Abstract
Hippocampal-dependent synaptic plasticity and memory are modulated by apamin-sensitive small conductance Ca2+-activated K+ (SK) channels. Transgenic mice overexpressing SK2 channels (SK2+/T mice) exhibit marked deficits in hippocampal memory and synaptic plasticity, as previously reported. Here, we examined whether SK2 overexpression affects the encoding or retention of contextual memory. Compared with wild-type littermates, SK2+/T mice exhibited significantly less context-dependent freezing 10 min and 24 h after conditioning. Interestingly, this contextual memory impairment was eliminated if SK2+/T mice were permitted longer pre-exposure to the conditioning chamber. These data support converging evidence that SK2 channels restrict the encoding of hippocampal memory.
The hippocampus is a critical neurobiological substrate for several forms of explicit memory (Eichenbaum 2000). The activation of N-methyl-D-aspartate (NMDA) receptors has been shown to be critical for forms of hippocampal synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD) (Malenka and Nicoll 1999), and for hippocampal memory (Bannerman et al. 1995; Cravens et al. 2006; Niewoehner et al. 2007). Several types of potassium (K+) channels influence hippocampal neuronal excitability and modulate NMDA receptor-dependent synaptic plasticity. In particular, small conductance Ca2±activated K+ (KCa (SK)) channels regulate hippocampal neuronal excitability, hippocampal synaptic plasticity, and memory encoding (Behnisch and Reymann 1998; Stocker et al. 1999; Stack-man et al. 2002; Kramar et al. 2004; Ris et al. 2007). In addition, SK channels appear to regulate synaptically evoked glutamatergic excitatory postsynaptic potentials (EPSPs) in the hippocampus; blockade of SK channels with apamin potentiates glutamatergic EPSPs (Faber et al. 2005; Ngo-Anh et al. 2005). These findings suggest that SK channels may negatively regulate NMDA receptor-dependent synaptic plasticity and memory via a feedback loop that depresses synaptic NMDA receptor activity.
All three SK channel subtypes (KCa2.1, KCa2.2, and KCa2.3 [SK1, SK2 and SK3]) are expressed in the rodent hippocampus (Kohler et al. 1996), yet in layers CA1 and CA3, SK1 and SK2 channels predominate over SK3 channels (Stocker and Pedarzani 2000; Sailer et al. 2002, 2004). We recently reported that transgenic mice that overexpress SK2 channels (SK2+/T mice) exhibit fourfold larger apamin-sensitive hyperpolarizing currents, and attenuated subthreshold glutamatergic EPSPs and synaptic plasticity in hippocampal slices compared with that of wild-type (WT) littermates (Hammond et al. 2006). These physiological alterations were matched by substantial deficits of SK2+/T mice in spatial learning and memory in the Morris water maze task and deficits in contextual fear memory after delay fear conditioning. These data indicate that SK2 channels modulate hippocampal synaptic plasticity and memory. However, it is of interest to define the precise memory processes that are affected by SK channels.
Apamin enhances the encoding of hippocampal-dependent spatial and object memory in male C57BL/6J mice by reducing the amount of training required to establish lasting memory in the Morris water maze and a spontaneous novel object recognition task (Stackman et al. 2002). Together, these data suggest that blocking SK channels with apamin affects the initial encoding of memory likely by influencing the patterns of neuronal activation and synaptic plasticity necessary for the storage of new information (Tzounopoulos and Stackman 2003). Initial assessments of SK2+/T mice indicated that the overexpression of SK2 channels impaired hippocampal memory (Hammond et al. 2006). We conducted the present studies to determine whether SK2 overexpression affects the encoding or retention of contextual memory processes.
The production and complete characterization of SK2+/T mice was reported previously (Hammond et al. 2006). We first examined whether SK2 overexpression impairs retention of the memory for the context–footshock association after delay fear conditioning in naïve male WT and SK2+/T mice, aged 8−10 wk. WT (n = 12) and SK2+/T (n = 14) mice were first exposed to the conditioning chamber (Freeze Monitor, 20 × 25 × 12.5 cm, San Diego Instruments) for a 5-min context pre-exposure session. There were no significant differences in freezing during the context pre-exposure session (Fig. 1A; t24 = 1.33, n.s.) indicating similar levels of motor behavior among the two groups of mice before conditioning. During the delay conditioning session 24 h later (Fig. 1B), mice were returned to the same chamber and received three pairings of a 30-sec auditory tone (CS, 68 dB, 30 Hz) and a 1-sec 0.5 mA footshock (US). A two-factor ANOVA on percent freezing data, with genotype as a between-group factor and CS–US pairing as a repeated measure revealed a significant effect of CS–US pairing, F(2,48) = 5.28; P < 0.008, a significant main effect of genotype, F(1,24) = 5.94; P < 0.025, and a nonsignificant interaction of genotype X session, F(2,48) = 0.46; n.s. An analysis of data from the final CS–US pairing indicated no difference in percent freezing between the two groups of mice, t24 = 1.76, n.s. The retention of contextual fear memory was tested by returning the mice to the same chamber for a 5-min context test at 10 min and 24 h after conditioning. Both genotypes exhibited conditioned fear to the context when tested at 10 min and 24 h postconditioning, expressed as an increase in percent freezing relative to the pre-exposure session (Fig. 1C). Analysis of context test freezing measures by two-factor ANOVA yielded a significant effect of session, F(2,48) = 24.66; P < 0.001, a significant interaction of genotype X session, F(2,48) = 6.54; P < 0.003, and a significant effect of genotype, F(1,24) = 42.37; P < 0.001. Post-hoc comparisons revealed that SK2+/T mice exhibited significantly less percent freezing than WT mice during the context test at 10 min (t24 = 3.56; P < 0.003) and at 24 h (t24 = 4.67; P < 0.001). These data indicate that SK2 overexpression impairs contextual fear conditioning, consistent with our previous report (Hammond et al. 2006). The fact that SK2+/T mice froze significantly less than WT mice during a context test just 10 min after conditioning suggests that the memory for the context may not have been sufficiently encoded during the pre-exposure session or that the contextual memory was not consolidated after the pre-exposure session. Either failure would have rendered the contextual memory unavailable for association with the footshock during the conditioning session. It is also possible that the contextual memory was established during the pre-exposure session but was not sufficiently reactivated during the conditioning session to permit its association with the aversive footshock stimulus.
Figure 1.
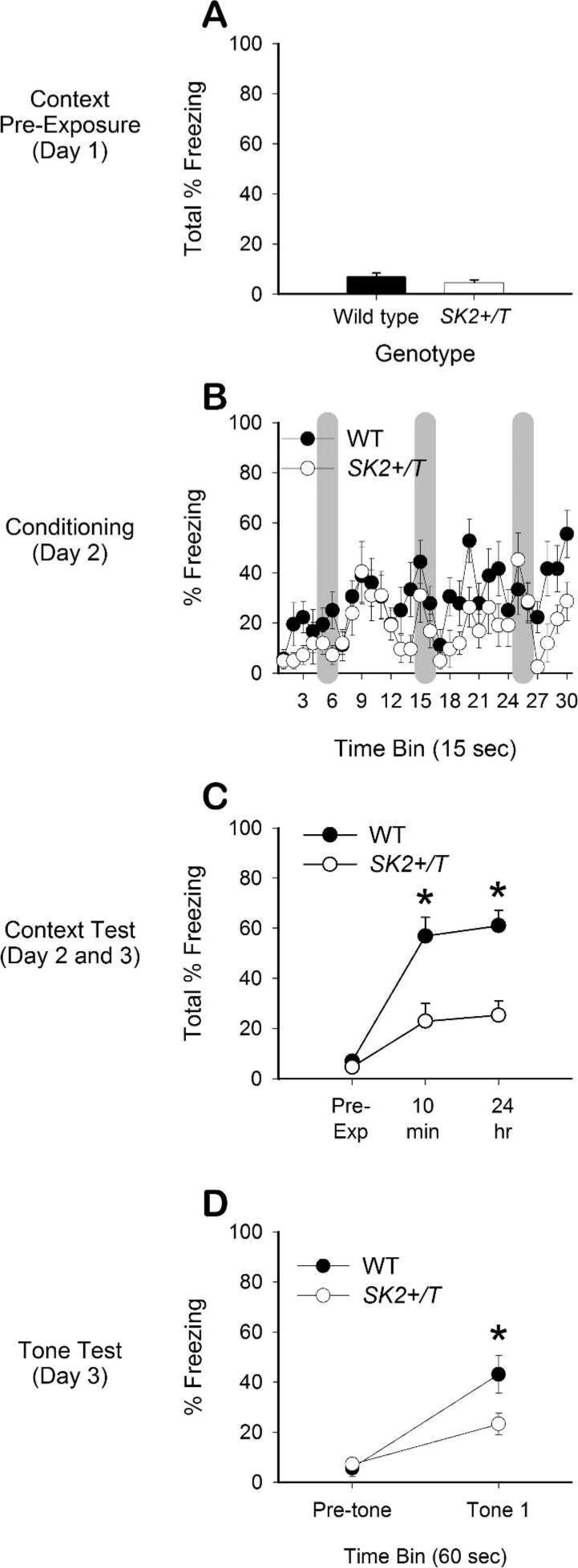
Contextual and cued fear conditioning is impaired in mice that overexpress SK2 channels. (A) Levels of percent time freezing did not differ between WT and SK2+/T mice during the 5-min context pre-exposure session on Day 1. (B) Both groups of mice exhibited similar levels of freezing during the conditioning session on Day 2. The gray vertical bars indicate the 30-sec periods during each auditory tone CS. (C) Retention of contextual fear memory was tested at 10 min (test of short-term memory) and 24 h (test of long-term memory) after conditioning. The respective freezing data from the pre-exposure session are plotted here for comparison. Both genotypes exhibited conditioned fear demonstrated by an increase in freezing relative to the pre-exposure session. However, the SK2+/T mice exhibited significantly less percent freezing compared with WT littermates during both retention tests, indicating impaired contextual conditioning. (D) Retention of cued fear memory was tested ∼1 h after the context test on Day 3. There were no differences in percent freezing between genotypes before the tone was presented (Pre-tone). However, SK2+/T mice exhibited significantly less freezing in response to the tone compared with WT mice, indicating that SK2 over expression also impaired cued fear conditioning. All error bars, SEM. Significant differences are indicated by an asterisk (* P < 0.05; see text for details).
Both genotypes exhibited conditioned freezing when the tone was presented in an environment distinct from the original training context (Fig. 1D). Analysis by two-factor ANOVA revealed a significant effect of minutes (min 1, the pre-tone interval; min 2, the post-tone interval), F(1,24) = 32.06; P < 0.001, a significant interaction of genotype X min, F(2,24) = 5.13; P < 0.035, and a near-significant main effect of genotype, F(1,24) = 5.13; P = 0.05. Post-hoc analyses revealed that percent freezing did not differ between genotypes during the pretone interval (min 1), t24 = 0.69; n.s. However, SK2+/T mice froze significantly less in response to the tone (min 2) than did the WT mice, t24 = 2.39; P < 0.03 consistent with our previous report (Hammond et al. 2006). In light of the recent demonstration that auditory stimuli in the range used in the present study produced auditory brainstem responses in SK2+/T mice that were not different from those of WT mice (Maison et al. 2007), we conclude that the deficits of the SK2+/T mice in cued fear conditioning are not due to sensory impairments.
Together, these data suggest that SK2 overexpression impairs both short- and long-term retention of contextual fear memory, an effect perhaps due to a weak association of the contextual representation with the footshock during the conditioning session. However, a more conservative interpretation of the impaired retention at 10 min post-training might be that the impairment is attributed to the state of the contextual representation encoded during context pre-exposure. Specifically, if the contextual memory was not encoded sufficiently during pre-exposure, then only a weak contextual memory would be available to be associated with the footshock stimulus during conditioning.
It is possible that SK2+/T mice might require more time to fully encode the contextual memory. To test this, we examined the effects of increased context pre-exposure on subsequent retention of contextual fear memory. Naïve mice received one 5-min (1 × 5-min; WT, n = 14, and SK2+/T, n = 13) or three 5-min (3 × 5-min; WT, n = 17, and SK2+/T, n = 17) context pre-exposure sessions on Day 1. Mice that received three context pre-exposure sessions were returned to their home cages after each session to permit thorough cleaning of the conditioning chambers. The intersession interval was ∼10 min. Conditioning and test sessions were conducted exactly as described for the experiment above.
There were no significant differences between WT and SK2+/T mice in freezing during the context pre-exposure sessions (Fig. 2A). During the delay conditioning session 24 h later (Fig. 2B), mice were returned to the same chamber and received three CS–US pairings. A two-factor ANOVA on percent freezing data, with genotype and condition (1 × 5-min, 3 × 5-min) as between-group factors and CS–US pairing as a repeated measure revealed a significant effect of CS–US pairing, F(2,114) = 9.70; P <0.001, a significant main effect of genotype, F(1,57) = 6.04; P <0.02, a non-significant effect of condition, F(1,57) = 0.90; n.s., a nonsignifi-cant interaction of genotype X CS-US pairing, F(2,114) = 1.24; n.s., a nonsignificant interaction of genotype X condition, F(2,114) = 2.71; n.s., and a nonsignificant interaction of genotype X condition X CS–US pairing, F(2,114) = 0.69; n.s. Overall, these results indicate that there was a significant genotypic difference in freezing during the conditioning session, although the degree to which each group acquired the conditioned freezing response did not differ. Furthermore, there was no difference in levels of freezing during the conditioning session between mice that received one or three 5-min context pre-exposure sessions.
Figure 2.
Effects of extending context pre-exposure time on retention of contextual fear memory. (A) The level of percent time freezing did not differ between WT and SK2+/T mice whether the mice received either one 5-min context pre-exposure or three 5-min context pre-exposure sessions on Day 1. The data for the mice that received one 5-min context pre-exposure session are plotted in S1 together with the data from the first context pre-exposure session of those mice that received three 5-min context pre-exposure sessions (S1, S2, and S3). Note the break in the ordinate axis. A repeated measures ANOVA on total percent time freezing for the 3 × 5-min pre-exposure mice yielded a significant effect of session, F(2,64) = 8.22; P < 0.002, a nonsignificant interaction of genotype X session, F(2,64) = 2.24; n.s., and a nonsignificant main effect of genotype, F(1,32) = 1.83; n.s. These data indicate that while freezing increased over the course of the three 5-min exposures to the conditioning chambers, there was no genotypic difference in freezing behavior prior to conditioning. The increase in amount of time the mice spent immobile would be expected to increase as the animals habituate to the locomotor activational effects of exposure to a novel environment. Genotypic differences in freezing behavior during the second and third 5-min pre-exposure sessions may reflect delayed habituation by the SK2+/T mice. (B) The freezing data from the conditioning trials presented on Day 2 are plotted separately for the 1 × 5-min group (left) and 3 × 5-min group (right) for clarity. Both groups of mice exhibited similar levels of freezing during the conditioning session. The gray vertical bars indicate the 30-sec periods during the presentation of each auditory tone CS. (C) Retention of contextual fear memory was tested at 24 h after conditioning. (C-1) SK2+/T mice that received one 5-min context pre-exposure session exhibited significantly less freezing during the context test than did comparably trained WT mice. This result is consistent with the data of Figure 1C. In contrast, SK2+/T mice that received three 5-min context pre-exposure sessions exhibited freezing that was comparable to the WT mice. (C-2) The freezing measures for the two groups of mice that received three 5-min pre-exposure sessions were calculated in 30-sec bins and plotted accordingly to examine the dynamic of freezing over the course of the 5-min context test. These data suggest that the rate and degree of extinction of context fear memory was equivalent across the two groups of mice. (D) Retention of cued fear memory was tested ∼1 h after the context test on Day 3. There were no differences in percent freezing between genotypes before the tone was presented (Pre-tone), and SK2+/T mice exhibited significantly less freezing in response to the tone compared with WT mice, regardless of context pre-exposure condition. All error bars, SEM. Significant differences are indicated by an asterisk (* P < 0.05; see text for details).
All mice exhibited conditioned fear to the context when tested 24 h after conditioning, expressed as an increase in percent freezing relative to the pre-exposure session (Fig. 2C-1). Analyses of context test freezing by a two-factor ANOVA yielded a significant main effect of genotype, F(1,57) = 14.64; P < 0.001, a significant main effect of condition, F(1,57) = 7.66; P < 0.009, and a nonsignificant interaction of genotype X condition, F(1,57) = 1.97; n.s. SK2+/T mice that received only one 5-min context pre-exposure session exhibited weak freezing during the context session. However, SK2+/T mice that received three 5-min context pre-exposure sessions exhibited freezing during the context test that was comparable to that of both groups of WT mice. These data indicate that retention of contextual fear memory was improved in the SK2+/T mice that received more extensive exposure to the context prior to conditioning.
Given that the context test is an extinction trial, we also examined genotypic differences in freezing responses over the context test for the 3 × 5-min pre-exposure mice, since these two groups exhibited equivalent total percent freezing scores. Figure 2, C-2, depicting percentage freezing measures for each 30-sec bin of the context test, reveals an equivalent within session decline in freezing responses for WT and SK2+/T mice. Analyses by a two-factor repeated measures ANOVA yielded a significant main effect of 30-sec bin, F(9,153) = 5.58; P < 0.001, but a nonsignificant interaction of genotype X condition, F(9,153) = 1.36; n.s., and a nonsignificant main effect of genotype, F(1,17) <1; n.s.
Both genotypes exhibited conditioned fear to the tone when presented in a novel environment, yet again, SK2+/T mice exhibited significantly less tone-elicited freezing than the WT mice (Fig. 2D). Analyses revealed a significant effect of minutes, F(1,57) = 89.70; P < 0.001, a significant interaction of genotype X min, F(1,57) = 9.77; P < 0.004, a significant main effect of genotype, F(1,57) = 6.55; P < 0.014, but a nonsignificant main effect of condition, F(1,57) = 0.27; n.s., and nonsignificant interactions of genotype X condition, F(1,57) = 0.1; n.s., and genotype X condition X min, F(1,57) = 1.08; n.s.
The hippocampal-dependent memory of the context is encoded during exploration of the conditioning chamber during context pre-exposure (Rudy et al. 2002; Matus-Amat et al. 2004). Provided it was consolidated, the contextual representation is then reactivated and associated with the aversive footshock stimulus during conditioning. Freezing or the expression of fear during the context test reflects the successful retention of the context + footshock association over the 24-h retention interval. Contextual fear memory was impaired in the SK2+/T mice during tests given 10 min after and 24 h after fear conditioning, suggesting that the SK2+/T mice had not sufficiently encoded the memory of the context. Tripling the amount of time that SK2+/T were allowed to explore the conditioning chambers during context pre-exposure was sufficient to alleviate the contextual fear memory deficit of the transgenic mice. Presumably, the additional opportunity to explore the conditioning chamber enabled the mice to completely encode the context memory. Thus, while the WT mice can encode a context representation during a single 5-min exposure, the SK2+/T mice require a longer period of encoding to produce a stable representation of the context that can be associated 24 h later with an aversive footshock stimulus. This result is similar to the recent report showing that increased pre-exposure to the context improves contextual fear conditioning in rats with lesions of the dorsal hippocampus (Wiltgen et al. 2006). This interpretation is consistent with the view that SK2 overexpression delays, but does not completely block contextual fear conditioning.
Overtraining may have enabled SK2+/T mice to use alternative learning pathways or mechanisms. Such alternative mechanisms likely mediate acquisition of conditioned contextual fear in rats after excitotoxic lesion of the dorsal hippocampus (Maren et al. 1997; Wiltgen et al. 2006). Specifically, rodents with compromised hippocampal function would be capable of acquiring an association of the footshock with one stimulus element of the context, rather than forming an association of the footshock with a configural representation of the context. The elemental stimulus+footshock association would permit those rodents to then display what would appear to be “context”-dependent freezing during the context test, provided that that stimulus element was available during the test session. Thus, SK2 overexpression may impair the use of a hippocampal-based pathway for encoding a configural representation of the context, but spare an alternative pathway's ability to form a configural representation or permit learning an elemental association.
The finding that increasing context pre-exposure was sufficient to eliminate the deficit in contextual fear memory in the SK2+/T mice suggests that SK2 overexpression does not affect memory retention or consolidation and memory retrieval. This interpretation is based on the inference that the increased context pre-exposure permitted SK2+/T mice to form a configural representation of the context on Day 1. This context memory was successfully retained over 24 h, when it was then reactivated and associated with the footshock stimulus on Day 2. The subsequent retention of this context + footshock memory over 24 h enabled the SK2+/T mice to demonstrate strong conditioned fear to the context on Day 3. Thus, we interpret these findings as evidence that SK2 overexpression affects contextual memory encoding, but not retention over 24-h intervals. Simply, SK2 over-expression impairs, but does not abolish encoding of contextual memory. Once encoded, the contextual memory was consolidated and then retrieved during conditioning and associated with the footshock stimulus. The lack of effect of SK2 overexpression on memory retention or retrieval is consistent with our previous report that SK channel blockade improved spatial and object memory encoding, but did not significantly affect memory retention (Stackman et al. 2002). The equivalent context test freezing responses of WT and SK2+/T mice that received 3 × 5-min context pre-exposure (Fig. 2C-2), suggest that the strength of the context memory decays in a similar manner for both groups of mice during extinction. We are now explicitly testing the influence of SK2 overexpression on long-term retention of contextual fear memory as part of a larger effort to define the differential influence of SK channels on distinct memory processes.
The memory-encoding deficits of SK2+/T mice likely represent a functional consequence of elevated SK2 channel-mediated attenuation of hippocampal physiological processes necessary for learning. The dendritic SK channel-mediated attenuation of glutamatergic EPSPs (Faber et al. 2005; Ngo-Anh et al. 2005) is significantly greater in hippocampal slices from SK2+/T mice (Hammond et al. 2006). Thus, it is possible that the increased dampening of hippocampal glutamatergic activity might slow, but not completely block context memory encoding in the SK2+/T mice. The induction of LTP at CA3–CA1 synapses in hippocampal slices from SK2+/T mice is impaired with moderate (50 Hz) stimulation, but not with a more intense, 100-Hz stimulation (Hammond et al. 2006). Likewise, blocking SK channels can facilitate LTP/LTD induction in response to stimulation that fails to induce lasting plasticity under control conditions (Stackman et al. 2002; Kramar et al. 2004; Ris et al. 2007). These data demonstrate that SK channels constrain but do not fully prevent hippocampal synaptic plasticity. These data are reminiscent of reports that weak to moderate blockade of hippocampal NMDA receptors produced a deficit of spatial learning that could be overcome with additional training (Butcher et al. 1990; Davis et al. 1992).
It is important to also consider the contribution of amygdaloid SK2 channels to the fear-conditioning deficits of the SK2+/T mice. SK channels limit glutamatergic EPSPs and synaptic plasticity in the lateral amygdala (Faber et al. 2005). Therefore, the deficits in contextual and cued fear memory of SK2+/T mice may be a consequence of the overexpression of SK2 channels in the lateral and basal nuclei of the amygdala, since these nuclei are fundamental for acquiring both elemental and configural associations (Phillips and LeDoux 1992; Muller et al. 1997; Maren 1998). Interestingly, overtraining can alleviate basolateral amygdala lesion-induced deficits in contextual fear memory (Ponnusamy et al. 2007), but not deficits in cued fear memory (Maren 1998). From these results, one might conclude that overtraining enabled the SK2+/T mice to encode a hippocampal-dependent configural representation of the context, rather than an amygdala-dependent elemental representation, since overtraining does not improve amygdala-dependent association of footshock and an elemental stimulus. It would be interesting to test whether overtraining SK2+/T mice during conditioning (e.g., presenting nine CS–US pairings, rather than three,) eliminates their deficit in cued fear memory 24 h later.
The present data are consistent with the view that increased SK2 channel activity impairs contextual memory encoding. It has been argued that age-related memory impairments may be due in part to changes in intracellular Ca2+ buffering during aging. The consequences of such age-related changes in Ca2+ buffering appear to be an increase in the Ca2+-dependent afterhyperpolarization (Norris et al. 1998) and in the activation of SK channels (Norris et al. 1998; Blank et al. 2003). It will be of interest to determine whether SK2 channel protein expression increases with age and whether blocking SK2 channels can limit age-related memory impairment.
Acknowledgments
We thank Dr. Rebecca Hammond for helpful discussions regarding the design of these experiments, Kyle Vick for comments on the manuscript, and Laurie Tull and Naomi Yoneyama for technical support. A preliminary report of these data was presented at the 36th annual Society for Neuroscience meeting in Atlanta, GA (October 2006). This research is based upon work supported by the National Science Foundation under Grant # IBN-0630522 awarded to R.W.S. and NIH 2R01NS038880-05 awarded to J.P.A.
References
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Behnisch T, Reymann KG. Inhibition of apamin-sensitive calcium dependent potassium channels facilitate the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci. Lett. 1998;253:91–94. doi: 10.1016/s0304-3940(98)00612-0. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Kye MJ, Radulovic J, Spiess J. Small-conductance, Ca2+-activated K+ channel SK3 generates age-related memory and LTP deficits. Nat. Neurosci. 2003;6:911–912. doi: 10.1038/nn1101. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Davis S, Morris RG. A dose-related impairment of spatial learning by the NMDA receptor antagonist, 2-amino-5-phosphonovalerate (AP5). Eur. Neuropsychopharmacol. 1990;1:15–20. doi: 10.1016/0924-977x(90)90005-u. [DOI] [PubMed] [Google Scholar]
- Cravens CJ, Vargas-Pinto N, Christian KM, Nakazawa K. CA3 NMDA receptors are crucial for rapid and automatic representation of context memory. Eur. J. Neurosci. 2006;24:1771–1780. doi: 10.1111/j.1460-9568.2006.05044.x. [DOI] [PubMed] [Google Scholar]
- Davis S, Butcher SP, Morris RG. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J. Neurosci. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat. Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J. Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of θ-induced long-term potentiation by brain-derived neurotrophic factor. J. Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Parker LL, Young L, Adelman JP, Zuo J, Liberman MC. Overexpression of SK2 channels enhances efferent suppression of cochlear responses without enhancing noise resistance. J. Neurophysiol. 2007;97:2930–2936. doi: 10.1152/jn.01183.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J. Neurosci. 1998;18:3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J. Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav. Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat. Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Niewoehner B, Single FN, Hvalby O, Jensen V, Meyer Zum Alten Borgloh S, Seeburg PH, Rawlins JN, Sprengel R, Bannerman DM. Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. Eur. J. Neurosci. 2007;25:837–846. doi: 10.1111/j.1460-9568.2007.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J. Neurosci. 1998;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Ponnusamy R, Poulos AM, Fanselow MS. Amygdala-dependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience. 2007;147:919–927. doi: 10.1016/j.neuroscience.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris L, Capron B, Sclavons C, Liegeois JF, Seutin V, Godaux E. Metaplastic effect of apamin on LTP and paired-pulse facilitation. Learn. Mem. 2007;14:390–399. doi: 10.1101/lm.571007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav. Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J. Neurosci. 2002;22:9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol. Cell. Neurosci. 2004;26:458–469. doi: 10.1016/j.mcn.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol. Cell. Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc. Natl. Acad. Sci. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Stackman RW. Enhancing synaptic plasticity and memory: A role for small conductance Ca2+-activated K+ channels. Neuroscientist. 2003;9:434–439. doi: 10.1177/1073858403259282. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J. Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]



