Abstract
Objective
To investigate evidence for the interplay between cytokines, angiotensin II and nNOS in the paraventricular nucleus (PVN), for regulating sympathetic outflow in a rat model of CHF.
Methods and results
Heart failure was induced in Sprague-Dawley rats by coronary artery ligation. One group of rats was treated with pentoxifylline (PTX, 30 mg/kg IP), a cytokine blocker, or vehicle, for 5 weeks. Another group of rats was pre-treated with PTX before coronary ligation to study prior cytokine blocking effect on survival. Both groups were combined in the analysis. Echocardiography demonstrated an increase in LV end-diastolic pressure and Tei index after 5-weeks in CHF rats. ELISA revealed a significant increase in plasma TNF-α and IL-1β in CHF rats. Inducible NOS (iNOS) and angiotensin receptor-type 1 (AT-1R) mRNA expressions were increased, while neural NOS (nNOS) was decreased in the PVN of CHF rats; these changes were reversed by PTX. PTX treatment also decreased plasma norepinephrine and epinephrine levels and improved baroreflex control of renal sympathoexcitation in CHF rats. Immunohistochemistry revealed elevated 3-nitrotyrosine formation in the heart and the PVN of CHF rats, but not in PTX treated rats.
Conclusion
PTX decreased both peripheral and central cytokine expression, alleviated nitric oxide dysregulation, and inhibited the formation of peroxynitrite in the PVN resulting in decreased sympathoexcitation in CHF rats.
Keywords: congestive heart failure, cytokines, sympathoexcitation, neuronal nitric oxide synthase, AT-1 receptor, peroxynitrite
1. Introduction
Sympathetic hyperactivity is a striking feature of the syndrome of congestive heart failure (CHF). Initially after myocardial injury, there is increased sympathetic activity even before the onset of overt heart failure [1]. When this sympathetic hyperactivity fails to restore the functioning of the injured myocardium, it results in generalized sympathoexcitation leading to increased vasoconstriction and ventricular remodelling.
Regulation of sympathetic activity is a complex process that involves the activation of several neurohormones, including those of the renin angiotensin system (RAS). Blockade of the AT-1R in the paraventricular nucleus (PVN) restores sympathetic activity in CHF [2]. In a previous study we showed that elevated TNF-α and IL-1β in the PVN of rats with acute myocardial infarction (MI) is mediated through cardiac sympathetic afferents [3]. Furthermore, we demonstrated that elevated cytokines in the PVN induce production of reactive oxygen species (ROS) [4]. ROS, produced in the neurons of the brain, in turn, are cytotoxic, further perpetuating sympathoexcitatory effects [5]. Studies from this and other labs have shown that pentoxifylline (PTX), a cytokine synthesis blocker, reduced the central and peripheral production of cytokines and attenuated the production of ROS, renal sympathetic nerve activity (RSNA), as well as plasma norepinephrine levels, an indirect measure of sympathetic activity in CHF rats [3, 4, 6]. Additionally, several studies report a cross-talk between pro-inflammatory cytokines and the RAS in both humans and animals. These studies show that treatment with angiotensin II (AngII) resulted in elevation of TNF-α in isolated heart preparations [7], while pre-treatment with losartan, an AT-1R blocker, attenuated the TNF-α biosynthesis induced by AngII [8], suggesting that AT-1R expression is closely related to that of TNF-α in the heart [8, 9]. These studies explain an apparent interaction between cytokines and AngII in the periphery. However, the cytokine-AngII interaction in the PVN of CHF animals is currently unexplored.
Studies by Patel et al. showed that rats with CHF had a decreased message for neuronal nitric oxide synthase (nNOS), and consequently, decreased nitric oxide (NO) in the PVN [10, 11], indicating loss of regulation of sympathetic tone. Furthermore, AngII-induced sympathetic hyperactivity disrupts the antagonistic mechanism of NO, while increasing superoxide production. This emphasizes the cross-talk between ROS and RAS mechanisms [12]. However, it is unknown whether increased cytokines interact with AT-1Rs within the PVN to modulate nNOS and contribute to sympathetic hyperactivity in CHF rats.
In the current study, we examined the hypothesis that increased cytokines in the PVN up-regulate AT-1R expression and deplete nNOS, contributing to exaggerated sympathetic activity in CHF rats. We used PTX to block cytokine synthesis in CHF rats, as this phosphodiesterase inhibitor has been documented to cross the blood-brain barrier (BBB) rapidly and efficiently after systemic administration [13], and inhibit production of TNF-α and IL-1β [14].
2. Methods
Studies were performed in male Sprague-Dawley rats weighing 250–300 g. To study the effect of PTX on survival of CHF rats, the study was conducted in two phases. In phase I, rats were pre-treated with PTX (30 mg/kg IP), or its vehicle (10% ethanol, IP), 24 h prior to induction of CHF or Sham surgery, to study the effects on survival of pre-treatment with a cytokine synthesis blocker. In Phase II, rats were subjected to CHF or Sham surgery and subsequently treated with PTX (30 mg/kg IP, daily) or vehicle for a period of 5-weeks. No significant difference was observed in the molecular and biochemical parameters analyzed between PTX pre-treated and post-treated CHF groups. Therefore, the results from both of these groups were combined and presented as CHF+PTX.
All procedures on animals in this study were approved by the Louisiana State University Institutional Animal Care and Use Committee, and were in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.1. CHF model
Heart failure was induced under ketamine+xylazine (90 and 10 mg/kg IP) anaesthesia by coronary artery ligation. In brief, the trachea was intubated, and the rat was placed on an Anesthesia Work Station (Hallowell EMC). A left thoracotomy was performed, the heart was exteriorized and the left anterior descending coronary artery was ligated. Sham-operated rats underwent thoracotomy and manipulation of the heart, except for the ligation of the coronary artery. All rats received analgesics (Buprex, 1 ml/kg SC) following the surgery.
2.2. Assessment of LV function
Echocardiography
Echocardiography was performed 24h after coronary artery ligation or Sham surgery under ketamine (25 mg/kg IP) sedation. Infarct size was estimated by planimetric measurement of the percentage of the LV that demonstrated systolic akinesis. Rats with infarct size ≥50% were selected and thereafter treated with PTX or vehicle for 5-weeks. At the end of the 5-week study, a second echocardiographic assessment was performed. Percent ischaemic zone (%IZ), LV ejection fraction (EF), LV end-diastolic volume (LVEDV), and LV end-diastolic volume-to-mass ratio, all indexes of severity of CHF, were determined from short- and long-axis images of the left ventricle (LV). LV mass and volume were calculated using the area length method. After completion of two-dimensional imaging, pulse-wave Doppler interrogation of mitral inflow was performed to determine heart rate (HR). Cardiac output (CO) was calculated as the product of HR and stroke volume (SV). From mitral inflow, isovolumetric relaxation time and isovolumetric contraction time were measured. The Tei index was calculated as described previously [4].
Morphological parameters
At the end of the study, the rats were sacrificed under deep anaesthesia using a carbon dioxide chamber. Lung and left and right ventricular masses were recorded, and the respective indices were obtained by dividing with body mass.
2.3. Survival
To calculate the survival in pre- and post-PTX treated groups, rats were monitored for mortality following induction of MI.
2.4. Measurement of circulating TNF-α
Circulating levels of TNF-α were quantified in the plasma samples using a commercially available rat TNF-α ELISA kit (Biosource, Camarillo, CA) as described previously [15, 16].
2.5. Estimation of circulating catecholamine levels
Plasma norepinephrine (NE) and epinephrine (EPI) were measured in plasma samples using an Eicom HTEC-500 system fitted with an HPLC-ECD using HPLC-EC as described previously [4].
2.6. Measurement of renal sympathetic nerve activity (RSNA)
RSNA was measured in Sham and CHF rats anesthetized with pentobarbital, as described previously [4]. Following equilibration, the maximum change (increase) in RSNA in response to an intravenous bolus injection of sodium nitroprusside (SNP; 100 µg/kg) was measured in each animal. The raw nerve activity, integrated nerve activity, mean arterial pressure (MAP), and HR were recorded on a Biopac Acknowledge system. At the end of the experiment, the background noise, defined as the signal recorded post-mortem, was measured and subtracted from the actual RSNA recorded, and subsequently expressed as percent change in RSNA from baseline (in response to SNP).
2.7. Extraction of PVN by Laser Capture Microscopy (LCM)
PVN was captured from frozen brain sections by LCM, as described previously [4]. All the parameters were set constant to attain a relatively equal amount of input RNA for comparative real-time RT-PCR analyses and protein for western blotting.
2.8. RNA isolation and real-time RT PCR
Total RNA was extracted from the tissues as described previously [4]. Real-time RT–PCR (qRT–PCR) was performed in 384-well PCR plates, using Bio-Rad PCR Master Mix (The iTaq SYBR™ Green Supermix with ROX) to study the expression levels of TNF-α (X 66539), IL-1β (NM 031512), iNOS (NM 012611), nNOS (NM 052799), AT-1R (NM 031009), and 18S (NR 003278) as the housekeeping gene using the ABI Prism 7900 sequence detection system (Applied Biosystems).
2.9. NADPH diaphorase staining for nNOS
At the end of week 5, the rats for NADPH diaphorase staining were anaesthetized and perfused transcardially with heparinized saline, followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PBS, pH 7.4). The brains were removed and post-fixed at 4°C for 4 h in 4% paraformaldehyde solution and then placed in 20% sucrose at 4°C for 24h. Brains were blocked in the coronal plane, and 30-µm-thick sections were cut with a cryostat. The sections were collected in 0.1 M PBS, containing 0.3% Triton X-100, 0.1 mg/ml nitroblue tetrazolium and 1.0 mg/ml β-NADPH, and were then placed in an incubator at 37°C for 1 hr. After incubation, the sections were rinsed in PBS (pH 7.4) and mounted on glass slides. NADPH-diaphorase positive neurons in the PVN of three adjacent sections at the same coronal level were counted as described by Zheng et al [17].
2.10. Immunohistochemistry
Immunohistochemistry was performed in formalin fixed sections of the brain as described previously [4]. The primary antibodies against nNOS and 3-nitrotyrosine (3-NT) (Santa Cruz, CA) (1:100) were incubated overnight at 4°C. The sections were then washed twice in PBS, and incubated with a peroxidase conjugated 2° IgG antibody for 30 minutes. Bound antibodies were detected with a streptavidin-peroxidase complex using 3, 3'-diaminobenzidine tetrahydrochloride in PBS containing 0.003% hydrogen peroxide.
2.11. Western blot analysis
Frozen LV, PVN and hypothalamus proteins were prepared and fractionated in 7.5–10% polyacrylamide gel and transferred to an Immobilon membrane, as described previously [4]. Due to limitation in the quantity of PVN, we used the hypothalamus for protein analysis of AT-1R. The membranes were blocked in 1% Casein in Tris-buffered solution containing 0.1% (v/v) Tween-20 for 1 h and then incubated overnight with antibodies (1:1000) against TNF-α, AT-1R, or nNOS (Santa Cruz, CA), followed by a peroxidase-conjugated goat anti-mouse IgG antibody (1:10,000). The signal was detected using an enhanced chemiluminescence immunoblotting detection system and the net intensity was determined and expressed in relative arbitrary units by normalizing the protein intensity to that of anti-GAPDH antibody.
2.12. Statistical analysis of data
All results are expressed as mean ± SEM. For statistical analysis of the data, student’s t test, or one-way ANOVA followed by Bonferroni's post hoc test were used. Survival among treated and untreated groups was analyzed using the Kaplan-Meier analysis of survival followed by the Log-rank test (GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego, CA, USA). Values of p<0.05 were considered significant.
3. Results
3.1. Effect of PTX treatment on the survival of CHF rats
As shown in table 1, pre-treatment with PTX significantly improved 24h survival after induction of CHF as compared to the post-treatment group. None of the Sham animals died during the entire study protocol.
Table I. Echocardiographic and cardiac morphological parameters.
Data are mean ± SEM. HR, heart rate; MAP, Mean arterial pressure; PP, Pulse pressure; LVEDV and LVEDP, left ventricular end-diastolic volume and pressure, respectively; SV, Stroke volume; CO, cardiac output; EF%, percent ejection fraction; IZ%, percent ischaemic zone; LVM and RVM, left and right ventricular mass, respectively; LVMI and RVMI, left and right ventricular mass index, respectively; LM, lung mass; LMI, lung mass index. Mass Index= mass of the organ/body weight.
| Sham | Sham+PTX | CHF | CHF+PTX | |
|---|---|---|---|---|
| Survival | ||||
| PTX pre-treatment | ||||
| n | 7 | 7 | 14 | 15 |
| 24h survival | 7 (100%) | 7 (100%) | 10 (71.4%)* | 14 (93.4%)*,# |
| 5-week survival | 7 (100%) | 7 (100%) | 9 (64.8%)* | 12 (80%)*,# |
| PTX post-treatment | ||||
| n | 7 | 7 | 10 | 10 |
| 24h survival | 7 (100%) | 7 (100%) | 7 (70%) | 7 (70%) |
| 5-week survival | 7 (100%) | 7 (100%) | 7 (70%) | 7 (70%) |
| Echocardiography | ||||
| n | 7 | 7 | 10 | 10 |
| HR (bpm) | 415.2 ± 17.19 | 410.0 ± 4.88 | 407.0 ± 14.09 | 410.7 ± 16.42 |
| MAP (mmHg) | 109±6 | 108±7 | 104±6 | 102±5 |
| PP (mmHg) | 39±4 | 37±3 | 26±4* | 27±2* |
| LVEDP (mmHg) | 4.19±0.08 | 4.52±0.35 | 24.38±1.23* | 14.17±2.7*,# |
| LVEDV (µl) | 548.4 ± 88.56 | 517.3 ± 56.87 | 759.1 ± 24.3* | 738.7 ± 52.49* |
| Mass (mg) | 763.4 ± 58.23 | 807.8 ± 33.77 | 939.3 ± 28.76* | 872.0 ± 46.0 |
| Vol/Mass (µl/mg) | 0.62 ± 0.09 | 0.52 ± 0.07 | 0.82 ± 0.03* | 0.86 ± 0.08* |
| SV (µl) | 473.2 ± 77.15 | 320.6 ± 41.5 | 261.7 ± 15.59* | 250.6 ± 31.2* |
| CO (ml/min) | 192.6 ± 22.0 | 157.3 ± 16.9 | 106.5 ± 7.7* | 114.6 ± 16.3* |
| EF (%) | 0.84 ± 0.01 | 0.82 ± 0.01 | 0.35 ± 0.02* | 0.36 ± 0.03* |
| IZ (%) | 0 | 0 | 54.42 ± 1.25* | 56.26 ± 0.89* |
| Tei index | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.65 ± 0.02* | 0.49 ± 0.02*,# |
| Morphology | ||||
| Bodyweight (g) | 360±15 | 359±23 | 355±12 | 353±18 |
| LVM (mg) | 0.87±0.03 | 0.88±0.05 | 1.00±0.08 | 0.95±0.07 |
| LVMI (mg/g) | 2.42±0.09 | 2.45±0.21 | 2.81±0.17 | 2.73±0.19 |
| RVM (mg) | 0.23±0.02 | 0.24±0.03 | 0.46±0.04* | 0.35±0.04*,# |
| RVMI (mg/g) | 0.64±0.09 | 0.67±0.07 | 1.31±0.15* | 0.99±0.11*,# |
| LM (g) | 1.49±0.064 | 1.52±0.09 | 4.53±0.31* | 3.17±0.19*,# |
| LMI (mg/g) | 4.15±0.13 | 4.23±0.10 | 12.78±0.84* | 8.99±0.39*,# |
p<0.05 vs. Sham
p<0.05 vs. CHF rats
3.2. Effect of blocking cytokines on sympathetic activity
Plasma norepinephrine (NE) and epinephrine (EPI) levels were significantly increased in the CHF rats as compared to those of Sham (Fig. 1A). Following PTX treatment, these catecholamine levels were decreased significantly. However, the NE and EPI levels in the Sham rats treated with PTX were not different from those of Sham operated rats.
Fig.1. Assessment of sympathetic activity.
(A) Norepinephrine (NE) and epinephrine (EPI) levels in the plasma, samples were analysed using HPLC-ECD. ***p<0.001 (n=5 per group). (B) Percent change in RSNA from baseline in rats in response to SNP IV bolus. (C) Percent change in MAP from baseline in response to IV SNP bolus. Note that although IV SNP produced a similar decrease in MAP between vehicle-treated Sham and CHF rats (respective baseline values; 127.2 ±5.5 mmHg and 124.2 ± 4.1 mmHg), the magnitude increase in RSNA to IV SNP was blunted in the CHF rats when compared to Sham rats. As compared to respective vehicle treated groups, IV SNP produced a greater decrease in MAP in PTX-Sham and PTX-CHF animals (respective baseline values; 112.0 ± 6.7 mmHg and 127.0 ± 4.1 mmHg, respectively). In CHF rats, PTX treatment also augmented the baroreflex control of RSNA in response to IV SNP. *p<0.05; **p<0.01 (n for Sham=9; Sham+PTX=5; CHF=6; CHF+PTX=11)
As shown in figures 1B and 1C, in Sham rats, IV SNP produced a marked decrease in MAP and a concurrent increase in RSNA. The hypotensive and sympathoexcitatory responses to IV SNP were further augmented in Sham rats treated with PTX. In CHF rats, IV SNP also decreased MAP to a level similar to that observed in Sham rats; however, the magnitude increase in RSNA evoked by SNP at this dose was significantly blunted. Of merit, IP PTX treatment in CHF rats not only enhanced the hypotensive response to IV SNP, but also augmented/restored the renal sympathoexcitatory response to this hypotensive stimulus.
3.3. Effect of PTX treatment on cardiac function and morphology in HF
Compared to the Sham rats, CHF rats demonstrated increased LVEDV, LVEDP, LV mass, mass/volume ratio and Tei index, and a decreased SV, CO and EF. However, treatment with PTX attenuated only Tei index. The carotid artery was catheterized to measure MAP and pulse pressure (PP). There were no differences in the baseline HR, MAP and PP between Sham and CHF groups (Table 1).
Compared with Sham rats, CHF rats had increased right ventricle mass index and lung mass index, which were decreased with PTX treatment, indicating less pulmonary vascular congestion and hypertrophy. No significant difference was observed in the left ventricular mass index.
3.4. Effect of PTX treatment on cytokine expression levels
As shown in figure 2, plasma TNF-α and IL-1β levels at 5 weeks remained at, or around, baseline levels during the entire study in the Sham groups. In contras, levels were significantly elevated in the CHF group, levels were attenuated by PTX treatment.
Fig.2. Plasma TNF-α and IL-1β levels from Sham and CHF rats treated with vehicle or PTX Data are mean ± SEM.
***p<0.001. For each group n=6.
At 5 weeks, the mRNA expression of TNF-α (Fig. 3A) and IL-1β (Fig. 3B) was significantly increased both in the LV and PVN of CHF rats. PTX treatment significantly decreased these increased levels. PTX treatment in the Sham group had no effect on the myocardial or PVN cytokine mRNA expression. Figure 3C illustrates that the elevated TNF-α protein levels in the LV and PVN, as analyzed by Western blot, were significantly attenuated by PTX treatment.
Fig.3. Expression of cytokines in the LV and the PVN.
(A) mRNA expression of TNF-α in the LV and the PVN as estimated by real-time RT-PCR. Values are mean ± SEM. (n=6 per group) (B) mRNA expression of IL-1β in the LV and the PVN. Values are mean ± SEM. (n=6 per group) (C) Western blot analysis of anti-TNF-α antibody in the LV and PVN. Bar graphs are mean ± SEM values of band intensities representing four independent experiments. **p<0.01; ***p<0.001
3.5. Effect of PTX treatment on AT-1R expression
The AT-1R expression was increased significantly in the LV and PVN of CHF rats compared to that of Sham rats, while treatment with PTX restored these levels to normal (Fig. 4A). Similarly, the elevated protein levels of AT-1R in the LV and the hypothalamus were restored to normal in PTX treated CHF rats (Fig. 4B).
Fig.4. AT-1R levels in the LV and the PVN.
(A) AT-1R mRNA expression in the LV and the PVN. ***p<0.001. (n=6 per group) (B) Western blot analysis of anti-AT-1R antibody in the LV and the hypothalamus. Bar graphs are mean ± SEM values of band intensities representing four independent experiments. *p<0.05 vs. Sham; #p<0.05 vs. CHF group
3.6. Effect of PTX treatment on NOS levels
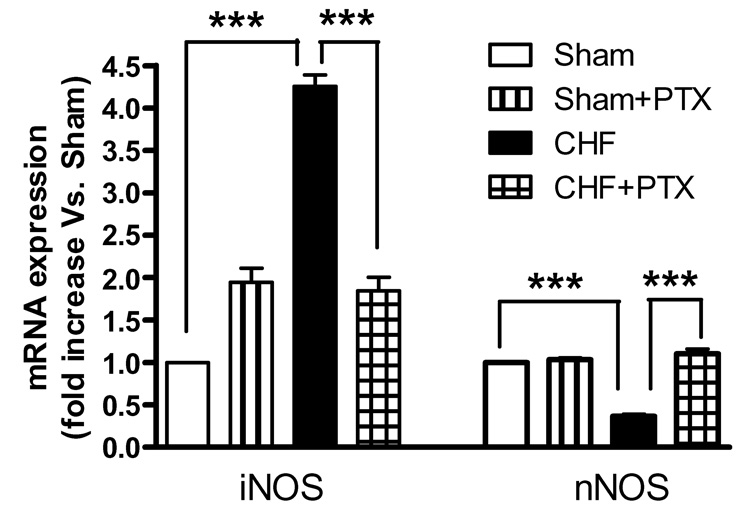
The mRNA expression of iNOS was significantly increased, while that of nNOS was significantly reduced in the PVN of CHF rats as compared to those of Sham rats. PTX normalized the expression of iNOS and nNOS within the PVN of CHF rats. No significant change in the nNOS expression was noticed in the PTX treated Sham rats (Fig. 5).
Fig.5. mRNA expression of iNOS and nNOS in the PVN Values are means ± SEM. (n=6 per group).
***p<0.001
Figure 6A illustrates the PVN stained positive for NADPH-diaphorase activity. The number of NOS-positive cells in the PVN of CHF rats was significantly less than that of the Sham group. In CHF rats, treatment with PTX restored the number of nNOS-positive neurons in the PVN to a level similar to that observed in the Sham group (Fig. 6B). Comparably, the protein expression of nNOS, as determined by Western blot, showed a significant decrease in CHF rats compared to both Sham rats and PTX treated CHF rats. PTX treated Sham rats showed nNOS levels comparable to that of the vehicle treated Sham rats (Fig. 6C). Immunohistochemical staining for nNOS protein showed that compared to Sham rats and PTX treated CHF rats, a significant decrease in the number of neurons positively stained for nNOS was observed in CHF rats (Fig. 6D).
Fig.6. Protein expression of nNOS in the PVN.
(A) nNOS activity detected by NADPH diaphorase staining in the PVN of Sham, PTX treated and untreated CHF rats. (Magnification shown 200X). (B) Quantification of nNOS positive neurons from the NADPH diaphorase staining. Data are mean ± SEM. (n=10 per group). (C) Western blot analysis of anti-nNOS antibody in the PVN. Bar graphs are mean ± SEM values of band intensities representing four independent experiments. **p<0.01; ***p<0.001 (D) Immunostaining of the PVN for anti-nNOS antibody (Magnification in the upper panel=400X; lower panel=200X).
3.7. Effect of blockade of cytokines on 3-nitrotyrosine (3-NT) staining
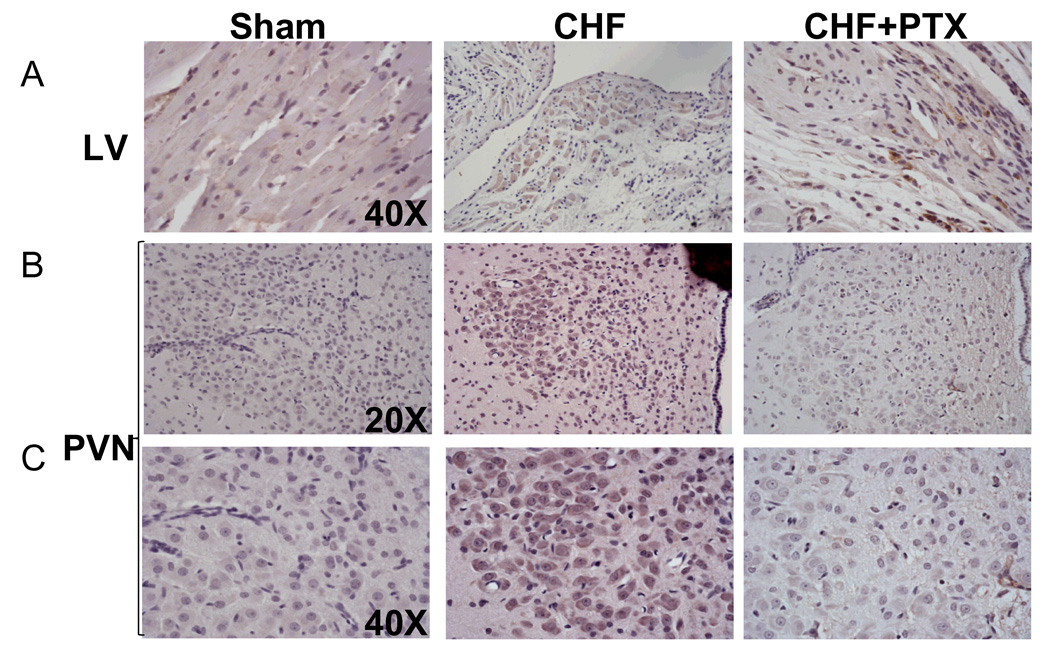
Diffuse positive immunostaining for 3-NT, an indicator of peroxynitrite, was observed in the surviving cardiomyocytes of the peri-infarct region of the LV of CHF rats (Fig. 7A). The PVN of CHF rats also demonstrated an increased staining for 3-NT (Fig. 7B and 7C). Treatment with PTX significantly reversed these changes both in the LV and in the PVN of CHF rats.
Fig.7. Micrograph showing immunostaining for anti-3-nitrotyrosine in (A) the LV and (B, C) the PVN.
Note the evident increase in anti-3-NT signalling in the PVN of vehicle treated CHF rats compared to those of Sham and PTX treated CHF rats.
4. Discussion
The novel findings of the present study are 1) Pre-treatment with a cytokine blocker improved survival and LV function in CHF rats; 2) CHF is associated with an increase in TNF-α and IL-β, and a depletion of nNOS, within the PVN. Our molecular and biochemical findings indicate that blockade of cytokine production by treatment with PTX restored the nNOS levels in the PVN and reduced sympathoexcitation in CHF; 3) AT-1 receptor levels were significantly elevated in the PVN of CHF rats, but not in those treated with PTX, suggesting a cross-talk between cytokines and the RAS in the PVN of HF rats.
Taken together, these results suggest that cytokines contribute to deleterious cardiac effects and decreases in NO bioavailability in the PVN, contributing to enhanced sympathoexcitation and poor survival rate, which is likely mediated via the AT-1R, ultimately suggesting a cross-talk between cytokines and RAS.
Increased sympathetic activity [18] and inflammatory cytokines [19] result in potentially serious ventricular arrhythmias, the main cause of mortality in CHF. Our results demonstrate that the increased LVEDP, lung weight and right ventricle mass in CHF were significantly lowered after PTX, indicating a selective effect on LV diastolic function which is comparable with decreased LVEDV. The increased Tei index in CHF was also significantly decreased by PTX. These results indicate an improved LV diastolic and systolic function. In addition, compared to the Sham group, CHF rats demonstrated a blunted sympathoexcitatory response to IV SNP. This finding is consistent with the notion that in CHF, there is an impaired baroreflex control of RSNA [20, 21]. Of interest, IP PTX treatment in CHF rats enhanced not only the hypotensive response to IV SNP, but also the magnitude of renal sympathoexcitation to this stimulus. These findings suggest that in CHF, PTX treatment improved the baroreflex mechanisms that influence central sympathoexcitatory outflow to the kidneys. Recent evidence also suggests that blocking the production of cytokines in CHF rats, decreases sympathetic activity [4, 22]. These adverse effects, including LV dysfunction and remodelling resulting in the progression of HF, might be due to a sustained increase in TNF-α [23, 24]. Thus, treatment with PTX improved cytokine-induced diastolic and systolic dysfunction and reduced sympathetic hyperactivity resulting in improved LV function.
Nevertheless, more complex mechanisms are associated with increased sympathetic activity in CHF than TNF-α alone. Recent studies underscore the importance of the interaction between cytokines and the RAS in cardiac remodelling and increased sympathetic activity in the progression of CHF. AngII and TNF-α can potentiate the effects of each other, resulting in a vicious cycle towards CHF[7–9]. The brain RAS also plays an important role in sympathetic hyperactivity and cardiac remodelling in CHF [25]. In the present study, treatment with PTX significantly decreased the elevated expression of AT-1R in the heart and the PVN, improved LV function, and decreased plasma catecholamines alongside a decrease in cytokine levels. These results further reinforce the cross-talk between cytokines and AngII in the PVN in CHF.
Besides its interaction with cytokines, AngII also interacts with NO in the PVN of CHF animals. A reduction in NO in CHF may mediate an amplification of the AngII signal to further increase sympathetic activity [26]. Positive nNOS neurons of the PVN are important in regulating central sympathetic outflow [27] and increased sympathetic activity in CHF is attributed, at least in part, by decreased nNOS neurons in the PVN [28]. The blunted baroreflex in CHF also causes central AngII to augment sympathetic activity in CHF [29]. Apart from the intrinsic AngII of the brain, circulating AngII molecules can cross the blood brain barrier (BBB) at the circumventricular organs (CVOs), which express AT-1Rs and project multiple neurons into the PVN [30]. Upon entry into the brain, these AngII molecules can potentiate the AT-1R as well as TNF-α expression. In addition, MI-activated cytokines within the myocardium that exceed the limit for utilization by the local cellular receptors in autocrine/paracrine functions become blood-borne and enter the systemic circulation. These cytokines enter the brain through a saturable transport mechanism or a passive transport mechanism via the CVOs, further potentiating TNF-α and AngII, while attenuating nNOS expression. Interestingly, results from the current study also demonstrate that IP PTX treatment normalized the increased levels of AT-1R and decreased levels of nNOS in the PVN of CHF rats, while improving the baroreflex control of RSNA to a hypotensive stimulus. This clearly corroborates our hypothesis that a cross-talk exists between cytokines, AngII and NO within the PVN, contributing to sympathoexcitation in CHF rats.
We also recently reported that increased oxidative stress in the PVN by cytokines is one possible reason for increased sympathetic activity in CHF rats [4]. ROS-induced cytotoxicity in the RVLM was shown to result in sympathoexcitation [5]. AngII also exerts a positive feed-forward mechanism in the production of AngII and superoxide, which are further sympathoexcitatory [12]. The decreased nNOS expression, and the increased iNOS expression and formation of peroxynitrite in the PVN, explain NO dysregulation in CHF rats. In the present study, treatment of CHF rats with PTX decreased iNOS expression and prevented the formation of peroxynitrite, thereby reducing the exaggerated sympathetic activity in CHF.
In addition to the blood-borne cytokines crossing the BBB, cardiac sympathetic afferents also activate hypothalamic synthesis of cytokines in rats [3]. Similarly, the elevated AT-1R expression in the heart and PVN suggests that both peripheral and central AngII could possibly play a role in depleting nNOS positive neurons in the PVN, possibly via sympathetic afferents. Restoration of nNOS levels by treatment with a cytokine blocker indicates a cross-talk between cytokines, AngII and NO. This ultimately implies that cytokines might decrease NO either directly, or indirectly, via a pathway involving the AT-1R, the action of which results in sympathetic hyperactivity in CHF (Figure 8).
Fig 8. Mode of action of TNF-α in modulating ROS production in the PVN and contributing to sympathoexcitation.
The MI-activated cytokines in the myocardium that become blood-borne, as well as the sympathetic afferent activation, potentiate the expression of TNF-α in the PVN that in turn up-regulates AT-1R expression in the PVN. TNF-α and AngII feed forward each others’ effects in the PVN, and together contribute to increased superoxide and decreased bioavailability of NO via peroxynitrite (ONOO·) formation, thus contributing to sympathoexcitation and increased LV dysfunction.
There are a few limitations to this study. In addition to blocking cytokines PTX has a positive inotropic effect on the heart; it is not known at this point whether the effects exerted by PTX are by virtue of its phosphodiesterase inhibitory activity. Nevertheless, we chose this drug due to its effect as a general cytokine blocker. The concept we are introducing in this study is that there is an interaction between cytokines, RAS and nitric oxide in the sympathoexcitatory process observed in CHF. Clearly, further studies are required to specifically delineate the mechanism by which the RAS and NO system interact within the neurons of the PVN in the presence or absence of cytokines.
Acknowledgements
The authors thank Elizabeth McIlwain, Jeffrey P Cardinale, Sherry Ring and Julie Millard for their excellent technical assistance.
Funding sources
NHLBI (HL080544 to J.F., HL62222 to K.P.P. and HL71212 to D.R.K.); the Board of Regents (LEQSF (2005-07)-RD-A-06 to J.F.); the NIDDK (DK43337); and the NIH National Center for Research Resources (P20 RR018766 to D.R.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 2.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. American journal of physiology. 2002;283:H423–H433. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]
- 3.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. American journal of physiology. 2004;286:H2264–H2271. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- 4.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, et al. TNF-{alpha} blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. American journal of physiology. 2007;293:H599–H609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 5.Dawson VL, Dawson TM. Nitric oxide neurotoxicity. Journal of chemical neuroanatomy. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 6.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, et al. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circulation research. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- 7.Frolkis I, Gurevitch J, Yuhas Y, Iaina A, Wollman Y, Chernichovski T, et al. Interaction between paracrine tumor necrosis factor-alpha and paracrine angiotensin II during myocardial ischemia. Journal of the American College of Cardiology. 2001;37:316–322. doi: 10.1016/s0735-1097(00)01055-x. [DOI] [PubMed] [Google Scholar]
- 8.Gurlek A, Kilickap M, Dincer I, Dandachi R, Tutkak H, Oral D. Effect of losartan on circulating TNFalpha levels and left ventricular systolic performance in patients with heart failure. Journal of cardiovascular risk. 2001;8:279–282. doi: 10.1177/174182670100800506. [DOI] [PubMed] [Google Scholar]
- 9.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure. Journal of the American College of Cardiology. 2000;35:714–721. doi: 10.1016/s0735-1097(99)00594-x. [DOI] [PubMed] [Google Scholar]
- 10.Patel KP, Zhang K, Zucker IH, Krukoff TL. Decreased gene expression of neuronal nitric oxide synthase in hypothalamus and brainstem of rats in heart failure. Brain research. 1996;734:109–115. [PubMed] [Google Scholar]
- 11.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. The American journal of physiology. 1998;275:R728–R734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 12.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. The Journal of clinical investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 14.Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M. Effects of phosphodiesterase inhibitors on cytokine production by microglia. Mult Scler. 1999;5:126–133. doi: 10.1177/135245859900500210. [DOI] [PubMed] [Google Scholar]
- 15.Francis J, Beltz T, Johnson AK, Felder RB. Mineralocorticoids act centrally to regulate blood-borne tumor necrosis factor-alpha in normal rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1402–R1409. doi: 10.1152/ajpregu.00027.2003. [DOI] [PubMed] [Google Scholar]
- 16.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. Journal of cardiology. 1995;26:357–366. [PubMed] [Google Scholar]
- 17.Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. American journal of physiology. 2005;288:H2332–H2341. doi: 10.1152/ajpheart.00473.2004. [DOI] [PubMed] [Google Scholar]
- 18.Aronson D, Burger AJ. Concomitant beta-blocker therapy is associated with a lower occurrence of ventricular arrhythmias in patients with decompensated heart failure. Journal of cardiac failure. 2002;8:79–85. doi: 10.1054/jcaf.2002.32946. [DOI] [PubMed] [Google Scholar]
- 19.Kowalewski M, Urban M, Mroczko B, Szmitkowski M. [Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias] Polskie archiwum medycyny wewnetrznej. 2002;108:647–651. [PubMed] [Google Scholar]
- 20.Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. American journal of physiology. 2004;287:H2138–H2146. doi: 10.1152/ajpheart.00112.2004. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Patel KP, Cornish KG, Channon KM, Zucker IH. nNOS gene transfer to RVLM improves baroreflex function in rats with chronic heart failure. American journal of physiology. 2003;285:H1660–H1667. doi: 10.1152/ajpheart.00239.2003. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, et al. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circulation research. 2007;101:304–312. doi: 10.1161/CIRCRESAHA.107.148940. [DOI] [PubMed] [Google Scholar]
- 23.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circulation research. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 24.Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart (British Cardiac Society) 2004;90:464–470. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Huang BS, Ganten D, Leenen FH. Prevention of sympathetic and cardiac dysfunction after myocardial infarction in transgenic rats deficient in brain angiotensinogen. Circulation research. 2004;94:843. doi: 10.1161/01.res.0000120864.21172.5a. [DOI] [PubMed] [Google Scholar]
- 26.Liu JL, Murakami H, Zucker IH. Angiotensin II-nitric oxide interaction on sympathetic outflow in conscious rabbits. Circulation research. 1998;82:496–502. doi: 10.1161/01.res.82.4.496. [DOI] [PubMed] [Google Scholar]
- 27.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. American journal of physiology. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 28.Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (NOS) positive neurons in the hypothalamus of rats with heart failure. Brain research. 1998;786:219–225. doi: 10.1016/s0006-8993(97)01449-2. [DOI] [PubMed] [Google Scholar]
- 29.Barron KW, Trapani AJ, Gordon FJ, Brody MJ. Baroreceptor denervation profoundly enhances cardiovascular responses to central angiotensin II. The American journal of physiology. 1989;257:H314–H323. doi: 10.1152/ajpheart.1989.257.1.H314. [DOI] [PubMed] [Google Scholar]
- 30.McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ. Interaction of circulating hormones with the brain: the roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clinical and experimental pharmacology & physiology. 1998;25:S61–S67. doi: 10.1111/j.1440-1681.1998.tb02303.x. [DOI] [PubMed] [Google Scholar]