, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol -Regul Integ Comp Physiol. 2001;281:R1401–R1410. doi: 10.1152/ajpregu.2001.281.5.R1401. [DOI] [PubMed] [Google Scholar]
- 14.Million M, Maillot C, Saunders P, Rivier J, Vale W, Taché Y. Human urocortin II, a new CRF-related peptide, displays selective CRF2-mediated action on gastric transit in rats. Am J Physiol - Gastrointest Liver Physiol. 2002;282:G34–G40. doi: 10.1152/ajpgi.00283.2001. [DOI] [PubMed] [Google Scholar]
- 15.Coste SC, Quintos RF, Stenzel-Poore MP. Corticotropin-releasing hormone-related peptides and receptors: emergent regulators of cardiovascular adaptations to stress. Trends Cardiovasc Med. 2002;12:176–182. doi: 10.1016/s1050-1738(02)00157-3. [DOI] [PubMed] [Google Scholar]
- 16.Chen C-Y, Doong M-L, Rivier JE, Taché Y. Intravenous urocortin II decreases blood pressure through CRF2 receptor in rats. Regul Pept. 2003;113:125–130. doi: 10.1016/s0167-0115(03)00003-x. [DOI] [PubMed] [Google Scholar]
- 17.Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 18.Wiley KE, Davenport AP. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. Br J Pharmacol. 2004;143:508–514. doi: 10.1038/sj.bjp.0705985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International union of pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 20.Rivier C, Grigoriadis D, Rivier J. Role of corticotropin-releasing factor receptors type 1 and 2 in modulating the rat ACTH response to stressors. Endocrinology. 2003;144:2396–2403. doi: 10.1210/en.2002-0117. [DOI] [PubMed] [Google Scholar]
- 21.Million M, Wang L, Wang Y, Adelson DW, Yuan P, Maillot C, Coutinho SV, McRoberts JA, Bayati A, Mattsson H, Wu VS, Wei JY, Rivier J, Vale W, Mayer EA, Taché Y. CRF2 receptor activation prevents colorectal distension-induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–181. doi: 10.1136/gut.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivier J, Lahrichi SL, Gulyas J, Erchegyi J, Koerber SC, Craig AG, Corrigan A, Rivier C, Vale W. Minimal-size, constrained corticotropin releasing factor agonists with i-(i+3) Glu-Lys and Lys-Glu bridges. J Med Chem. 1998;41:2614–2620. doi: 10.1021/jm980164e. [DOI] [PubMed] [Google Scholar]
- 23.Miranda A, Koerber SC, Gulyas J, Lahrichi S, Craig AG, Corrigan A, Hagler A, Rivier C, Vale WW, Rivier JE. Conformationally restricted competitive antagonists of human/rat corticotropin-releasing factor. J Med Chem. 1994;37:1450–1459. doi: 10.1021/jm00036a010. [DOI] [PubMed] [Google Scholar]
- 24.Miranda A, Lahrichi SL, Gulyas J, Koerber SC, Craig AG, Corrigan A, Rivier C, Vale WW, Rivier JE. Constrained corticotropin releasing factor (CRF) antagonists with i-(i+3) Glu---Lys bridges. J Med Chem. 1997;40:3651–3658. doi: 10.1021/jm970311t. [DOI] [PubMed] [Google Scholar]
- 25.Grant G, Vale WW. Methods in Enzymology, Hormones and Cyclic Nucleotides. Academic Press; New York: 1974. Pituitary receptor binding assay of hypothalamic releasing factors; pp. 213–219. [DOI] [PubMed] [Google Scholar]
- 26.Perrin MH, Sutton SW, Cervini L, Rivier JE, Vale WW. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of CRF receptors. J Pharmacol Exper Ther. 1999;288:729–734. [PubMed] [Google Scholar]
- 27.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41 residue ovine hypothalamic peptide that stimulates the secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 28.Cervini L, Theobald P, Corrigan A, Craig AG, Rivier C, Vale W, Rivier J. Corticotropin releasing factor (CRF) agonists with reduced amide bonds and Ser7 substitutions. J Med Chem. 1999;42:761–768. doi: 10.1021/jm980607e. [DOI] [PubMed] [Google Scholar]
- 29.Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin releasing factor: Effect on ACTH secretion in the rat. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- 30.Miranda A, Lahrichi S, Gulyas J, Rivier C, Koerber S, Miller C, Corrigan A, Sutton S, Craig AG, Vale W, Rivier J. Competitive antagonists of the corticotropin releasing factor (CRF) scanned with an i-(i+3) Glu Lys Bridge. Fifteenth American Peptide Symposium; Nashville, TN. 1997. pp. 2–164. [Google Scholar]
- 31.Rivier J, Gulyas J, Corrigan A, Craig AG, Martinez V, Taché Y, Vale W, Rivier C. Astressin analogues (CRF antagonists) with extended duration of action in the rat. J Med Chem. 1998;41:5012–5019. doi: 10.1021/jm980426c. [DOI] [PubMed] [Google Scholar]
- 32.Yuan P, Million M, Rivier J, Pambukchian K, Taché Y. Stressin 1, A specific agonist of corticotropin releasing factor (CRF) receptor type 1 (CRF1), activates submucosal neurons in ileum and myenteric neurons in colon and stimulates colonic motor function in conscious rats. 35th Annual Meeting of the Society for Neuroscience; Abstract Viewer/Itinerary Planner: Washington, D.C.. 2005. online. [Google Scholar]
- 33.Nozu T, Martínez V, Rivier J, Taché Y. Peripheral urocortin delays gastric emptying: role of CRF 2 receptors. Am J Physiol: Gastrointest Liver Physiol. 1999;39:G867–G874. doi: 10.1152/ajpgi.1999.276.4.G867. [DOI] [PubMed] [Google Scholar]
- 34.Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569–1579. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]