Abstract
Altered microbial exposure is a possible explanation for the increase of allergies in the Western world. However, genetic factors influence microbially induced immune responses. We have investigated the TLR4(Asp299Gly) gene polymorphism and its possible association with receptor expression of circulating peripheral blood monocytes and the in vitro cytokine responses and phosphorylation of intracellular signaling proteins in peripheral blood mononuclear cells (PBMC) stimulated with lipopolysaccharide (LPS) from Escherichia coli and Salmonella enterica serotype Typhimurium. We studied 34 of the predominant haplotype TLR4 Asp299 (AA) and 8 heterozygote Asp299Gly (AG) individuals. TLR4 expression levels were similar in the two genotype groups. Serovar Typhimurium LPS induced interleukin-12p70 from PBMC, and the degree of phosphorylation of the intracellular signaling protein IκBα in PBMC was lower in the AG than the AA group (P = 0.03 and P = 0.04, respectively). These results were not seen, however, when PMBC were stimulated with E. coli-derived LPS. Based on these results, we propose that TLR4(Asp299Gly) gene polymorphism and the bacterial origin of LPS should be considered when environmental LPS exposure is evaluated in disease risk or protection.
The incidence of airway allergic diseases, i.e., atopic asthma and allergic rhinoconjunctivitis, has increased in the last few decades, particularly in industrialized countries (2), and it has been suggested that this phenomenon may be due in part to increased environmental cleanliness, reflecting decreased or altered microbial exposure (3).
The priming of immune responses to microorganisms is initiated by the interaction of microbe-derived molecules with pattern recognition receptors, e.g., Toll-like receptors (TLRs), present on cells of the innate immune system. TLRs are an evolutionarily conserved group of pattern recognition receptors, and there are currently 10 different TLRs that have been described in human subjects (12). TLR2 has been found to recognize lipoproteins, lipoteichoic acid, and zymosan, whereas TLR4 seems to be the exclusive TLR for lipopolysaccharide (LPS), a major component of the outer cell membrane of gram-negative bacteria (11). TLR4 activation can lead to two intracellular signaling pathways. One is dependent on the recruitment of the adaptor protein MyD88 and the other on the adaptor protein TRIF (reviewed in reference 13). The MyD88-dependent pathway leads to the activation of nuclear factor kappa B (NF-κB) and subsequently to the transcription of proinflammatory genes and the production of cytokines, such as interleukin-12 (IL-12) and gamma interferon. The TRIF-dependent pathway leads to the activation of genes that lead to the production of interferons. Important proteins in the activation of these signaling pathways are IκB, mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase, the phosphorylation of which takes place in cell activation.
The genes encoding TLRs show high variability in human populations (14), but to what extent these genetic variations modify interaction with microbial molecules and thereby immune responses or risk of immune-mediated diseases is not fully understood. The TLR4(Asp299Gly) gene polymorphism that we studied was first reported to be associated with endotoxin airway hyporesponsiveness in humans by Arbour et al. (1). The authors found that inhaled LPS resulted in decreased airway tissue responsiveness and that individuals heterozygotic for the mutation showed a low level of expression of TLR4 in airway epithelia. We have reported that the TLR4(Asp299Gly) polymorphism was independently associated with decreased LPS-induced IL-12p70 and IL-10 responses of peripheral blood mononuclear cells (PBMC) (9). Furthermore, we found a relationship between the TLR4(Asp299Gly) polymorphism and asthma, especially atopic asthma, among Swedish children (9), whereas such a relationship was not found in other studies (16, 19, 28). The underlying molecular mechanisms resulting in reduced LPS-induced responses in individuals with the TLR4(Asp299Gly) gene polymorphism need to be further elucidated.
The aim of the study was to investigate the possible association of TLR4(Asp299Gly) gene polymorphism with the ex vivo expression of monocyte surface molecules, LPS-induced phosphorylation of intracellular signaling molecules, and cytokine production in relation to airway allergic diseases.
MATERIALS AND METHODS
Study subjects.
Individuals with the TLR4(Asp299Gly) polymorphism and airway allergic disease and age-matched nonallergic controls were selected from a population participating in a former study (9) or from medical students at Linköping University. The participants answered a questionnaire regarding their allergic history, present allergic symptoms, and allergy medication. The characteristics of the participants are shown in Table 1. Ongoing allergic symptoms were defined as doctor-diagnosed allergic rhinoconjunctivitis or asthma, with typical symptoms and use of medication for these symptoms during the previous 12 months. Venous blood samples were drawn into heparinized tubes (Becton Dickinson, Stockholm, Sweden). Blood sampling was conducted out of the pollen season, i.e., between September and April. Allergen-specific immunoglobulin E antibodies were analyzed in plasma with UniCap, Pharmacia CAP System, Phadiatop (Pharmacia Diagnostics, Uppsala, Sweden), including 11 common airborne allergens. All 14 allergic individuals and 9 of the 27 nonallergic individuals had positive Phadiatop results. The study group consisted of 8 individuals with the heterozygote TLR4(Asp299Gly) AG genotype, and the other 34 had the haplotype AA genotype. There were no GG individuals in our material. The genotype exists but is rare (1). Two of the AG individuals had asthma and allergic rhinoconjunctivitis. Three of the six AG individuals with no allergic symptoms were Phadiatop positive. The age and sex distributions were similar in the allergic and the nonallergic groups (Table 1).
TABLE 1.
Characteristics of study participants
| Characteristica | Value for group with TLR4 phenotype:
|
|
|---|---|---|
| Asp299 (AA) | Asp299Gly (AG) | |
| No. of participants with: | ||
| Asthma only | 2 | 0 |
| Asthma and ARC | 7 | 2 |
| ARC only | 3 | 0 |
| Positive Phadiatop results | 18 | 5 |
| Mean age (yr) | 19 | 22 |
| No. of participants | ||
| Female | 17 | 5 |
| Total | 34 | 8 |
ARC, allergic rhinoconjunctivitis.
TLR4 gene polymorphism detection.
DNA was isolated from whole blood by using the Qiagen Blood MiniKit (VWR, Stockholm, Sweden) according to the manufacturer's instructions. Genotyping of the TLR4(Asp299Gly) polymorphism was performed by amplification of genomic DNA by PCR using the forward primer 5′ TAGAGGGCCTGTGCAATTTGA 3′ and the reverse primer 5′ CTAATTCTAAATGTTGCCATCC 3′, resulting in a 172-bp fragment. The PCR was performed with approximately 50 ng of template DNA, 200 μM deoxynucleoside triphosphate, 2 mmol/liter MgCl2, 1 μmol/liter of each primer, and a final concentration of 20 mmol/liter (NH4)2SO4, 0.01% Tween 20, 75 mmol/liter Tris HCl (pH 9.0), and 0.5 U Taq DNA polymerase (Gibco BRL, Life Technologies, Scotland, United Kingdom). The cycling conditions were 94°C for 2 min and 35 cycles at 94°C for 30 s, annealing at 61°C for 30 s, and extension at 72°C for 30 s in a thermal cycler (PTC-100; MJ Research, Watertown, MA). DNA sequencing was performed using a MegaBace1000 DNA sequencer (Amersham Biosciences, California) and a DYEnamic ET terminator kit (Amersham).
Analyses of monocyte-related cell surface markers.
Heparinized whole blood was stained according to the manufacturer's instructions. Briefly, antibodies were added to tubes with 50 μl blood. After 15 min of incubation at room temperature, 50 μl Optilyse B (Beckman Coulter, Bromma, Sweden) was added to each tube and the tubes were vortexed vigorously. After 10 min, 0.5 ml H2O was added. The following fluorochrome-conjugated monoclonal antibodies were used: the phycoerythrin-conjugated mouse anti-human monoclonal antibodies anti-CD80 (Becton Dickinson [BD] Biosciences, San Jose, CAA), anti-CD58 (BD Biosciences), anti-CCR2 (R&D systems, Minneapolis, MN), anti-TLR4 (Insight Biotechnology, Wembley, United Kingdom), and anti-HLA-DR (BD Pharmingen); the fluorescein isothiocyanate-conjugated mouse anti-human monoclonal antibodies anti-CCR5 (BD Pharmingen), anti-CXCR4 (R&D systems), anti-TLR2 (Insight Biotechnology), and anti-CD86 (Caltag, Burlingame, CA); and the peridin chlorophyll protein-conjugated mouse anti-human monoclonal antibody anti-CD14 (BD Biosciences). The isotype control antibodies used were fluorescein isothiocyanate- and phycoerythrin-conjugated γ1 and γ2a (BD Biosciences) and peridin chlorophyll protein-conjugated γ1 (BD Biosciences). The samples were analyzed on a four-color FACSCalibur (BD, San Jose, CA), and the acquired data were analyzed with CellQuest software (BD, San Jose, CA). The monocyte population was gated according to forward and side scatter, and 5,000 CD14+ cells were counted. The limit for positivity was set with isotype controls, and only CD14+ cells were included in the analyses, except for the analyses of CD14 expression, in which the cells were gated only according to size and granularity.
Cell culture.
PBMC were isolated on a Ficoll Paque density gradient (Pharmacia Biotech, Sollentuna, Sweden) and washed three times in RPMI 1640 (Gibco, Invitrogen, Paisley, Scotland, United Kingdom) supplemented with 5% fetal calf serum (Invitrogen, Carlsbad, CA). The cells were then cryopreserved according to standard methodology in 10% dimethyl sulfoxide (Sigma-Aldrich, Stockholm, Sweden), 50% fetal calf serum, and 40% RPMI 1640 (Life Technologies AB, Täby, Sweden). After thawing, the cells were washed and diluted to 1 × 106 cells/ml and suspended in AIM-V serum-free medium (Life Technologies AB) with 20 μM mercaptoethanol (Sigma-Aldrich).
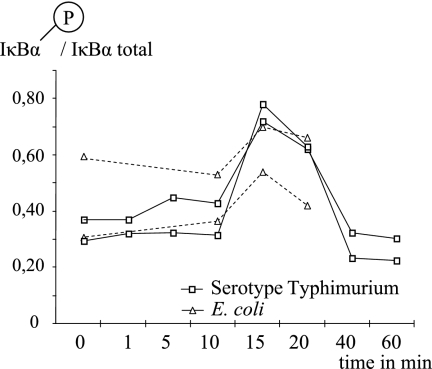
For analyses of phosphorylation of intracellular signaling proteins, 5 × 106 PBMC were incubated with 100 ng/ml of either LPS from Salmonella enterica serotype Typhimurium (referred to herein as serotype Typhimurium) or Escherichia coli serotype O26:B6 (referred to herein as E. coli; Sigma-Aldrich) for 15 min at 37°C with 5% CO2 (Forma CO2 incubator model 3862; Forma Scientific Inc., Marietta, OH). The cell culture conditions were optimized based on the highest degree of IκBα phosphorylation, and the tested time points were 0, 1, 5, 10, 15, 20, 40, and 60 min for LPS from serotype Typhimurium and 10, 15, and 20 min for LPS from E. coli. The highest degree of IκBα phosphorylation was found after 15 min of activation using both sorts of LPS (Fig. 1). The LPS concentration used was chosen based on earlier evaluations of optimal cytokine secretion (9). Cell activation was stopped by the addition of ice-cold sterile phosphate-buffered saline (Medicago AB, Uppsala, Sweden), and the cells were then placed on ice. The supernatants were aspirated after 5 min of centrifugation at 300 × g at 4°C, and the cells were lysed by the addition of 1.5 ml lysis solution (Bio-Rad Laboratories, Hercules, CA). The cell lysates were rotated at 4°C for 20 min and then stored at −20°C until they were analyzed.
FIG. 1.
The time-dependent response of LPS (100 ng/ml)-induced IκBα phosphorylation in PBMC was evaluated in two individuals. Both E. coli- and serotype Typhimurium-derived LPS-induced IκBα phosphorylation rates were highest after 15 min of LPS stimulation.
Total-protein quantification of the cell lysates was performed with a Bio-Rad DC Protein Assay kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. A standard curve with seven standard points was created with bovine serum albumin (Sigma, Saint Louis, MO) diluted in phosphate-buffered saline buffer (Medicago AB, Uppsala, Sweden). The assays were performed in a Costar 3595 plate (Costar Inc., Corning, NY), and the absorbance was measured with a Versamax microplate reader (Global Medical Instrumentation Inc., Ramsey, MN). All samples were diluted in lysis buffer to the same total protein concentration, i.e., 604 ng/ml.
Total and phosphorylated IκBα, ERK-2, and p38 MAPK protein contents were analyzed in the cell lysates with a BioPlex kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Lysed tumor necrosis factor alpha-treated HeLa cells, epidermal growth factor-treated HEK293 cells, and lysed UV-treated HEK293 cells served as reference samples for phosphorylated IκBα, phosphorylated ERK2, and phosphorylated p38 MAPK, respectively. Lysed untreated HeLa cells were used as a reference sample for quantification of total protein and as a negative control for analysis of phosphorylated protein. Lysis buffer was used as a blank. The analysis was performed on a Luminex 100 (Luminex, Austin, TX), and the data were processed with the StarStation 2.0 program. The mean fluorescence intensity (MFI) for the phosphorylated protein was divided by the MFI value for the positive control for that protein. The MFI for the total protein content was calculated in a similar manner. The results are presented as the quotient of the phosphorylated protein divided by the total protein content.
For cytokine analysis, 1 × 106 PBMC were cultured in the presence of 100 ng/ml of either LPS from serotype Typhimurium or LPS from E. coli. The samples were incubated at 37°C with 5% CO2 for 48 h. Then, the samples were centrifuged at 400 × g for 20 min, and the supernatants were aspirated and stored at −70°C until they were analyzed. The levels of IL-12p70 in the cell supernatants were determined with a commercial human IL-12 Quantikine high-sensitivity enzyme-linked immunosorbent assay kit (R&D systems, Minneapolis, MN) according to the manufacturer's instructions. The levels of IL-10 in the cell supernatants were detected by means of enzyme-linked immunosorbent assay with a human IL-10 PeliPair reagent set kit (Sanquin, Amsterdam, The Netherlands) according to the manufacturer's instructions, except that nonspecific protein binding sites were blocked by using low-fat cow's milk (Arla, Stockholm, Sweden). Comparisons of LPS-induced cytokine responses were made after the control value, i.e., responses from cells cultured in medium alone, was withdrawn. The sensitivity limits for quantitative determinations were 0.63 pg/ml for IL-12p70 and 4.7 pg/ml for IL-10. Due to the limited volume of supernatant, IL-10 and IL12p70 were the only cytokines measured.
Statistical analyses.
To enable statistical analyses, samples with cytokine levels below the sensitivity limit were given half the value of the sensitivity limit. As the analyzed parameters were not normally distributed, nonparametric statistical tests, corrected for ties, were used. Unpaired groups were analyzed with the Mann-Whitney U test and paired groups with the Wilcoxon signed-rank test. A difference together with a P value below 0.05 was considered statistically significant. The statistical analyses were performed with the statistical package Statview 5.0 for PC (SAS institute Inc., Cary, NC).
Ethical considerations.
The study was approved by the Human Research Ethics Committee of the Medical Faculty at Linköping University. All participants gave their written informed consent.
RESULTS
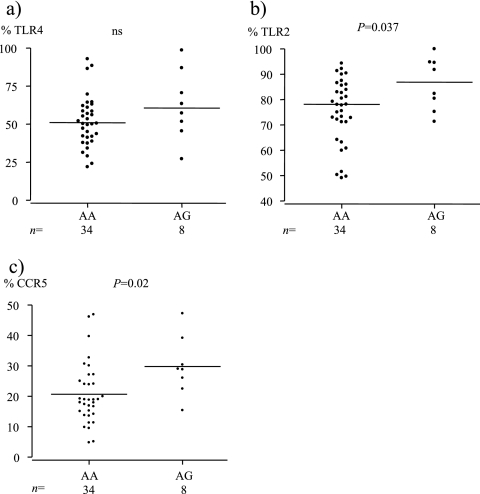
The percentages of CD14+ cells and the intensities of CD14 expression on cells in the monocyte gate, defined according to size (forward scatter) and granularity (side scatter), were similar in TLR4 (with Asp299) homozygotes (AA) and TLR4(Asp299Gly) heterozygotes (AG). Both the frequencies of CD14+ cells expressing TLR4 (Fig. 2a) and the levels of TLR4 expression were similar in the two genotype groups (MFI median, 17.5, and range, 11.6 to 26.9, versus 17.7 and 12.8 to 51.8 for AA and AG individuals, respectively). The percentage of circulating CD14+ cells expressing TLR2 was higher among heterozygote AG than AA individuals, however (Fig. 2b), and a similar result was observed for the expression of CCR5 (Fig. 2c). The levels of the surface expression of TLR2 and CCR5 were similar in the two genotype groups. The expression of all other cell surface markers analyzed (e.g., CD80, CD58, CCR2, HLA-DR, CXCR4, and CD86) were similar in the two genotype groups.
FIG. 2.
Levels of TLR4 (a), TLR2 (b), and CCR5 (c) expression on CD14-positive monocytes in whole-blood samples from individuals with (AG) and without (AA) the TLR4(Asp299Gly) polymorphism. The median value for each group is indicated by a horizontal line. ns, not significant.
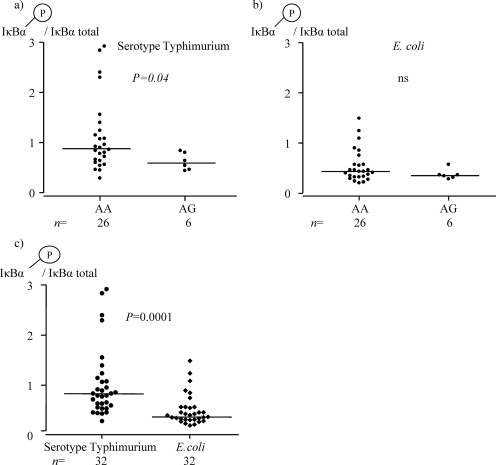
The degree of IκBα phosphorylation after serotype Typhimurium LPS stimulation was lower in individuals heterozygous for TLR4(Asp299Gly) polymorphism (AG) than in TLR4 (Asp299) (AA) individuals (Fig. 3a). Such a difference was not observed after stimulation with E. coli-derived LPS, however (Fig. 3b). Both serotype Typhimurium- and E. coli-derived LPS induced upregulated phosphorylation of IκBα, although the responses were higher using LPS from serotype Typhimurium (Fig. 3c). Under the culture conditions used, no LPS-induced phosphorylation of ERK-2 or p38 MAPK was observed (data not shown).
FIG. 3.
(a) The degree of IκBα phosphorylation in PBMC after serotype Typhimurium-derived LPS stimulation was lower in individuals with (AG) than in those without (AA) the TLR4(Asp299Gly) polymorphism. (b) The degrees of IκBα phosphorylation in PBMC after E. coli-derived LPS stimulation were similar in the two genotype groups. (c) The degree of IκBα protein phosphorylation in PBMC was higher using LPS from serotype Typhimurium than using LPS from E. coli. The graphs show results from 15 min of stimulation of PBMC and present the quotient of phosphorylated IκBα divided by the total amount of IκBα. The median value for each group is indicated by a horizontal line. ns, not significant.
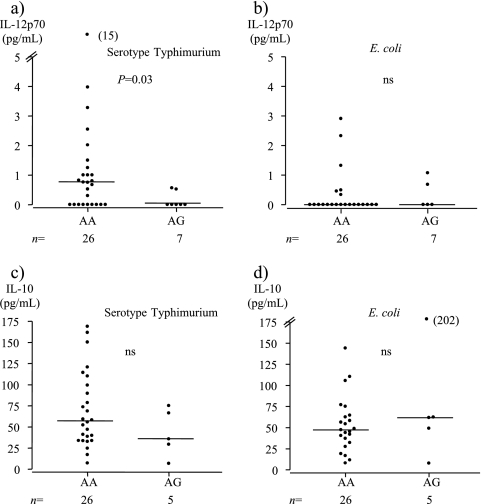
Serotype Typhimurium-derived LPS-induced IL-12p70 secretion was significantly lower in the AG genotype individuals than in AA individuals (Fig. 4a), whereas no difference between the genotypes was seen after stimulation with E. coli-derived LPS (Fig. 4b). The results persisted when only nonallergic AG and AA individuals were compared (data not shown). No clear relationship between the TLR4(Asp299Gly) polymorphism and LPS-induced IL-10 secretion was observed (Fig. 4c and d).
FIG. 4.
(a) Serotype Typhimurium-derived LPS induced lower IL-12p70 secretion from PBMC in individuals with (AG) than in those without (AA) the TLR4(Asp299Gly) polymorphism. (c) No difference in IL-10 secretion was observed between the genotype groups. (b and d) E. coli-derived LPS-induced IL-12p70 (b) and IL-10 (d) cytokine secretion from PBMC did not differ between the two genotype groups. The median value for each group is indicated by a horizontal line. ns, not significant.
Upregulated IL-12p70 secretion was more commonly observed after stimulation with serotype Typhimurium-derived LPS (19/32; 59%) than after stimulation with LPS from E. coli (8/27; 30%), and the secretion level was also higher (4a and b). All individuals responded with upregulated IL-10 secretion to LPSs from both bacterial strains, and the levels were similar (4c and d).
Serotype Typhimurium LPS-induced IκBα phosphorylation correlated strongly with serotype Typhimurium LPS-induced IL12p70 secretion (r = 0.67; P = 0.0006), and a similar correlation was seen for LPS induced IL-10 secretion (r = 0.34; P = 0.08).
Serotype Typhimurium-derived LPS-induced IκBα phosphorylation was lower in allergic individuals than in nonallergic individuals with a negative Phadiatop result. Since our results showed that the AG genotype affects cytokine secretion, we compared the AA individuals separately and the result persisted (median, 0.6, and range, 0.3 to 1.1, versus 1.1 and 0.5 to 2.9 in allergic and nonallergic individuals, respectively; P = 0.028). The number of individuals with AG was too low to compare allergic and nonallergic individuals separately. The expression levels of the analyzed monocyte surface markers and LPS-induced cytokine responses were similar in allergic (asthma and/or allergic rhinoconjunctivitis) and nonallergic individuals (data not shown).
DISCUSSION
Here, we confirmed our previous findings (9) that heterozygotes for the TLR4(Asp299Gly) gene polymorphism have lower LPS-induced IL-12 secretion. This reduced sensibility to LPS in TLR4(Asp299Gly) AG individuals seems to be attributed to impaired intracellular activation of the NF-κB pathway rather than divergent TLR4 expression on the cell surface.
Stimulation through TLRs leads to the activation of a cytoplasmic Toll-IL-1 receptor domain; to subsequent activation of several signaling proteins, including MyD88; and further to the activation of transcription factors, like NF-κB (reviewed in reference 22). Upon activation of the NF-κB complex, the inhibitory protein IκBα is rapidly phosphorylated and degraded. The degradation of IκBα enables nuclear translocation of the NF-κB dimer and initiation of, e.g., cytokine production (reviewed in reference 27). Analysis of phosphorylated IκBα protein is consequently an indirect measurement of NF-κB translocation and activation (23).
Monocytic cell lines transfected with the TLR4(Asp299Gly) allele show impaired NF-κB activity after LPS stimulation (1). Further, reduced LPS-induced phosphorylation of IκBα in PBMC of individuals heterozygous for TLR4(Asp299Gly) has recently been reported (24). The resulting amino acid change (aspartic acid to glycine) in TLR4(Asp299Gly) heterozygotes is believed to alter the extracellular domain of the TLR4 receptor (20). The consequences may be that the final receptor does not recognize the CD14-LPS complex as efficiently as the receptor coded by the predominant AA gene haplotype. Interestingly the degree of IκBα phosphorylation was higher after cell activation with LPS from serotype Typhimurium than with that from E. coli, and impaired IκBα phosphorylation among TLR4(Asp299Gly) heterozygotes was observed only after stimulation with serotype Typhimurium LPS. Differences in the structure of lipid A, such as the number and position of acyl chains, have been suggested to affect the binding and signaling properties of LPS; the lipid A portion of Salmonella consists of seven acyl groups, whereas E. coli has six (21). Alternatively, low responsiveness to E. coli LPS may be due to the continuous exposure to E. coli in our environment in contrast to Salmonella. Besides lower IκBα phosphorylation, E. coli LPS also induced lower cytokine secretion than serotype Typhimurium LPS. This may be one explanation for the controversial results regarding the effect of the TLR4(Asp299Gly) gene polymorphism on LPS sensibility (1, 5, 8, 10, 17, 25, 26). LPSs from E. coli strains are preferably used in studies investigating immune responses induced via TLR4 (8, 10, 17, 25). The reduced cytokine responses among TLR4(Asp299Gly) heterozygotes were observed only when the cells were activated with serotype Typhimurium-derived LPS. The serotype Typhimurium-derived LPS-induced IκBα phosphorylation correlated with serotype Typhimurium-derived LPS-induced IL-12 and IL-10 secretion, emphasizing that an impaired NF-κB pathway is the explanatory mechanism behind the reduced cytokine response in individuals with the TLR4(Asp299Gly) polymorphism.
The expression of TLR4 on circulating monocytes analyzed in whole blood was not associated with the TLR4(Asp299Gly) gene polymorphism. In contrast, lower TLR4 expression on cryopreserved PBMC (24) and on human airway epithelia (1) in Asp299Gly individuals has been reported. The discrepancy might be due to the different cell types studied and the handling of the cells. However, individuals heterozygous for the TLR4(Asp299Gly) gene polymorphism had higher percentages of TLR2- and CCR5-expressing monocytes in our study. This may be due to immunological cross talk between different TLRs. Mu et al. showed that pretreating mouse macrophages with anti-TLR4 antibodies resulted in the upregulation of TLR2 and increased cytokine secretion after stimulation with superantigen (18). Intact TLR4 signaling was important for downregulation of TLR2 expression. In endotoxin tolerance, rechallenge of cells with LPS results in impaired responsiveness, but other pattern recognition molecules may become activated instead.
Airway allergy was associated with decreased serotype Typhimurium LPS-induced IκBα phosphorylation, leading to lower cytokine production. This association was not observed using E. coli-derived LPS, however. We have previously reported low LPS-induced IL-10 and IL-12 secretion in individuals with airway allergy using LPSs from both serotype Typhimurium (9) and E. coli (7). Notably, the LPS concentration used in the latter study was 10,000 times higher than the concentration in the present study. Also, in another study claiming lower LPS-induced IL-10 secretion in individuals with asthma, higher LPS concentrations were used (4). The LPS source is not stated in this study but is presumably E. coli. This suggests that the endotoxin levels and bacterial origin of LPS should be considered when environmental endotoxin exposure is evaluated in disease risk or protection.
We could not detect the previously reported LPS activation of ERK-2 and p38 MAPK signaling pathways (6, 15), possibly due to the time and dose of LPS stimulation chosen, which were optimized for detection of IκBα phosphorylation.
In conclusion, we suggest that there is a failure of LPS-induced TLR4 signal transduction in individuals with the TLR4(Asp299Gly) polymorphism or airway allergy, resulting in impaired cytokine transcription and secretion. The bacterial origin of the LPS seems to be important for the magnitude of induced immune responses in circulating human cells. Most interestingly, the association of TLR4(Asp299Gly) polymorphism with LPS response in vitro is dependent on the origin of the LPS. Accordingly, not only TLR4 polymorphism, but also the bacterial origin of LPS, should be considered when the endotoxin exposure is an environmental modulator of disease risk or protection.
Acknowledgments
We thank the participants and also the research nurses Lena Lindell and Kicki Helander for excellent technical assistance.
The Swedish Asthma and Allergy Association's Research Fund and The County Council of Östergötland are acknowledged for financial support.
Footnotes
Published ahead of print on 15 October 2008.
REFERENCES
- 1.Arbour, N. C., E. Lorenz, B. C. Schutte, J. Zabner, J. N. Kline, M. Jones, K. Frees, J. L. Watt, and D. A. Schwartz. 2000. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25:187-191. [DOI] [PubMed] [Google Scholar]
- 2.Asher, M. I., S. Montefort, B. Björkstén, C. K. Lai, D. P. Strachan, S. K. Weiland, and H. Williams. 2006. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368:733-743. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield, S. F., R. Stanwell-Smith, R. W. Crevel, and J. Pickup. 2006. Too clean, or not too clean: the hygiene hypothesis and home hygiene. Clin. Exp. Allergy 36:402-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borish, L., A. Aarons, J. Rumbyrt, P. Cvietusa, J. Negri, and S. Wenzel. 1996. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 97:1288-1296. [DOI] [PubMed] [Google Scholar]
- 5.Calvano, J. E., D. J. Bowers, S. M. Coyle, M. Macor, M. T. Reddell, A. Kumar, S. E. Calvano, and S. F. Lowry. 2006. Response to systemic endotoxemia among humans bearing polymorphisms of the Toll-like receptor 4 (hTLR4). Clin. Immunol. 121:186-190. [DOI] [PubMed] [Google Scholar]
- 6.Carter, A. B., M. M. Monick, and G. W. Hunninghake. 1999. Both Erk and p38 kinases are necessary for cytokine gene transcription. Am. J. Respir. Cell Mol. Biol. 20:751-758. [DOI] [PubMed] [Google Scholar]
- 7.Casas, R., S. Skarsvik, A. Lindström, O. Zetterström, and K. Duchén. 2007. Impaired maturation of monocyte-derived dendritic cells from birch allergic individuals in association with birch-specific immune responses. Scand. J. Immunol. 66:591-598. [DOI] [PubMed] [Google Scholar]
- 8.Erridge, C., J. Stewart, and I. R. Poxton. 2003. Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the Toll-like receptor 4 gene show no deficit in lipopolysaccharide signalling. J. Exp. Med. 197:1787-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagerås Böttcher, M., M. Hmani-Aifa, A. Lindström, M. C. Jenmalm, X. M. Mai, L. Nilsson, H. A. Zdolsek, B. Björkstén, P. Söderkvist, and O. Vaarala. 2004. A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12(p70) responses in Swedish children. J. Allergy Clin. Immunol. 114:561-567. [DOI] [PubMed] [Google Scholar]
- 10.Heesen, M., B. Bloemeke, and D. Kunz. 2003. The cytokine synthesis by heterozygous carriers of the Toll-like receptor 4 Asp299Gly polymorphism does not differ from that of wild type homozygotes. Eur. Cytokine Netw. 14:234-237. [PubMed] [Google Scholar]
- 11.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 12.Imler, J. L., and J. A. Hoffmann. 2001. Toll receptors in innate immunity. Trends Cell Biol. 11:304-311. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan, J., K. Selvarajoo, M. Tsuchiya, G. Lee, and S. Choi. 2007. Toll-like receptor signal transduction. Exp. Mol. Med. 39:421-438. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus, R., D. Vercelli, L. J. Palmer, W. J. Klimecki, E. K. Silverman, B. Richter, A. Riva, M. Ramoni, F. D. Martinez, S. T. Weiss, and D. J. Kwiatkowski. 2002. Single nucleotide polymorphisms in innate immunity genes: abundant variation and potential role in complex human disease. Immunol. Rev. 190:9-25. [DOI] [PubMed] [Google Scholar]
- 15.Lee, J. C., and P. R. Young. 1996. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J. Leukoc. Biol. 59:152-157. [DOI] [PubMed] [Google Scholar]
- 16.LeVan, T. D., S. Von Essen, D. J. Romberger, G. P. Lambert, F. D. Martinez, M. M. Vasquez, and J. A. Merchant. 2005. Polymorphisms in the CD14 gene associated with pulmonary function in farmers. Am. J. Respir. Crit. Care Med. 171:773-779. [DOI] [PubMed] [Google Scholar]
- 17.Michel, O., T. D. LeVan, D. Stern, M. Dentener, J. Thorn, D. Gnat, M. L. Beijer, P. Cochaux, P. G. Holt, F. D. Martinez, and R. Rylander. 2003. Systemic responsiveness to lipopolysaccharide and polymorphisms in the toll-like receptor 4 gene in human beings. J. Allergy Clin. Immunol. 112:923-929. [DOI] [PubMed] [Google Scholar]
- 18.Mu, H. H., N. D. Pennock, J. Humphreys, C. J. Kirschning, and B. C. Cole. 2005. Engagement of Toll-like receptors by mycoplasmal superantigen: downregulation of TLR2 by MAM/TLR4 interaction. Cell Microbiol. 7:789-797. [DOI] [PubMed] [Google Scholar]
- 19.Raby, B. A., W. T. Klimecki, C. Laprise, Y. Renaud, J. Faith, M. Lemire, C. Greenwood, K. M. Weiland, C. Lange, L. J. Palmer, R. Lazarus, D. Vercelli, D. J. Kwiatkowski, E. K. Silverman, F. D. Martinez, T. J. Hudson, and S. T. Weiss. 2002. Polymorphisms in toll-like receptor 4 are not associated with asthma or atopy-related phenotypes. Am. J. Respir. Crit. Care Med. 166:1449-1456. [DOI] [PubMed] [Google Scholar]
- 20.Rallabhandi, P., J. Bell, M. S. Boukhvalova, A. Medvedev, E. Lorenz, M. Arditi, V. G. Hemming, J. C. Blanco, D. M. Segal, and S. N. Vogel. 2006. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J. Immunol. 177:322-332. [DOI] [PubMed] [Google Scholar]
- 21.Rietschel, E. T., H. W. Wollenweber, R. Russa, H. Brade, and U. Zähringer. 1984. Concepts of the chemical structure of lipid A. Rev. Infect. Dis. 6:432-438. [DOI] [PubMed] [Google Scholar]
- 22.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 23.Traenckner, E. B., H. L. Pahl, T. Henkel, K. N. Schmidt, S. Wilk, and P. A. Baeuerle. 1995. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 14:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tulic, M. K., R. J. Hurrelbrink, C. M. Prele, I. A. Laing, J. W. Upham, P. Le Souef, P. D. Sly, and P. G. Holt. 2007. TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide. J. Immunol. 179:132-140. [DOI] [PubMed] [Google Scholar]
- 25.van der Graaf, C., B. J. Kullberg, L. Joosten, T. Verver-Jansen, L. Jacobs, J. W. Van der Meer, and M. G. Netea. 2005. Functional consequences of the Asp299Gly Toll-like receptor-4 polymorphism. Cytokine 30:264-268. [DOI] [PubMed] [Google Scholar]
- 26.von Aulock, S., N. W. Schroder, K. Gueinzius, S. Traub, S. Hoffmann, K. Graf, S. Dimmeler, T. Hartung, R. R. Schumann, and C. Hermann. 2003. Heterozygous toll-like receptor 4 polymorphism does not influence lipopolysaccharide-induced cytokine release in human whole blood. J. Infect. Dis. 188:938-943. [DOI] [PubMed] [Google Scholar]
- 27.Wang, T., X. Zhang, and J. J. Li. 2002. The role of NF-κB in the regulation of cell stress responses. Int. Immunopharmacol. 2:1509-1520. [DOI] [PubMed] [Google Scholar]
- 28.Yang, I. A., S. J. Barton, S. Rorke, J. A. Cakebread, T. P. Keith, J. B. Clough, S. T. Holgate, and J. W. Holloway. 2004. Toll-like receptor 4 polymorphism and severity of atopy in asthmatics. Genes Immun. 5:41-45. [DOI] [PubMed] [Google Scholar]