Abstract
Dysregulation of the immune system drives HIV pathogenesis. As we develop new ways to treat HIV and AIDS, we encounter new clinical ramifications of our treatment on regulatory components of the immune system. HIV-associated Immune Reconstitution Inflammatory Syndrome (IRIS) occurs after initiation of anti-retroviral therapy (ART) with inappropriate and dysbalanced restoration of the immune system resulting in pathologic inflammatory reactions with significant morbidity. IRIS is most commonly associated with latent, occult, or past infections, including tuberculosis, Cryptococcus neoformans, and Mycobacterium avium-complex. We discuss common clinical presentations, new diagnostic modalities, current hypotheses of IRIS pathogenesis, and future directions of IRIS-related research, focusing on the identification of biomarkers that can be used to predict and diagnose IRIS.
Keywords: Immune reconstitution inflammatory syndrome, IRIS, IRD, HIV/AIDS, cytokines, T cells, HAART, biomarkers
Introduction
HIV immune reconstitution inflammatory syndrome (IRIS) is a newly described clinical syndrome which occurs after initiation of antiretroviral therapy (ART), resulting in clinical deterioration due to pathological inflammatory reactions. Biomarkers to predict or diagnosis IRIS are limited, and the pathogenesis is poorly understood. As access to HIV treatment has expanded, there have been remarkable increases in survival and decreases in morbidity worldwide. Yet, new challenges have emerged, such as IRIS. IRIS events are often an exaggerated, inflammatory response to a known pathogen. The clinical symptoms may mimic that of an active infection or relapse of a prior infection, but microbiological cultures are often negative. IRIS events occur as the immune system recovers from an anergic state with the reconstitution of T-cell antigen-specific immunity. IRIS appears to be an antigen-specific exaggerated inflammatory response in which the normal immune regulatory mechanisms that usually limit the degree of inflammation are lacking. Although IRIS is a clinical syndrome, studies directed at understanding the pathogenesis of IRIS have led to the identification of candidate biomarkers that could be used to diagnose IRIS and predict which patients are at risk.
Categorization
IRIS is often categorized by the associated pathogen and the clinical scenario. Pathogens commonly associated with IRIS include, Mycobacterium tuberculosis, Cryptococcus neoformans, Mycobacterium avium-complex, and cytomegalovirus (CMV). The most common clinical scenarios are: 1) unmasking IRIS and 2) paradoxical IRIS (Table 1). In either scenario, an essential feature is dysregulated restoration of antigen-specific immunity leading to a pathological, exaggerated inflammatory response. The primary difference between these clinical scenarios is whether the infection is newly identified after initiation of ART (unmasked) or was previously treated but clinical worsening developed after initiation of ART (paradoxical). In unmasking IRIS, the typical clinical course is often greatly accelerated with abrupt atypical manifestations in both disease severity and location.
Table 1.
Common Pathologic IRIS Scenarios
| “Unmasking” IRIS |
| • Occult, subclinical opportunistic infection |
| • Unmasked by ART |
| • Infectious pathogens present |
| “Paradoxical” IRIS |
| • Clinical recrudescence of a successfully treated infection |
| • Symptomatic relapse despite microbiologic treatment success. |
| • Antigen driven immune activation |
| • Sterile cultures, typically* |
Cultures are often negative; however, this depends on the nature of the infection and the timing of the event. Though cultures are negative, histopathology stains, antigen, or DNA based testing is frequently positive.
At present, it is unclear if these two forms of IRIS occur through a general, universal mechanism or whether distinct pathogenic mechanisms are involved in the different clinical scenarios (unmasking vs. paradoxical). As well, it is unclear whether pathophysiology differs by pathogen. Current knowledge suggests there may indeed be distinct pathogenic mechanisms for different organisms, which may also be related to this distinction between paradoxical and unmasking IRIS. Thus, an emphasis has been made for IRIS researchers to use these categorizations when describing the epidemiology and investigating the pathogenesis. Examples of the common IRIS clinical scenarios are presented in Table 2 by pathogen. The terminology of ‘IRIS’, ‘Immune Reconstitution Disease’ or ‘Immune restoration disease’ (IRD), and ‘Immune Reconstitution Syndrome’ are often used interchangeably.
Table 2.
Frequent Clinical and Laboratory Features of IRIS by Pathogen
| Condition | Clinical Features | Laboratory Features |
|---|---|---|
| CMV | blurred vision | Vitritis, uveitis |
| Cryptococcus | Aseptic meningitis, lymphadenitis, pneumonitis | Sterile cultures, decreased CRAG, elevated CSF pressure in ~75%. |
| Herpes viruses | exaggerated dermatologic lesions of HSV or VZV of various intensity | PCR positive |
| Hepatitis B | acute hepatitis | Increased LFTs. |
| Hepatitis C | rapid development of cirrhosis difficult to distinguish from hepatotoxicity | HBV/HCV viral loads typically decreased. |
| M. avium complex | lymphadenitis, draining sinuses Pulmonary-thoracic disease Abdominal lymphadenopathy | Granulomatous reaction, paucity of organisms, rare hypercalcemia Fever 66%, Prior diagnosis 25% |
| M. tuberculosis | lymphadenitis, pneumonitis | Paucity of AFB, PCR positive |
A major diagnostic challenge for clinicians is distinguishing IRIS from recurrence or relapse of an infection, as often the clinical presentation is similar. At present, IRIS remains a diagnosis of exclusion which necessitates eliminating the possibility of disease relapse, drug resistance, non-compliance, and alternative diagnoses. While IRIS is frequent within the first 12 weeks of ART, IRIS can occur at anytime as can alternative diagnoses.
Potential Biomarkers predictive of IRIS
The four principal factors associated with an increased risk of developing IRIS are: 1) low baseline CD4+ T-cell count, 2) excellent virologic response to ART, 3) increased antigen burden of an opportunistic infection (OI), 4) early initiation of ART after an OI. These factors have been identified in some retrospective case-control and cohort trials; however, these risk factors are by no means universal. For example, extrapolating from a case series on TB-IRIS may not be informative for defining the risk of hepatitis B IRIS. Of course, the statistical risk for a group does not perfectly predict the outcome for an individual patient, either.
Baseline CD4 Profiles
IRIS occurs more frequently in patients who have advanced HIV/AIDS when they start ART and in those who are previously ART naïve [1,2]. When CD4+ cells are <200 cells/µL prior to ART initiation, patients are more likely to develop IRIS [1,3,4]. The degree of immunosuppression likely affects IRIS risk in two ways. First, the lower the CD4+ count is, the greater the risk of an OI. Secondly, progressive damage to the immune system disrupts the homeostatic regulatory mechanisms.
IRIS rarely occurs in persons starting ART with relatively high CD4+ counts >350 cells/µL [5]. This observation has particular implications for populations in resource-limited regions where persons are more likely to have more advanced AIDS, more co-infection with OIs, and have lower CD4+ counts when they initiate treatment. In resource-limited regions, the incidence of IRIS appears to be 20–25% [6,7,8].
The degree of absolute CD4+ rise with ART is not a risk factor [2,7,9]. Earlier case definitions used CD4+ increase after start of ART as a criterion [10]; however, IRIS can occur without an appreciable CD4+ increase. Some cohort studies have reported that IRIS is associated with an increase in the CD4 percentage or the ratio of CD4 to CD8 cells. One reported risk factor with the highest positive predictive value, based on the available data, is an increase in the CD4 percentage of ≥12% in the first 30 days of ART.11 This occurred in nearly half of patients with HIV-associated IRIS due to TB in France, but in none of patients without IRIS [11]. Similarly, a rapid increase in the CD4 to CD8 ratio of >0.33 in the first 30 days of ART in patients with TB co-infection appeared to be strongly correlated with IRIS [11,12].
Higher levels of CD8+CD25+ cells prior to ART initiation may be a predictive biomarker for later IRIS events [13]. In a cohort study of 50 Spanish patients with <100 CD4 cells/µL, those developing IRIS (number and IRIS-type not specified) had 17% ±32% CD8+CD25+ cells versus those without IRIS who had 4%±6.8% (P=0.07) and rapid decreases to 3–4% within the first month of ART. This study illustrates the problematic nature of the existing data on IRIS pathogenesis; the available data are derived from relatively small cohorts with a wide inter-personal heterogeneity. Thus, while a biomarker may be seem to be predictive within a specific study population, the results may not be generalizable to individuals.
Virologic Response
The non-modifiable IRIS risk factors include being ART naïve and having an excellent response to ART. For example, patients with a > 2 log drop in HIV-1 RNA viral load at 90 days of ART are at higher risk for IRIS [2,14]. The virologic response may be more important as a risk factor for IRIS in heavily ART pretreated populations with pre-existing HIV virologic resistance where only those patients who have a good response to ART are at high risk for IRIS [2]. In ART naïve populations, the virologic response is usually good whether or not patients develop IRIS [7,8]. Overall, those lacking a virologic response are unlikely to achieve a meaningful immune recovery, and thus, are at lower risk of IRIS. In populations with extensive virologic resistance, ineffective ART regimens are more likely to place populations at risk for new AIDS events rather than IRIS events. Thus, in retrospective cohorts composed of both ART naive and treatment experienced populations, being ART naive is often a risk factor for IRIS.
Antigenic Burden
Because of the link between IRIS and prior OIs, patients who have OIs that were identified prior to HIV therapy may benefit from delayed initiation of ART until they complete a period of therapy for the OI. The decision about when to start ART should be made on a case by case basis, depending on the type of OI and the severity of the infection. The antigen burden of an OI may be a indicator of IRIS risk. In a retrospective cohort study of TB patients, those with disseminated TB or extra-pulmonary TB had greater incidence of IRIS compared to those with pulmonary infection only [15,16,17]. In patients with disseminated cryptococcal fungemia, rates of IRIS were 6-fold higher compared to those with cryptococcal infection but without cryptococcemia. Higher CSF cryptococcal antigen titers at initial meningitis diagnosis and persistence of positive cultures after treatment were both risk factors for later development of IRIS upon commencing ART [3,18].
Timing of Starting ART after an OI
After an OI, persons starting ART within 2 months of an OI appear to have anywhere from zero to up to a ten-fold increase in the risk of IRIS [14,19,20,21]. The recently completed ACTG A5164 trial prospectively randomized 282 persons with an OI to starting ART immediately (<14 days, median 12 days) vs. delayed (>28 days, median 45 days) [21]. In this cohort, the median CD4 count was 29 cells/µL. The cohort consisted principally of patients with Pneumocystis jirovecii pneumonia (PCP) (63%), and the study found earlier initiation of ART resulted in fewer deaths and fewer new AIDS events (14% immediate ART vs. 24% deferred ART; P=.035) [21]. In this ACTG study, rates of IRIS were low, but importantly, did not differ by timing of ART initiation (5.6% immediate vs. 8.5% deferred ART) [21].
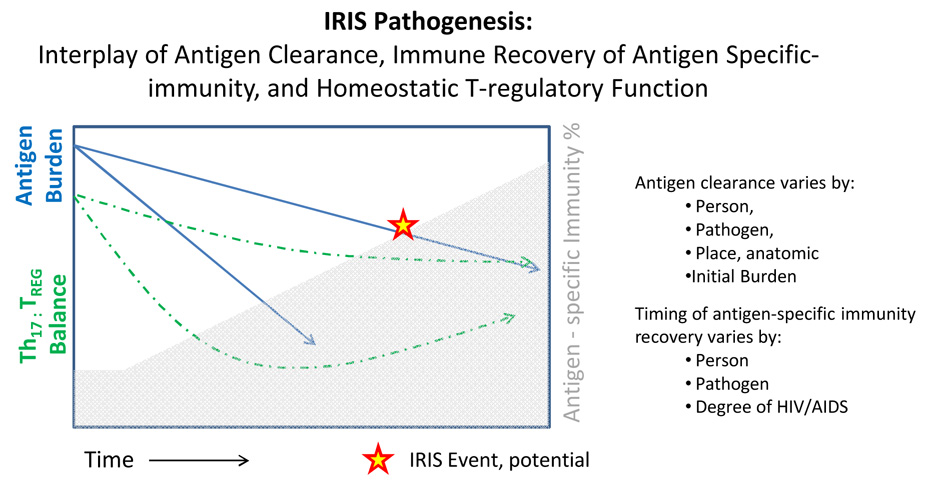
There are caveats, however, to the broad generalization of this study to other populations. First, two-thirds of those with PCP (50% of the cohort) were receiving corticosteroids and this may have prevented the development of IRIS. While corticosteroids are part of standard PCP treatment regimens, there is evidence that steroids blunt over-all thymic recovery after ART initiation. Second, patients with TB were excluded from the study, and TB is the most common IRIS-associated infection. Finally, other OIs that have been associated with IRIS, such as cryptococcal meningitis, occurred infrequently, and therefore this study was statistically underpowered for detecting differences in IRIS rates. Thus, the optimal timing for starting ART in patients with recent OIs has not yet been determined for most clinical scenarios. The interplay between the antigen burden and antigen clearance with the recovery of antigen-specific immunity and recovery of T-cell subsets is a complex dynamic (Figure 1).
Figure 1.
The conditions for IRIS are a complex interplay between host and pathogen. The recovery of the host’s immune system is critical to IRIS pathogenesis. With ART, persons gradually reconstitute their antigen-specific immunity to a pathogen (Shaded in Gray). Yet, the timing of reconstitution of T-cell subsets is variable. During reconstitution, the normal 2:1 balance between the pro-inflammatory (Th-17 subset) and the anti-inflammatory (T-regulatory subset) of the immune system (Dashed Line) may become unbalanced leading to the possibility of dysregulated responses.
Secondly, following an infection, antigen clearance is variable (Solid Line). Pathogens with rapid antigen clearance are less likely to generate an IRIS event, such as pneumococcal pneumonia. However, pathogens, such as TB and cryptococcus, in which antigens persist for months are at higher risk of developing IRIS (~33%). Overall, with HIV therapy, the immune system’s reversal from an anergic state in the presence of abundant antigen and in the absence of normal homeostatic regulatory mechanisms create the potential for an IRIS event.
Other Potential Demographic Risk Factors
There are other postulated risk factors for IRIS which may include sex, age, or specific ART regimen. Sex is unlikely to be an independent risk factor, but some retrospective studies have reported men to have a 2–3 fold higher risk of IRIS [3,14,22], yet in other prospective cohorts, sex has no association with IRIS [7,9,17]. The current belief is that sex is not an independent risk factor for IRIS. Age has not been established as a consistent risk for IRIS [2], though some retrospective case-control studies have matched for age [23]. In a U.K. cohort, the median age of those experiencing IRIS was 33.7 years vs. 35.6 years without IRIS [24]. In a Texas cohort, average age at time of ART initiation did not differ at 38.5 years with IRIS vs. 38.9 years without IRIS [14]. In 169 U.S. patients with TB prospectively enrolled, there was no association between age and risk of TB-IRIS [17]. Lastly, protease inhibitors have been suggested as a risk factor for IRIS [2], yet other cohorts have not replicated this finding [14,17,21]. Once again, extrapolating across all IRIS conditions may be incorrect, and age and gender may be specific risk factors in specific IRIS scenarios with specific pathogens.
Cell Surface Biomarkers related to IRIS Pathogenesis
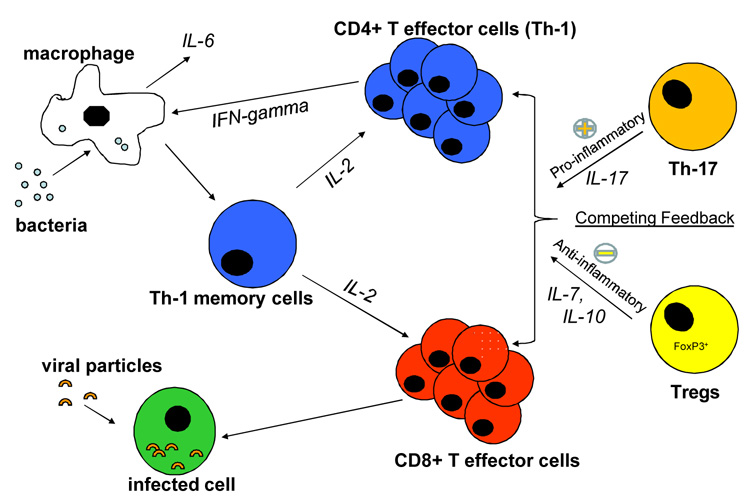
The clinical features of IRIS and its association with specific OIs suggests that IRIS is an antigen-driven process in which normal immune homeostasis is dysregulated. Inflammatory responses in IRIS are exaggerated and the mechanisms that normally limit inflammation seem to be broken. These features implicate particular components of the immune system in IRIS pathogenesis, namely those components which normally maintain homeostasis and modulate the function of the “effector” cells of the immune system [25]. Damage to these homeostatic control mechanisms may allow “overreaction” to certain antigen triggers, causing pathological inflammatory responses in IRIS patients. Figure 2 displays the major components of the immune system involved in immune regulation and effector functions [26]. Antigens, such as M. tuberculosis, activate dendritic cells and phagocytic macrophages, which seek out and consume foreign material. These cells then activate Th1 memory cells, promoting their differentiation into CD4+ Th1 effector cells.
Figure 2.
Immune cells normally interact in a state of homeostasis mediated by T regulatory cells (TREGs). Alteration in the balance between these components of the immune system and in their functionality may lead to IRIS. Adapted from Kestens et al.[26].
Functional T helper (TH) cells are classified into subsets such as interferon-γ (IFN- γ) producing TH1 and interleukin (IL)-4 producing TH2 cells which have long been known to be important mediators of host defense. TH1 cells are crucial for clearance of intracellular pathogens, such as M. tuberculosis, while TH2 cells are crucial for clearance of extracellular organisms. Recently, the discovery of TH17 cells, a new lineage of helper T cells preferentially produce IL-17, IL-17F, and IL-22 as signature cytokines, appear to play a critical role in sustaining inflammatory responses [27,28]. A reciprocal relationship has been described between pro-inflammatory TH17 lineage and anti-inflammatory, regulatory T-cells [29].
Regulatory T-cells (TREGs)
The maintenance of a homeostatic state is made possible by TREGs. TREGs suppress the proliferation of effector CD4+ and CD8+ cells and suppress their cytokine production, thus limiting the immune response to microbial antigens. The differentiation of naïve helper T cells (Th0) into TREGs is via signaling by TGF-β, IL-7, and IL-10. Disruption of TREG signaling may upset the delicate balance between an appropriately controlled immune response to an infectious pathogen and the development of exaggerated inflammatory states which are harmful. A critically important cytokine in this balance is IL-6. In the presence of TGF-β, IL-6 preferentially induces TH17 cells, and in the absence of IL-6, TREG differentiation can occur [29,30].
Normally, there is an approximately 2:1 ratio between pro-inflammatory TH17 cells and the TREGs. During immune reconstitution, various subsets may reconstitute at differing rates, and this ratio may become unbalanced. Altered or absent TREGs function may be an integral part of the cellular and cytokine dysregulation leading to IRIS. Such defects in modulatory function may occur because of alterations in TREG production, or changes in their functional capacity.
One finding has been increased ratios of TREG to effector/memory cells in patients with IRIS [31]. Seddiki noted a significant numeric expansion of the CD4+ CD25+ CD127+ FoxP3+ subset of TREGs after ART initiation in patients who developed IRIS, compared with HIV-negative controls and HIV patients who did not develop IRIS. One would expect increased TREG numbers to correlate with increased anti-inflammatory activity. However, Seddiki also found that the actual functional ability of these TREGs to suppress inflammation appears to be diminished in TB IRIS patients. In the experiments, TREGs showed blunted ability to suppress the release of the pro-inflammatory cytokines IL-6, IL-4, TNF-α, and IFN-γ, resulting in increased expression [31]. On the other hand, levels of IL-10, an inhibitory cytokine released directly by TREGs in effector cell modulation, were lower in some IRIS patients, supporting the concept of unbalanced homeostasis leaning too far in favor of immune activation (Seddiki, personal communication). This result might not be generalizable to all forms of IRIS; in cryptococcal meningitis patients, we found no differences in IL-10 production in the serum from patients who did or did not develop IRIS. Though there may be greater numbers of TREGs, these cells may produce less effective homeostatic regulation in persons with IRIS.
Cytokines as Biomarkers of Inflammation
All of these cellular interactions are mediated by signaling molecules, including the interleukins and other immunologic cytokines. Some cytokines are pro-inflammatory and others have anti-inflammatory / suppressive / homeostatic functions. Finding consistent patterns in cytokine activity during IRIS events will likely be the key to understanding the mechanisms by which some people to develop severe IRIS symptoms and others have none. A number of studies have found elevations in IL-6, one of the general pro-inflammatory cytokines, at IRIS events. In our prospective experience in Uganda, IL-6 levels in serum are 3-fold higher in IRIS patients than in comparable control patients without IRIS matched by CD4+, OI-status, and time of ART. A similar retrospective observation of increased plasma IL-6 after IRIS events was reported among persons with a history of a variety of IRIS events in Australia [33]. Many cells produce and modulate IL-6, though macrophages, in particular, are implicated in IL-6 hyperproduction associated with IRIS [32,33]. As well, in a case-control study of one person with nontuberculous mycobacterial IRIS manifesting as abdominal lymphadenopathy reported a temporal increase in IL-6 levels at time of IRIS event, returning toward levels of controls thereafter [56].
In our experience in Uganda, patients without a known active OI, who then develop IRIS after initiating ART, predominantly developed the unmasking form of IRIS manifest as dermatologic conditions, such as Herpes Zoster, Herpes simplex, and genital warts. The cytokines which were up-regulated at time of IRIS events in these IRIS patients were IFN-γ, TNF-α, IL-6, and MIP-1α. Similarly, among human herpesvirus-related IRIS, bio-available IL-6 was increased [34].
IRIS-associated Infections
As we develop more refined understanding of the cytokine/immune cell interactions occurring in particular infections, we may find common underlying mechanisms of IRIS pathogenesis. Because of the heterogeneity of IRIS-associated infections, however, most current studies examining the molecular underpinnings of IRIS have focused on specific infectious pathogens. Thus, we will organize our next discussion around the most common IRIS-associated microbes; M. tuberculosis and other mycobacteria, Cryptococcus, viral pathogens (including CMV, VZV and HSV), and Pneumocystis.
Biomarkers for Tuberculosis (TB) IRIS
Tuberculosis was one of the first identified pathogens to be associated with IRIS. As discussed above, it can occur either as a “paradoxical” IRIS event to previously treated TB or as “unmasking” of underlying latent or occult infection. With ART, TB-antigen-specific immunity reconstitutes; however the timing is variable and not consistent [35]. Those with TB-IRIS have increased numbers of TB-specific CD4+ T cells when measured by ELISpot interferon-release assay [36]. In TB-IRIS, Th1 cytokines appear to be up-regulated at the time of IRIS, with increases in interleukin (IL)-2, IL-12, and IFN-gamma as well as chemokines such as interferon-inducible protein 10 (IP-10/CXCL10) and monokine-induced by gamma interferon (MIG/CXCL9) [36]. Unpublished data also suggest C-reactive protein (CRP) is frequently elevated at time of TB-IRIS events [37]. Morlese et al also reported elevated CRP and IL-6 in a person with unmasking of abdominal TB [38].
Clinically, TB-associated IRIS most often occurs in the early phase after ART initiation, when memory T cells redistribute [39]. Th-1 cells are integral to the immune component of TB granuloma formation, and IL-6 may play a role in development of TB-associated IRIS responses, as well [40,41]. Other studies investigating ex-vivo stimulation of PBMCs report increased production of interferon-gamma (IFN-γ), human interferon-inducible protein-10 (IP-10), and monokine induced by IFN-γ (MIG) in patients with TB-associated IRIS events [42].
A different hypothesis by Shankar et al. postulates that patients may be protected by tolerance to the most antigenic component of TB, bacterial lipopolysaccharides. Having this tolerance, either because of long-term immune exposure or up-regulated TREG function, may decrease the likelihood of developing IRIS. Having diminished TREG function or a very high antigen burden may then predispose a patient to developing IRIS after they start ART [43].
Biomarkers for Mycobacterium avium-complex IRIS
In one case series, 30% of patients with M. avium-complex (MAC) subsequently developed IRIS upon ART initiation with a cumulative incidence rate of 15 episodes per 100 patient years [44]. The incidence of MAC in North America and Europe has decreased considerably in the ART era. In a Canadian case series from 1993 through 2004, the cumulative incidence of IRIS among those starting ART with CD4 counts <100 cells/µL or <50 cells/µL was 3.5% and 3.6% respectively [45]. MAC IRIS primarily presents as lymphadenitis, but fever of unknown origin (FUO), hepatosplenomegaly, abscess, or hypercalcemia have also been reported [14]. Both unmasking and paradoxical IRIS events are possible.
Diagnosis is primarily based on histopathology. Once again, the most serious differential diagnosis is treatment failure due to the development of drug resistance [46]. To differentiate IRIS from resistance, obtaining tissue for AFB staining, culture, and resistance testing is essential. IRIS lymphadenitis is marked by copious granulomatous inflammation with a paucity of organisms, at times no organisms may be seen, and indentifying the inciting pathogen can be difficult. Mycobacterial antigens persist long after culture sterility. Among HIV-seronegative persons treated for TB, 25% have PCR-positive sputum after two and four months of anti-mycobacterial therapy [47]. PCR probes may help delineate the inciting pathogen, but PCR cannot distinguish IRIS from persistent infection.
In a small U.K. case series of unmasking MAC-related IRIS presenting at an average of 11.5 months, Imami et al found that many patients lacked antigen-specific responses [48]. Similar unpublished findings have been reported by Elliot and colleagues in Australia in that restoration of antigen-specific responses can vary by person as well as by different antigens of a pathogen. For example, persons may selectively respond to the M. tuberculosis antigens of ESAT-6 or CFP-10 or PPD during immune recovery. The response by peripheral blood mononuclear cells (PBMCs) to antigen when measured from peripheral blood during an IRIS event can be either overwhelming or undetectable (unpublished). Sampling the compartment where the relevant inflammation is occurring, such as the lungs or lymph nodes, is not always possible to determine the composition of the antigen specific response. Imami et al reported patients with MAC unmasking, who had failed standard therapy, responded to adjuctive therapy with IL-2 and GM-CSF. The combination of IL-2 and GM-CSF supplementation was also associated with increased proliferative responses and increased IL-2 production [48].
Biomarkers for Cryptococcal Meningitis (CM)
There have been three retrospective and one prospective cohort studies of cryptococcal meningitis IRIS conducted among patients in France, Uganda, Houston, and Thailand [3, 9,18,49]. Among the 255 patients cumulatively described, the incidence of IRIS is near 30%. The common triad for CM-IRIS is: 1) treated CM, 2) recently started HAART, and 3) new aseptic meningitis [9]. The presentation of the aseptic meningitis of IRIS is clinically indistinguishable from cryptococcal treatment failure and/or relapse. In IRIS, a culture-negative meningitis occurs which appears to be triggered by the presence of residual antigen in the CSF. The spectrum of cryptococcal disease in IRIS is not always restricted to the meninges and can also manifest as atypical disseminated disease, such as cutaneous lesions, lymphadenopathy, mediastinitis, pneumonitis with ARDS, and CNS cryptococcomas [9,18,50,51,52]. In these cases, a paucity of organisms is usually recovered but copious granulomatous inflammation exists.
At time of a CM-IRIS event, a CSF WBC pleocytosis and elevated protein are often present, but these levels can overlap with what is observed in CM relapse. Of persons with CM-IRIS, 80% will have a CSF WBC cell count <100 cells/µL [18]. The CSF glucose level is usually in the low normal range. In general, an increased intracranial pressure (ICP) of >20 cm H2O is very common in patients with CM. An elevated ICP occurs in 50% of individuals and pressures >35 cm H2O occur in 25% of individuals with CM in the U.S [53]. In resource limited regions, the ICP has been reported to be higher, with a median CSF opening pressure of 33 cm H2O and with 81% having elevated pressure (≥20 cm) in patients with CM [9]. In cases of IRIS, the ICP is equally or even more elevated than with culture positive cryptococcal meningitis. Greater than 75% of patients with CM IRIS will have a CSF opening pressure >30 cm H2O, and the average is near 45 cm H2O at the time of IRIS events [9,18].
Obtaining a culture is critical to differentiate treatment failure or relapse from IRIS [54]. In cases of IRIS, the CSF cryptococcal antigen (CRAG) titer usually has decreased dramatically following initiation of ART and the initial anti-cryptococcal therapy [18]. The average level of the CSF CRAG titer in patients with culture positive cryptococcal meningitis is 1:2048. In CM-IRIS with negative CSF cultures, the CSF CRAG titers average 1:128 [18]. In patients with IRIS, 75% will have a CSF CRAG titer ≤ 1:256, whereas in culture positive meningitis 75% of CRAG titers are ≥1:256 [18].
In a Ugandan cohort [9], we identified heterogeneous cytokine profiles in patients with CM-IRIS marked by increases in IL-2, IL-6, IL-9, IL-17, and TNF-α in the serum of individual patients. Notably, interferon-γ, IP-10, and MIG in serum were not increased at time of IRIS events compared to those without IRIS. Additionally, at time of IRIS events, preliminary data suggest serum CRP levels are often, but not always, markedly elevated (mean 170 ± 100 mg/L). At time of initial CM diagnosis, CSF cytokine profiles are similar and are not predictive of future IRIS events. Based on published studies, predictive biomarkers of CM IRIS have not been identified.
Zheng et al have found increased macrophage phagocytosis of C. neoformans in response to increased IFN-γ production by CD4+ T cells. The CD4+ cells themselves had direct cytotoxicity against C. neoformans through secretion of granulysin [55]. GM-CSF also plays a critical role in the phagocytosis of Cryptococcus by macrophages [56,57,58], and may play an important role in the rate of antigen clearance. In HIV patients, this process of antigen clearance appears to be impaired, but could potentially improve after initiation of ART and play a role in the association between IRIS and more rapid expansion of CD4+ cells.
Biomarkers for Viral-related IRIS
The understanding of viral related IRIS is very limited at present. In part, this comes from the poor understanding of the immunology and pathogenesis of many viral related infections. One particular limitation is in understanding the basis between asymptomatic viral shedding and symptomatic disease; specifically, whether the manifestations of ‘disease’ are caused by a viral cytopathic affect or the activation of an immune response.
Hepatitis B virus (HBV) or hepatitis C virus co-infection with HIV are common conditions worldwide, especially in Asia or among illicit drug use populations. Differentiating between what is hepatotoxicity due to ART versus an IRIS event is difficult. At present, there are no reliable biomarkers to distinguish the two, and hepatitis-associated IRIS is probably under-recognized. Moderate to severe hepatitis flares after starting ART are fairly common, affecting 10–12% of patients who start ART; however, most of these flares are self-resolving [59,60]. In the setting of IRIS, HBV viral loads are decreased from pre-ART baseline with the restoration of CD4+ HBV-specific immunity [61], causing an elevation of liver enzymes that has the same clinical appearance as hepatotoxicity.
Kaposi sarcoma (KS) is an AIDS defining condition that typically improves with immune reconstitution; however clinical flares and worsening can occur shortly after starting ART [62]. The timing of onset of KS IRIS appears to be similar to other forms of IRIS, and usually occurs within the first 2–3 months of ART. In patients with KS IRIS, cutaneous KS lesions may initially expand or become painful; visceral KS-associated IRIS may be fatal. The diagnosis of KS IRIS is clinical, and specific biomarkers have not been identified.
Although numerous viral infections are associated with IRIS, we presently do not know if these diverse clinical diseases are due to common pathophysiological mechanisms, but we hypothesize that dysregulated immune homeostasis plays a significant role.
Summary
HIV patients starting on anti-retroviral therapy have profound changes in the function of components of their immune system as immune recovery occurs. Our previous understanding of immune recovery treated the immune system and its cellular and protein components as a single unit, which gradually improved as HIV diminished. We now find that ‘immune reconstitution’ happens at different rates for different subcomponents of the immune system, and reconstituted immune function does not always mimic normal immune function. IRIS develops when the immune response is exaggerated due to lack of homeostatic regulation. Elucidating the subtle patterns within specific cell populations in the recovering immune system and the intrinsic immune modulatory pathways will give us further insight the pathogenesis of this clinically important phenomenon. In the future, a better understanding of IRIS pathogenesis will lead to development of new biomarkers to identify those patients at greatest risk and to develop specific treatments to return the immune system to homeostasis following initiation of HIV therapy.
Future Perspectives
The importance of biomarkers in the future of medicine cannot be overemphasized. From the early 19th century, various laboratory-based tests have been unveiled with a positive impact on the practice of medicine. We envision the use of biomarkers in predicting who is at risk of developing IRIS. However, until then, the next few years will require dedicated scientists working to better define the pathogenesis of IRIS.
Over the next decade, increasing numbers of patients will require ART, especially in Sub-Saharan Africa. For example, in Uganda, there are 1.5 million people living with HIV. But only about 70,000 currently receive ART. With the increasing roll out of ART and consolidation of established ART programs in Africa, IRIS has emerged as an issue that cannot be ignored.
The early development of solutions to the problem of IRIS will depend on the establishment of collaborative research networks responsible for the convergence of research ideas. In order to be successful, multi-disciplinary research teams will need to combine investigators skilled in clinical, epidemiological, and basic science research to define the pathophysiology of IRIS and the clinical management of IRIS.
One limitation of IRIS research has been the paucity of data from prospective cohorts. Most of the IRIS data to date have been generated from retrospective cohorts using a variety of non-conforming case definitions. A major task is to define IRIS in a generalizable way that can be used by all researchers in the field. The International Network for the Study of HIV-associated IRIS (INSHI), composed of researchers from Australia, Europe, South Africa, Uganda, and the United States will produce IRIS definitions and likely play a significant role in research on the pathogenesis, treatment, and prevention of IRIS. Such networks will also lead to the development of biomarkers that can be used to diagnose IRIS and will lead to better definitions in the future. As various IRIS- related events are studied, one of the questions to be answered will be whether the pathophysiology is pathogen-specific or if general pathways of immune regulation are involved in all forms of IRIS through a common mechanism. Thus, biomarkers for diagnosis and prediction of IRIS may be general to all forms of IRIS or may be pathogen-specific.
A major ongoing dilemma is the adequate characterization and categorization of populations and IRIS conditions being studied. Differentiating unmasking from paradoxical IRIS events is critical to correctly studying the epidemiology and pathophysiology of IRIS. Extrapolating across pathogens is likely unwise in comparing pathophysiology of IRIS in the near term.
Genetic profiles
An active area of current research focuses on the genetic variations associated with IRIS. Patients with CMV-related IRIS have been found to have increased frequency of HLA-B44 haplotypes [63]. Specific regulatory cytokine gene polymorphisms have been reported to be protective against herpes virus-associated IRIS (IL12B-3’UTR) and against mycobacterial-associated IRIS (TNFα-308*2 and IL6-174*G) [64]. These alleles play a role in decreasing cytokine production. IL-6, IL-12, and TNF-α polymorphisms have been implicated in other studies in development of mycobacterial-associated IRIS. In the future, we may be able to exploit these types of genetic markers to determine genetic susceptibility to IRIS prior to initiating anti-retroviral therapy.
Genomic Biomarkers of Gene Expression
Our research group has used microarray technology to investigate the genome-wide differences in gene expression among HIV-infected persons with and without IRIS events in two prospective cohorts: those with AIDS but without an active OI and those with recent cryptococcal meningitis when starting ART in Sub-Saharan Africa. Our preliminary data suggest that expression of numerous genes involved in immune activation and anti-viral responses can be used as diagnostic and predictive biomarkers of IRIS. Thus, this research direction shows great promise.
Although traditional epidemiologic and HIV parameters have had limited capability to predict IRIS on an individual level, biomarkers of immune activation and anti-viral responses may predict the development of IRIS before the clinical syndrome becomes evident. The early identification of patients who are at high risk of developing IRIS will allow better strategies for prevention and treatment of IRIS.
The discovery of biomarkers can be expensive, tedious and time consuming. There are however, newer technologies that will become widely available in the future. These include Luminex technology which can be used for cytokine profiling as well as microarrays used to profile gene expression at the mRNA level. The use of gene sequencing and newer technology to identify genomic polymorphisms on a genome-wide scale will also play a role in identifying specific genetic polymorphisms that may be useful to identify patients at risk for IRIS. These powerful methods will become increasingly available and utilized in the next 5– 10 years and will likely revolutionize the discovery of biomarkers.
Executive Summary
HIV Immune Reconstitution Inflammatory Syndrome (IRIS) is a common complication of antiretroviral therapy
IRIS typically occurs in persons with advanced HIV/AIDS with CD4+ counts <200 cells/µL.
Incidence of IRIS is 10–15% among persons with AIDS in North America & Europe. IRIS is more frequent in resource-limited regions (20–25%).
Persons with recent opportunistic infections (OIs) are a greater risk of IRIS. Rates of IRIS following tuberculosis (TB) or cryptococcal meningitis are near 33%.
Two major clinical scenarios of IRIS occur
Paradoxical IRIS is the symptomatic relapse of a previously treated infection. Inflammation is driven by dysregulated, immunologic mechanisms despite microbiologic treatment success.
Unmasking IRIS is the accelerated presentation of a new opportunistic infection which was occult or latent when starting HIV therapy. Enhanced screening may detect this OI prior to starting HIV therapy.
Pathophysiology of IRIS is poorly understood but hypothesized to be due to a dysregulated, pathologic immune response
Conditions for IRIS require: antigen, restoration of antigen-specific immunity, dysregulated immune response which is hypothesized to be functional imbalance in pro-inflammatory (Th1, Th17) versus anti-inflammatory (TREG) T-cell populations.
Cytokine profiles of IRIS events are heterogeneous based on the inciting pathogen (e.g. viral, fungal, mycobacterial, etc) with marked individual variation.
Pro-inflammatory cytokines are generated at time of mycobacterial and fungal IRIS events, such as IL-6, IL-17, IFN-γ, and TNF-α.
C-reactive protein is frequently elevated at time of mycobacterial and fungal-associated IRIS events.
Genetic and genomic studies are ongoing to elucidate the pathophysiology of IRIS
Genetic polymorphisms of IL-6, IL-12, and TNF-α have been implicated as possible factors in risk and protection from IRIS.
Differences in gene expression exist prior to beginning HIV therapy which may predict the risk of subsequent IRIS. The genes differentially expressed involve immune activation and anti-viral responses.
Ongoing prospective cohort studies are validating these biomarkers.
IRIS Research is rapidly developing with ongoing prospective cohort studies
In the next 5–10 years, a much greater understanding of IRIS should develop.
Ongoing international cohort studies are investigating the pathophysiology of IRIS through evaluation of: cell surface markers of peripheral blood mononuclear cells (PBMCs), cytokine profiles, genetic DNA polymorphisms, and RNA gene expression.
Future prospective cohort studies and clinical trials will be needed to validate prevention and treatment strategies.
Acknowledgements
Financial Support: This work was supported in part by the Academic Alliance Foundation (DBM), University of Minnesota Academic Health Center (PRB,DBM), National Institute of Allergy and Infectious Diseases T32AI055433 (SB, DRB); L30AI066779; K12RR023247: (DRB), Minnesota Medical Foundation (PRB, DRB, DBM), University of Minnesota Office of International Programs (DRB).
Abbreviations
- ART
anti-retroviral therapy
- CM
Cryptococcal meningitis
- CMV
cytomegalovirus
- IRIS
Immune reconstitution syndrome
- OI
opportunistic infection
- TB
Tuberculosis
- TREG
regulatory T-cells
Footnotes
Conflicts of Interest: No conflicts of interest exist.
References
- 1.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 2.Manabe YC, Campbell JD, Sydnor E, Moore RD. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J Acquir Immune Defic Syndr. 2007;46:456–462. doi: 10.1097/qai.0b013e3181594c8c. [DOI] [PubMed] [Google Scholar]
- 3.Lortholary O, Fontanet A, Memain N, et al. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19:1043–1049. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 4.French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107–115. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 5.Srikantiah P, Walusimbi MN, Kayanja HK, et al. Early virological response of zidovudine/lamivudine/abacavir for patients co-infected with HIV and tuberculosis in Uganda. AIDS. 2007;21:1972–1974. doi: 10.1097/QAD.0b013e32823ecf6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huruy K, Mulu A, Mengistu G, et al. Immune Reconstitution Inflammatory Syndrome among HIV/AIDS Patients during Highly Active Antiretroviral Therapy in Addis Ababa, Ethiopia. Jpn J Infect Dis. 2008;61:205–209. [PubMed] [Google Scholar]
- 7.Murdoch DM, Venter WDF, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 8.Meya DB, Boulware DR, Rhein J, et al. Immune Reconstitution Inflammatory Syndrome in HIV Infected Ugandans. Abstract 920 Presented at: Infectious Disease Society of America Annual Conference; San Diego, CA. 2007. Oct 5, [Google Scholar]
- 9.Kambugu A, Meya DB, Rhein J, et al. Outcomes of Cryptococcal Meningitis in Sub-Saharan Africa in the Era of Antiretroviral Therapy Availability. Clin Inf Dis. 2008;46:1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson J, Meier M, Wall J, Ying J, Fichtenbaum CJ. Immune reconstitution syndrome in HIV: validating a case definition and identifying clinical predictors in persons initiating antiretroviral therapy. Clin Inf Dis. 2006;42:1639–1646. doi: 10.1086/503903. [DOI] [PubMed] [Google Scholar]
- 11.Breton G, Duval X, Estellat C, et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Inf Dis. 2004;39:1709–1712. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 12.Barry SM, Lipman MC, Deery AR, et al. Immune reconstitution pneumonitis following Pneumocystis carinii pneumonia in HIV-infected subjects. HIV Med. 2002;3:207–211. doi: 10.1046/j.1468-1293.2002.00115.x. [DOI] [PubMed] [Google Scholar]
- 13.Cianchetta-Sivori M, Raso S, Fernandez-Guerrero M, Górgolas M, García R. Do CD8(+)CD25(+) cells predict immune reconstitution syndrome in HIV-positive patients who begin HAART? AIDS. 2007;21:2347–2349. doi: 10.1097/QAD.0b013e3282f125da. [DOI] [PubMed] [Google Scholar]
- 14.Shelburne SA, Visnegarwala F, Darcourt J, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 15.McCormack JG. Miliary tuberculosis with paradoxical expansion of intracranial tuberculomas complicating HIV infection in a patient receiving highly active antiretroviral therapy. Clin Infec Dis. 1998;26:1008–1009. doi: 10.1086/517636. [DOI] [PubMed] [Google Scholar]
- 16.Wendel KA, Alwood KS, Gachuhi R, Chaisson RE, Bishai WR, Sterling TR. Paradoxical worsening of tuberculosis in HIV-infected persons. Chest. 2001;120:193–197. doi: 10.1378/chest.120.1.193. [DOI] [PubMed] [Google Scholar]
- 17.Burman W, Weis S, Vernon A, et al. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int J Tuberc Lung Dis. 2007;11:1282–1289. [PubMed] [Google Scholar]
- 18.Shelburne SA, Darcount J, White CA, et al. The Role of Immune Reconstitution Inflammatory Syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Inf Dis. 2005;40:1049–1053. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 19.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect.Dis. 2005;5:361–373. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 21.Zolopa A, Andersen J, Komarow L, et al. Immediate vs Deferred ART in the Setting of Acute AIDS-related Opportunistic Infection: Final Results of a Randomized Strategy Trial, ACTG A5164. Abstract 142 Presented at: 15th Conference on Retroviruses and Opportunistic Infections (CROI) 2008; Boston. 2008. Feb 6, [Google Scholar]
- 22.Olalla J, Pulido F, Rubio R, Costa MA, et al. Paradoxical responses in a cohort of HIV-1-infected patients with mycobacterial disease. Int J Tuberc Lung Dis. 2002;6:71–75. [PubMed] [Google Scholar]
- 23.Park WB, Choe PG, Jo JH, et al. Immune reconstitution inflammatory syndrome in the first year after HAART: influence on long-term clinical outcome. AIDS. 2006;20:2390–2392. doi: 10.1097/QAD.0b013e328010f201. [DOI] [PubMed] [Google Scholar]
- 24.Ratnam I, Chiu C, Kandala NB, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418–427. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 25.Munier ML, Kelleher AD. Acutely dysregulated, chronically disabled by the enemy within: T-cell responses to HIV-1 infection. Immunol.Cell Biol. 2007;85:6–15. doi: 10.1038/sj.icb.7100015. [DOI] [PubMed] [Google Scholar]
- 26.Kestens L, Seddiki N, Bohjanen PR. Immunopathogenesis of immune reconstitution disease in HIV patients responding to antiretroviral therapy. Curr Opin HIV AIDS. 2008;3:419–424. doi: 10.1097/COH.0b013e328302ebbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stockinger B, Veldhoen M, Martin B. Th17 T cells: Linking innate and adaptive immunity. Semin Immunol. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Wing K, Miyara M. Regulatory T cells - a brief history and perspective. Eur J Immunol. 2007;37:S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 29.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 30.Yao Z, Fanslow WC, Seldin MF, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 31.French MA. Pathogenesis of Immune Reconstitution Inflammatory Syndrome. Presented at: 14th Conference on Retroviruses and Opportunistic Infections (CROI); Los Angeles, CA. 2007. Feb 28, [Google Scholar]
- 32.French MA, Price P, Stone SF. Immune Restoration Disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 33.Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV.Med. 2002;3:21–27. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 34.Stone SF, Price P, Brochier J, French MA. Plasma bioavailable interleukin-6 is elevated in human immunodeficiency virus-infected patients who experience herpesvirus-associated immune restoration disease after start of highly active antiretroviral therapy. J Infect Dis. 2001;184:1073–1077. doi: 10.1086/323599. [DOI] [PubMed] [Google Scholar]
- 35.Schluger NW, Perez D, Liu YM. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest. 2002;122:597–602. doi: 10.1378/chest.122.2.597. [DOI] [PubMed] [Google Scholar]
- 36.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 37.Meintjes Graeme. personal communication. Kampala, Uganda: TB-IRIS Workshop; 2006. Nov 28–30, [Google Scholar]
- 38.Morlese JF, Orkin CM, Abbas R, et al. Plasma IL-6 as a marker of mycobacterial immune restoration disease in HIV-1 infection. AIDS. 2003;17:1411–1413. doi: 10.1097/00002030-200306130-00025. [DOI] [PubMed] [Google Scholar]
- 39.Bucy RP, Hockett RD, Derdeyn CA, et al. Initial increase in blood CD4+(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J.Clin.Invest. 1999;103:1391–1398. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol.Cell Biol. 2007;85:103–111. doi: 10.1038/sj.icb.7100027. [DOI] [PubMed] [Google Scholar]
- 41.Saunders BM, Frank AA, Orme IM, Cooper AM. CD4+ is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell Immunol. 2002;216:65–72. doi: 10.1016/s0008-8749(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 42.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 43.Shankar EM, Vignesh R, Murugavel KG, et al. Immune reconstitution inflammatory syndrome in association with HIV/AIDS and tuberculosis: Views over hidden possibilities. AIDS Res.Ther. 2007;4:29. doi: 10.1186/1742-6405-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Harthi L, Voris J, Patterson BK, et al. Evaluation of the impact of highly active antiretroviral therapy on immune recovery in antiretroviral naive patients. HIV Med. 2004;5:55–65. doi: 10.1111/j.1468-1293.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 45.Phillips P, Bonner S, Gataric N, et al. Nontuberculous Mycobacterial immune reconstitution syndrome in HIV-infected patients: spectrum of disease and long term follow-up. Clin Inf Dis. 2005;41:1483–1497. doi: 10.1086/497269. [DOI] [PubMed] [Google Scholar]
- 46.Heifets L. Susceptibility testing of Mycobacteriumavium complex isolates. Antimicrobial Agents and Chemotherapy. 1996;40:1759–1767. doi: 10.1128/aac.40.8.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velayati AA, Bakayev VV, Bahrmand AR. Use of PCR and culture for detection of Mycobacterium tuberculosis in specimens from patients with normal and slow responses to chemotherapy. Scand J Infect Dis. 2002;34:163–166. doi: 10.1080/00365540110080106. [DOI] [PubMed] [Google Scholar]
- 48.Pires A, Nelson M, Pozniak AL, et al. Mycobacterial immune reconstitution inflammatory syndrome in HIV-1 infection after antiretroviral therapy is associated with deregulated specific T-cell responses: Beneficial effect of IL-2 and GM-CSF immunotherapy. J. Immune Based Ther. Vaccines. 2005;3:7. doi: 10.1186/1476-8518-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sungkanuparph S, Jongwutiwes U, Kiertiburanakul S. Timing of cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in patients with AIDS and cryptococcal meningitis. J Acquir Immune Defic Syndr. 2007;45:595–596. doi: 10.1097/QAI.0b013e318061b5eb. [DOI] [PubMed] [Google Scholar]
- 50.Trevenzoli M, Cattelan AM, Rea F, et al. Mediastinitis due to cryptococcal infection: a new clinical entity in the HAART era. J Infect. 2002;45:173–179. doi: 10.1016/s0163-4453(02)91052-2. [DOI] [PubMed] [Google Scholar]
- 51.Cattelan AM, Trevenzoli M, Sasset L, Lanzafame M, Marchioro U, Meneghetti F. Multiple cerebral cryptococcomas associated with immune reconstitution in HIV-1 infection. AIDS. 2004;18:349–351. doi: 10.1097/00002030-200401230-00034. [DOI] [PubMed] [Google Scholar]
- 52.York J, Bodi I, Reeves I, Riordan-Eva P, Easterbrook PJ. Raised intracranial pressure complicating cryptococcal meningitis: immune reconstitution inflammatory syndrome or recurrent cryptococcal disease. J Inf. 2005;51:165–171. doi: 10.1016/j.jinf.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 53.The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. Clin Infect Dis. 2000;30:47–54. doi: 10.1086/313603. [DOI] [PubMed] [Google Scholar]
- 54.Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43:1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- 55.Zheng CF, Ma LL, Jones GJ, et al. Cytotoxic CD4+T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood. 2007;109:2049–2057. doi: 10.1182/blood-2006-03-009720. [DOI] [PubMed] [Google Scholar]
- 56.de Silva TI, Cope A, Goepel J, Greig JM. The use of adjuvant granulocyte-macrophage colony-stimulating factor in HIV-related disseminated atypical mycobacterial infection. J Infect. 2007;54:e207–e210. doi: 10.1016/j.jinf.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Joshi PC, Raynor R, Fan X, Guidot DM. HIV-1 Transgenic Expression in Rats Decreases Alveolar Macrophage Zinc Levels and Phagocytosis. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2007-0344OC. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen GH, Olszewski MA, McDonald RA, et al. Role of granulocyte macrophage colony-stimulating factor in host defense against pulmonary Cryptococcus neoformans infection during murine allergic bronchopulmonary mycosis. Am J Pathol. 2007;170:1028–1040. doi: 10.2353/ajpath.2007.060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 60.John M, Flexman J, French MA. Hepatitis C virus-associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune restoration disease? AIDS. 1998;12:2289–2293. doi: 10.1097/00002030-199817000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Baker JV, Boulware DR, Bohjanen PR. A case for treating high hepatitis B DNA levels before starting HIV therapy. AIDS. 2006;20:2402–2403. doi: 10.1097/QAD.0b013e3280110aef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leidner RS, Aboulafia DM. Recrudescent Kaposi's sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care Stds. 2005;19:635–644. doi: 10.1089/apc.2005.19.635. [DOI] [PubMed] [Google Scholar]
- 63.Price P, Keane NM, Stone SF, Cheong KY, French MA. MHC haplotypes affect the expression of opportunistic infections in HIV patients. Hum.Immunol. 2001;62:157–164. doi: 10.1016/s0198-8859(00)00239-1. [DOI] [PubMed] [Google Scholar]
- 64.Price P, Morahan G, Huang D, et al. Polymorphisms in cytokine genes define subpopulations of HIV-1 patients who experienced immune restoration diseases. AIDS. 2002;16:2043–2047. doi: 10.1097/00002030-200210180-00009. [DOI] [PubMed] [Google Scholar]