Abstract
This review proposes a new taxonomy of automatic and controlled attention. The taxonomy distinguishes among the role of the attendee (puppet and robot, critic and actor), the attention process (stimulus orienting vs. response control), and the attention operation (activation vs. inhibition vs. adjustment), and identifies cognitive phenotypes by which attention is overtly expressed. We apply the taxonomy to four childhood attention disorders: attention deficit hyperactivity disorder, spina bifida meningomyelocele, traumatic brain injury, and acute lymphoblastic leukemia. Variations in attention are related to specific brain regions that support normal attention processes when intact, and produce disordered attention when impaired. The taxonomy explains group differences in behavioral inattention, hyperactivity, and impulsiveness, as well as medication response. We also discuss issues relevant to theories of the cognitive and neural architecture of attention: functional dissociations within and between automatic and controlled attention; the relative importance of type of brain damage and developmental timing to attention profile; cognitive-energetic models of attention and white matter damage; temporal processing deficits, attention deficits and cerebellar damage; and the issue of cognitive phenotypes as candidate endophenotypes.
Keywords: Attention deficit hyperactivity disorder, Spina bifida, Traumatic brain injuries, Acute lymphoblastic leukemia, Mesencephalon, Frontal lobe
INTRODUCTION
Attention is unobservable, so models of attention and its components are based on inferences about how an individual perceives, thinks, and acts. This article presents a new, three-dimensional functional taxonomy that organizes contingent relationships among perception, cognition, and movement into a framework for understanding attention and its disorders in children. Herein, we:
Describe the architecture of the taxonomy in terms of three unobservable constructs and an observable cognitive phenotype.
Apply the taxonomy to four childhood attention disorders, making functional comparisons within each disorder.
Demonstrate compatibility of the taxonomy with the neurobiology of attention.
Compare behavioral profiles of inattention, impulsivity, and hyperactivity across disorders and predict treatment responsiveness.
Discuss some theoretical issues pertaining to attention and predict attention profiles in disorders not yet studied.
ATTENTION TAXONOMY
Stimulus Orienting Versus Response Control
Models of attention include both stimulus orienting and response control. Stimulus orienting is the automatic capture of attention by salient sensations (James, 1890; more recently, automatic disengaging and shifting attention, Mirsky et al., 1991; Posner & Peterson, 1990; filtering of salience information, Knudsen, 2007; or creating saliency maps, Treue, 2003). Response control is the voluntary direction of a motor response, corresponding to Posner and Peterson’s (1990) anterior attention system and to Knudsen’s (2007) response selection. Being an unobservable construct, attention is inferred in both stimulus orienting and response control from manipulations of the relation between sensation and movement. In stimulus orienting, inhibition of return (IOR) is inferred from the contingencies between motor engaging and disengaging. In response control, stopping an ongoing action is inferred from the relation between go and stop actions.
A new taxonomy (Figure 1) was prompted by considerations relevant to the specific aims. First, we aimed to integrate stimulus orienting and response control into a single taxonomy. Some models of attention used in childhood clinical disorders focus on only one process; for example, Barkley’s (1997) model of attention considers only response control. Second, we wanted to distinguish among operations within stimulus orienting and response control. Some earlier models of attention that considered both processes (e.g., Mirsky et al., 1991; Posner & Peterson, 1990) have not separated activation, inhibition, and adjustment. Third, we aimed to incorporate recent parsings of attendee roles (e.g., the distinction in striatal learning between a “critic” who evaluates performance and an “actor” who performs) and cognitive phenotypes (e.g., the distinction in response control between restraining a prepotent response and canceling an ongoing response). Fourth, we wanted to integrate the three attention dimensions into a factorially structured taxonomy to identify both functional assets and deficits. Such an integration will facilitate functional comparisons both within and across clinical disorders and generate not only descriptions, but also principled inferences about the neural and behavioral correlates of attention.
Fig. 1.
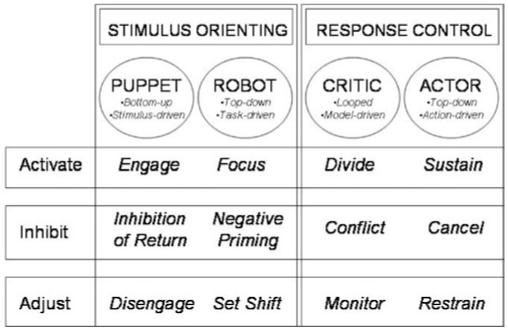
Attention taxonomy.
Puppet Versus Robot Versus Critic Versus Actor
Within stimulus orienting, the attendee may be a puppet or robot. We devised the puppet metaphor to capture the idea of a passive attendee whose attention is driven by bottom-up, stimulus-driven orienting to exogenous (external) stimuli, such as a flash of light. We devised the robot metaphor to capture the idea of an active attendee whose orienting is top-down and task-driven, in accord with endogenous (internal) programs, scripts, or symbols, such as an arrow.
Within response control, the attendee may be a critic or an actor. The critic metaphor captures the idea of looped, model-driven responding, involving an active attendee who evaluates performance options and reward contingencies in light of an existing model of desired behavior. The actor metaphor captures the idea of a top-down, action-driven, active attendee responding in accordance with an instructional set and attention priorities. We adopted the actorcritic metaphors from habit-driven learning (Dayan & Balleine, 2002; O’Reilly & Frank, 2006) and animal studies separating a dorsal striatum (actor) role in performance from a ventral striatum (actor and critic) role in performance and learning (Atallah et al., 2007).
Activation Versus Inhibition Versus Adjustment
The attendee is involved in activation, inhibition, or adjustment. Activation enables directed attention towards the attended material. Inhibition refers to diverse attention processes, some of which concern automatic avoidance of previously attended locations (e.g., IOR), and some of which involve voluntary acts of inhibitory control (e.g., stopping an ongoing action in response to a signal). Adjustment refers to a number of evaluative-regulative processes by which previous attention modifies subsequent attention (Larson et al., 2007), including error detection, performance monitoring, and adjusting the contingency between present action and future reward (Holroyd & Coles, 2002; Nigg et al., 2005; Sagvolden et al., 1998).
Attention Measures Express Cognitive Phenotypes
The italicized entries in Figure 1 are cognitive phenotypes, which are overt and measurable expressions of the attention constructs. For each cognitive phenotype, we identify a representative task measure, below.
The attendee as puppet
Activation enables automatic stimulus engagement. The engage cognitive phenotype may be measured by the timeto orient to a target following an exogenous cue, such as a flash of light (Posner & Peterson, 1990). Inhibition enables the attendee to avoid orienting to a previously attended location or stimulus. The IOR cognitive phenotype is indexed by a longer time to return to a previously attended cue location (Klein, 2000). Adjustment enables a disengage process to withdraw attention from one stimulus so that it may be moved to another. The disengagement cognitive phenotype may be measured by disengagement cost, the time to detach from an exogenous cue conditionalized on engage time (Dennis et al., 2005a).
The attendee as robot
Activation enables a voluntary focus on the stimulus. The focus cognitive phenotype may be measured by time to attend to a target following an endogenous cue, such as a symbol or an arrow (Posner & Peterson, 1990). Inhibition slows responses to recently ignored stimulus relative to new stimuli. The negative priming (NP) cognitive phenotype may be measured by a longer time to attend to recently ignored stimuli (Tipper, 1992). Adjustment enables set shift to redirect attention. The shift cognitive phenotype may be measured by shifting attention in response to an endogenous cue (Schmitter-Edgecombe & Langill, 2006).
The attendee as critic
Activation enables a voluntary allocation of attention. The divide cognitive phenotype may be measured by the ability to activate concurrent attention streams (e.g., Manly et al., 1999). Inhibition enables the suppression of one of two interfering schemata or behaviors. The conflict cognitive phenotype may be measured by the ability to perform a controlled act while inhibiting a prepotent or competing response (e.g., to say “Day” for a moon picture, Gerstadt et al., 1994, or to inhibit an automatic process like word decoding in favor of a controlled process like color naming, Stroop, 1935). Adjustment enables monitoring of response conflict, errors, and top-down response control (Larson et al., 2007). The conflict cognitive phenotype may be measured by error detection and performance adjustment (Holroyd & Coles, 2002).
The attendee as actor
Activation concerns response control. The sustain cognitive phenotype may be measured by the slope of the curve of attentional vigilance over time (Seidel & Joschko, 1990). Inhibition enables cancellation or withdrawal of a response being executed. The cancel cognitive phenotype may be measured by canceling an act being executed (e.g., the stopsignal task, Logan, 1994). Adjustment enables withholding or delaying a prepotent response. The restrain cognitive phenotype may be measured by restraining a response (e.g., Axelrod et al., 1978) or delaying an action (e.g., Gordon, 1983).
Interrelation of Attention Processes
The taxonomy parses a set of attention processes and roles that normally work together in a complex choreography (Mesulam, 1990). Bottom-up, top-down, and looped operations operate in the same attention space. Stimulus orienting serves as a circuit breaker for response control (as when a flash of lightning draws attention from a book and we orient to the window). Dissociable inhibitory processes work together; for example, IOR not only aids visual search, but also helps adjust behavioral demands in a dynamic environment (Ivanoff & Taylor, 2006). Arousal influences the level of activation. Controlled attention helps maintain instructional set, even for stimulus orienting tasks.
While the key to the taxonomy is attendee roles in stimulus orienting and response control, time is a thread throughout, and the terms time binding (Dennis, 2006) and intertemporal competence (Barkley, 1997) characterize temporal processes in attention, such as time estimation, time management, and rule maintenance (Barkley et al., 1997). The taxonomy does not explicitly address working memory, which is the product of currently activated attention, including the activated subset that can be manipulated or inhibited (i.e., the products of the right side of Figure 1). Although it is not an account of executive function, the taxonomy does address executive constructs like response control, conflict, and monitoring.
APPLYING THE TAXONOMY TO CHILDHOOD ATTENTION DISORDERS
Overview
Disordered attention characterizes several childhood conditions, including attention deficit hyperactivity disorder considered as a primary form of adaptive impairment (P-ADHD, Barkley, 1997), and other congenital brain malformations such as spina bifida meningomyelocele (SBM; Dennis et al., 2005a, 2005b) and Sotos syndrome (a haploinsufficiency of the Nuclear receptor Set Domain containing protein 1 gene, NSD 1; de Boer et al., 2006; Kurotaki et al., 2002). Impaired attention is also a consequence of acquired brain insults, including traumatic brain injury (TBI; Levin et al., 2007), childhood brain tumors (Dennis et al., 1998), and acute lymphoblastic leukemia (ALL; Schatz et al., 2004).
Using the taxonomy in Figure 1, we now review attention in four disorders that vary in prevalence, etiology, and developmental course: P-ADHD, TBI, SBM, and ALL. Attention deficits define P-ADHD, and occur in a subset of children with the three other disorders, in which the presenting problem is explicit brain injury. In P-ADHD and SBM, attention is disordered early in development; in contrast, in TBI and ALL, disordered attention is acquired after some period of normal development.
To facilitate the review, we consider two paradigmatic tasks for stimulus orienting (covert orienting, Figure 2) and response control (stop signal, Figure 3). Most components of Figure 1 can be understood in the context of these two tasks. We also summarize the review in Table 1.
Fig. 2.
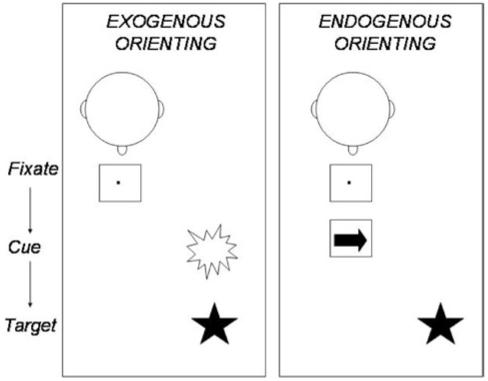
Covert stimulus orienting paradigms. In exogenous orienting (left), the participant maintains central fixation and then an exogenous cue, such as a luminance change, appears to one side of fixation, followed by a target, to which the participant must respond. In this example, the brightness cue will facilitate target detection because it draws attention to the side on which the target will appear (a misleading cue would have appeared on the side opposite to the upcoming target). In endogenous orienting (right), the participant maintains central fixation, which is then replaced by a central endogenous cue, such as an arrow, followed by a target, to which the participant must respond. In this example, the arrow cue will facilitate target detection because it draws attention to the side on which the target will appear (a misleading arrow would have directed attention to the side opposite to the upcoming target).
Fig. 3.
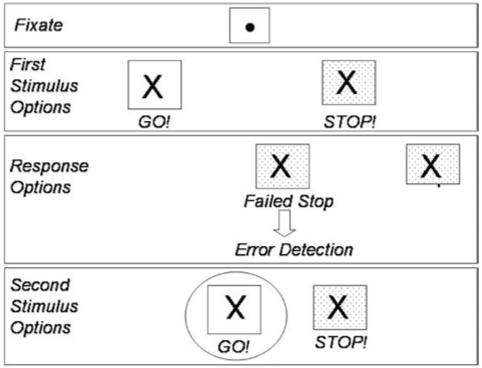
Stop signal paradigm. The participant fixates a central dot and then a go stimulus appears, either an X indicating a left hand response (shown in figure) or an O indicating a right hand response. One-third of the trials involve a stop signal (a background color change) following the go signal to indicate that the participant should not respond. Because of the adaptively manipulated delay interval between go and stop signals, each participant will fail to stop on half of the stop trials. Failed stop trials activate the error detection system, so that go trials that follow a failed stop trial (circled in figure) will be slower than go trials that do not follow failures to stop.
Table 1.
Robots, puppets, critics, and actors: The fractionation of attention in developmental disorders
| Puppet | Robot | Critic | Actor | |
|---|---|---|---|---|
| P-ADHD | ||||
| Activate | Intact | Intact | Impaired | Impaired |
| Inhibit | Intact | Intact | Impaired | Impaired |
| Adjust | Intact | Intact | Impaired | Impaired |
| SBM | ||||
| Activate | Impaired | Impaired | ? | Intact |
| Inhibit | Impaired | Intact | Intact | Intact |
| Adjust | Impaired | Intact | Intact | ? |
| TBI | ||||
| Activate | Intact | ? | Impaired | Impaired |
| Inhibit | ? | Intact | Impaired | Impaired |
| Adjust | Intact | Intact | Impaired | Impaired |
| ALL | ||||
| Activate | Impaired | Impaired | ? | Intact |
| Inhibit | ? | ? | ? | Intact |
| Adjust | Impaired | Impaired | ? | Intact |
Note. Bold entries are from strong, clear data; regular text entries represent a less clear, more limited, or more qualified database, or data from related tasks or age group. Intact or impaired refer to group, not individual, data.
Primary Attention Deficit Hyperactivity Disorder
P-ADHD affects 4-7% of children worldwide (Szatmari, 1992) and is defined by hyperactive, impulsive, inattentive, and0or maladaptive behavior (Barkley, 2004; Goldman et al., 1998; Jensen et al., 1999). P-ADHD persists into adulthood, although heterogeneity in ADHD is considerable throughout development (Barkley et al., 2008), with hyperactivity being more common in children than in adults (Biederman et al., 2000).
Puppet and robot
Meta-analysis of 14 studies on exogenous stimulus orienting (Figure 2) in children with P-ADHD show preserved engage and move operations (Huang-Pollock & Nigg, 2003). Children with P-ADHD have normal IOR (Li et al., 2003). Neither adolescents (Pritchard et al., 2007) nor adults (Nigg et al., 2002) with P-ADHD exhibit difficulties with NP. Children with P-ADHD show no set shifting deficits (Piek et al., 2007; Riccio et al., 2006).
Critic and actor
Children with P-ADHD often have response inhibition deficits (Barkley, 1997; McLean et al., 2004; Nigg, 2003; Pennington & Ozonoff, 1996; Rhodes et al., 2005; Schachar et al., 1995; Westerberg et al., 2004), especially on the stop signal task (Figure 3; Willcutt et al., 2005). Children with P-ADHD have deficits both in the ability to cancel a prepared response with a signal to stop (stop signal presented with a variable delay after go signal) and to restrain a strong response tendency pending a signal to stop (stop and go signals presented concurrently) (Schachar et al., 2007). These children cannot delay responding to achieve a motivationally salient outcome (Kuntsi et al., 2001; Neef et al., 2001; Schweitzer & Sulzer-Azaroff, 1995; Sonuga-Barke, 1994; Sonuga-Barke et al., 1996; Tripp & Alsop, 2001).
Divided attention is impaired in children with P-ADHD, whether treated or drug-naïve (Pasini et al., 2007; Tucha et al., 2006). Children and adults with P-ADHD are impaired on Stroop interference (Lansbergen et al., 2007), suggesting conflict impairment. Children with P-ADHD fail to slow after errors, indicating difficulties in monitoring errors, post-error adjustment, or conflict management (Schachar et al., 2004b).
Children with P-ADHD struggle to sustain attention over time (Seidel & Joschko, 1990); they show increased reaction time (RT) over trial blocks and commit more omission errors (Anderson et al., 2006; Brewer et al., 2001; but see Huang-Pollock & Nigg, 2003). Increased performance variability includes transient temporal fluctuations in attention (Teicher et al., 2004), more variable RT to primary task stimuli (Alderson et al., 2007), lower response predictability (Aase et al., 2006; Aase & Sagvolden, 2005), higher intraindividual variability (Douglas, 1999; Klein et al., 2006), and increased RT, especially in the slow tail of the distribution (Hervey et al., 2004, 2006; Leth-Steensen et al., 2000).
Spina Bifida Meningomyelocele
Spina bifida is a common disabling birth defect, occurring in about 19.3 per 100,000 live births in North America (Martin et al., 2006). The most severe form, spina bifida meningomyelocele (SBM), occurs in 90% of cases (Detrait et al., 2005). Compared to siblings or to normative samples, children with SBM are more distractible and less attentive (Ammerman et al., 1998; Burmeister et al., 2005; Colvin et al., 2003; Rose & Holmbeck, 2007; Vachha & Adams, 2005) and around one-quarter to one-third is inattentive (Burmeister et al., 2005; Davidovitch et al., 1999; Fletcher et al., 2005). It is not clear whether attention deficits extend into adulthood.
Puppet and robot
Children with SBM show accurate but slow covert orienting to both exogenous and endogenous cues (a deficit in the engage and focus processes; Figure 2), but increased disengagement costs only to exogenous cues (a deficit in the disengage0move process, Dennis et al., 2005a). Those with beaking of the midbrain tectum show attenuated IOR (Dennis et al., 2005b). Their normal disengagement costs for endogenously cued information suggests intact NP.
Critic and actor
Children with SBM have not been evaluated on tests of divided attention. They exhibit difficulties with speed of response rather than conflict; for example, they show deficient naming speed but not poorer Stroop interference (Fletcher et al., 1996a,b). Some monitor skills appear intact: They adapt to prismatic distortion (Colvin et al., 2003), respond to error information with corrective saccades (Salman et al., 2006b), and recalibrate movement after forced ballistic movement errors (Dennis et al., 2006).
Children with SBM can sustain attention over time, and their RT does not increase over time (Brewer et al., 2001; Swartwout et al., in press). Studies of commission errors are not consistent even on similar tasks, with some finding decreased (Colvin et al., 2003) and others finding increased error rates (Swartwout et al., in press), so the nature of restraint is unclear, especially in the absence of prototypic response control tasks (see Figure 3). Delay has not been studied.
Traumatic Brain Injury
Traumatic brain injury (TBI) is frequent in children and adolescents (180 in 100,000 youths annually, Kraus & McArthur, 1996; Kraus et al., 1990), and long-term neuropsychological deficits include inattention (Yeates, 2000). Premorbid P-ADHD contributes to post-TBI attention symptoms (Gerring et al., 1998, 2000; Levin et al., 2007; Max et al., 1998), although some 15-20% of TBI survivors with noADHD history meet diagnostic criteria for secondaryADHD (S-ADHD; Gerring et al., 1998, 2000; Herskovits et al., 1999; Max et al., 2005b, 2005c; Slomine et al., 2005; Yeates et al., 2005), and even more develop subthreshold attention symptoms.
S-ADHD is associated not only with severe TBI (Max et al., 2004) but also with mild or moderate TBI (Gerring et al., 1998, 2000; Herskovits et al., 1999; Max et al., 1998; Max et al., 2005a, 2005b; Schachar et al., 2004a; Slomine et al., 2005), as well as with high levels of preinjury behavioral difficulties (Schachar et al., 2004a), maladaptive function, or psychosocial adversity (Gerring et al., 1998; Max et al., 2004, 2005b, 2005c). S-ADHD manifests as early as 3 months post-TBI (Gerring et al., 1998, 2000) and persists over years (Gerring et al., 1998; Levin et al., 2007; Max et al., 2005c). Inattentive symptoms peak at six months post-injury and later decline, whereas hyperactive0impulsive symptoms fluctuate for two years (Levin et al., 2007) and then diminish (Levin et al., 2007; Max et al., 2005b, 2005c). Although poor inhibitory control improves over time (Leblanc et al., 2005), children with TBI with or without S-ADHD exhibit impaired long-term attention (e.g., Dennis et al., 1995; Konrad et al., 2000a, 2000b; Schachar et al., 2004a).
Puppet and robot
Young adults with severe TBI have intact engage and disengage0move processes (Bate et al., 2001). Adults with TBI shift set as well as controls (Schmitter-Edgecombe & Langill, 2006) and show no NP deficits (Ries & Marks, 2005; Simpson & Schmitter-Edgecombe, 2000), suggesting intact endogenous stimulus orienting.
Critic and actor
Children with mild or severe TBI slow less than controls after making an error (Ornstein et al., in press), suggesting monitor impairment. Children with severe TBI have divided attention deficits, especially as task demands increase (Catroppa et al., 2007). Conflict difficulty characterizes TBI: Stroop errors occur more frequently in both the short term and long term in children and adolescents with mild TBI complicated by abnormalities detected by computed tomography (CT) scans (Levin et al., 2008) or with moderatesevere TBI (Chadwick et al., 1981; Nolan & Mathieu, 2000; Ward et al., 2006). Children with TBI have difficulty sustaining attention (Dennis et al., 1995), and those with S-ADHD tend to have longer RT (Slomine et al., 2005). Children with TBI, or children with S-ADHD and TBI, exhibit longer stop signal RT than controls (Konrad et al., 2000a, 2000b; Leblanc et al., 2005), especially those with more severe injury and a de novo diagnosis of S-ADHD (Schachar et al., 2004a), suggesting cancel difficulty. Children with TBI make more commission errors (Konrad et al., 2000a; Levin et al., 2004), and exhibit an inability to delay their motor responses, with or without reward (Dennis et al., 1995; Konrad et al., 2000a), suggesting difficulty with cancel0restrain processes.
Acute Lymphoblastic Leukemia
Of the 3,000-6,000 people diagnosed with acute lymphoblastic leukemia (ALL) each year in the United States, two-thirds are children (Cortes & Kantarjian, 1995; Parker et al., 1997), and 80% will survive (Parker et al., 1997). ALL is associated with cognitive deficits, including poor attention, after treatment with cranial radiation therapy (CRT) and0or chemotherapy, (Christie et al., 1995; Cousens et al., 1988; Fletcher & Copeland, 1988; Goff et al., 1980; Hertzberg et al., 1997; Mennes et al., 2005), especially with early exposure and longer time since treatment (Cousens et al., 1988; Jankovic et al., 1994). Attention problems are evident at least 7-14 years following ALL treatment (Lockwood et al., 1999; Schatz et al., 2004), especially in females and in children treated with CRT at a younger age (Lockwood et al., 1999; Schatz et al., 2004).
Puppet and robot
Children with ALL treated with CRT, especially those radiated in early childhood, have difficulties with stimulus orienting (Lockwood et al., 1999). ALL participants treated with CRT demonstrate an exaggerated cue validity effect, suggesting disengage difficulties (Schatz et al., 2004). Compared to controls, children with ALL have slower but equally accurate focused attention (Mennes et al., 2005). Set shifting is compromised in children with ALL, even those with chemotherapy but no CRT (Buizer et al., 2005), although intensive chemotherapy (Buizer et al., 2005) and CRT (Lockwood et al., 1999) both exacerbate deficits.
Childhood survivors of ALL are slow but accurate when focusing attention (Lahteenmaki et al., 2001), especially if treated with methotrexate (Mennes et al., 2005). IOR and negative priming are yet to be studied.
Critic and actor
Divided attention, monitoring, and conflict have not been studied in ALL. Some studies report intact sustained attention in children with ALL (Mennes et al., 2005; Rodgers et al., 2003; but see Spiegler et al., 2006). However, Lockwood et al. (1999) found that children with ALL had poor sustained attention, especially if female, diagnosed at a younger age, treated with higher doses of methotrexate (Buizer et al., 2005), or treated with CRT at a younger age.
Children with ALL treated with methotrexate chemotherapy are slower and more variable than (but as accurate as) controls on response inhibition tasks (Mennes et al., 2005). CRT does not exacerbate inhibition problems (Lockwood et al., 1999). Children with ALL treated with chemotherapy differ from age norms in delayed response tasks (Spiegler et al., 2006). The sustain process appears intact in many, if not all children with ALL, and response inhibition (whether cancel or restrain0delay) processes are sometimes unimpaired.
NEUROBIOLOGY OF ATTENTION
The taxonomy is function-based, but we next demonstrate that: a) the elements of the taxonomy are compatible with the neurobiology of stimulus orienting and response control; and b) within each of the childhood attention disorders, functional deficits are present when the putative neural substrates are impaired and absent when they are intact.
Stimulus Orienting and the Posterior Cortex, Midbrain, and Superior Colliculus
Deficits in disengaging (not shifting attentional set) are associated with superior colliculus and posterior cortex lesions in adults (Posner et al., 1984). IOR deficits in adults are associated with superior colliculus lesions (Rafal et al., 1989, 1991; Sapir et al., 1999) and midbrain lesions such as those in progressive supranuclear palsy (Rafal & Grimm, 1981; Rafal et al., 1988).
SBM involves malformations of the cerebellum, hindbrain, and midbrain (Fletcher et al., 2005; Salman et al., 2006a). The midbrain abnormality, tectal beaking (Barkovich et al., 2005; Fletcher et al., 2005), is a mechanical consequence of brain development in a small posterior fossa (McLone & Knepper, 1989). Brain growth in SBM affects posterior more than anterior cortical structures, producing selective reduction in posterior cortical volume (Dennis et al., 1981; Fletcher et al., 1996a,b; Juranek et al., 2008) and impairments in white matter integrity (Hasan et al., 2008a), with relative preservation, even hypertrophy, of anterior cortex (Juranek et al., 2008) and some cortical and subcortical structures (Hasan et al., 2008b; Miller et al., 2008).
In SBM, deficits in disengaging and shifting are associated with tectal beaking and posterior brain volume loss (Dennis et al., 2005a), and attenuated IOR is associated with tectal beaking (Dennis et al., 2005b). Individual differences in stimulus orienting deficits are correlated with individual differences in structural brain damage observed in children with SBM in the posterior cortex, midbrain, and superior colliculus.
The midbrain is involved in dopamine dysfunction in children with P-ADHD. Ernst et al. (1999) found that accumulation of [F-18]DOPA, a measure of dopa decarboxylase activity in synaptic terminals of dopaminergic-rich regions, was nearly 50% higher in the right midbrain in children with ADHD, measures being correlated with symptom severity. However, this excess of midbrain dopamine does not disrupt stimulus orienting in P-ADHD.
We predict that structural or neurochemical damage to the midbrain should produce stimulus-orienting deficits at any age. For example, childhood acquired midbrain tumors, such as pineal germinomas, should be associated with impaired stimulus orienting.
Response Control and Frontal-Striatal Circuits
Response control develops throughout childhood (Davidson et al., 2006; Rubia et al., 2006) and involves two principal dopamine-modulated pathways: a prefrontal-dorsal striatum circuit and an orbitofrontal-ventral striatum circuit. Activation of the superior, medial, and inferior frontal gyri as well as midline networks are associated with withholding and monitoring motor responses (Chevrier et al., 2007; Rubia et al., 2001). The withdrawal or canceling of responses has been shown to activate the right inferior frontal gyrus, the right anterior cingulate cortex (ACC), supplementary motor cortex, and caudate (Chevrier et al., 2007; Rubia et al., 2001). These data are congruent with the idea of distinct brain systems for intentional actions and the withholding of intended actions (Brass & Haggard, 2007). Frontal regions are also implicated in conflict management: Better interference control is associated with activity in the right middle frontal gyrus and left inferior frontal gyrus (Bunge et al., 2001).
There is ongoing debate about the neural basis of error detection and performance monitoring. The extensive limbic connections of the ACC contribute to the modulation of emotional expression (Critchley, 2005), and the dorsal ACC is directly involved in higher-order cognitive functions such as attention and response selection (e.g., Bush et al., 2000). Although the striatum has an important role as critic, the ACC also supports evaluative aspects of performance monitoring (Dias & Aggleton, 2000) that require integration of cognitive and limbic input. It is activated preceding errors (Li et al., 2007), responds to both internal and external error signals (Holroyd et al., 2004), and is more activated by error responses than by correct responses and by error feedback than by correct feedback (Holroyd et al., 2004). The dorsal ACC is particularly activated by error detection invoked by failed inhibition trials of the stop signal task (Chevrier et al., 2007), and by changes in task “sets” that signal adaptive changes in response style (Woodward et al., 2008). The role of the ACC in conflict monitoring may be domain-general because dorsal ACC activity is related to a cognitive conflict between major and minor musical modes (Mizuno & Sugishita, 2007).
Individuals with P-ADHD show selective cortical thinning of networks serving attention and executive control (Makris et al., 2007). Structural imaging of children with P-ADHD has identified volume reduction in the whole cerebrum (Castellanos et al., 2002), cortical gray matter, caudate nucleus, globus pallidus, prefrontal cortex, ACC (Castellanos et al., 1996; Filipek et al., 1997; Seidman et al., 2006), and especially, the right inferior frontal cortex (Durston et al., 2004; Sowell et al., 2003), with relatively lesser involvement of the posterior cortex, midbrain, and cerebellum (Berquin et al., 1998). Various brain regions have been implicated in P-ADHD symptoms (Seidman et al., 2005), including dorsolateral prefrontal cortex and orbitofrontal cortex (Spencer et al., 2007), and dysfunction has been identified in dorsolateral and orbitofrontal cortices, ACC, inferior frontal gyri, and striatal-pallidal-thalamic circuits (e.g., Bush et al., 2005; Spencer et al., 2007).
Inhibitory control deficits associated with P-ADHD are correlated with functional alterations in a frontostriatal network. Poor interference control in children with P-ADHD is associated with decreased activation of the left inferior frontal gyrus (Vaidya et al., 2005), and hypoactivation of the dorsal ACC occurs in adults with P-ADHD (Bush et al., 1999). During inhibition trials, children with P-ADHD fail to activate the right inferior frontal or precentral gyri (Vaidya et al., 2005), exhibit abnormal activity in the dorsal ACC (no activation, Durston et al., 2003, hypoactivation, Tamm et al., 2004), and exhibit striatal dysfunction, including decreased left caudate activity (Durston et al., 2003; Vaidya et al., 1998). Poor cancellation by P-ADHD patients is associated with hypoactive mesial prefrontal cortex (i.e., near the dorsal ACC) and ventrolateral prefrontal cortex, and decreased left caudate activity (Rubia et al., 1999).
TBI is frequently associated with dysfunction of the prefrontal and frontal cortex (Levin et al., 1996; Wilde et al., 2005), and corpus callosum and frontal lobe white matter (Levin et al., 2000), with some cerebellar involvement (Spanos et al., 2007). S-ADHD is associated with damage to neural regions known to be abnormal in P-ADHD, including the orbitofrontal gyrus (Max et al., 2005b), thalamus (Gerring et al., 2000), and basal ganglia (Gerring et al., 2000; Herskovits et al., 1999; Max et al., 2005a; and see Max et al., 2005c). Children with TBI0S-ADHD also exhibit response inhibition deficits, although there is no clear association with lesion site (Leblanc et al., 2005; Levin et al., 2004). ACC activity in adults with TBI is abnormal during conflict tasks (Soeda et al., 2005), possibly because of diffuse axonal damage disrupting frontal-cortical and subcortical networks, leading to reduced post-error slowing (Larson et al., 2007). In children with TBI, inhibitory impairments are correlated with prefrontal injury. Restraining a prepotent response on go0no-go tasks is related to volume of left prefrontal lesions (Levin et al., 1993, 2004), as well as to injury severity (Konrad et al., 2000a; Levin et al., 2004, but see Leblanc et al., 2005).
Children with P-ADHD and children with TBI have deficits in response control, with individual differences being correlated with individual differences in prefrontal-dorsal striatum and orbitofrontal-ventral striatum circuits. The caudate, putamen, and globus pallidus are part of a striatalpallidal-thalamic circuit. In feedforward and feedback relations with the cortex, this circuit modulates inhibitory control and sensitivity to reward (Alexander et al., 1986), which is consistent with the idea that dopaminergic and0or noradrenergic medications affect ADHD by increasing inhibitory effects of frontal cortical activity on subcortical brain regions (Zametkin & Rapoport, 1987). The thalamus is part of two subcortical networks that are implicated in P-ADHD symptomatology as well as response inhibition (Gerring et al., 2000), and damage to the thalamus produces distractibility and disinhibition (Gentilini et al., 1987). To be sure, there is considerable heterogeneity in the brain systems associated with inhibition. Furthermore, the link of S-ADHD or P-ADHD to the thalamus is less clear than that of either of these disorders to the caudate, putamen, or inferior frontal gyrus.
CROSS-DISORDER COMPARISONS OF INATTENTION, IMPULSIVITY, AND HYPERACTIVITY
Inattentive and0or hyperactive-impulsive behaviors are features of P-ADHD, and exhibited by some children with TBI, SBM, and ALL. The association of inattention, impulsivity, and hyperactivity is strongest in P-ADHD. Between 65% and 90% of children with P-ADHD are hyperactive (Morgan et al., 1996; Paternite et al., 1995; Spencer et al., 2007; Wolraich et al., 1996), although the recession of hyperactivity with age may imply an age-dependent association. In TBI and S-ADHD, inattention and impulsivity, but not hyperactivity, are associated, in that most children with S-ADHD do not show hyperactivity, although they may exhibit inattention and impulsivity (Max et al., 2005a, 2005b, 2005c). Children with ALL exhibit some hyperactive symptoms, with approximately one-third of children with ALL achieving a mildly atypical score on the Conners’ Parent Rating Scale-Hyperactivity Index (T-score . 60; Conklin, personal communication, November 26, 2007). Unlike those with P-ADHD or S-ADHD, children with SBM rarely show hyperactivity or impulsivity (Fletcher et al., 2005).
The cross-disorder comparison of inattentiveness, hyperactivity, and impulsivity prompts three testable predictions about stimulant mediation treatment. Methylphenidate blocks the dopamine transporter (Dresel et al., 2000), which has a greater effect on the striatum than on the prefrontal cortex (Durston et al., 2005), given that the dopamine transporter gene is more highly expressed in the striatum than in the cortex, where other means of dealing with intrasynaptic dopamine predominate. The first prediction is that methylphenidate should affect response control more than stimulus orienting. This prediction is supported by the data reviewed in this article and also by evidence that methylphenidate specifically affects sustained attention and top-down control, rather than arousal (Johnson et al., 2008).
P-ADHD is a disorder of neurotransmission (synthesis, release, reuptake, effect) mediated by genes that code for dysfunctional or suboptimally functioning dopamine, whereas S-ADHD arises from damaged neural networks, which may or may not include striatal dysfunction. Given the different underlying mechanisms in the two conditions, the second prediction is that methylphenidate will be more effective in treating response control in P-ADHD than in S-ADHD. Methylphenidate also improves behavioral (not cognitive) hyperactivity0impulsiveness in S-ADHD, although improvement occurs acutely and is less robust than that in children with P-ADHD (Jin & Schachar, 2004).
Combined inattention and hyperactivity may reflect striatal and prefrontal-striatal dysfunction, whereas inattention may be primarily a problem of the prefrontal cortex and the prefrontal-parietal circuit (Diamond, 2005). This distinction, made within P-ADHD, prompts a third prediction across disorders, that treatment responsiveness should be positively correlated with hyperactivity and impulsiveness (specifically, should be highest in P-ADHD, moderate in S-ADHD, lower in ALL, and lowest in SBM). Stimulants have effectively treated P-ADHD for over more than three decades of randomized clinical trials, with 65% to 75% of individuals with P-ADHD being clinical responders, compared to 4% to 30% of individuals treated with placebo (Greenhill, 2002). Granted that a smaller proportion of children with S-ADHD (7%; Levin et al., 2007) than P-ADHD (56%; Visser et al., 2007) receive stimulant treatment, methylphenidate does improve attention in children after TBI (Mahalick et al., 1998). Methylphenidate ameliorates attention problems in ALL (Conklin et al., 2007), and both moderate and low doses of methylphenidate improve parent and teacher attention ratings of ADHD symptoms, as well as teacher ratings of social-academic competence (Mulhern et al., 2004). Inattention in children with SBM does not respond robustly to methylphenidate treatment. In a large double blind, placebo-controlled trial of methylphenidate medication in a clinic population of children with SBM, Davidovitch et al. (1999) found no statistically significant medication effects (but see positive medication effects in a smaller, heterogeneous sample in Mayes et al., 1994).
INFERENCES AND PREDICTIONS
The application of the taxonomy to childhood disorders of attention has produced descriptive comparisons within and between disorders. New information has been added for each condition: for P-ADHD, the diversity of deficits in response control and the intactness of stimulus orienting; for SBM and ALL, the intactness of response control; and for S-ADHD, the similarity to P-ADHD with respect to deficits in response control and adaptive regulation. Individual children with P-ADHD are known to have uncorrelated deficits in multiple processes (e.g., delay intolerance is independent of inhibitory dyscontrol, Solanto et al., 2001; Sonuga-Barke et al., 1994), and we show this to be true for other childhood attention disorders. In addition, our data prompt inferences about theoretical questions and predictions about attention in disorders not yet studied.
Double Dissociation Between Automatic and Controlled Attention
Within- and between-disorder comparisons in childhood conditions have provided functional and neurobiological evidence of a double dissociation between automatic and controlled forms of attention. This is congruent with a long history of adult lesion studies, and also with new evidence from markers of event-related potentials. For example, listening to Mozart’s D major sonata K.448 has opposite effects on event-related potentials markers of involuntary and voluntary attention (Zhu et al., 2008).
Inhibition Is a Diverse Attention Construct
Inhibition constitutes a diverse and often uncorrelated group of functions. In children with P-ADHD, the puppet is intact (normal IOR), but the actor is impaired (poor restrain). Different conditions have the same inhibitory impairment (P-ADHD and TBI both show impaired actor role and have restrain deficits). Adult disorders show differently dissociated forms of inhibition; for example, patients with Parkinson’s disease demonstrate intact IOR but impaired endogenously evoked inhibition (NP) (Grande et al., 2006).
Dissociations also exist within and between top-down and bottom-up processes. Children with P-ADHD are impaired on top-down response inhibition of the actor but not on endogenous top-down stimulus orienting of the robot. In children with SBM, bottom-up exogenous stimulus orienting deficits in the puppet are uncorrelated with intact top-down endogenous stimulus orienting in the robot.
Brain Injury Trumps Age at Onset
Anatomy trumps age at onset with respect to the puppet in stimulus orienting, in that SBM and ALL share deficits in this domain despite differences in age at onset, although stimulus-orienting deficits may have different anatomical origins in these two conditions. Anatomy certainly trumps age at onset for response control. Despite differences in age at onset, response control is largely unimpaired in SBM and ALL, and impaired in P-ADHD and TBI0S-ADHD, high-lighting the role of anterior brain regions and the subcortical dopaminergic system in the production of response control deficits. Age at onset seems relevant to persistence of deficits in the two congenital conditions and the relative volatility over time of deficits in acquired conditions.
Although both P-ADHD and SBM are congenital conditions, they have distinct attentional and neurobiological profiles. Children with SBM have difficulties with error detection, although not with post-error adjustment. It is possible that they share error detection difficulties with children with P-ADHD because of shared midbrain dysfunction, perhaps related to mesolimbic dopamine (Di Chiara, 1998), or mesencephalic function more generally, affecting dorsal ACC and right frontal function. We predict some error detection deficits in children with other forms of structural or neurochemical midbrain dysfunction (e.g., children with pontine tumors).
Temporal Stability of Attention Deficits
Both P-ADHD and SBM have attention deficits that persist over time and, at least for P-ADHD, into adult life. Their common pattern of stability of attention problems over time is consistent with their shared status as genetically based life-long developmental conditions with no period of normal development.
Compared to P-ADHD, children with TBI and S-ADHD have more volatile attention symptoms over time. Children with P-ADHD are evaluated in the school-aged years, and are already into the chronic stage of their attention disorder. It is unclear whether the picture of enhanced symptom volatility in children with TBI and S-ADHD arises because they have been studied in relatively short terms in relation to injury onset. Perhaps S-ADHD recovers over time, while formative brain anomalies ensure that P-ADHD will persist. However, one issue is that the time frame for resolution of cognitive deficits matches long-term TBI degenerative processes (e.g., loss of axonal connections and secondary focal brain atrophy) better than the short-term recovery mechanisms known to be operative (e.g., reduction in inflammatory processes and brain swelling).
Based on our data, we predict that attention deficits, whatever their form, will be more stable over time in developmental conditions (e.g., Sotos syndrome) than in acquired conditions (e.g., childhood strokes).
Cognitive-Energetic Models, White Matter Damage, and Activating the Actor
In their cognitive-energetic model of attention, Russell et al. (2006) propose that P-ADHD involves inefficient astrocyte function from deficient ATP production in neurons, over milliseconds, and deficient myelination of axons during development, over months and years. Poor formation and supply of lactate produces inefficient and inconsistent performance in P-ADHD and other white matter disorders (Russell et al., 2006).
Although ALL treatment produces cortical atrophy (Baron et al., 1995), CRT and0or chemotherapy (Moore, 2005) particularly disrupt white matter, disturbances of which range from folate deficiency and subacute myeloencephalopathy to leukoencephalopathy with myelin degeneration and white matter necrosis (Maria et al., 1993). Antimetabolites such as methotrexate involved in ALL treatment particularly affect white matter (Cole & Kamen, 2006). SBM involves two types of white matter disruption, the first involving abnormal white matter development and the second, persistent white matter degeneration with increased age. Diffusion tensor tractography in children with SBM reveals abnormal development in the association pathways (e.g., poor visualization of tracts, impairment in myelination) as well as acquired abnormalities in intrinsic axonal characteristics (Hasan et al., 2008a).
The cognitive-energetic model of Russell and colleagues (2006) predicts increased response variability with compromised white matter, congenital or acquired. Children with SBM or ALL have little increased variability on sustained attention tasks despite impaired white matter, so our data provide no support for this prediction.
Temporal Processing and the Cerebellum
Temporal processing has been proposed as a key deficit in P-ADHD (e.g., Barkley et al., 1997), with a central importance being given to cerebellar abnormalities (Berquin et al., 1998). The cerebellar abnormalities in P-ADHD are minor compared to the massive cerebellar compromise in SBM, which is associated with abnormalities (in 98%) and volume reductions in the lateral cerebellum (Fletcher et al., 2005) and with upward and downward displacement of the cerebellar vermis (Salman et al., 2006a). Children with S-ADHD exhibit the typical P-ADHD attention profile, and are less likely than children with SBM to have cerebellar damage.
Perception of brief time intervals (around 400 ms) is consistently impaired in childhood cerebellar disease (SBM, Dennis et al., 2004), adolescents with cerebellar degenerative disease (ataxia-telangiactiasia, Mostofsky et al., 2000), and adult survivors of childhood cerebellar tumors (Hetherington et al., 2000). Temporal estimation tasks, involving cognitive estimations of longer durations ranging from 4 minutes to a half hour, is intact in adult survivors of childhood cerebellar tumors (Hetherington et al., 2000). Children with P-ADHD cannot estimate long durations, around 4 sec, although they accurately perceive brief durations (Radonovich & Mostofsky, 2004), suggesting that the temporal impairment in P-ADHD involves failure to monitor temporal information in working memory rather than a cerebellar central timing deficit (Mahone et al., 2007).
Our data temper the view that the cerebellar temporal processing is of primary importance in P-ADHD. Not only is the degree of cerebellar damage unrelated to attention profiles, but children with P-ADHD have temporal estimation rather than timing deficits.
Can Puppets as Well as Actors be Candidate Endophenotypes?
Endophenotypes are hypothetical constructs intervening between genes and symptoms (Almasy & Blangero, 2001). Endophenotypes were originally proposed in the context of psychiatric disorders (Gottesman & Gould, 2003), but have more recently been used in neurodevelopmental disorders such as ADHD (Aron & Poldrack, 2005). Endophenotypes have more explanatory power than the cognitive phenotypes italicized in Figure 1, so we consider whether two of our cognitive phenotypes, response inhibition and IOR, might be considered as candidate endophenotypes. Crosbie et al. (2008) examined the theoretical rationale and a priori criteria for validating an endophenotype in P-ADHD and, with Biederman et al. (1995), propose that a candidate endophenotype should be common in affected individuals (sensitive), relatively unique to the disorder (specific), and relatively uncommon in the general population; they suggest, further, that a powerful way to establish endophenotype status would be to identify markers in cases with genetic, but not acquired, ADHD.
Response inhibition is a candidate endophenotype for P-ADHD (Aron & Poldrack, 2005; Crosbie et al., 2008). While response inhibition deficits are common in P-ADHD, but not in SBM or ALL, and are relatively uncommon in the general population, they also occur in S-ADHD, an acquired condition. A convincing non-genetic etiology for acquired conditions is difficult to demonstrate (Crosbie et al., 2008), and children with S-ADHD, considered as a group, have elevated rates of P-ADHD. Informative comparisons for invalidating response inhibition as a putative endophenotype would be a study of response inhibition in mild TBI, and a comparison of response inhibition deficits in children with TBI and a de novo S-ADHD diagnosis and those with TBI and preexisting P-ADHD.
Endophenotypes should be state-independent (i.e., should not vary with disease progression or treatment, Crosbie et al., 2008). Both acquired conditions, TBI and ALL, show cognitive phenotypes that fluctuate with disease characteristics, time since diagnosis, or treatment. Attention is worsened by severe injury in TBI and by adjuvant CRT treatment in ALL, in contrast to the relatively stable patterns of congenital conditions over the lifespan (in P-ADHD and, possibly, in SBM). Cognitive phenotypes in acquired conditions are perhaps less likely than those in congenital conditions to be endophenotypes marking genetic risk.
IOR may be a candidate endophenotype. Children of mothers with a genetic mutation in the folate metabolic pathway share the genetic mutation, have the upper spinal cord lesions (Volcik et al., 2000) associated with tectal beaking (Fletcher et al., 2005), and have IOR deficits primarily when they have tectal beaking (Dennis et al., 2005b). Children with ALL have a dual compromise of folate metabolism. Part of their treatment-related delayed neurotoxicity is a pharmacologic disruption of CNS folate physiology (Cole & Kamen, 2006), and they have a genotype involving a folate metabolic mutation linked to inattentive symptoms (Krull et al., 2008). While it is not clear that the identical mutations are involved (the C677T methylenetetrahydrofolate is the risk factor for upper spinal lesion level deficits in SBM, Volcik et al., 2000, the A1298C methylenetetrahydrofolate genotype is the predominant link to inattention in ALL, Krull et al., 2008), there is evidence for a link between folate mutations and inattention. More broadly, candidate endophenotypes for attention disorders might exist, not only in the actor and critic, but also in the puppet and robot.
CONCLUSIONS
Taxonomies are useful to the extent that they organize what is known and direct the search for the unknown. Our taxonomy describes attention within and across childhood attention disorders, is congruent with known neurobiology of attention, and predicts treatment responsiveness. It facilitates critical evaluation of some theoretical positions on attention and predicts specific attention deficits in clinical conditions not yet studied.
ACKNOWLEDGMENTS
The authors’ studies in this article were supported by grants from the US National Institutes of Health Program Project Grant P01 HD35946 (JMF, MD), National Institute of Neurological Diseases and Stroke Grant 2R011NS 21889-16 (MD), and grants from The Ontario Mental Health Foundation and The Physicians’ Services Research Institute Foundation (MD), and Canadian Institutes of Health Research, MOP 44070, 64277, 74699 and 82796 (RS). No financial conflicts of interest exist with respect to this manuscript. The information in this manuscript and the manuscript itself is an original work that is not currently under review elsewhere and has not been previously published in any form. We thank Brenda J. Spiegler, Ph.D., Arturo Hernandez, Ph.D., and the three JINS reviewers for helpful comments on the manuscript.
REFERENCES
- Aase H, Meyer A, Sagvolden T. Moment-to-moment dynamics of ADHD behaviour in South African children. Behavioral and Brain Functions. 2006;2:11. doi: 10.1186/1744-9081-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aase H, Sagvolden T. Moment-to-moment dynamics of ADHD behaviour. Behavioral and Brain Functions. 2005;1:12. doi: 10.1186/1744-9081-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit0hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: Rationale and study design. American Journal of Medical Genetics. 2001;105:42–44. [PubMed] [Google Scholar]
- Ammerman RT, Kane VR, Slomka GT, Reigel DH, Franzen MD, Gadow KD. Psychiatric symptomalogy and family functioning in children with spina bifida. Journal of Clinical Psychology in Medical Settings. 1998;5:449–465. [Google Scholar]
- Anderson V, Anderson D, Anderson P. Comparing attentional skills in children with acquired and developmental central nervous system disorders. Journal of the International Neuropsychological Society. 2006;12:519–531. doi: 10.1017/s135561770606067x. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit0hyperactivity disorder. Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Lopez-Paniagua D, Rudy JW, O’Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nature Neuroscience. 2007;10:126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Axelrod S, Leiber L, Noonan M. Classification of random forms and distortions presented to the left or right visual field. Perceptual and Motor Skills. 1978;47:615–621. doi: 10.2466/pms.1978.47.2.615. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Driving impairments in teens and adults with attention-deficit0hyperactivity disorder. The Psychiatric Clinics of North America. 2004;27:233–260. doi: 10.1016/S0193-953X(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Koplowitz S, Anderson T, McMurray MB. Sense of time in children with ADHD: Effects of duration, distraction, and stimulant medication. Journal of the International Neuropsychological Society. 1997;3:359–369. [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in Adults: What the Science Says. Guilford Press; New York: 2008. [Google Scholar]
- Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873–1887. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- Baron IS, Fennell EB, Voeller KK. Pediatric neuropsychology in the medical setting. Oxford University Press; New York: 1995. [Google Scholar]
- Bate AJ, Mathias JL, Crawford JR. The covert orienting of visual attention following severe traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 2001;23:386–398. doi: 10.1076/jcen.23.3.386.1190. [DOI] [PubMed] [Google Scholar]
- Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, Castellanos FX. Cerebellum in attention-deficit hyperactivity disorder: A morphometric MRI study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Mick E, Spencer T, Wilens T, Kiely K, Guite J, Ablon JS, Reed E, Warburton R. High risk for attention deficit hyperactivity disorder among children of parents with childhood onset of the disorder: A pilot study. American Journal of Psychiatry. 1995;152:431–435. doi: 10.1176/ajp.152.3.431. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. American Journal of Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P. To do or not to do: The neural signature of self-control. The Journal of Neuroscience. 2007;27:9141–9145. doi: 10.1523/JNEUROSCI.0924-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer VR, Fletcher JM, Hiscock M, Davidson KC. Attention processes in children with shunted hydrocephalus versus attention deficit-hyperactivity disorder. Neuropsychology. 2001;15:185–198. doi: 10.1037//0894-4105.15.2.185. [DOI] [PubMed] [Google Scholar]
- Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, Veerman AJ. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: Effect of treatment intensity. Pediatric Blood & Cancer. 2005;45:281–290. doi: 10.1002/pbc.20397. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Burmeister R, Hannay HJ, Copeland K, Fletcher JM, Boudousquie A, Dennis M. Attention problems and executive functions in children with spina bifida and hydrocephalus. Child Neuropsychology. 2005;11:265–283. doi: 10.1080/092970490911324. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit0hyperactivity disorder revealed by fMRI and the Counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:214–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit0hyperactivity disorder: A review and suggested future directions. Biological Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit0 hyperactivity disorder. The Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. Children’s attentional skills 5 years post-TBI. Journal of Pediatric Psychology. 2007;32:354–369. doi: 10.1093/jpepsy/jsl019. [DOI] [PubMed] [Google Scholar]
- Chadwick O, Rutter M, Shaffer D, Shrout PE. A prospective study of children with head injuries: IV specific cognitive deficits. Journal of Clinical Neuropsychology. 1981;3:101–120. doi: 10.1080/01688638108403117. [DOI] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Human Brain Mapping. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D, Leiper AD, Chessells JM, Vargha-Khadem F. Intellectual performance after presymptomatic cranial radiotherapy for leukaemia: Effects of age and sex. Archives of Disease in Childhood. 1995;73:136–140. doi: 10.1136/adc.73.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PD, Kamen BA. Delayed neurotoxicity associated with therapy for children with acute lymphoblastic leukemia. Mental Retardation and Developmental Disabilities Research Reviews. 2006;12:174–183. doi: 10.1002/mrdd.20113. [DOI] [PubMed] [Google Scholar]
- Colvin AN, Yeates KO, Enrile BG, Coury DL. Motor adaptation in children with myelomeningocele: Comparison to children with ADHD and healthy siblings. Journal of the International Neuropsychological Society. 2003;9:642–652. doi: 10.1017/S1355617703940045. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Khan RB, Reddick WE, Helton S, Brown R, Howard SC, Bonner M, Christensen R, Wu S, Xiong X, Mulhern RK. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: A randomized, double-blind, cross-over trial. Journal of Pediatric Psychology. 2007;32:1127–1139. doi: 10.1093/jpepsy/jsm045. [DOI] [PubMed] [Google Scholar]
- Cortes JE, Kantarjian HM. Acute lymphoblastic leukemia: A comprehensive review with emphasis on biology and therapy. Cancer. 1995;76:2393–2417. doi: 10.1002/1097-0142(19951215)76:12<2393::aid-cncr2820761203>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cousens P, Waters B, Said J, Stevens M. Cognitive effects of cranial irradiation in leukaemia: A survey and meta-analysis. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1988;29:839–852. doi: 10.1111/j.1469-7610.1988.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Pérusse S, Barr CL, Schachar RJ. Validating psychiatric endophenotypes: Inhibitory control and attention deficit hyperactivity disorder. Neuroscience and Biobehavioural Reviews. 2008;32:40–55. doi: 10.1016/j.neubiorev.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Davidovitch M, Manning-Courtney P, Hartmann LA, Watson J, Lutkenhoff M, Oppenheimer S. The prevalence of attentional problems and the effect of methylphenidate in children with myelomenigocele. Pediatric Rehabilitation. 1999;3:29–35. doi: 10.1080/136384999289658. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- de Boer L, Roder I, Wit JM. Psychosocial, cognitive, and motor functioning in patients with suspected Sotos syndrome: A comparison between patients with and without NSD1 gene alterations. Developmental Medicine and Child Neurology. 2006;48:582–588. doi: 10.1017/S0012162206001228. [DOI] [PubMed] [Google Scholar]
- Dennis M. Prefrontal cortex: Typical and atypical development. In: Risberg J, Grafman J, editors. The frontal lobes: Development, function and pathology. Cambridge University Press; New York: 2006. pp. 128–162. [Google Scholar]
- Dennis M, Edelstein K, Copeland K, Frederick J, Francis DJ, Hetherington R, Blaser S, Kramer LA, Drake JM, Brandt ME, Fletcher JM. Covert orienting to exogenous and endogenous cues in children with spina bifida. Neuropsychologia. 2005a;43:976–987. doi: 10.1016/j.neuropsychologia.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Dennis M, Edelstein K, Copeland K, Frederick JA, Francis DJ, Hetherington R, Blaser S, Kramer LA, Drake JM, Brandt ME, Fletcher JM. Space-based inhibition of return in children with spina bifida. Neuropsychology. 2005b;19:456–465. doi: 10.1037/0894-4105.19.4.456. [DOI] [PubMed] [Google Scholar]
- Dennis M, Edelstein K, Hetherington R, Copeland K, Frederick J, Blaser SE, Kramer LA, Drake JM, Brandt M, Fletcher JM. Neurobiology of perceptual and motor timing in children with spina bifida in relation to cerebellar volume. Brain. 2004;127:1292–1301. doi: 10.1093/brain/awh154. [DOI] [PubMed] [Google Scholar]
- Dennis M, Fitz CR, Netley CT, Sugar J, Harwood-Nash DC, Hendrick EB, Hoffman HJ, Humphreys RP. The intelligence of hydrocephalic children. Archives of Neurology. 1981;38:607–615. doi: 10.1001/archneur.1981.00510100035004. [DOI] [PubMed] [Google Scholar]
- Dennis M, Hetherington CR, Spiegler BJ. Memory and attention after childhood brain tumors. Medical and Pediatric Oncology. 1998;(Suppl 1):25–33. doi: 10.1002/(sici)1096-911x(1998)30:1+<25::aid-mpo4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Dennis M, Jewell D, Edelstein K, Brandt ME, Hetherington R, Blaser SE, Fletcher JM. Motor learning in children with spina bifida: Intact learning and performance on a ballistic task. Journal of the International Neuropsychological Society. 2006;12:598–608. doi: 10.1017/S1355617706060772. [DOI] [PubMed] [Google Scholar]
- Dennis M, Wilkinson M, Koski L, Humphreys R. Attention deficits in the long term after childhood head injury. In: Broman S, Michel ME, editors. Traumatic head injury in children. Oxford University Press; New York: 1995. pp. 165–187. [Google Scholar]
- Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicology and Teratology. 2005;27:515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Attention-deficit disorder (attention-deficit0 hyperactivity disorder without hyperactivity): A neurobiologically and behaviorally distinct disorder from attention-deficit0 hyperactivity disorder (with hyperactivity) Development and Psychopathology. 2005;17:807–825. doi: 10.1017/S0954579405050388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: Differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. The European Journal of Neuroscience. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. Journal of Psychopharmacology. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Douglas VI. Cognitive control processes in attention-deficit0hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of disruptive behavior disorders. Plenum; New York: 1999. pp. 105–138. [Google Scholar]
- Dresel S, Krause J, Krause KH, LaFougere C, Brinkbaumer K, Kung HF, Hahn K, Tatsch K. Attention deficit hyperactivity disorder: Binding of [99mTc] TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. European Journal of Nuclear Medicine. 2000;27:1518–1524. doi: 10.1007/s002590000330. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, Steenhuis MP, Minderaa RB, Buitelaar JK, Kahn RS, van Engeland H. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Molecular Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, Kahn RS, van Engeland H. Magnetic resonance imaging of boys with attention-deficit0hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. The American Journal of Psychiatry. 1999;156:1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Brookshire BL, Landry SH, Bohan TP, Davidson KC, Francis DJ, Levin HS, Brandt ME, Kramer LA, Morris RD. Attentional skills and executive functions in children with early hydrocephalus. Developmental Neuropsychology. 1996a;12:53–76. doi: 10.1037//0894-4105.12.4.578. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Copeland DR. Neurobehavioral effects of central nervous system prophylactic treatment of cancer in children. Journal of Clinical and Experimental Neuropsychology. 1988;10:495–537. doi: 10.1080/01688638808408255. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Copeland K, Frederick JA, Blaser SE, Kramer LA, Northrup H, Hannay HJ, Brandt ME, Francis DJ, Villarreal G, Drake JM, Laurent JP, Townsend I, Inwood S, Boudousquie A, Dennis M. Spinal lesion level in spina bifida: A source of neural and cognitive heterogeneity. Journal of Neurosurgery. 2005;102:268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, McCauley SR, Brandt ME, Bohan TP, Kramer LA, Francis DJ, Thorstad K, Brookshire BL. Regional brain tissue composition in children with hydrocephalus. relationships with cognitive development. Archives of Neurology. 1996b;53:549–557. doi: 10.1001/archneur.1996.00550060093022. [DOI] [PubMed] [Google Scholar]
- Gentilini M, De Renzi E, Crisi G. Bilateral paramedian thalamic artery infarcts: Report of eight cases. Journal of Neurology, Neurosurgery, and Psychiatry. 1987;50:900–909. doi: 10.1136/jnnp.50.7.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerring J, Brady K, Chen A, Quinn C, Herskovits E, Bandeen-Roche K, Denckla MB, Bryan RN. Neuroimaging variables related to development of secondary attention deficit hyperactivity disorder after closed head injury in children and adolescents. Brain Injury. 2000;14:205–218. doi: 10.1080/026990500120682. [DOI] [PubMed] [Google Scholar]
- Gerring JP, Brady KD, Chen A, Vasa R, Grados M, Bandeen-Roche KJ, Bryan RN, Denckla MB. Premorbid prevalence of ADHD and development of secondary ADHD after closed head injury. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:647–654. doi: 10.1097/00004583-199806000-00015. [DOI] [PubMed] [Google Scholar]
- Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: Performance of children 3.5-7 years old on a Stroop-like day-night test. Cognition. 1994;53:129–153. doi: 10.1016/0010-0277(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Goff JR, Anderson HR, Jr., Cooper PF. Distractibility and memory deficits in long-term survivors of acute lymphoblastic leukemia. Journal of Developmental and Behavioral Pediatrics. 1980;1:158–163. [PubMed] [Google Scholar]
- Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit0hyperactivity disorder in children and adolescents. The Journal of the American Medical Association. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- Gordon M. The Gordon diagnostic system. Gordon Systems; De Witt, NY: 1983. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. The American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grande LJ, Crosson B, Heilman KM, Bauer RM, Kilduff P, McGlinchey RE. Visual selective attention in Parkinson’s disease: Dissociation of exogenous and endogenous inhibition. Neuropsychology. 2006;20:370–382. doi: 10.1037/0894-4105.20.3.370. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Stimulant medication treatment of children with attention deficit hyperactivity disorder. In: Jensen PS, Cooper JR, editors. Attention deficit hyperactivity disorder: State of science best practices. Civic Research Institute; Kingston, NJ: 2002. pp. 9–27. [Google Scholar]
- Hasan KM, Eluvathingal TJ, Kramer LA, Ewing-Cobbs L, Dennis M, Fletcher JM. White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: A diffusion tensor tractography study of the association pathways. Journal of Magnetic Resonance Imaging. 2008a;27:700–709. doi: 10.1002/jmri.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Sankar A, Halphen C, Kramer LA, Ewing-Cobbs L, Dennis M, Fletcher JM. Quantitative diffusion tensor imaging and intellectual outcomes in spina bifida. Journal of Neurosurgery: Pediatrics. 2008b;2:75–82. doi: 10.3171/PED/2008/2/7/075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits EH, Megalooikonomou V, Davatzikos C, Chen A, Bryan RN, Gerring JP. Is the spatial distribution of brain lesions associated with closed-head injury predictive of subsequent development of attention-deficit0 hyperactivity disorder? Analysis with brain-image database. Radiology. 1999;213:389–394. doi: 10.1148/radiology.213.2.r99nv45389. [DOI] [PubMed] [Google Scholar]
- Hertzberg H, Huk WJ, Ueberall MA, Langer T, Meier W, Dopfer R, Skalej M, Lackner H, Bode U, Janssen G, Zintl F, Beck JD. CNS late effects after ALL therapy in childhood. Part I: Neuroradiological findings in long-term survivors of childhood ALL-an evaluation of the interferences between morphology and neuropsychological performance. The German late effects working group. Medical and Pediatric Oncology. 1997;28:387–400. doi: 10.1002/(sici)1096-911x(199706)28:6<387::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit0hyperactivity disorder: A meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene AL, Keith CC, Hinshaw SP, Swanson JM, Hechtman L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychology. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Hetherington R, Dennis M, Spiegler B. Perception and estimation of time in long-term survivors of posterior fossa tumors. Journal of the International Neuropsycholgical Society. 2000;6:682–692. doi: 10.1017/s1355617700666067. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, Cohen JD. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature Neuroscience. 2004;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT. Searching for the attention deficit in attention deficit hyperactivity disorder: The case of visuospatial orienting. Clinical Psychology Review. 2003;23:801–830. doi: 10.1016/s0272-7358(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Ivanoff J, Taylor T. Inhibition of return promotes stop-signal inhibition by delaying responses. Visual Cognition. 2006;13:503–512. [Google Scholar]
- James W. Principles of psychology. Holt; New York: 1890. [Google Scholar]
- Jankovic M, Brouwers P, Valsecchi MG, Van Veldhuizen A, Huisman J, Kamphuis R, Kingma A, Mor W, Van Dongen-Melman J, Ferronato L, Mancini MA, Spinetta JJ, Masera G, ISPACC. International study group on psychosocial aspects of childhood cancer Association of 1800 cGy cranial irradiation with intellectual function in children with acute lymphoblastic leukaemia. Lancet. 1994;344:224–227. doi: 10.1016/s0140-6736(94)92997-1. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Kettle L, Roper MT, Sloan MT, Dulcan MK, Hoven C, Bird HR, Bauermeister JJ, Payne JD. Are stimulants overprescribed? Treatment of ADHD in four U.S. communities. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:797–804. doi: 10.1097/00004583-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Jin C, Schachar R. Methylphenidate treatment of attention-deficit0hyperactivity disorder secondary to traumatic brain injury: A critical appraisal of treatment studies. CNS Spectrums. 2004;9:217–226. doi: 10.1017/s1092852900009019. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Barry E, Bellgrove MA, Cox M, Kelly SP, Dáibhis A, Daly M, Keavey M, Watchorn A, Fitzgerald M, McNicholas F, Kirley A, Robertson IH, Gill M. Dissociation in response to methylphenidate on response variability in a group of medication naïve children with ADHD. Neuropsychologia. 2008;46:1532–1541. doi: 10.1016/j.neuropsychologia.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Juranek J, Fletcher JM, Hasan KM, Breier JI, Cirino PT, Alvarez PP, Diaz JD, Ewing-Cobbs L, Dennis M, Papanicolaou A. Neocortical reorganization in spina bifida. Neuroimage. 2008;40:1516–1522. doi: 10.1016/j.neuroimage.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annual Review of Neuroscience. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Scholl M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit0hyperactivity disorder (ADHD) Brain Injury. 2000a;14:859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Scholl M. Lack of inhibition: A motivational deficit in children with attention deficit0hyperactivity disorder and children with traumatic brain injury. Child Neuropsychology. 2000b;6:286–296. doi: 10.1076/chin.6.4.286.3145. [DOI] [PubMed] [Google Scholar]
- Kraus JF, McArthur DL. Epidemiologic aspects of brain injury. Neurologic Clinics. 1996;14:435–450. doi: 10.1016/s0733-8619(05)70266-8. [DOI] [PubMed] [Google Scholar]
- Kraus JF, Rock A, Hemyari P. Brain injuries among infants, children, adolescents, and young adults. American Journal of Diseases of Children. 1990;144:684–691. doi: 10.1001/archpedi.1990.02150300082022. [DOI] [PubMed] [Google Scholar]
- Krull KR, Brouwers P, Jain N, Zhang L, Bomgaars L, Dreyer Z, Mahoney D, Bottomley S, Okcu MF. Folate pathway genetic polymorphisms are related to attention disorders in childhood leukemia survivors. Journal of Pediatrics. 2008;152:101–105. doi: 10.1016/j.jpeds.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2001;42:199–210. [PubMed] [Google Scholar]
- Kurotaki N, Imaizumi K, Harada N, Masuno M, Kondoh T, Nagai T, Ohashi H, Naritomi K, Tsukahara M, Makita Y, Sugimoto T, Sonoda T, Hasegawa T, Chinen Y, Tomita Ha HA, Kinoshita A, Mizuguchi T, Yoshiura Ki K, Ohta T, Kishino T, Fukushima Y, Niikawa N, Matsumoto N. Haploinsufficiency of NSD1 causes Sotos syndrome. Nature Genetics. 2002;30:365–366. doi: 10.1038/ng863. [DOI] [PubMed] [Google Scholar]
- Lahteenmaki PM, Holopainen I, Krause CM, Helenius H, Salmi TT, Heikki LA. Cognitive functions of adolescent childhood cancer survivors assessed by event-related potentials. Medical and Pediatric Oncology. 2001;36:442–450. doi: 10.1002/mpo.1108. [DOI] [PubMed] [Google Scholar]
- Lansbergen M, Kenemans JL, van Engeland H. Stroop interference and attention0deficit disorder: A review and meta-analysis. Neuropsychology. 2007;21:251–262. doi: 10.1037/0894-4105.21.2.251. [DOI] [PubMed] [Google Scholar]
- Larson CL, Aronoff J, Stearns JJ. The shape of threat: Simple geometric forms evoke rapid and sustained capture of attention. Emotion. 2007;7:526–534. doi: 10.1037/1528-3542.7.3.526. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Chen S, Swank PR, Ewing-Cobbs L, Barnes M, Dennis M, Max J, Levin H, Schachar R. Response inhibition after traumatic brain injury (TBI) in children: Impairment and recovery. Developmental Neuropsychology. 2005;28:829–848. doi: 10.1207/s15326942dn2803_5. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Levin HS, Benavidez DA, Verger-Maestre K, Perachio N, Song J, Mendelsohn DB, Fletcher JM. Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology. 2000;54:647–653. doi: 10.1212/wnl.54.3.647. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Mendelsohn D, Lilly MA, Bruce D, Fletcher JM, Chapman SB, Harward H, Eisenberg HM. Cognition in relation to magnetic resonance imaging in head-injured children and adolescents. Archives of Neurology. 1993;50:897–905. doi: 10.1001/archneur.1993.00540090008004. [DOI] [PubMed] [Google Scholar]
- Levin HS, Fletcher JM, Kusnerik L, Kufera JA, Lilly MA, Duffy FF, Chapman S, Mendelsohn D, Bruce D. Semantic memory following pediatric head injury: Relationship to age, severity of injury, and MRI. Cortex. 1996;32:461–478. doi: 10.1016/s0010-9452(96)80004-9. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Max J, Li X, Swank P, Ewing-Cobbs L, Dennis M, Menefee DS, Schachar R. Symptoms of attention-deficit0hyperactivity disorder following traumatic brain injury in children. Journal of Developmental and Behavioral Pediatrics. 2007;28:108–118. doi: 10.1097/01.DBP.0000267559.26576.cd. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Roberson G, Li X, Ewing-Cobbs L, Dennis M, Chapman S, Max J, Hunter J, Schachar R, Luerssen TG, Swank P. Abnormal CT after mild traumatic brain injury predicts cognitive sequelae in school aged children. Journal of Neurosurgery. Pediatrics. 2008;1:461–470. doi: 10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Zhang L, Swank PR, Hunter J. Selective impairment of inhibition after TBI in children. Journal of Clinical and Experimental Neuropsychology. 2004;26:589–597. doi: 10.1080/13803390409609783. [DOI] [PubMed] [Google Scholar]
- Li CS, Chang HL, Lin SC. Inhibition of return in children with attention deficit hyperactivity disorder. Experimental Brain Research. 2003;149:125–130. doi: 10.1007/s00221-002-1362-8. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood KA, Bell TS, Colegrove RW., Jr. Longterm effects of cranial radiation therapy on attention functioning in survivors of childhood leukemia. Journal of Pediatric Psychology. 1999;24:55–66. [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego: 1994. pp. 189–240. [Google Scholar]
- Mahalick DM, Carmel PW, Greenberg JP, Molofsky W, Brown JA, Heary RF, Marks D, Zampella E, Hodosh R, von der Schmidt E., 3rd Psychopharmacologic treatment of acquired attention disorders in children with brain injury. Pediatric Neurosurgery. 1998;29:121–126. doi: 10.1159/000028705. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Prahme MC, Ruble K, Mostofsky SH, Schwartz CL. Motor and perceptual timing deficits among survivors of childhood leukemia. Journal of Pediatric Psychology. 2007;32:918–925. doi: 10.1093/jpepsy/jsm028. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser K, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit0hyperactivity disorder. Cerebral Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Manly T, Robertson IH, Anderson V, Nimmo-Smith I. Test of everyday attention for children. Thames Valley; Bury St. Edmonds, UK: 1999. [Google Scholar]
- Maria BL, Dennis M, Obonsawin M. Severe permanent encephalopathy in acute lymphoblastic leukemia. The Canadian Journal of Neurological Sciences. 1993;20:199–205. [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: Final data for 2004. National Vital Statistics Report. 2006 September 29;55(1) [PubMed] [Google Scholar]
- Max JE, Lansing AE, Koele SL, Castillo CS, Bokura H, Schachar R, Collings N, Williams KE. Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Developmental Neuropsychology. 2004;25:159–177. doi: 10.1080/87565641.2004.9651926. [DOI] [PubMed] [Google Scholar]
- Max JE, Lindgren SD, Knutson C, Pearson CS, Ihrig D, Welborn A. Child and adolescent traumatic brain injury: Correlates of disruptive behaviour disorders. Brain Injury. 1998;12:41–52. doi: 10.1080/026990598122845. [DOI] [PubMed] [Google Scholar]
- Max JE, Manes FF, Robertson BA, Mathews K, Fox PT, Lancaster J. Prefrontal and executive attention network lesions and the development of attention-deficit0 hyperactivity symptomatology. Journal of the American Academy of Child and Adolescent Psychiatry. 2005a;44:443–450. doi: 10.1097/01.chi.0000156661.38576.0f. [DOI] [PubMed] [Google Scholar]
- Max JE, Schachar RJ, Levin HS, Ewing-Cobbs L, Chapman SB, Dennis M, Saunders A, Landis J. Predictors of attention-deficit0hyperactivity disorder within 6 months after pediatric traumatic brain injury. Journal of the American Academy of Child and Adolescent Psychiatry. 2005b;44:1032–1040. doi: 10.1097/01.chi.0000173293.05817.b1. [DOI] [PubMed] [Google Scholar]
- Max JE, Schachar RJ, Levin HS, Ewing-Cobbs L, Chapman SB, Dennis M, Saunders A, Landis J. Predictors of secondary attention-deficit0hyperactivity disorder in children and adolescents 6 to 24 months after traumatic brain injury. Journal of the American Academy of Child and Adolescent Psychiatry. 2005c;44:1041–1049. doi: 10.1097/01.chi.0000173292.05817.f8. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Crites DL, Bixler EO, Humphrey FJ, 2nd, Mattison RE. Methylphenidate and ADHD: Influence of age, IQ and neurodevelopmental status. Developmental Medicine and Child Neurology. 1994;36:1099–1107. doi: 10.1111/j.1469-8749.1994.tb11811.x. [DOI] [PubMed] [Google Scholar]
- McLean A, Dowson J, Toone B, Young S, Bazanis E, Robbins TW, Sahakian BJ. Characteristic neurocognitive profile associated with adult attention-deficit0 hyperactivity disorder. Psychological Medicine. 2004;34:681–692. doi: 10.1017/S0033291703001296. [DOI] [PubMed] [Google Scholar]
- McLone DG, Knepper PA. The cause of the Chiari II malformation: A unified theory. Pediatric Neuroscience. 1989;15:1–12. doi: 10.1159/000120432. [DOI] [PubMed] [Google Scholar]
- Mennes M, Stiers P, Vandenbussche E, Vercruysse G, Uyttebroeck A, De Meyer G, Van Cool SW. Attention and information processing in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Pediatric Blood and Cancer. 2005;44:478–486. doi: 10.1002/pbc.20147. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Miller E, Widjaja E, Blaser S, Dennis M, Raybaud C. The old and the new: Supratentorial MR findings in Chiari II malformation. Child’s Nervous System. 2008;24:563–575. doi: 10.1007/s00381-007-0528-x. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: A neuropsychological approach. Neuropsychology Review. 1991;2:109–145. doi: 10.1007/BF01109051. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Sugishita M. Neural correlates underlying perception of tonality-related emotional contents. Neuroreport. 2007;18:1651–1655. doi: 10.1097/WNR.0b013e3282f0b787. [DOI] [PubMed] [Google Scholar]
- Moore BD., 3rd. Neurocognitive outcomes in survivors of childhood cancer. Journal of Pediatric Psychology. 2005;30:51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- Morgan AE, Hynd GW, Riccio CA, Hall J. Validity of DSM-IV ADHD predominantly inattentive and combined types: Relationship to previous DSM diagnoses0subtype differences. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:325–333. doi: 10.1097/00004583-199603000-00014. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Kunze JC, Cutting LE, Lederman HM, Denckla MB. Judgment of duration in individuals with ataxia-telangiectasia. Developmental Neuropsychology. 2000;17:63–74. doi: 10.1207/S15326942DN1701_04. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Khan RB, Kaplan S, Helton S, Christensen R, Bonner M, Brown R, Xiong X, Wu S, Gururangan S, Reddick WE. Short-term efficacy of methylpheni-date: A randomized, double-blind, placebo-controlled trial among survivors of childhood cancer. Journal of Clinical Oncology. 2004;22:4795–4803. doi: 10.1200/JCO.2004.04.128. [DOI] [PubMed] [Google Scholar]
- Neef NA, Bicard DF, Endo S. Assessment of impulsivity and the development of self-control in students with attention deficit hyperactivity disorder. Journal of Applied Behavior Analysis. 2001;34:397–408. doi: 10.1901/jaba.2001.34-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. Response inhibition and disruptive behaviors: Toward a multiprocess conception of etiological heterogeneity forADHD combined type and conduct disorder early-onset type. Annals of the New York Academy of Sciences. 2003;1008:170–182. doi: 10.1196/annals.1301.018. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Butler KM, Huang-Pollock CL, Henderson JM. Inhibitory processes in adults with persistent childhood onset ADHD. Journal of Consulting and Clinical Psychology. 2002;70:153157. doi: 10.1037//0022-006x.70.1.153. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit0hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Nolan P, Mathieu F. Deficits de l’attention et de la vitesse du traitement de l’information chez des enfants ayant subi un traumatisme craniocerebral leger. Annales de Réadaptation et de Médecine Physique. 2000;43:236–245. [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: A computational model of learning in the prefrontal cortex and basal ganglia. Neural Computation. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Levin HS, Chen C, Hanten G, Ewing-Cobbs L, Dennis M, Max J, Logan GD, Schachar R. Performance monitoring in children following traumatic brain injury. Journal of Child Psychology and Psychiatry. doi: 10.1111/j.1469-7610.2008.01997.x. in press. [DOI] [PubMed] [Google Scholar]
- Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA: A Cancer Journal for Clinicians. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- Pasini A, Paloscia C, Alessandrelli R, Porfirio MC, Curatolo P. Attention and executive functions profile in drug naive ADHD subtypes. Brain and Development. 2007;29:400–408. doi: 10.1016/j.braindev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Paternite CE, Loney J, Roberts MA. External validation of oppositional disorder and attention deficit disorder with hyperactivity. Journal of Abnormal Child Psychology. 1995;23:453–471. doi: 10.1007/BF01447208. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Piek JP, Dyck MJ, Francis M, Conwell A. Working memory, processing speed, and set-shifting in children with developmental coordination disorder and attention-deficit-hyperactivity disorder. Developmental Medicine and Child Neurology. 2007;49:678–683. doi: 10.1111/j.1469-8749.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- Posner MI, Peterson SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. The Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard VE, Neumann E, Rucklidge JJ. Interference and negative priming effects in adolescents with attention deficit hyperactivity disorder. The American Journal of Psychology. 2007;120:91–122. [PubMed] [Google Scholar]
- Radonovich KJ, Mostofsky SH. Duration judgments in children with ADHD suggest deficient utilization of temporal information rather than general impairment in timing. Child Neuropsychology. 2004;10:162–172. doi: 10.1080/09297040409609807. [DOI] [PubMed] [Google Scholar]
- Rafal RD, Calabresi PA, Brennan CW, Sciolto TK. Saccade preparation inhibits reorienting to recently attended locations. Journal of Experimental Psychology. 1989;15:673–685. doi: 10.1037//0096-1523.15.4.673. [DOI] [PubMed] [Google Scholar]
- Rafal RD, Grimm RJ. Progressive supranuclear palsy: Functional analysis of the response to methysergide and anti-parkinsonian agents. Neurology. 1981;31:1507–1518. doi: 10.1212/wnl.31.12.1507. [DOI] [PubMed] [Google Scholar]
- Rafal RD, Henik A, Smith J. Extrageniculate contribution to reflex visual orienting in normal humans: A temporal hemifield advantage. Journal of Cognitive Neuroscience. 1991;3:351–358. doi: 10.1162/jocn.1991.3.4.322. [DOI] [PubMed] [Google Scholar]
- Rafal RD, Posner MI, Friedman JH, Inhoff AW, Bernstein E. Orienting of visual attention in progressive supranuclear palsy. Brain. 1988;111:267–280. doi: 10.1093/brain/111.2.267. [DOI] [PubMed] [Google Scholar]
- Rhodes SM, Coghill DR, Matthews K. Neuropsychological functioning in stimulant-naive boys with hyperkinetic disorder. Psychological Medicine. 2005;35:1109–1120. doi: 10.1017/s0033291705004599. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Homack S, Jarratt KP, Wolfe ME. Differences in academic and executive function domains among children with ADHD predominantly inattentive and combined types. Archives of Clinical Neuropsychology. 2006;21:657–667. doi: 10.1016/j.acn.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Ries M, Marks W. Selective attention deficits following severe closed head injury: The role of inhibitory processes. Neuropsychology. 2005;19:476–483. doi: 10.1037/0894-4105.19.4.476. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Marckus R, Kearns P, Windebank K. Attentional ability among survivors of leukaemia treated without cranial irradiation. Archives of Disease in Childhood. 2003;88:147–150. doi: 10.1136/adc.88.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose BM, Holmbeck GN. Attention and executive functions in adolescents with spina bifida. Journal of Pediatric Psychology. 2007;32:983–994. doi: 10.1093/jpepsy/jsm042. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: A study with functional MRI. The American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: Conjunctive brain activations across different versions of go0no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell VA, Oades RD, Tannock R, Killeen PR, Auerbach JG, Johansen EB, Sagvolden T. Response variability in attention-deficit0hyperactivity disorder: A neuronal and glial energetics hypothesis. Behavioral and Brain Functions. 2006;2:30–54. doi: 10.1186/1744-9081-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Zeiner P, Berger D. Altered reinforcement mechanisms in attention-deficit0hyperactivity disorder. Behavioural Brain Research. 1998;94:61–71. [PubMed] [Google Scholar]
- Salman MS, Blaser SE, Sharpe JA, Dennis M. Cerebellar vermis morphology in children with spina bifida and Chiari Type II malformation. Child’s Nervous System. 2006a;22:385–393. doi: 10.1007/s00381-005-1180-y. [DOI] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Eizenman M, Lillakas L, To T, Westall C, Steinbach M, Dennis M. Saccadic adaptation in Chiari Type II malformation. Canadian Journal of Neurological Sciences. 2006b;33:372–378. doi: 10.1017/s0317167100005321. [DOI] [PubMed] [Google Scholar]
- Sapir A, Soroker N, Berger A, Henik A. Inhibition of return in spatial attention: Direct evidence for collicular generation. Nature Neuroscience. 1999;2:1053–1054. doi: 10.1038/15977. [DOI] [PubMed] [Google Scholar]
- Schachar R, Levin HS, Max JE, Purvis K, Chen S. Attention deficit hyperactivity disorder symptoms and response inhibition after closed head injury in children: Do preinjury behavior and injury severity predict outcome? Developmental Neuropsychology. 2004a;25:179–198. doi: 10.1080/87565641.2004.9651927. [DOI] [PubMed] [Google Scholar]
- Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and cancellation: Multiple inhibition deficits in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2007;35:229–238. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Schachar R, Tannock R, Marriott M, Logan G. Deficient inhibitory control in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 1995;23:411–437. doi: 10.1007/BF01447206. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, Pakulak A. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2004b;32:285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Schatz J, Kramer JH, Ablin AR, Matthay KK. Visual attention in long-term survivors of leukemia receiving cranial radiation therapy. Journal of the International Neuropsychological Society. 2004;10:211–220. doi: 10.1017/S1355617704102075. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Langill M. Costs of a predictable switch between simple cognitive tasks following severe closed-head injury. Neuropsychology. 2006;20:675–684. doi: 10.1037/0894-4105.20.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer JB, Sulzer-Azaroff B. Self-control in boys with attention deficit hyperactivity disorder: Effects of added stimulation and time. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1995;36:671–686. doi: 10.1111/j.1469-7610.1995.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Seidel WT, Joschko M. Evidence of difficulties in sustained attention in children with ADHD. Journal of Abnormal Child Psychology. 1990;18:217–229. doi: 10.1007/BF00910732. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit0hyperactivity disorder. Biological Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Seidman L, Valera E, Makris N, Monuteaux D, Boriel D, Kelkar K, Kennedy SN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit0hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Simpson A, Schmitter-Edgecombe M. Intactness of inhibitory attentional mechanisms following severe closed-head injury. Neuropsychology. 2000;14:310–319. doi: 10.1037//0894-4105.14.2.310. [DOI] [PubMed] [Google Scholar]
- Slomine BS, Salorio CF, Grados MA, Vasa RA, Christensen JR, Gerring JP. Differences in attention, executive functioning, and memory in children with and without ADHD after severe traumatic brain injury. Journal of the International Neuropsychological Society. 2005;11:645–653. doi: 10.1017/S1355617705050769. [DOI] [PubMed] [Google Scholar]
- Soeda A, Nakashima T, Okumura A, Kuwata K, Shinoda J, Iwama T. Cognitive impairment after traumatic brain injury: A functional magnetic resonance imaging study using the Stroop task. Neuroradiology. 2005;47:501–506. doi: 10.1007/s00234-005-1372-x. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD0HD: A supplement to the NIMH multimodal treatment study of AD0HD. Journal of Abnormal Child Psychology. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. On dysfunction and function in psychological theories of childhood disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1994;35:801–815. doi: 10.1111/j.1469-7610.1994.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Houlberg K, Hall M. When is “impulsiveness” not impulsive? The case of hyperactive children’s cognitive style. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1994;35:1247–1253. doi: 10.1111/j.1469-7610.1994.tb01232.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Williams E, Hall M, Saxton T. Hyperactivity and delay aversion. III: The effect on cognitive style of imposing delay after errors. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1996;37:189–194. doi: 10.1111/j.1469-7610.1996.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Spanos GK, Wilde EA, Bigler ED, Cleavinger HB, Fearing MA, Levin HS, Li X, Hunter JV. Cerebellar atrophy after moderate-to-severe pediatric traumatic brain injury. American Journal of Neuroradiology. 2007;28:537–542. [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E. Attention-deficit0 hyperactivity disorder: Diagnosis, lifespan, comorbidities, and neurobiology. Journal of Pediatric Psychology. 2007;32:631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- Spiegler BJ, Kennedy K, Maze R, Greenberg ML, Weitzman S, Hitzler JK, Nathan PC. Comparison of longterm neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. Journal of Clinical Oncology. 2006;24:3858–3864. doi: 10.1200/JCO.2006.05.9055. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Swartwout MD, Cirino PT, Hampson AW, Fletcher JM, Brandt ME, Dennis M. Sustained attention in children with two etiologies of early hydrocephalus. Neuropsychology. doi: 10.1037/a0013373. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P. The epidemiology of attention-deficit hyperactivity disorders. In: Weiss G, editor. Attention-deficit hyperactivity disorder. Vol. 1. Saunders; Philadelphia: 1992. pp. 361–371. [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit0 hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Lowen SB, Polcari A, Foley M, McGreenery CE. Novel strategy for the analysis of CPT data provides new insight into the effects of methylphenidate on attentional states in children with ADHD. Journal of Child and Adolescent Psychopharmacology. 2004;14:219–232. doi: 10.1089/1044546041648995. [DOI] [PubMed] [Google Scholar]
- Tipper SP. Selection for action:The role of inhibitory mechanisms. Current Directions in Psychological Science. 1992;1:1–4. [Google Scholar]
- Treue S. Visual attention: The where, what, how and why of saliency. Current Opinion in Neurobiology. 2003;13:428–432. doi: 10.1016/s0959-4388(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward delay in children with attention deficit hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2001;42:691–698. [PubMed] [Google Scholar]
- Tucha O, Walitza S, Mecklinger L, Sontag TA, Kubber S, Linder M, Lange KW. Attentional functioning in children with ADHD-predominantly hyperactive-impulsive type and children with ADHD-combined type. Journal of Neural Transmission. 2006;113:1943–1953. doi: 10.1007/s00702-006-0496-4. [DOI] [PubMed] [Google Scholar]
- Vachha B, Adams R. Myelomeningocele, temperament patterns, and parental perceptions. Pediatrics. 2005;115:58–63. doi: 10.1542/peds.2004-0797. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2005;162:1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit0hyperactivity disorder. Pediatrics. 2007;119:S99–106. doi: 10.1542/peds.2006-2089O. [DOI] [PubMed] [Google Scholar]
- Volcik KA, Blanton SH, Tyerman GH, Jong TS, Rott EJ, Page TZ, Romaine NK, Northrup H. Methylenetetrahydrofolate reductase and spina bifida: Evaluation of level of defect and maternal genotypic risk in Hispanics. American Journal of Medical Genetics. 2000;95:21–27. [PubMed] [Google Scholar]
- Ward H, Shum D, McKinlay L, Baker S, Wallace G. Prospective memory and pediatric traumatic brain injury: Effects of cognitive demand. Child Neuropsychology. 2006;13:219–239. doi: 10.1080/09297040600910003. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Hirvikoski T, Forssberg H, Klingberg T. Visuo-spatial working memory span: A sensitive measure of cognitive deficits in children with ADHD. Child Neuropsychology. 2004;10:155–161. doi: 10.1080/09297040409609806. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, Fearing MA, Cleavinger HB, Li X, Swank PR, Pedroza C, Roberson GS, Bachevalier J, Levin HS. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2005;22:333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit0hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wolraich ML, Hannah JN, Pinnock TY, Baumgaertel A, Brown J. Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a county-wide sample. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:319–324. doi: 10.1097/00004583-199603000-00013. [DOI] [PubMed] [Google Scholar]
- Woodward TS, Metzak PD, Meier B, Holroyd CB. Anterior cingulate cortex signals the requirement to break inertia when switching tasks: A study of the bivalency effect. Neuroimage. 2008;40:1311–1318. doi: 10.1016/j.neuroimage.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Yeates KO. Closed-head injury. In: Yeates KO, Taylor HG, Ris MD, editors. Pediatric neuropsychology: Research, theory, and practice. Guilford Press; New York: 2000. pp. 92–116. [Google Scholar]
- Yeates KO, Armstrong K, Janusz J, Taylor HG, Wade S, Stancin T, Drotar D. Long-term attention problems in children with traumatic brain injury. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:574–584. doi: 10.1097/01.chi.0000159947.50523.64. [DOI] [PubMed] [Google Scholar]
- Zametkin AJ, Rapoport JL. Neurobiology of attention deficit disorder with hyperactivity: Where have we come in 50 years? Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26:676–686. doi: 10.1097/00004583-198709000-00011. [DOI] [PubMed] [Google Scholar]
- Zhu W, Zhao L, Zhang J, Ding X, Liu H, Ni E, Ma Y, Zhou C. The influence of Mozart’s sonata K.448 on visual attention: An ERPs study. Neuroscience Letters. 2008;434:35–40. doi: 10.1016/j.neulet.2008.01.043. [DOI] [PubMed] [Google Scholar]


