Abstract
Background
The association between kidney function and cognitive impairment has not been assessed in a national sample with a wide spectrum of kidney disease severity.
Study Design
Cross-sectional.
Setting & Participants
23,405 participants [EF1](mean age 64.9 ± 9.6 years) with baseline measurements of creatinine and cognitive function participating in the REGARDS (REasons for Geographic And Racial Differences in Stroke) Study, a study of stroke risk factors in a large national sample.
Predictor
Estimated glomerular filtration rate (eGFR).
Outcome
Cognitive impairment.
Measurements
Chronic kidney disease (CKD) was defined as an eGFR <60 ml/min/1.73m2 Kidney function was analyzed in 10 ml/min/1.73 m2 increments among those with CKD, and in exploratory analyses, across the range of kidney function. Cognitive function was assessed using the Six-item Screener and participants with a score ≤4 were considered to have cognitive impairment.
Results
CKD was associated with an increased prevalence of cognitive impairment, independent of confounding factors (odds ratio (OR) 1.23, 95% confidence interval (95% CI) 1.06, 1.43). Among those with CKD, each 10 ml/min/1.73m2 decrease in eGFR below 60 ml/min/1.73m2 was associated with an 11% increased prevalence of impairment (OR 1.11, 95% CI 1.04, 1.19). Exploratory analyses revealed a non-linear association between eGFR and the prevalence of cognitive impairment, with a significant, increased prevalence of impairment among those with eGFR <50 and ≥100 ml/min/1.73m2.
Limitations
Longitudinal measures of cognitive function were not available.
Conclusions
Among US adults, lower levels of kidney function are associated with an increased prevalence of cognitive impairment. The prevalence of impairment appears to increase early in the course of kidney disease; therefore screening for impairment should be considered among all adults with CKD.
Index words: CKD, cognitive function, cognitive impairment
Chronic kidney disease (CKD) and dementia are common conditions among the elderly that have growing public health significance as the United States population ages. Persons with CKD have an increased risk of stroke and a high burden of cardiovascular risk factors such as diabetes, hypertension, and hyperlipidemia, which in turn, are linked with cognitive impairment 1–3. Studies in selected populations suggest CKD is an independent risk factor for cognitive impairment and dementia4–6; however these studies were largely restricted to elderly cohorts with limited representation of African Americans[EF2], had small numbers of participants with advanced CKD, and used serum creatinine alone as a proxy for glomerular filtration rate (GFR). Conversely, most large epidemiological studies of cardiovascular risk factors and cognitive impairment did not include kidney function as a covariate 1–3. The goals of this study are to assess the association between kidney function and cognitive impairment in a population-based cohort across a wide spectrum of kidney disease severity, and to determine whether the associations are independent of traditional cardiovascular risk factors.
METHODS
Study design
The REGARDS (REasons for Geographic and Racial Differences in Stroke) Study is a nationally representative sample of adults aged 45 years and older in the United States (US) population. Recruitment of the REGARDS cohort has been previously described 7. Briefly, participants are identified from commercially available lists of residents and recruited through an initial mailing followed by telephone contact. The cooperation rate in REGARDS is 64.6% and the participation rate is 44.7%; both are comparable to rates in other cohort studies 8, 9. The sample is designed so that approximately one-half of the participants will be African American and one-half white, and one-half will be men and one-half women. Individuals living in the stroke belt are over sampled. Recruitment began in January 2003; upon completion of recruitment the REGARDS cohort will consist of a sample of 30,000 participants with follow-up extending for up to four years7.
Data
The data used in these analyses are obtained from two sources, a telephone interview and a subsequent in-home examination conducted by a nurse or other health professional. During the telephone interview demographic characteristics, self report of health conditions and use of antihypertensive or diabetes medications are obtained. During the subsequent in-home examination anthropometric measurements and an ECG are obtained. Blood pressure is measured twice with the participant seated, and the average of the measurements employed. Blood is drawn in the fasting state with samples shipped to a central laboratory for determination of serum markers including creatinine, total and high density lipoprotein (HDL) cholesterol and glucose.
Cardiovascular risk factors
For these analyses, we included cardiovascular and stroke risk factors previously identified to be associated with CKD, cognitive impairment, or both. Hypertension was defined as a systolic blood pressure >140 mm Hg, a diastolic blood pressure >90 mm Hg, or self-reported current treatment for hypertension. Diabetes was defined as fasting glucose ≥126 mg/dL (glucose in mg/dL may be converted to mmol/L by multiplying by 0.05551), non-fasting glucose ≥200 mg/dL, or self-reported current treatment for diabetes. Elevated cholesterol was defined as a total cholesterol ≥240 mg/dL (cholesterol in mg/dL may be converted to mmol/L by multiplying by 0.02586) or self-reported current treatment for elevated cholesterol. Obesity was defined as a body mass index ≥30 kg/m2. Tobacco use was categorized as current or past use versus never use. Left ventricular hypertrophy and atrial fibrillation were ascertained by electrocardiogram (ECG). Prevalent cerebrovascular disease was defined as self-report of a diagnosed stroke or transient ischemic attack (TIA). Prevalent coronary heart disease was defined as ECG evidence of a myocardial infarction or self-report of a myocardial infarction, coronary artery bypass surgery, coronary angioplasty or coronary stenting. Education level was classified as less than high school education, high school education, or some post-high school education, geographic region was categorized as stroke belt or buckle versus other regions, as previously described 7.
Kidney function
In 2007 after completion of REGARDS recruitment, the REGARDS laboratory at the University of Vermont changed creatinine reagents to a method traceable to creatinine determined by isotope dilution mass spectrometry (IDMS). Fifty samples were run in duplicate comparing the original method to the IDMS traceable method, yielding the following calibration equation: IDMS traceable creatinine = −0.06 + 0.953*creatinine. In addition, the Vermont calibration was confirmed with 200 serum samples that were sent to the Cleveland Clinic, resulting in the following calibration equation: calibrated creatinine = −0.06 + 0.98*REGARDS creatinine. As these two equations were nearly identical, the Vermont equation was used to convert the original REGARDS creatinine values to standardized, IDMS traceable values for estimation of GFR, using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation10:
estimated GFR = 175 × standardized creatinine(−1.154) × age(−0.203) × 1.212 (if black) × 0.742 (if female).
This approach was used to obtain the estimated GFR values for the current paper, and will be used in future publications concerning the REGARDS cohort.
Cognitive function
Starting on January 1, 2004, a six item cognitive screening examination was incorporated into the REGARDS baseline telephone interview and administered to all participants enrolled on or after that date. Designed for either in-person or telephone administration, the Six-item Screener is a test of global cognitive function that includes recall and temporal orientation items derived from the widely used Mini-Mental State Exam (Appendix A)11, 12. Scores range from 0–6; a score ≤4 has been shown to have a sensitivity of 74.2–84.0% and a specificity of 80.2 to 85.3% in community and clinical samples for a diagnosis of cognitive impairment11.
Statistical analysis
We used standard descriptive statistics to assess baseline characteristics and to test differences in characteristics between participants with and without CKD and with and without cognitive impairment. We used multivariable logistic regression models to determine the association (expressed as an odds ratio (OR) and 95% confidence interval (95% CI)) between kidney function and cognitive impairment. We first modeled kidney function in two ways: as the binary variable CKD (defined by National Kidney Foundation guidelines13 as a GFR <60 ml/min/1.73 m2) (GFR in mL/min/1.73m2 may be converted to mL/s/1.73m2 by multiplying by 0.01667) and as an ordinal term for estimated GFR in 10 ml/min/1.73 m2 intervals (using those with an estimated GFR ≥60 ml/min/1.73 m2 as the reference category).Models were adjusted for demographic characteristics (age, sex, race, education and region), prevalent cardiovascular disease (stroke/TIA and coronary heart disease), and individual cardiovascular risk factors. We included interaction terms for kidney function with race, age and cardiovascular risk factors to determine if effect modification was present. To confirm the results, we performed several sensitivity analyses. First, we adjusted for depressive symptoms (assessed with the Center for Epidemiological Studies-Depression 4-item scale14) in addition to cardiovascular risk factors. Second, we performed the analysis using multiple imputation techniques for missing data. In exploratory analyses, we then evaluated these associations across a wide range of estimated GFR values to determine the association between kidney function and cognitive impairment among those typically classified as having normal kidney function. For these models we incorporated linear and quadratic terms for GFR, or estimated GFR strata ranging from 10–19 ml/min/1.73 m2 to ≥100 ml/min/1.73 m2. A p-value <0.05 was considered statistically significant.
RESULTS
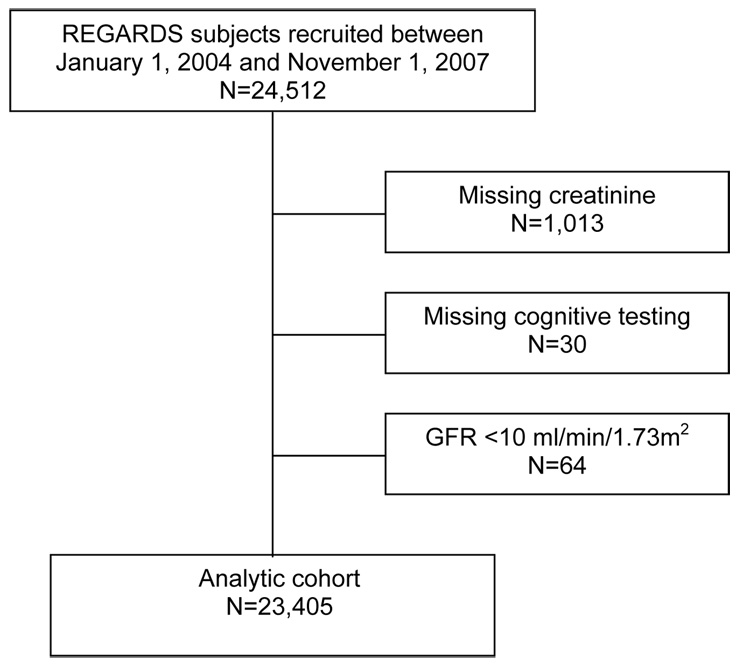
There were 24,512 participants who had been recruited into the cohort between January 1, 2004 and November 1, 2007. Among these participants 23,499 had a non-missing serum creatinine measurement, and of these participants, 23,469 underwent cognitive function testing (Figure 1). We excluded 64 individuals with an estimated GFR <10 ml/min/1.73m2, leaving 23,405 participants in the analytic cohort.
Figure 1.
Derivation of the analytic cohort
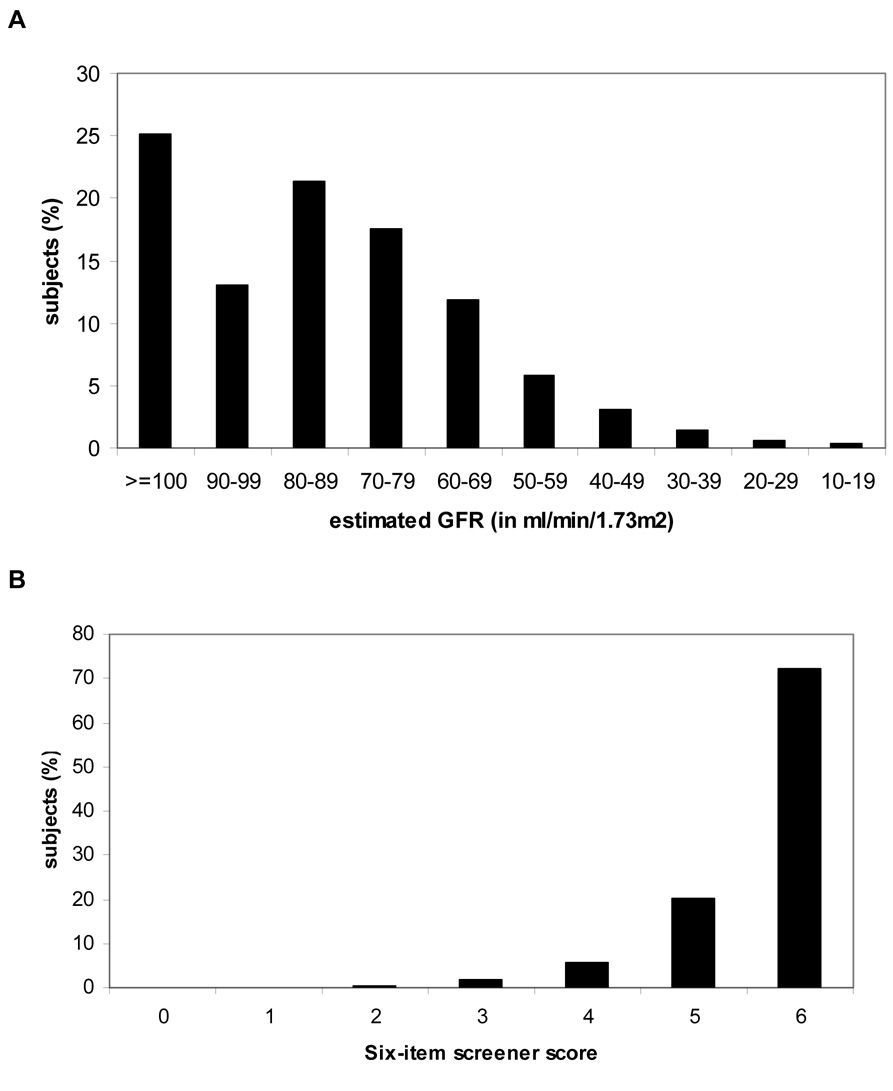
The participants included in our analyses had a mean (SD) age of 64.9 (9.6) years; 41.0% were African American and 40.5% were men (Table 1). CKD was present in 11.0% (n=2,586) of the sample (Figure 2a). Compared to participants without CKD, participants with CKD were older and were more likely to be women and white. Participants with CKD had a higher prevalence of stroke/TIA, coronary heart disease, diabetes, hypertension, obesity, left ventricular hypertrophy and atrial fibrillation, and a lower prevalence of elevated cholesterol. Approximately 8% of the sample (n=1830) had a score of four or less on the Six-item screener indicative of impaired cognitive function (Figure 2b).
Table 1.
Baseline characteristics of the study population, for all included participants, and by chronic kidney disease (CKD) status
| All Participants N=23,405 |
CKD (GFR <60) N=2586 |
No CKD (GFR ≥60) N=20,819 |
P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years)* | 64.9 (9.6) | 71.1 (9.3) | 64.1 (9.4) | <0.001 |
| Men | 9469 (40.5%) | 991 (38.3%) | 8478 (40.7%) | 0.02 |
| African American | 9588 (41.0%) | 986 (38.1%) | 8602 (41.3%) | 0.002 |
| Region | 0.008 | |||
| Stroke Belt | 8218 (35.1%) | 841 (32.5%) | 7377 (35.5%) | |
| Stroke Buckle | 5045 (21.6%) | 600 (23.2%) | 4445 (21.4%) | |
| Other region | 10,127 (43.3%) | 1143 (44.2%) | 8984 (43.2%) | |
| Education | <0.001 | |||
| Less than high school | 2736 (11.7%) | 427 (16.5%) | 2309 (11.1%) | |
| High school | 6106 (26.1%) | 712 (27.6%) | 5394 (25.9%) | |
| Post high school/Professional | 14,543 (62.1%) | 1445 (55.9%) | 7320 (63.0%) | |
| Comorbidity | ||||
| Prior Stroke or TIA | 2197 (9.5%) | 475 (18.6%) | 1722 (8.3%) | <0.001 |
| Coronary Heart Disease | 3049 (13.0%) | 645 (24.9%) | 2404 (11.6%) | <0.001 |
| Diabetes | 4780 (20.5%) | 851 (33.0%) | 3929 (19.0%) | <0.001 |
| Hypertension | 13,407 (57.6%) | 2051 (79.7%) | 11,356 (54.8%) | <0.001 |
| Elevated cholesterol | 12,725 (54.9%) | 1156 (45.1%) | 11,569 (56.1%) | <0.001 |
| Tobacco use (ever vs. never) | 12,419 (53.3%) | 1342 (52.0%) | 11,077 (53.4%) | 0.2 |
| Obese | 8978 (38.6%) | 1065 (41.7%) | 7913 (38.3%) | <0.001 |
| Left ventricular hypertrophy | 1459 (6.3%) | 219 (8.7%) | 1240 (6.1%) | <0.001 |
| Atrial fibrillation | 2020 (8.8%) | 354 (14.1%) | 1666 (8.2%) | <0.001 |
| Serum creatinine (mg/dl)* | 1.00 (0.3) | 1.6 (0.6) | 0.9 (0.2) | <0.001 |
| Estimated GFR | 85.9 (23.7) | 47.7 (10.4) | 90.7 (20.3) | <0.001 |
| (ml/min/1.73m2)* | ||||
mean (SD)
Abbreviations: GFR - glomerular filtration rate, TIA - transient ischemic attack
Note: To convert serum creatinine in mg/dL to µmol/L, multiply by 88.4; GFR in mL/min/1.73m2 to mL/s/1.73m2, multiply by 0.01667.
Figure 2.
A Distribution of estimated glomerular filtration rate (GFR). B. Distribution of scores on Six-item Screener
In unadjusted analyses, CKD, as well as several other cardiovascular risk factors, was associated with a significant, increased prevalence of cognitive impairment (Table 2).The association remained significant after additional adjustment for demographic characteristics, prevalent cardiovascular disease and cardiovascular risk factors (OR 1.23, 95% CI 1.06, 1.43).When estimated GFR was modeled as an ordinal variable among those with CKD, each 10 ml/min/1.73m2 decrement in GFR below 60 ml/min/1.73m2 was associated with an 11% increased prevalence of impairment (OR 1.11, 95% CI 1.04, 1.19) after accounting for confounding factors (Table 3).
Table 2.
Unadjusted association of chronic kidney disease (CKD) and cardiovascular risk factors with cognitive impairment
| Cardiovascular risk factor | Cognitive impairment (N=1830) |
No Cognitive impairment (N=21,566) |
P-value |
|---|---|---|---|
| Age (years) | <0.001 | ||
| 45–55 | 8.0% | 15.9% | |
| 56–65 | 26.2% | 37.8% | |
| 66–75 | 35.2% | 31.0% | |
| 76–85 | 25.2% | 13.7% | |
| >85 | 5.4% | 1.6% | |
| Male | 46.7% | 39.9% | <0.001 |
| Education | <0.001 | ||
| Less than high school | 25.1% | 10.6% | |
| High school | 30.5% | 25.7% | |
| Post high school/Professional | 44.4% | 63.7% | |
| CKD | 17.3% | 10.5% | <0.001 |
| Prior Stroke or TIA | 15.7% | 8.9% | <0.001 |
| Coronary Heart Disease | 18.4% | 12.6% | <0.001 |
| Diabetes | 28.0% | 19.9% | <0.001 |
| Hypertension | 66.1% | 56.9% | <0.001 |
| Elevated cholesterol | 55.7% | 54.8% | 0.5 |
| Tobacco use (ever vs. never) | 54.7% | 53.1% | 0.2 |
| Obese | 37.4% | 38.8% | 0.2 |
| Left ventricular hypertrophy | 11.3% | 5.9% | <0.001 |
| Atrial fibrillation | 9.8% | 8.8% | 0.1 |
Abbreviation: TIA - transient ischemic attack.
Table 3.
Adjusted association between reduced kidney function and prevalence of cognitive impairment
| Kidney function | Adjusted odds ratio (and 95% confidence interval) 1 |
|---|---|
| CKD vs. no CKD | 1.23 (1.06, 1.43) |
| per 10 ml/min/1.73m2 decrease in GFR | 1.11 (1.04, 1.19) |
| (reference = GFR ≥60 ml/min/1.73m2) |
Model adjusted for age, sex, race, education, region, prevalent stroke/TIA, coronary heart disease diabetes, hypertension, elevated cholesterol, smoking, obesity, left ventricular hypertrophy, and atrial fibrillation.
To convert GFR in mL/min/1.73m2 to mL/s/1.73m2, multiply by 0.01667.
The association between kidney function and cognitive impairment did not vary by age, race, or cardiovascular risk factors such as diabetes or prior stroke (all p-values for interaction terms were not significant). For example, among participants below the median age of 65, there was an 11% increased risk of impairment for each 10 ml/min/1.73m2 decrement in estimated GFR below 60 ml/min/1.73m2 (OR 1.11, 95% CI 0.97–1.26). Among participants 65 and older, there was a 17% increased risk of impairment for each 10 ml/min/1.73m2 decrement in estimated GFR below 60 ml/min/1.73m2 (OR 1.17, 95% CI 1.09–1.23).
To confirm the primary findings, we performed several additional analyses. When we adjusted for depressive symptoms in addition to cardiovascular risk factors, the association between low GFR and cognitive impairment remained robust (OR 1.11, 95% CI 1.04, 1.19). We also performed the analyses using multiple imputations for missing data. The results were materially unchanged.
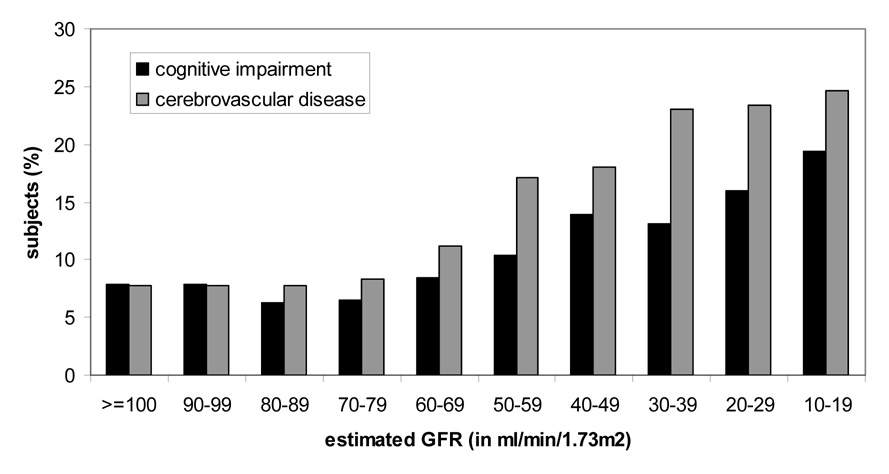
We then evaluated the prevalence of cognitive impairment across a wide range of estimated GFR rather than focusing on those with CKD. There was a non-linear or U-shaped association between estimated GFR and cognitive impairment, with an inflection point around 70 ml/min/1.73m2 (Figure 3). After multivariable adjustment, there were significant associations between estimated GFR strata <50 ml/min/1.73m2 and cognitive impairment, as well as a trend towards a modest increase in impairment among those with an estimated GFR 50–70 ml/min/1.73m2.The relationship seemed to differ from that of GFR with cerebrovascular disease, where prevalence increased below a GFR threshold of 70 ml/min/1.73m2 In contrast, while the inflection point appeared to be similar, there was also a significant, increased prevalence of cognitive impairment among those with estimated GFR ≥100 ml/min/1.73m2 (Table 4), which persisted after multivariable adjustment (p-value for GFR quadratic term <0.001). Inclusion of linear and quadratic terms for body mass index rather than the binary variable obesity did not alter these associations.
Figure 3.
Unadjusted prevalence of cognitive impairment and of cerebrovascular disease by estimated glomerular filtration rate (GFR, in ml/min/1.73m2).
Table 4.
Odds ratios (and 95% confidence intervals) for cognitive impairment by level of estimated glomerular filtration rate (GFR, in ml/min/1.73m2)
| GFR Category | Unadjusted | Demographics adjusted1 |
Multivariable adjusted2 |
|---|---|---|---|
| ≥ 100 | 1.26 (1.08, 1.47) |
1.20 (1.02, 1.41) |
1.18 (1.01, 1.39) |
| 90–99 | 1.26 (1.05, 1.51) |
1.13 (0.94, 1.36) |
1.13 (0.94, 1.36) |
| 80–89 | 1.00 (Referent) |
1.00 (Referent) |
1.00 (Referent) |
| 70–79 | 1.04 (0..87, 1.24) |
1.05 (0.88, 1.25) |
1.04 (0.87, 1.24) |
| 60–69 | 1.37 (1.14, 1.64) |
1.16 (0.96, 1.40) |
1.14 (0.94, 1.37) |
| 50–59 | 1.69 (1.36, 2.10) |
1.27 (1.01, 1.59) |
1.22 (0.97, 1.53) |
| 40–49 | 2.26 (1.75, 2.91) |
1.51 (1.16, 1.97) |
1.44 (1.10, 1.89) |
| 30–39 | 2.34 (1.65, 3.32) |
1.51 (1.05, 2.18) |
1.44 (1.00, 2.08) |
| 20–29 | 2.99 (1.82, 4.90) |
1.80 (1.08, 3.02) |
1.61 (0.95, 2.71) |
| 10–19 | 3.51 (1.75, 7.06) |
2.16 (1.06, 4.44) |
1.86 (0.90, 3.82) |
Model adjusted for age, sex, race, education, and region.
Model adjusted for age, sex, race, education, region, prevalent stroke/TIA, coronary heart disease, diabetes, hypertension, elevated cholesterol, smoking, obesity, left ventricular hypertrophy, and atrial fibrillation.
To convert GFR in mL/min/1.73m2 to mL/s/1.73m2 multiply by 0.01667.
DISCUSSION
In a large national sample of African American and white adults, individuals with lower levels of kidney function were more likely to have cognitive impairment compared to individuals with normal kidney function, independent of prevalent cardiovascular disease and cardiovascular risk factors. These results suggest that CKD, in addition to other modifiable cardiovascular risk factors, may be an important marker of cognitive impairment in US adults.
These results confirm and extend previous studies in elderly populations that had limited representation of African Americans and of individuals with advanced CKD. In the Health, Aging and Body Composition Study, we found that elderly individuals with CKD, defined similarly to the current study, had a 1.3 to 2.4-fold higher risk of cognitive decline over four years of follow-up, even after accounting for a number of confounding factors 4. In a cross-sectional study of menopausal women participating in the Heart Estrogen/progestin Replacement Study (HERS), a lower estimated GFR was associated with poorer performance on tests of global cognition, executive function, language, and memory5. When HERS participants were stratified by estimated GFR, only women with an estimated GFR <30 ml/min/1.73m2 had a significantly elevated prevalence of impairment. In the Cardiovascular Health Study, Seliger et al. found an association between serum creatinine concentration and the risk of incident dementia over a median six years of follow-up that was dependent on self-reported health status6. Among older individuals with good or excellent health, an elevated serum creatinine concentration was associated with a 62% increased risk of dementia; however there was no association between kidney function and incident dementia in the subgroup of individuals with poor or fair self-reported health. Recently, Hailpern et al. reported that moderate CKD, defined as an estimated GFR of 30–59 ml/min/1.73m2, was associated with poorer concentration and attention among 20–59 year old National Health And Nutrition Examination Survey (NHANES) participants15.
Compared to these previous studies, the large sample size of REGARDS allowed us to examine the association between GFR and cognitive impairment across a wider spectrum of kidney function. Our results confirm that CKD is associated with an increased prevalence of cognitive impairment, and suggest that impairment may occur earlier in the course of kidney disease than previously recognized. Indeed, we found a significant increase in the prevalence of impairment for those with an estimated GFR <50 ml/min/1.73m2, and a trend towards an increased prevalence of impairment among those typically classified as having normal or near-normal kidney function (estimated GFR 50–70 ml/min/1.73m2), relative to participants with an estimated GFR 80–89 ml/min/1.73m2. This finding should be interpreted with caution since these were exploratory analyses and the MDRD Study equation is known to be less accurate in this range16. If confirmed in future studies, this would suggest that even small reductions in kidney function are associated with clinically significant consequences for cognitive functioning.
We also noted a significant, non-linear association between estimated GFR and the prevalence of cognitive impairment. Whether this observation reflects misclassification due to confounding from malnutrition or other factors is unclear. Nevertheless, it is worth noting since studies which do not account for this non-linear association may underestimate the true association between CKD and cognitive impairment. Future studies utilizing cystatin-c, a novel marker of kidney function which is currently being assayed in REGARDS17, may clarify the association between kidney function and cognitive function among those with normal estimated GFR.
Individuals with CKD are frequently prescribed cumbersome medical regimens, and they must understand and weigh complex medical choices including the decision to undergo kidney transplantation, the decision to initiate dialysis, and the choice of dialysis modality. In addition to other factors, the high prevalence of cognitive impairment among persons with CKD may explain why practice guidelines for blood pressure management, preemptive vascular access placement, and other clinical targets remain difficult to achieve. While screening for cognitive impairment is generally not a routine part of CKD care, accurate identification and treatment of persons with cognitive impairment may facilitate improved adherence with dietary and pharmacologic therapies and aid in dialysis and long-term care planning.
The causes of cognitive impairment in persons with CKD cannot be determined from the current study, although these results suggest that traditional cardiovascular and stroke risk factors do not fully account for the association between reduced kidney function and cognitive impairment. In addition to traditional risk factors, CKD is also associated with a number of novel cardiovascular risk factors, including inflammation, oxidative stress, anemia, vascular calcification and hyperhomocysteinemia that may play an important role in the development and progression of cognitive impairment18–20.
REGARDS participants were selected using population-based sampling strategies, therefore the results of this study should be broadly generalizable to the US adult population. Nevertheless, several limitations should be noted. These analyses were cross-sectional; therefore whether CKD is a marker for other factors that lead to cognitive impairment or a true causal risk factor cannot be concluded. Ongoing longitudinal studies of CKD and cognitive function in REGARDS and other cohorts will provide additional support for the hypothesis that CKD independently contributes to cognitive impairment. The Six-item Screener is a relatively insensitive measure of cognitive function and does not test different domains of cognitive function. The finding of such a strong association using a relatively insensitive measure of cognitive function only underscores the true strength of the association. The use of more sensitive measures of cognitive function, and in particular, measures of executive function associated with vascular causes of cognitive impairment, may have strengthened the associations reported here. Such measures were recently added to REGARDS follow-up assessments and will be available for future studies. Finally, while we adjusted for a large number of cardiovascular risk factors and for the presence of cardiovascular disease, residual confounding may still exist due to unmeasured comorbidity or misclassification.
Among African American and white US adults, lower levels of kidney function are associated with an increased prevalence of cognitive impairment, independent of traditional cardiovascular risk factors. The prevalence of impairment appears to increase early in the course of kidney disease; thus screening for impairment should be considered among all adults with CKD.
Given the high prevalence of CKD among US adults and the adverse consequences of cognitive impairment, future clinical trials to improve cognitive function should consider targeting this high risk population.
Acknowledgements
The authors acknowledge the participating investigators and institutions: University of Alabama at Birmingham, Birmingham, Alabama (Study PI, Data Coordinating Center, Survey Research Unit): George Howard, Leslie McClure, Virginia Howard, Libby Wagner, Virginia Wadley, Rodney Go; University of Vermont (Central Laboratory): Mary Cushman; Wake Forest University (ECG Reading Center): Ron Prineas; Alabama Neurological Institute (Stroke Validation Center, Medical Monitoring): Camilo Gomez, David Rhodes, Susanna Bowling; University of Arkansas for Medical Sciences (Survey Research): LeaVonne Pulley; Examination Management Services Incorporated (In-Home Visits): Andra Graham; National Institute of Neurological Disorders and Stroke, National Institutes of Health (funding agency): Claudia Moy.
Support: Dr. Kurella-Tamura is supported by a research grant from the Amgen Nephrology Institute Junior Faculty Research Support Program. This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. The Renal REGARDS study is supported by an unrestricted educational grant from AMGEN Corporation (B. Newsome, Principal Investigator).
Financial Disclosure: None.
Appendix A
The Six-Item Screener
Instructions for the patient: I would like to ask you some questions that ask you to use your memory. I am going to name three objects. Please wait until I say all three words, and then repeat them. Remember what they are because I am going to ask you to name them again in a few minutes, but please do not write anything down. Please repeat these words for me: APPLE - TABLE - PENNY. (Interviewer may repeat names 3 times if necessary, but repetition not scored.)
| Did the patient correctly repeat all three words? | Yes | No |
| Thank you. Now, | ||
| Incorrect | Correct | |
| 1. Without looking at a calendar or a watch, what year is this? | ____ (0) | ____ (1) |
| 2. Without looking at a calendar or a watch, what month is this? | ____ (0) | ____ (1) |
| 3. Without looking at a calendar or a watch, what is the day of the week? | ____ (0) | ____ (1) |
| What are the three objects I asked you to remember? (any order is acceptable) | ||
| 4. Apple | ____ (0) | ____ (1) |
| 5. Table | ____ (0) | ____ (1) |
| 6. Penny | ____ (0) | ____ (1) |
| Score (sum of items 1–6): | _________ (0–6) | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001;56(12):1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 2.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 3.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 4.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16(7):2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 5.Kurella M, Yaffe K, Shlipak MG, Wenger NK, Chertow GM. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45(1):66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15(7):1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 7.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 8.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 9.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 14.Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educational and Psychological Measurement. 2003;53:1117–1125. [Google Scholar]
- 15.Hailpern SM, Melamed ML, Cohen HW, Hostetter TH. Moderate Chronic Kidney Disease and Cognitive Function in Adults 20 to 59 Years of Age: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2007;18(7):2205–2213. doi: 10.1681/ASN.2006101165. [DOI] [PubMed] [Google Scholar]
- 16.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 18.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 19.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 20.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119(4):327–334. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]