Abstract
Campylobacter jejuni is a major cause of diarrhea in humans. A mouse lung model of infection was previously established for C. jejuni. We used this model to study cytokine production in the lungs and correlated it with pathological changes. C. jejuni strain 81-176 or sterile phosphate-buffered saline was intranasally inoculated into adult BALB/c mice. The levels of proinflammatory cytokines (gamma interferon, tumor necrosis factor alpha, interleukin-1β [IL-1β], IL-2) and anti-inflammatory cytokines (IL-4, IL-10), in addition to those of IL-6, were assessed on days 1, 3, and 5 postinfection by enzyme-linked immunosorbent assay, and the ratios of proinflammatory cytokines to anti-inflammatory cytokines were calculated. Since IL-6 is unique in that it is both a proinflammatory cytokine and a TH2 cytokine, it was considered to be both in the determination of these ratios. The significance of the cytokine levels and ratios were determined by the Mann-Whitney U test (P ≤ 0.05). The induction of proinflammatory cytokines in the lungs of infected mice, as indicated by the cytokine levels and ratios, coincided with the accumulation of neutrophils and activated macrophages, in addition to the clearance of the bacterial load and bacteriumlike structures that we have previously shown in the same groups of mice. This was followed by increased levels of anti-inflammatory cytokines and the resolution of inflammation and pathology in the lungs. This study demonstrates the dynamics of cytokine production and their correlation with tissue inflammation and the resolution of infection. This model is useful for further studies of the pathogenesis of C. jejuni infection and vaccine evaluation.
Campylobacter jejuni is a major food-borne pathogen and cause of diarrhea worldwide (46, 47). It predominantly produces inflammatory diarrhea in individuals in developed countries (50) but produces watery diarrhea in individuals in developing countries (51). Although C. jejuni infections are not often associated with a systemic illness, extraintestinal manifestations are increasingly being reported, especially in the immunocompromised population (47). The organism is reported to possess a variety of putative virulence factors, including the ability to produce toxins and invade epithelial cells (24, 40, 53). Obviously, both bacterial and host factors contribute to the differences in the clinical manifestations of the disease in different populations (53).
Cytokines play an important role in the pathogenesis of the disease and in immunity to infection. The induction of proinflammatory and anti-inflammatory cytokines is important in determining whether the immune system is successful in providing protection against specific pathogenic organisms (21, 29). Cytokine responses have been measured in a variety of cell lines infected with C. jejuni, but such studies cannot represent the myriad of different cells of the whole animal (4, 5, 22, 25, 26, 33, 45). Mouse intraperitoneal and oral models have been used to study cytokine responses to C. jejuni (7, 54, 55). However, these models do not result in disease but result in colonization only (6). Recently, mice that were NF-κB−/−, MyD88−/−, IL-10−/− or that had limited enteric flora were used to study the pathogenesis of C. jejuni (11, 18, 34, 58). However, the lack of widespread availability and the high cost of these models might limit their usefulness.
The mouse intranasal model has been used to study the pathogenesis and immunity of enteric pathogens, such as Shigella flexneri (41, 52) and Vibrio cholerae (19, 20). This model has successfully been adapted for the study of C. jejuni infection by some investigators (7). Of the different strains of mice tested, the BALB/c mouse was found to be the most susceptible to C. jejuni infection (7, 49). The lungs and gut are part of a common mucosal system. It is known that oral infections induce immunity that protects other mucosal sites, such as the lungs (30). Recently, we used this mouse lung model to characterize the pathology and ultrastructural lesions in the lung after infection with C. jejuni (3). There are no data on the cytokine response to C. jejuni infection and its contribution to pathogenesis in a suitable animal model. This prompted us to determine the cytokine responses in the lungs of the same groups of mice with which pathological studies were done. We confined our investigation to the local effects of cytokines in the lungs.
MATERIALS AND METHODS
Mice.
Six-week old, female, outbred BALB/c mice from the Animal Resource Centre, Faculty of Medicine, Kuwait University, Jabriya, Kuwait, were used in the studies. The mice were housed in cages with sterile bedding made of wood shavings and were given a standard pellet diet (Special Diet Services, United Kingdom) and filtered tap water. The studies were conducted according to institutional guidelines.
Bacteria.
C. jejuni 81-176, which caused an outbreak of diarrhea in schoolchildren (31), was kindly provided by P. Guerry, Enteric Diseases Department, Naval Medical Research Center, Silver Spring, MD. It was cultured on 7% sheep blood agar (BA; Oxoid, Basingstoke, Hampshire, England), Campy-BAP agar (Oxoid), or charcoal agar (Oxoid) by incubation of the plates at 42°C for 48 h in a microaerobic atmosphere generated with a Campy GasPak system (Oxoid) in a jar with activated palladium catalyst.
Unless mentioned otherwise, C. jejuni was grown in a microaerobic atmosphere at 42°C.
In vivo passage of C. jejuni 81-176 in mice.
It has been found that C. jejuni 81-176 does not cause natural infection in laboratory mice and therefore first needs to be adapted to establish a consistent infection in mice (7, 16). We did not succeed in adapting the strain by the intravenous route, as carried out by Baqar et al. (7), since we failed to recover the organism from the blood after inoculation (3). Therefore, the strain was passaged four times through the ileal loop and then three times through the lungs of adult BALB/c mice as follows. C. jejuni was cultured on BA, suspended in phosphate-buffered saline (PBS; pH 7.2) to ∼1010 CFU/ml, and injected into the ileal loops of fasted adult BALB/c mice. One day later, the material that accumulated within the loops was injected into other mice, and this was repeated until the strain underwent four passages (12). Then, C. jejuni was recovered on Campy-BAP agar and subcultured on BA. This organism was then passaged through the intranasal route (7). The lungs of 6- to 8-week-old BALB/c mice were collected 24 h postinfection, homogenized in PBS, and cultured on BA. The resulting growth was then used to inoculate other mice, and this was repeated until the strain underwent three passages. The lung homogenates of the last set of mice were plated on BA and charcoal agar to detect C. jejuni. Colonies identified as C. jejuni were stocked in brucella broth (BBL, Baltimore, MD) with 15% glycerol (Sigma, St. Louis, MO) at −70°C.
Intranasal inoculation of mice.
The mouse-adapted C. jejuni 81-176 isolate was cultured on BA for 24 h. The growth was suspended in 1 ml of brain heart infusion broth (Oxoid) supplemented with 1% yeast extract (Becton Dickinson, Sparks, MD). The suspension was used to inoculate a biphasic medium with a brain heart infusion agar slant and brain heart infusion broth supplemented with 1% yeast extract for 24 h. The bacteria were pelleted by centrifugation at 2,147 × g at 4°C for 30 min in a benchtop centrifuge with a 1.19 rotor (GS-6R; Beckman, Fullerton, CA) and resuspended in PBS (pH 7.2) to yield ∼4 × 109 CFU in a 30-μl volume.
Six- to 8-week-old BALB/c mice were anesthetized via an intraperitoneal injection of xylazine and ketamine (19). The test mice were intranasally inoculated with 30 μl of the C. jejuni culture, and control mice were intranasally inoculated with an equal volume of sterile PBS (pH 7.2).
Organ harvesting and processing.
Our previous studies have shown that the bacterial inoculum from the lungs is detectable on day 1 but not at subsequent time points. Intracytoplasmic bacteriumlike structures were observed in the lung tissues of infected mice on days 3 and 5 but not at subsequent time points. The histopathological lesions in the lungs were maximal on days 3 and 5 postinfection (3). Therefore, we decided to study the cytokine levels in the lungs on days 1, 3, and 5 postinfection. The cytokine levels in four test mice and four control mice were estimated on each of these days. While the mouse was under anesthesia, the mouse skin was decontaminated with a 70% alcohol wipe in a laminar-flow hood. The mouse was then killed by neck dislocation, and the lungs were collected in preweighed, sterile vials (Eppendorf AG, Hamburg, Germany) and placed on ice. The weights of the lungs were recorded on a sensitive, electronic top-loading scale (PJ-400; Mettler, Switzerland).
The lungs were cut into pieces and then homogenized in 1 ml PBS (pH 7.2) in a pestle and mortar. Triton X-100 solution (Sigma) was added to the homogenates to make a 1% solution. The homogenates were kept on ice for 30 min and then centrifuged at 19,319 × g at 4°C for 10 min in a Beckman J2-MI centrifuge with a JA 20.1 rotor (Beckman). The supernatant was stored at −80°C until it was assayed for cytokines.
Cytokine ELISA.
The cytokines in the lungs were assayed by enzyme-linked immunosorbent assay (ELISA). These included proinflammatory cytokines (tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], interleukin-1β [IL-1β], IL-2) and anti-inflammatory cytokines (IL-4, IL-10), in addition to IL-6. Antibody-coated ELISA kits for mouse IFN-γ, TNF-α, IL-4, IL-10, and IL-1β were obtained from Endogen (Pierce Biotechnology, Rockford, IL), and ELISA kits for mouse IL-2 and IL-6 were obtained from Biosource International (Invitrogen, Camarillo, CA). The cytokine levels in duplicate samples were assayed according to the instructions of the manufacturers. Absorbance values were read at a λ value of 450 nm with an ELISA reader (Labsystems Multiscan MS, Finland). The intensity of color in the wells was proportional to the amount of cytokine present in the sample. Standard curves were plotted for each cytokine by using reference values, and the cytokine level in the sample was obtained by extrapolation of the optical density of the sample to the standard curve. The sensitivities of the assays were as follows: 3 pg/ml for IL-6 and IL-1β, 5 pg/ml for IL-4, 8 pg/ml for IL-2, 9 pg/ml for TNF-α, and 12 pg/ml for IL-10. The cytokine levels were expressed in pg/100 mg of lung tissue as follows: (pg/ml × volume of homogenate [in ml])/(weight [in grams] × 10). The following ratios of proinflammatory cytokines to anti-inflammatory cytokines were calculated for each sample: TNF-α/IL-4, TNF-α/IL-10, TNF-α/IL-6, IFN-γ/IL-4, IFN-γ/IL-10, IFN-γ/IL-6, IL-2/IL-4, IL-2/IL-10, IL-2/IL-6, IL-6/IL-4, IL-6/IL-10, IL-1β/IL-4, IL-1β/IL-6, and IL-1β/IL-10. It should be noted that IL-6 is an atypical cytokine with dual functions. It is a proinflammatory cytokine that is important in the TH2 differentiation process (13, 14, 28). In most cases, it is not regarded as an anti-inflammatory cytokine, even though its anti-inflammatory contributions have been suggested by some researchers (28, 32, 59). Therefore, we decided to explore its role in C. jejuni infection by considering its dual role in these ratios and comparing the trends of the ratios with the trends presented by the ratios of the other cytokines.
The studies were conducted with a total of 24 mice, half of which were test animals and the other half of which were controls. Four test mice and an equal number of control mice were each killed on the required days postinfection (days 1, 3, and 5) for the measurement of cytokine levels. These were the same animals whose histopathologies were determined previously (3).
Statistical analysis.
Since the data were not normally distributed, they were expressed as the median and the range for each group of mice. The Mann-Whitney U test was used for nonparametric comparison of the median cytokine values by the use of SPSS software. Differences were considered significant if the P value was ≤0.05.
RESULTS AND DISCUSSION
Mouse lung model of infection.
Recovery of the test organism from the lungs following intranasal inoculation suggests that the bacteria reached the lungs. A previous study of Baqar et al. (7) has demonstrated colonization of the lungs for up to 7 days postinfection with C. jejuni strain 81-176 and a mouse mortality rate of up to 72%, depending on the inoculum size. However, in our study, the organism was recoverable from the lungs only on the first day postinfection and there was no mortality associated with the infection. Differences in the passage history of the strain (passage in the ileal loop followed by intranasal passage in our study versus passage by the intravenous route in the study of Baqar et al. [7]) might have influenced the outcome of the resultant infection. Multiple in vitro passages of C. jejuni are known to affect its colonizing ability (17). When we received the strain, we did not know its passage history. Other workers have also noted the rapid clearance from the lungs of other pathogens, such as a Shigella sp. (52). Campylobacteriosis in humans is not a lethal infection per se (48), and a significant proportion of human cases of campylobacteriosis result in mild manifestations or remain asymptomatic (47). Thus, severe illness scores are not a prerequisite for the model of C. jejuni infection (3).
Even though C. jejuni could not be cultured from the lungs after the first day of infection in our study, intracytopasmic bacteriumlike structures were observed in the lung tissues of infected mice on day 3 and day 5 but not at subsequent time points. The histopathological lesions were maximal on days 3 and 5 postinfection. The lesions were characterized by the initial predominance of polymorphonuclear leukocytes, followed by the accumulation of macrophages and, later, the predominance of epithelioid cells. Focal peribronchial pneumonia appeared on day 3 postinfection, a granulomalike reaction appeared on day 4 postinfection, and bronchopneumonia appeared on day 5 postinfection. The lungs of the control mice showed focal nonexpansion (3).
C. jejuni induces proinflammatory cytokines in the lungs of infected mice.
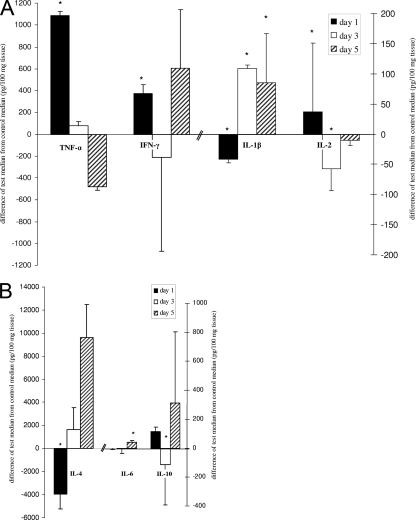
The levels of TNF-α and IFN-γ were significantly higher in the test mice than in the control mice on day 1. However, the IFN-γ levels tended to be lower in the test mice than in the control mice on day 3, and the TNF-α levels in the test mice tended to be lower than those in the control mice on day 5 (Fig. 1A). Since bacteria were not detectable in the lungs after day 1 but the pathology was maximal on days 3 to 5 postinfection, this would indicate that the pathology was due to inflammation caused by proinflammatory cytokines. The pathology and the proinflammatory cytokine production in the lungs of control mice were probably related to the aspiration of the normal upper respiratory flora (from above the larynx) and were not related to infection of the mouse colony (3). This has been observed in other studies, too (19, 52). In support of this, we observed gram-positive cocci and bacilli representing the normal upper respiratory flora on culture of mouse lung tissue. The pathology and cytokine production in the test mice were therefore due to the combined effects of both the test organism and the aspirated normal flora. Therefore, the inclusion of mice to test for the effect of the aspiration flora alone served as an appropriate control. TNF-α is mainly produced by activated mononuclear phagocytes and antigen-stimulated T cells. IFN-γ is a major macrophage-activating cytokine that is produced by activated TH1 cells and cytotoxic cells as well as by NK cells (49). It is known to induce the expression of many adhesion proteins on the vascular endothelium to potentiate the function of TNF-α and to promote T-lymphocyte infiltration at the sites of infection. Thus, the induction of high levels of TNF-α and IFN-γ indicated the activation of macrophages and TH1 cells and is related to polymorphonuclear cell accumulation in the lungs of infected mice. This was followed by the infiltration of activated macrophages (3). Similarly, TNF-α and IFN-γ were found to be the major components of the immune response to intranasal infection with V. cholerae and S. flexneri (19, 20, 52).
FIG. 1.
Differences in median cytokine levels in the test mice and the control mice (in pg/100 mg tissue), where the median control levels are zeros on the y axis. (A) TNF-α, IFN-γ, IL-1β, and IL-2; (B) IL-4, IL-6, and IL-10. Each bar represents the values for four test mice compared to the values for four control mice at that time point. Black bars, white bars, and bars with slanted black lines, data for day 1, day 3, and day 5 postinfection, respectively (*, P < 0.05, Mann-Whitney U test). Error bars represent ranges (maximum or minimum test value minus median control value).
Other proinflammatory cytokines were induced at later time points. In the lungs of the infected mice, the levels of IL-1β were significantly higher than those in the control mice on days 3 and 5 and the levels of IL-6 were significantly higher than those in the controls on day 5 (Fig. 1A and B). C. jejuni was shown to induce the production of IL-1β in monocyte cell lines (26, 45) and dendritic cell lines (25). IL-1β is produced by a variety of cells (macrophages, neutrophils, epithelial and endothelial cells) and, together with TNF-α, acts as a mediator of local inflammation (21, 43, 49), whereby B and T cells are activated and the cytokine IL-6 is induced (21, 49). IL-1β- and TNF-α-mediated neutrophil recruitment in the lungs has been demonstrated previously (49, 57).
It is difficult to determine the source of IL-1β in the lungs. The infiltration of lymphocytes and epithelioid cells (activated macrophages) and the development of granulomatous lesions seemed to coincide with the enhanced levels of proinflammatory cytokines, such as IL-1β. This chronic inflammatory response correlated well with the persistence of the inflammatory stimulus caused by the presence of bacteria (prior to day 3) and bacteriumlike structures (beyond day 3) (3).
IL-6 is an atypical cytokine with dual functions (13, 14, 28, 32). The importance of IL-6 as a proinflammatory cytokine is suggested by the concomitant increase in the levels of IL-1β. IL-1β and TNF-α are known to induce the production of IL-6 (1, 21). Thus, it is not unlikely that the production of IL-6 is a mechanism that perpetuates the inflammatory response to C. jejuni infection. However, the level of IL-6 increased on day 5, similar to the levels of other TH2 cytokines (IL-4 and IL-10). This may indicate its dual role.
Previous studies have indicated that an enterotoxigenic strain of C. jejuni could stimulate the production of IL-2, while it lacked the ability to induce the production of macrophage-derived cytokines (38, 39). C. jejuni 81-176 could induce significantly higher levels of IL-2 in the lungs of infected mice (on day 1) (Fig. 1A). IL-2 is an immunomodulatory cytokine produced by TH1 cells, and its increased levels indicate enhanced T-cell and B-cell activation. Its proinflammatory association is suggested by the concomitant increase in the levels of other proinflammatory macrophage-derived cytokines, TNF-α and IL-1β, at the same and later time points, respectively. IL-2 also plays a role in controlling C. jejuni infection upon rechallenge (8).
The induction of proinflammatory cytokines in the lungs of infected mice is related to the resulting lung pathology and bacterial clearance.
C. jejuni is known to elicit proinflammatory cytokines, as determined in a number of in vitro studies (4, 5, 23, 25, 26, 45), as well as in intraperitoneal models (2, 38). The induction of proinflammatory cytokines was associated with the active invasion of bacteria in tissue culture models (4, 27) and was related to the acute phase of Campylobacter gastroenteritis and the associated inflammatory pathology in the intestine in monkeys (44). Proinflammatory cytokines were also associated with the acute phase of shigellosis in humans (43) and the acute phase of inflammatory diarrhea in humans due to a group of invasive pathogens, including Campylobacter, although specific data for individual pathogens were not given (15). Similarly, we observed a correlation between the increased levels of TNF-α and IFN-γ and neutrophil accumulation in the lungs of the test mice during the early phase of infection. Phagocytic activity might have been occurring on day 1 without the significant production of IL-1β, as demonstrated by the intraperitoneal inoculation of mice with C. jejuni (38).
At later time points, there were increased levels of IL-1β and IL-6 which coincided with the infiltration of activated macrophages and lymphocytes, as well as the clearance of the bacterial load and the appearance of bacteriumlike structures in the cytoplasm of infected cells. The role of IL-6 in minimizing intestinal colonization has been demonstrated previously (8), as it stimulates the production of IL-4 by naïve CD4+ T cells, their differentiation into effector TH2 cells, and, hence, the activation of humoral immunity, including the production of secretory immunoglobulin A in the intestinal fluid (8, 14).
It is likely that the intracellular bacterial structures (observed on days 3 and 5) might have provided the stimulus for the production of proinflammatory cytokines. The roles of lipopolysaccharides and enterotoxins in the induction of proinflammatory cytokines have been demonstrated previously (4, 9, 38). These factors might also lead to neutrophil infiltration in the lungs (49) through the stimulation of IL-1β and TNF-α (21). Thus, our results indicate that C. jejuni induces proinflammatory cytokine production and pathology in the lungs, similar to the manner in which it has been reported to do so in the intestine, and suggests the usefulness of the model for studying the roles of different virulence factors in eliciting inflammatory cytokine responses.
The induction of proinflammatory cytokines was followed by enhanced levels of anti-inflammatory cytokine levels in the lungs of infected mice.
The initial induction of proinflammatory cytokines was followed by increased levels of anti-inflammatory cytokines IL-4 and IL-10 on days 3 and 5, respectively (Fig. 1B). In vitro studies previously suggested the ability of C. jejuni to induce IL-4 and IL-10 from different cell types and suggested the roles of C. jejuni-induced IL-4 and IL-10 in controlling the infection (4, 25). These anti-inflammatory cytokines are known to play an important role in the downregulation of inflammatory reactions and host survival during gastrointestinal infection (21). Even though the induction of IL-4 and IL-10 did not dampen the levels of IL-1β, it seemed to reduce the level of inflammation by reducing the levels of TNF-α and IFN-γ and the accumulation of inflammatory cells (neutrophils and macrophages) in the lung tissue (38) and bronchoalveolar lavage fluid (36). This was also correlated with the apparent clearance of the bacterial load in the lungs and the gradual clearance of bronchopneumonia. Thus, these cytokines seem to play a role in controlling the inflammatory response (36), controlling the infection (4), and protecting against further lung injury (36).
Increased levels of the anti-inflammatory cytokines IL-4 and IL-10, along with the inflammatory cytokine IL-1β, seemed to be important in ensuring the effective clearance of C. jejuni and bacteriumlike intracellular structures. Anti-inflammatory cytokines may serve to control the inflammatory process, while IL-1β may activate macrophages to clear the phagocytosed bacterial structures. In addition, the presence of IL-4 and IL-10 reflected the activation of TH2 cells and B cells, possibly for the production of the antibodies that are necessary for future encounters. We did not measure the levels of C. jejuni antibodies in these mice. The production of antibodies would confirm the role of anti-inflammatory cytokines in the recovery from inflammation and inhibition of further tissue damage, as suggested by other studies with C. jejuni (4) and a Shigella sp. (52).
A summary of the correlation between the histopathology of the lungs and the cytokine levels of the C. jejuni-infected mice is shown in Table 1.
TABLE 1.
Summary of histological findings in lungs of test mice and comparison of cytokine levels in the lungs of test and control mice
| Day postinfection | Histopathology
|
Median cytokine level (pg/100 mg tissue) in test mice compared with that in control micea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation | Histology | Intracellular bacteriumlike structures | TNF-α | IFN-γ | IL-1β | IL-2 | IL-4 | IL-6 | IL-10 | |
| 1 | Interstitial inflammation | Polymorphonuclear cells | No | 1,086* | 373* | −42* | 37* | −3,927* | −2 | 113 |
| 3 | Peribronchial pneumonia | Macrophages and epithelioid cells | Yes | 76 | −214 | 109* | −58* | 1,629 | −6 | −113* |
| 5 | Bronchopneumonia | Macrophages and epithelioid cells | Yes | −478 | 602 | 85* | −11 | 9,600 | 36* | 311 |
Difference in median test levels from median control levels (pg/100 mg tissue). A minus sign indicates that the median test levels were lower than the median control levels. An asterisk indicates the median test levels were significantly different from (i.e., significantly higher than or significantly lower than) the median control levels (P ≤ 0.05, Mann-Whitney U test).
The cytokine ratios reflected the general trends in the cytokine responses induced by C. jejuni after intranasal inoculation.
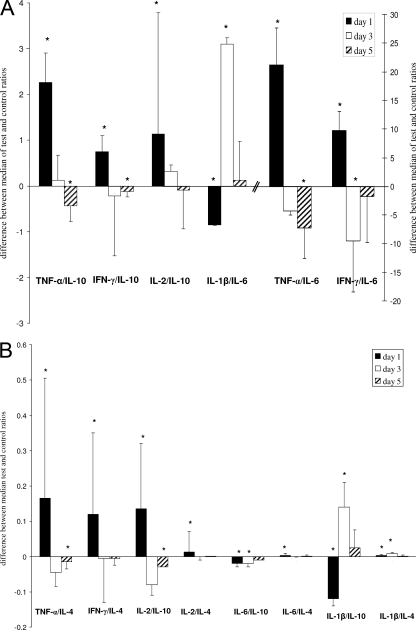
In an attempt to assess the levels of proinflammatory cytokines relative to the levels of anti-inflammatory cytokines and examine the difference between infected mice and the sham-treated controls, the ratios of proinflammatory cytokines to anti-inflammatory cytokines were calculated for each mouse. The differences between the medians of the ratios for the test group to those for the control group are shown in Fig. 2. Although all ratios showed a similar trend, some of the ratios (Fig. 2A) indicated a stronger trend than others (Fig. 2B). On day 1, most of the ratios for the test mice were higher than those for the controls; some were 2- to 3-fold higher (e.g., TNF-α/IL-10 and IFN-γ/IL-6), and some were 20-fold higher (e.g., TNF-α/IL-6). This is indicative of a strong TH1 bias on day 1.
FIG. 2.
Differences in median cytokine ratios in the test mice and the control mice, where the median control ratios are zeros on the y axis. (A) Ratios that differed by >1; (B) ratios that differed by <1. Each bar represents the values for four test mice compared to the values for four control mice at that time point. Black bars, white bars, and bars with slanted black lines, data for day 1, day 3, and day 5 postinfection, respectively (*, P < 0.05, Mann-Whitney U test). Error bars represent ranges (maximum or minimum test value minus median control value).
At later time points, the reduction in the levels of proinflammatory cytokines and the increase in the levels of anti-inflammatory cytokines in infected mice compared to those in control mice are reflected in the ratios of the proinflammatory cytokines to anti-inflammatory cytokines (Fig. 2). These ratios for the test mice became less than those for the control mice, as demonstrated by the ratios for IFN-γ/IL-6 and TNF-α/IL-10 on days 3 and 5 postinfection, respectively, which suggested a shift to an anti-inflammatory cytokine bias on days 3 and 5. However, the ratios involving IL-1β (e.g., IL-1β/IL-6 and IL-1β/IL-10) were lower for the test mice than for the control mice on day 1 but became significantly higher for the test mice than for the control mice on day 3 (Fig. 2). This also reflects the cytokine profile observed on days 3 and 5 postinfection, in which the IL-1β levels were significantly higher in the test mice than in the control mice (Fig. 1A).
These ratios also provide a mechanism whereby the contribution of IL-6 to the inflammatory response can be explored. We decided to consider it in the denominator to identify whether it indicates trends similar to those for other TH2 cytokines. Indeed, in most cases, the ratios of the proinflammatory cytokines to the anti-inflammatory cytokines (e.g., TNF-α/IL-10) gave a trend similar to that for TNF-α/IL-6, suggesting that the overall balance of IL-6 with TNF-α was similar to that for the other TH2 cytokines. The ratios for IL-6 (IL-6/IL-4 and IL-6/IL-10) were not similar to the ratios for IL-1β and TNF-α (e.g., IL-1β/IL-10 and TNF-α/IL-10). Therefore, our ratios presume that the proinflammatory cytokine IL-6 functions similarly to other TH2 cytokines in this model, even though IL-6 might not be an anti-inflammatory cytokine in most cases.
In addition to relating our observation to TH cells, we may need to extend our observation to other cells of the immune system, such as NK cells and macrophages, and refer to these responses as type 1 responses, regardless of whether these responses arose from T cells or not (56). In fact, our observation of the production of cytokines at as early as day 1 postinfection may suggest the involvement of non-T cells.
Concluding remarks.
Our results suggest that C. jejuni induces both proinflammatory and anti-inflammatory cytokines. The release of proinflammatory cytokines could well explain the observed neutrophil accumulation, followed by the activation and recruitment of macrophages into the infected lungs. This resulted in the apparent clearance of the bacterial load and the subsequent elimination of intracytoplasmic structures. In the later phases, the enhanced levels of IL-1-β and IL-6 were overlapped by the production of IL-4 and IL-10, which might have played a role in inhibiting the production of proinflammatory cytokines as well as resolving the inflammation and pathology in the lungs. This scenario has been suggested by in vitro work (5) and was similarly shown for human shigellosis (43). Our findings fit with the inflammatory nature of diarrhea in human campylobacteriosis in developed countries (10). However, a comparison with human infections cannot be easily made due to the absence of data regarding the cytokine responses in humans with campylobacteriosis.
Many aspects of pathogenesis and immunity to C. jejuni are poorly understood, due to the lack of a suitable animal model of human Campylobacter infection (37, 42, 49). Since there are many inherent histological and immunological similarities between the lung and the intestine, the mouse lung model has been used to study the pathogenesis of and immune responses to a number of intestinal pathogens (7, 19, 52). However, one dissimilarity between the lung and intestine must be stressed: whereas the lung is a sterile organ, the intestinal tract is filled with millions of microorganisms. The presence of microorganisms in the intestine might modulate the cytokine response in that organ. This difference should be kept in mind when parallels in the histopathological and cytokine responses in the lung versus those in the intestine are drawn. The intranasal model has not been used to study the cytokine responses or the pathologies caused by C. jejuni infection. Our study has filled this deficiency. The virulence factors that elicited cytokine production in the intranasal model remain to be determined. Several studies indicated the roles of lipooligosaccharides and toxins in pathogenesis (20, 23, 25, 35). This model could be used to study the roles of cytokines in the pathogenesis of C. jejuni diarrhea and Guillain-Barré syndrome-related C. jejuni diarrhea (60) and the specific bacterial antigens that induce the TH1 and TH2 subsets of lymphocytes.
The model described here is the first whole-animal model of C. jejuni infection in which the dynamics of a variety of proinflammatory and anti-inflammatory cytokines have been investigated. It augurs well that the profiles of the two categories of cytokines parallel the histopathological changes in the lung. This lends credence to the suitability of the model and shows that proinflammatory cytokines are involved in the induction of pathology and that anti-inflammatory cytokines are involved in its resolution. This model can be used to evaluate C. jejuni vaccines and their protective effect in controlling the production of proinflammatory cytokines, as has been done with a Shigella sp. (52). We must await the availability of intestinal models with small animals, such as mice, for validation of the data obtained with the lung model in this study. Even though some mouse intestinal colonization models have recently become available (17, 34, 58), these may not be suitable for cytokine studies, as they are developed with cytokine-knockout mice.
Acknowledgments
This study was partly supported by College of Graduate Studies, Kuwait University, Khaldiyah, Kuwait.
We thank Shilpa Haridas, Fawaz Azizieh, Parvez Raut, and Rahmatullah Al-Haj for their technical assistance. We are grateful to Sunny Ojoko of the Kuwait Animal Resource Centre, Kuwait University, for care of the animals.
Footnotes
Published ahead of print on 30 September 2008.
REFERENCES
- 1.Abbas, A., and A. Lichtman. 2003. Cellular and molecular immunology, 5th ed. W. B. Saunders, Philadelphia, PA.
- 2.Abram, M., D. Vučković, B. Wraber, and M. Dorić. 2000. Plasma cytokine response in mice with bacterial infection. Mediators Inflamm. 9:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Banna, N. A., T. A. Junaid, T. C. Mathew, R. Raghupathy, and M. J. Albert. 2008. Histopathological and ultrastructural studies of a mouse lung model of Campylobacter jejuni infection. J. Med. Microbiol. 57:210-217. [DOI] [PubMed] [Google Scholar]
- 4.Al-Salloom, F. S., A. Al Mahmeed, A. Ismaeel, G. A. Botta, and M. Bakhiet. 2003. Campylobacter-stimulated INT 407 cells produce dissociated cytokine profiles. J. Infect. 47:217-224. [DOI] [PubMed] [Google Scholar]
- 5.Bakhiet, M., F. S. Al-Salloom, A. Qareiballa, K. Bindayna, I. Farid, and G. A. Botta. 2004. Induction of α and β chemokines by intestinal epithelial cells stimulated with Campylobacter jejuni. J. Infect. 48:236-244. [DOI] [PubMed] [Google Scholar]
- 6.Baqar, S., L. A. Applebee, and A. L. Bourgeois. 1995. Immunogenicity and protective efficacy of a prototype Campylobacter whole-cell vaccine in mice. Infect. Immun. 63:3731-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baqar, S., A. L. Bourgeois, L. A. Applebee, A. S. Mourad, M. T. Kleinosky, Z. Mohran, and J. R. Murphy. 1996. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect. Immun. 64:4933-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baqar, S., N. D. Pacheco, and F. M. Rollwagen. 1993. Modulation of mucosal immunity against Campylobacter jejuni by orally administered cytokines. Antimicrob. Agents Chemother. 37:2688-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkholz, S., U. Knipp, C. Nietzki, R. J. Adamek, and W. Opferkuch. 1993. Immunological activity of lipopolysaccharide of Helicobacter pylori on human peripheral mononuclear blood cells in comparison to lipopolysaccharides of other intestinal bacteria. FEMS Immunol. Med. Microbiol. 6:317-324. [DOI] [PubMed] [Google Scholar]
- 10.Blaser, M. J. 1997. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 176(Suppl.):S103-S105. [DOI] [PubMed] [Google Scholar]
- 11.Chang, C., and J. F. Miller. 2006. Campylobacter jejuni colonization of mice with limited enteric flora. Infect. Immun. 74:5261-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chattopadhyay, U. K., R. S. Rathore, D. Pal, and M. S. Das. 1991. Enterotoxigenicity of human and animal isolates of Campylobacter jejuni in ligated rat ileal loops. J. Diarrhoeal Dis. Res. 9:20-22. [PubMed] [Google Scholar]
- 13.Clahsen, T., and F. Schaper. 2 September 2008, posting date. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J. Leukoc. Biol. doi: 10.1189/jlb.0308178. [Epub ahead of print.] [DOI] [PubMed]
- 14.Diehl, S., and M. Rincon. 2002. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 39:531-536. [DOI] [PubMed] [Google Scholar]
- 15.Enocksson, A., J. Lundberg, E. Weitzberg, A. Norrby-Teglund, and B. Svenungsson. 2004. Rectal nitric oxide gas and stool cytokine levels during the course of infectious gastroenteritis. Clin. Diagn. Lab. Immunol. 11:250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernie, D. S., and R. W. Park. 1977. The isolation and nature of campylobacters (microaerophilic vibrios) from laboratory and wild rodents. J. Med. Microbiol. 10:325-329. [DOI] [PubMed] [Google Scholar]
- 17.Field, L. H., J. L. Underwood, S. M. Payne, and L. J. Berry. 1991. Virulence of Campylobacter jejuni for chicken embryos is associated with decreased bloodstream clearance and resistance to phagocytosis. Infect. Immun. 59:1448-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox, J. G., A. B. Rogers, M. T. Whary, Z. Ge, N. S. Taylor, S. Xu, B. H. Horwitz, and S. E. Erdman. 2004. Gastroenteritis in NF-κB-deficient mice is produced with wild-type Campylobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72:1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullner, K. J., J. C. Boucher, M. A. Hanes, G. K. Haines III, B. M. Meehan, C. Walchle, P. J. Sansonetti, and J. J. Mekalanos. 2002. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J. Exp. Med. 195:1455-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haines, G. K., III, B. A. Sayed, M. S. Rohrer, V. Olivier, and K. J. Fullner Satchell. 2005. Role of Toll-Like receptor 4 in the proinflammatory response to Vibrio cholerae O1 El Tor strains deficient in production of cholera toxin and accessory toxins. Infect. Immun. 73:6157-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, B., S. Poole, and M. Wilson. 1998. Bacteria-cytokine interactions in health and disease. Portland Press, London, United Kingdom.
- 22.Hickey, T. E., S. Baqar, A. L. Bourgeois, C. P. Ewing, and P. Guerry. 1999. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT 407 cells. Infect. Immun. 67:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickey, T. E., G. Majam, and P. Guerry. 2005. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytolethal distending toxin. Infect. Immun. 73:5194-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu, L., and D. J. Kopecko. 2000. Interactions of Campylobacter with eukaryotic cells: gut luminal colonization and mucosal invasion mechanisms, p. 191-215. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology Press, Washington, DC.
- 25.Hu, L., M. D. Bray, M. Osorio, and D. J. Kopecko. 2006. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect. Immun. 74:2697-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, M. A., S. Totemeyer, J. M. Duncan, C. E. Bryant, and P. A. Barrow. 2003. Induction of proinflammatory responses in human monocytic cell line THP-1 by Campylobacter jejuni. Infect. Immun. 71:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung, S., S. Zimmer, E. Lüneberg, M. Frosch, H. Karch, T. Korn, and K. V. Toyka. 2005. Lipooligosaccharide of Campylobacter jejuni prevents myelin-specific enteral tolerance to autoimmune neuritis—a potential mechanism in Guillain-Barré syndrome? Neurosci. Lett. 381:175-178. [DOI] [PubMed] [Google Scholar]
- 28.Kamimura, D., K. Ishihara, and T. Hirano. 2003. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev. Physiol. Biochem. Pharmacol. 149:1-38. [DOI] [PubMed] [Google Scholar]
- 29.Kidd, P. 2003. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 8:223-246. [PubMed] [Google Scholar]
- 30.Kiyono, H. 2001. Mucosal immune system: close encounter in the uncharted world of immunology. Ophthalmologica 215(Suppl. 1):22-32. [DOI] [PubMed] [Google Scholar]
- 31.Korlath, J. A., M. T. Osterholm, L. A. Judy, J. C. Forfang, and R. A. Robinson. 1985. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J. Infect. Dis. 152:592-596. [DOI] [PubMed] [Google Scholar]
- 32.Kum, W. W., S. B. Cameron, R. W. Hung, S. Kalyan, and A. W. Chow. 2001. Temporal sequence and kinetics of proinflammatory and anti-inflammatory cytokine secretion induced by toxic shock syndrome toxin 1 in human peripheral blood mononuclear cells. Infect. Immun. 69:7544-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacCallum, A. J., D. Harris, G. Haddock, and P. H. Everest. 2006. Campylobacter jejuni-infected human epithelial cell lines vary in their ability to secrete interleukin-8 compared to in vitro-infected primary human intestinal tissue. Microbiology 152:3661-3665. [DOI] [PubMed] [Google Scholar]
- 34.Mansfield, L. S., J. A. Bell, D. L. Wilson, A. J. Murphy, H. M. Elsheikha, V. A. Rathinam, B. R. Fierro, J. E. Linz, and V. B. Young. 2007. C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect. Immun. 75:1099-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran, A. 1995. Biological and serological characterization of Campylobacter jejuni lipopolysacharides with deviating core and lipid A structures. FEMS Immun. Med. Microbiol. 11:121-130. [DOI] [PubMed] [Google Scholar]
- 36.Mulligan, M. S., M. L. Jones, A. A. Vaporciyan, M. C. Howard, and P. A. Ward. 1993. Protective effects of IL-4 and IL-10 against immune complex-induced lung injury. J. Immun. 151:5666-5674. [PubMed] [Google Scholar]
- 37.Newell, D. G. 2001. Animal models of Campylobacter jejuni colonization and disease and the lessons to be learned from similar Helicobacter pylori models. J. Appl. Microbiol. 90(Suppl.):57S-67S. [DOI] [PubMed] [Google Scholar]
- 38.Pancorbo, P. L., M. A. De Pablo, E. Ortega, A. M. Gallego, C. Alvarez, and G. A. De Cienfuegos. 1999. Evaluation of cytokine production and phagocytic activity in mice infected with Campylobacter jejuni. Curr. Microbiol. 39:129-133. [DOI] [PubMed] [Google Scholar]
- 39.Pancorbo, P. L., M. A. De Pablo, E. Ortega, M. A. Puertollano, A. M. Gallego, and G. A. Cienfuegos. 2001. Potential intervention of Campylobacter jejuni in the modulation of murine immune response. Curr. Microbiol. 43:209-214. [DOI] [PubMed] [Google Scholar]
- 40.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 41.Phalipon, A., M. Kaufmann, P. Michetti, J.-M. Cavaillon, M. Huerre, P. Sansonetti, and J.-P. Kraehenbuhl. 1995. Monoclonal immunoglobulin A antibody directed against serotype-specific of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J. Exp. Med. 182:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prendergast, M. M., D. R. Tribble, S. Baqar, D. A. Scott, J. A. Ferris, R. I. Walker, and A. P. Moran. 2004. In vivo phase variation and serologic response to lipooligosaccharide of Campylobacter jejuni in experimental human infection. Infect. Immun. 72:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raqib, B., A. A. Lindberg, B. Wretlind, P. K. Bardhan, U. Andersson, and J. Andersson. 1995. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect. Immun. 63:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sestak, K., C. K. Merritt, J. Borda, E. Saylor, S. R. Schwamberger, F. Cogswell, E. S. Didier, P. J. Didier, G. Plauche, R. P. Bohm, P. P. Aye, P. Alexa, R. L. Ward, and A. A. Lackner. 2003. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus monkeys. Infect. Immun. 71:4079-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegesmund, A. M., M. E. Konkel, J. D. Klena, and P. F. Mixter. 2004. Campylobacter jejuni infection of differentiated THP-1 macrophages results in interleukin-1β release and caspase-1 independent apoptosis. Microbiology 150:561-569. [DOI] [PubMed] [Google Scholar]
- 46.Skirrow, M. B., and M. J. Blaser. 1995. Campylobacter jejuni, p. 825-848. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, New York, NY.
- 47.Skirrow, M. B., and M. J. Blaser. 2000. Clinical aspects of Campylobacter infection, p. 69-88. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology Press, Washington, DC.
- 48.Smith, G. S., and M. J. Blaser. 1985. Fatalities associated with Campylobacter jejuni infections. JAMA 253:2873-2875. [PubMed] [Google Scholar]
- 49.Strierter, R. M., T. J. Standiford, M. W. Rolfe, J. P. Lynch III, A. P. Metinko, and S. L. Kunkel. 1992. Cytokines in pulmonary injury, p. 397-412. In S. L. Kunkel and D. G. Remick (ed.), Cytokines in health and disease. Marcel Dekker, New York, NY.
- 50.Tauxe, R. V. 1992. Epidemiology of Campylobacter jejuni infections in the United States and other industralized nations, p. 9-19. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology Press, Washington, DC.
- 51.Taylor, D. N. 1992. Campylobacter infections in developing countries, p. 20-30. In I. Nachamkin. M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology Press, Washington, DC.
- 52.Van De Verg, L. L., C. P. Mallett, H. H. Collins, T. Larsen, C. Hammack, and T. L. Hale. 1995. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect. Immun. 63:1947-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Vliet, A. H. M., and J. M. Ketley. 2001. Pathogenesis of enteric Campylobacter infection. J. Appl. Microbiol. 90(Suppl.):45S-56S. [DOI] [PubMed] [Google Scholar]
- 54.Vučković, D., M. Abram, M. Bubonja, B. Wraber, and M. Dorić. 2006. Host resistance to primary and secondary Campylobacter jejuni infections in C57BL/6 mice. Microb. Pathog. 40:35-39. [DOI] [PubMed] [Google Scholar]
- 55.Vučković, D., M. Abram, and M. Dorić. 1998. Primary Campylobacter jejuni infection in different mice strains. Microb. Pathog. 24:263-268. [DOI] [PubMed] [Google Scholar]
- 56.Wang, J., J. Wakeham, R. Harkness, and Z. Xing. 1999. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J. Clin. Investig. 103:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warren, J. S., K. R. Yabroff, D. G. Remick, S. L. Kunkel, S. W. Chensue, R. G. Kunkel, K. J. Johnson, and P. A. Ward. 1989. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J. Clin. Investig. 84:1873-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson, R. O., V. Novik, D. Hofreuter, M. Lara-Tejero, and J. E. Galán. 2007. A MyD88-deficient mouse model reveals a role for Nramp1 in Campylobacter jejuni infection. Infect. Immun. 75:1994-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing, Z., J. Gauldie, G. Cox, H. Baumann, M. Jordana, X. F. Lei, and M. K. Achong. 1998. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 101:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuki, N. 1997. Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain-Barré syndrome and Miller-Fisher syndrome. J. Infect. Dis. 176(Suppl.):S150-S153. [DOI] [PubMed] [Google Scholar]