Abstract
Recent efforts to improve the serologic diagnosis of Lyme disease have included the use of a synthetic peptide (C6) that reproduces the sequence of invariable region 6 of VlsE, the variable surface antigen of Borrelia burgdorferi. In the present study, the diagnostic performance of DiaSorin's recombinant VlsE-based chemiluminescence immunoassay in 1,947 human serum samples was evaluated. Sensitivity was determined using two serum panels from the CDC. For panel I, we observed sensitivities of 68.4% and 75.6% for subjects with early, localized (n = 19) or disseminated (n = 41) disease, respectively. For panel II, we observed sensitivities of 61.5% and 100% for subjects with early (n = 26) or late-stage (n = 11) disease, respectively. We observed a specificity of 99.5% for healthy donors (n = 600) living either in regions of the United States where the disease is endemic or in regions where it is not endemic. Overall, specificity among 207 potentially cross-reactive sera from subjects who had other spirochetal infections, nonspirochetal infections including bacterial and viral infections, or autoimmune or neurologic disease; who were positive for rheumatoid factor or anti-mouse antibodies; or who had been previously vaccinated for Lyme disease was 93.7%. In a direct comparison of 1,038 prospectively collected samples for Lyme disease testing we observed a relative sensitivity of 70%, a relative specificity of 99.1%, and an overall agreement of 97.1% between the DiaSorin recombinant VlsE chemiluminescence immunoassay and the Immunetics peptide-based C6 enzyme-linked immunosorbent assay.
Lyme borreliosis, or Lyme disease (LD), is the most common tick-borne disease in the United States, Europe, and parts of Asia (10, 58, 65). In 2006, 19,931 cases of LD were reported to the U.S. Centers for Disease Control and Prevention (CDC), yielding an incidence rate of 6.7/100,000. Although LD has been reported across most of the continental United States, nearly 95% of the cases are from 10 states of the northeast, mid-Atlantic, and north central regions, where the average incidence is 31.6/100,000 (http://www.cdc.gov/ncidod/dvbid/lyme/ld_rptdLymeCasesbyState.htm, CDC, January 2007, posting date). The causative agent of LD is a group of genetically diverse spirochetes belonging to the Borrelia burgdorferi sensu lato genogroup. Within the genogroup are three different species of spirochetes known to be pathogenic to humans; they are B. burgdorferi sensu stricto, Borrelia garinii, and Borrelia afzelii (63). In the United States, all of the LD cases are due to B. burgdorferi sensu lato. In Europe and parts of Asia, all three species have been found, with B. garinii and B. afzelii being more common than B. burgdorferi. Lyme disease is transmitted by ticks of the Ixodes ricinus species complex. In the United States, the principal tick vector in the northeast and north central states is Ixodes scapularis, whereas Ixodes pacificus is the principal host reservoir in the coastal northwest (54, 55). In Europe and Asia, the primary vectors are I. ricinus and Ixodes persulcatus, respectively (19, 24, 59).
There is significant variability in the presentation of LD (53). In the early phase, fatigue, fever, headache, and muscle and joint pain are common symptoms. Although erythema migrans (EM), commonly referred to as the bulls-eye rash, occurs at the site of tick bite in 60 to 80% of early cases, only 20 to 40% of patients recall being bitten by a tick (10). Headache, stiff neck, swollen lymph nodes, numbness and pain in limbs, facial paralysis, and meningitis are early indicators of dissemination and may occur days to weeks after infection. In late LD, the patient may experience intermittent or continuous episodes of dermatologic, neurologic, cardiac, and rheumatologic manifestations. Although clinical symptoms from LD cases worldwide are similar, there are well-documented differences due to the diverse pathogenic potentials of the various B. burgdorferi genotypes (45, 62, 63).
Diagnosis of LD is based upon a physician's review of clinical symptoms and the patient's exposure risk in an area where the disease is endemic. Laboratory tests may provide confirmation of diagnosis but should not be used in the absence of a positive clinical correlation or epidemiologic risk. Laboratory culture of B. burgdorferi offers the best evidence of disease causality; however, its success requires specialized media and days to weeks of incubation and is tissue and disease stage dependent. Although spirochete recovery rates of 60 to 80% or higher from EM biopsy samples and blood in cases of early disease have been reported, recovery of spirochetes from joint tissue, cerebrospinal fluid, or any other tissue in late disease is rare (29, 56). Molecular detection of spirochete DNA by PCR in early-disease cases has been frequently reported, but this diagnostic approach lacks methodological standardization, continues to be burdened by excessive false-positive results, and, like culture, appears insensitive in most late-disease cases (2, 44, 51). At present, serologic tests detecting antibody responses to B. burgdorferi proteins offer the most practical means for laboratory confirmation of LD (29).
Most first-generation serology tests used whole-cell lysates of B. burgdorferi strain B31 as antigens to capture anti-B. burgdorferi antibodies with formats such as enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence assay (IFA). The ELISA method is considerably more sensitive, less subjective, and better suited to laboratories handling large workloads (37). Both formats, however, have suffered from poor specificity due to cross-reactive antibodies and a lack of assay standardization. Indeed, the poor interlaboratory agreement reported in national proficiency programs was an important rationale behind the establishment of national laboratory guidelines for improving the serologic diagnosis of LD (7, 11). These guidelines centered on the establishment of a two-test protocol, in which a positive or equivocal ELISA or IFA is followed by confirmatory testing in separate immunoglobulin M (IgM) and IgG Western immunoblotting (WB), depending on the time from disease onset (11). While analogous in principle to the ELISA, WB combines the selectivity of gel electrophoresis with immunochemical specificity to allow antibodies against individual proteins to be detected and analyzed. Most WB assays (which also use whole-cell lysates of low-passage-number B31) have inherent drawbacks of their own. Depending on the conditions of the assay, multiple proteins may comigrate, immunoreactive band intensity may pose interpretive challenges, and the longevity of the IgM response, even after successful antibiotic treatment, can be difficult to interpret. Although the specificity of the two-test approach has resulted in improved performance compared to ELISA or IFA alone (13, 60), its sensitivity in cases of early LD remains relatively low (<40%) (5). In addition, WB adds considerable expense due to the complexity of the test, the time required for technical reading and scoring, and the associated reagent costs. For these reasons, efforts to improve the sensitivity and specificity of serologic tests continue.
Several approaches to enhance the discriminating ability of serologic tests have been described. These include the use of detergent extracts to isolate proteins of interest by molecular weight (9), preabsorption of sera with other bacteria to eliminate cross-reactive antibodies (64), and the use of purified proteins (22). More recently, third-generation serologic tests using sensitive synthetic peptides and recombinant protein-based techniques have been reported (23, 35, 36). One approach utilizes the recently identified Borrelia antigen VlsE (variable major protein-like sequence, expressed), an immunogenic 35-kDa outer surface lipoprotein (14). The VlsE protein contains conserved domains at both the amino and carboxyl termini separated by a variable domain. The variable domain contains six variable regions (VR) and six invariable regions (IR). The six IR are interspersed in the variable domain and are conserved among strains and genospecies of the B. burgdorferi sensu lato complex. During infection with B. burgdorferi, the six VR routinely undergo sequence variation by recombination, a process that is recognized as an important immune evasion mechanism. Of considerable significance is that the IR (particularly IR6) are also immunodominant. Studies using sera from patients with LD have demonstrated a strong immune response against VlsE in all stages of the disease, including the early stage (28, 30). In this report, we evaluate the performance characteristics of the Liaison Borrelia burgdorferi test, a chemiluminescence immunoassay (CLIA) based on recombinant VlsE, for the detection of total IgM/IgG antibodies to B. burgdorferi.
MATERIALS AND METHODS
Study population overview.
The ability of the Liaison CLIA to determine the presence or absence of specific antibodies (IgM/IgG) against B. burgdorferi in 1,947 human serum samples was evaluated. Independent sample procurement organizations were used to collect the serum samples from healthy donors, patients with non-LD etiologies, and individuals with a clinical diagnosis of LD. All samples were deidentified and stored at −20°C before analysis by the CDC or the Foundation for Blood Research. Institutional review board approval was obtained for the collection of all samples used in the study. Further descriptions are provided below.
Healthy donor sera.
Six hundred sera (from 172 females and 428 males) were collected to evaluate the comparative specificities of the LD assays. One group (n = 300) comprised prospectively collected single-serum samples from subjects in Pennsylvania, an area where LD is known to be endemic. The second group (n = 300) comprised prospectively collected single-serum samples from subjects in Arizona, where LD is not endemic.
Potentially cross-reactive sera.
To further evaluate the specificity of the assays, 196 samples from subjects who had other spirochetal infections, nonspirochetal bacterial or viral infections, or autoimmune diseases or who were seropositive for rheumatoid factor (RF) or human anti-mouse antibodies (HAMA) were tested. Within this study group were 18 samples from patients with syphilis, 10 with tick-borne relapsing fever, 20 with Epstein-Barr virus (EBV), 20 with cytomegalovirus (CMV), 20 with human immunodeficiency virus (HIV), and 20 with Helicobacter pylori. Also tested were sera from 20 patients with rheumatoid arthritis, 20 with systemic lupus erythematosus, 8 with multiple sclerosis, and 20 with various autoimmune diseases (e.g., systemic sclerosis, Sjögren's syndrome, etc.) and from 20 subjects (10 each) whose sera were seropositive for RF or HAMA.
LYMErix vaccine recipients.
Eleven samples from five adults, collected after various doses of the recombinant OspA (outer surface protein A) vaccine (LYMErix; previously distributed by SmithKline Beecham Pharmaceuticals, Philadelphia, PA), were also tested.
Characterized LD sera.
Two panels of sera from well-characterized LD patients were collected and assayed by the CDC. Panel I consisted of 60 sera from suspected LD patients presenting with EM in Westchester, NY. Clinical diagnosis of these patients was confirmed by culture isolation of B. burgdorferi from skin biopsy samples. Patients were classified as having early localized or early disseminated disease. Localized disease was defined as EM accompanied by no more than regional lymphadenopathy, fatigue, or minor headache; disseminated disease was defined by the presence of multiple secondary EM lesions, arthritis or arthralgias, abdominal pain or tenderness, generalized lymphadenopathy, or signs or symptoms of central nervous system infection (headache and neck stiffness, facial palsy, or dysesthesias). Acute phase sera (n = 19) were collected at baseline presentation, and convalescent phase sera (n = 41) were collected 1 to 3 weeks after presentation and initiation of antibiotic therapy. The second panel (II) comprised 42 sera. Five of these samples were from healthy donors, and 37 were from patients who had initially presented with early (n = 26) or late (n = 11) stage disease. All patients were treated promptly with appropriate antibiotics, and large-volume test samples were collected weeks to months later, often long after complete clinical cure. Lyme disease was confirmed by B. burgdorferi culture of samples collected prior to antibiotic treatment in 27 of 37 patients.
Prospective study sera.
For the prospective study, sera from 1,038 subjects with suspected LD (641 females and 397 males) were collected for routine LD testing at a regional clinical laboratory in Massachusetts. The sample set was divided in half, and the samples were distributed to the CDC and Foundation for Blood Research for comparative performance analysis of the Liaison B. burgdorferi assay and the predicate device described below.
Reproducibility study.
A panel of eight human serum samples was tested in quadruplicate for a period of 5 days according to the Clinical and Laboratory Standards Institute (CLSI) document EP15-A2 (12). The panel included the manufacturer's negative and positive assay controls, three negative samples, one sample in the equivocal zone, and two positive samples. All controls and samples were aliquoted and stored at −20°C prior to testing.
Chemiluminescence immunoassay.
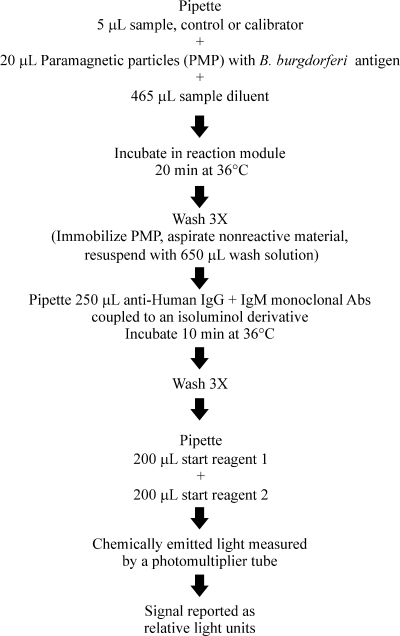
The Liaison system (DiaSorin Inc., Stillwater, MN) is a fully automated random-access analyzer which employs two-step CLIA technology (Fig. 1). The Borrelia burgdorferi assay uses recombinant Borrelia VlsE antigens from B. burgdorferi sensu stricto strain B31 and the PBi strain of B. garinii to coat solid phase paramagnetic particles. VlsE from B. burgdorferi sensu stricto B31 was prepared according to the method of Lawrenz and coworkers (28). VlsE from PBi was cloned and expressed as described by Goettner and coworkers (20). The purification process was modified by DiaSorin to include three chromatographic steps: chelating affinity chromatography, anion-exchange chromatography, and gel filtration. In brief, 5 μl of bilevel calibrators, controls (negative and positive), and patient serum is automatically pipetted into the reaction module, containing sample diluent and the antigen-bound particles. After a 20-min incubation period, unbound antibodies are removed through a wash step. This is followed by a 10-min incubation of conjugate reagent, containing monoclonal antibodies (anti-human IgM and anti-human IgG) coupled to an isoluminol derivative, which reacts with the specific IgM and IgG antibodies bound to the paramagnetic particles. After a final wash cycle, a flash chemiluminescence reaction is initiated by addition of a two-part starter reagent. The light signal produced is measured by a photomultiplier tube as relative light units and reflects the concentration of IgM and IgG antibodies to B. burgdorferi present in the specimen. Data reduction is performed using a master curve stored in the analyzer and a two-point recalibration method that adjusts for laboratory conditions during measurement. Results are reported in terms of an index value and graded as negative (<0.9), equivocal (0.9 to ≤1.1), or positive (>1.1). The negative and positive serum controls are tested on a daily basis to verify the assay's performance.
FIG. 1.
Overview of the automated Liaison chemiluminescence immunoassay.
Method comparison studies.
Performance of the recombinant VlsE assay was assessed by a direct comparison with an FDA-cleared predicate device from Immunetics (Boston, MA). The Immunetics C6 B. burgdorferi (Lyme) ELISA kit is a 96-microwell plate containing the synthetic C6 peptide antigen derived from the VlsE protein envelope. Like the VlsE assay from DiaSorin, the Immunetics C6 assay uses a bivalent (IgM/IgG) conjugate to detect Borrelia antibodies. Samples were assayed in accordance with the manufacturer's instructions; plates were read using either ELX808 or EL800 microplate readers equipped with KC4 software (version 3.0 or 2.5, respectively) from Bio-Tek Instruments, Inc. (Winooski, VT). Results were reported as negative (ELISA index score ≤ 0.90), equivocal (0.91 to 1.09), or positive (≥1.10).
Western blotting.
Serum samples from the prospectively collected healthy donors, the CDC panels, or the prospective LD suspect group that tested equivocal or positive with either the VlsE or C6 assay were further tested by WB using the MarDx B. burgdorferi (separate IgG and IgM) Marblot strip test system (Trinity Biotech, Carlsbad, CA). The Marblot assay is based on low-passage-number B31 antigens of B. burgdorferi. Each assay included a negative control, a weakly reactive control, and a serum band locator. The weakly reactive control was used as an internal standard for determining cutoff intensities of scored and unscored bands. The blot banding template provided by the manufacturer was used to identify the relative positions of the reactive IgM or IgG bands in the serum band locator, which was then used to facilitate the assignment of molecular weights for each unknown strip. Interpretation of the Western blot patterns was based on the CDC criteria for IgM and IgG antibodies (11). A positive IgM immunoblot was defined by reactivity to two or more of the following bands: 23 (OspC), 39 (BmpA), or 41 (Fla) kDa. An IgG immunoblot was considered positive if five or more of the following bands were observed: 18, 23 (OspC), 28, 30, 39 (BmpA), 41 (Fla), 45, 58, 66, and 93 kDa. Blots were read by observers with experience using this technique.
Statistical analysis.
Concordance between the VlsE and C6 assays within the different population samples was tested with McNemar's test for paired samples. Exact P values were calculated due to the small number of discordant pairs.
RESULTS
Test precision and specificity.
Table 1 summarizes the precision data observed for the VlsE assay. Within-run imprecision (coefficients of variation [CVs]) for the six serum samples and two controls ranged from 3.6 to 10.8%, whereas between-run and total imprecision CVs ranged from 3.4 to 12.0% and from 6.2 to 14.7%, respectively.
TABLE 1.
Precision data for the Liaison Borrelia burgdorferi assay
| Samplea | Mean Liaison index | CV (%)
|
||
|---|---|---|---|---|
| Within run | Between run | Total | ||
| Negative control | 0.06 | 10.8 | 12.0 | 14.7 |
| Positive control | 1.84 | 4.8 | 9.4 | 9.7 |
| 1 | 0.14 | 3.7 | 11.4 | 11.2 |
| 2 | 0.49 | 6.4 | 10.1 | 11.2 |
| 3 | 0.83 | 5.9 | 9.7 | 10.5 |
| 4 | 1.06 | 5.0 | 9.2 | 9.7 |
| 5 | 1.79 | 3.6 | 5.4 | 6.2 |
| 6 | 6.83 | 6.6 | 3.4 | 7.5 |
Serum samples (n = 20 for all samples) were collected from individuals previously tested for B. burgdorferi and determined to be negative (samples 1 to 3), equivocal (sample 4), or positive (samples 5 and 6). The negative and positive serum controls are provided by the manufacturer.
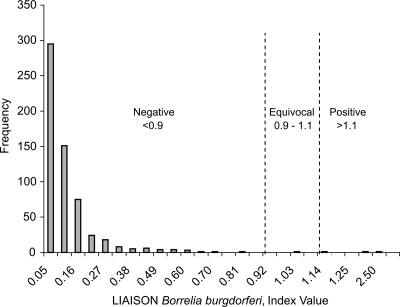
Sera from 600 healthy donors from areas where LD is endemic and areas where it is not were tested in both the VlsE and C6 assays in order to assess background reactivities in these populations (Table 2). A negative result was found for 99.5% (597/600) of these healthy controls in the VlsE assay compared to 98.5% (591/600) in the C6 assay (P = 0.146). The 12 separate reactive samples (3 from the VlsE assay and 9 from the C6 assay) were found to be negative by WB analysis for IgM or IgG antibodies (data not shown). The VlsE index value (mean ± 1 standard deviation) for donors living in an area where LD is endemic, 0.11 ± 0.19, was slightly higher than that for those living in an area where it is not, 0.09 ± 0.13; however, the difference was not statistically significant (P = 0.211). The distribution profile for the combined groups in the VlsE assay is displayed in Fig. 2.
TABLE 2.
Comparison of sensitivities and specificities for the VlsE and C6 assays for the detection of B. burgdorferi antibodies
| Patient group | n | VlsE assay
|
C6 assay
|
WB
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. with result of:
|
Sen.c (%) | Spe.d (%) | No. with result of:
|
Sen. (%) | Spe. (%) | No. with result of:
|
Sen. (%) | Spe. (%) | |||||
| + | − | + | − | + | − | ||||||||
| CDC panel I (culture confirmed) | |||||||||||||
| Early localized diseasea | 19 | 13 | 6 | 68.4 | 11 | 8 | 57.9 | 9 | 10 | 47.4 | |||
| Early disseminated diseaseb | 41 | 31 | 10 | 75.6 | 33 | 8 | 80.5 | 31 | 10 | 75.6 | |||
| CDC panel II | |||||||||||||
| Healthy | 5 | 0 | 5 | 100.0 | 0 | 5 | 100.0 | 0 | 5 | 100.0 | |||
| Early disease (<2 mo) | 26 | 16 | 10 | 61.5 | 20 | 6 | 76.9 | 17 | 9 | 65.4 | |||
| Late disease (>2 mo) | 11 | 11 | 0 | 100.0 | 11 | 0 | 100.0 | 11 | 0 | 100.0 | |||
| Non-LD | |||||||||||||
| CMV | 20 | 0 | 20 | 100.0 | 0 | 20 | 100.0 | ||||||
| EBV | 20 | 0 | 20 | 100.0 | 0 | 20 | 100.0 | ||||||
| HAMA positive | 10 | 2 | 8 | 80.0 | 1 | 9 | 90.0 | ||||||
| Healthy, nonendemice | 300 | 2 | 298 | 99.3 | 7 | 293 | 97.7 | ||||||
| Healthy, endemicf | 300 | 1 | 299 | 99.7 | 2 | 298 | 99.3 | ||||||
| Helicobacter pylori | 20 | 1 | 19 | 95.0 | 0 | 20 | 100.0 | ||||||
| HIV | 20 | 0 | 20 | 100.0 | 0 | 20 | 100.0 | ||||||
| LYMErix vaccine | 11 | 0 | 11 | 100.0 | 0 | 11 | 100.0 | ||||||
| Multiple sclerosis | 8 | 0 | 8 | 100.0 | 0 | 8 | 100.0 | ||||||
| Rheumatoid arthritis | 20 | 2 | 18 | 90.0 | 2 | 18 | 90.0 | ||||||
| RF positive | 10 | 0 | 10 | 100.0 | 0 | 10 | 100.0 | ||||||
| Syphilis patients | 18 | 0 | 18 | 100.0 | 0 | 18 | 100.0 | ||||||
| Systemic lupus erythematosus | 20 | 0 | 20 | 100.0 | 0 | 20 | 100.0 | ||||||
| Tick-borne relapsing fever | 10 | 7 | 3 | 30.0 | 4 | 6 | 60.0 | ||||||
| Various autoimmune diseases (e.g., systemic sclerosis, Sjögren's syndrome) | 20 | 1 | 19 | 95.0 | 1 | 19 | 95.0 | ||||||
| Total | 807 | 16 | 791 | 98.0 | 17 | 790 | 97.9 | ||||||
Nine collected at baseline presentation, 10 collected 1 to 3 weeks after presentation and treatment.
Twelve collected at baseline presentation, 29 collected 1 to 3 weeks after presentation and treatment.
Sen., sensitivity.
Spe., specificity.
Subjects from an area where LD is not endemic.
Subjects from an area where LD is endemic.
FIG. 2.
Distribution profile of Liaison index values among 600 healthy blood donors using the Borrelia burgdorferi IgM/IgG assay. The manufacturer's cutoffs are illustrated by the vertical lines. Results are graded as negative, equivocal, or positive. The overall frequency of positive or equivocal results in the normal population was 0.5% (3 out of 600).
Additional specificities for the VlsE and C6 assays in patients with disease etiologies other than LD are shown in Table 2. Specificities of 95 to 100% were observed in both assays for patients with CMV, EBV, HIV, H. pylori, or syphilis or who had various forms of autoimmune (e.g., systemic sclerosis, sicca syndrome) or neurologic disease (multiple sclerosis). Specificities of 90 and 100% were demonstrated in both assays for patients diagnosed with rheumatoid arthritis and for RF-positive patients, respectively. Lower specificities were observed in both the VlsE and C6 assays for patients who had either HAMA (80% versus 90%, respectively) or infection with the closely related spirochete agent of tick-borne relapsing fever, Borrelia hermsii (30% versus 60%, respectively). All 11 sera collected from individuals after vaccination with the LYMErix vaccine tested negative by both assays. For the combined sample sets (n = 807), the overall specificities for the VlsE and C6 tests were nearly identical: 98.0% and 97.8%, respectively.
Test sensitivity for LD patients.
Tabulated sensitivity results for the retrospective testing of samples in CDC's LD serum panels are presented in Table 2. Among patients presenting with early localized disease (panel I; n = 19), the VlsE assay was more sensitive (68.4%) than the C6 assay (57.9%) or WB (47.4%) in detecting antibodies to B. burgdorferi. For patients with early disseminated LD (panel I; n = 41), we observed sensitivities of 75.6% for VlsE, 80.5% for C6, and 75.6% for WB. None of these differences were statistically significant by McNemar's test for paired samples (P > 0.5). Overall sensitivities for the combined groups (n = 60) of the VlsE and C6 immunoassays were identical (77.3%) and higher than that observed for WB (66.7%). When the two-tier strategy was applied using either the VlsE assay or C6 assay as the first-tier test followed by WB of all equivocal or positive samples, the clinical sensitivity was reduced to 58.3% for both assays.
CDC panel II consisted of samples from patients who initially presented with early localized or disseminated disease and late stage disease. Samples were collected weeks to months after disease onset and antibiotic treatment. Among samples from subjects who presented with early disease in CDC panel II (n = 26), we observed sensitivities of 61.5% for VlsE and 76.9% for C6 (P = 0.125 by McNemar's test for paired samples), compared to 65.4% for WB. It should be noted, however, that many of the samples from early stage disease patients were collected months after antibiotic treatment and clinical cure, at a time when diminished reactivity to VlsE and C6 is reportedly a marker of cure (47). For samples from cases of late LD (n = 11), we observed a sensitivity of 100% for VlsE, C6, and WB. Overall sensitivities for the combined groups in panel II (n = 37) were 73.0% for VlsE, 83.8% for C6 (P = 0.125), and 75.6% for WB. When we applied the CDC-recommended two-tier strategy using the VlsE or C6 test as the screening test followed by WB, the observed clinical sensitivities were reduced to 59.5% and 62.1%, respectively.
We determined the relative sensitivity, specificity, and agreement of the VlsE assay with respect to the predicate device C6 assay for a group of 1,038 samples collected prospectively for LD serological testing (Table 3). In this group, the recombinant VlsE assay showed a relative specificity of 99.1%, a relative sensitivity of 70.0%, and an overall agreement of 97.1% with respect to the C6 assay. Of the 36 C6-positive samples that were also detected by VlsE, 61.1% (22/36) were confirmed by MarDx WB (6 were IgM positive, 12 were IgG positive, and 4 were positive for IgM and IgG antibodies). The remaining 14 cases were WB negative; however, five sera had reactivity to the 23-kDa protein OspC, one of the immunodominant antigens expressed in early stage disease. Of the 20 discrepant samples (C6 positive and VlsE negative [n = 14] or C6 negative and VlsE positive [n = 6]), 19 were negative by WB assays. In the former group, 1 of 14 was positive by WB for IgG reactivity whereas the remaining 13 sera (93%) were negative by WB, with 12 of the samples having only one detectable band or no detectable bands. The overall prevalence of antibodies to B. burgdorferi among the study group determined by the VlsE assay was 3.95%. The highest prevalence of anti-Borrelia antibodies was seen among those individuals <10 years of age (12.5%), with a gradual decline for individuals between 10 and 40 years of age (nadir, 2.0%) and a second peak (6.25%) among 60 to 69 year olds (data not shown).
TABLE 3.
Relative sensitivity, specificity, and agreement for the Liaison B. burgdorferi VlsE CLIA with respect to the Immunetics C6 ELISA for 1,038 prospectively collected samples for LD serological testinga
| Liaison VlsE CLIA result | No. with Immunetics C6 ELISA result of:
|
Total | ||
|---|---|---|---|---|
| Positive | Equivocal | Negative | ||
| Positive | 35 | 0 | 6 | 41 |
| Equivocal | 1 | 0 | 3 | 4 |
| Negative | 14 | 6 | 973 | 993 |
| Total | 50 | 6 | 982 | 1,038 |
Relative sensitivity, 70.0% (35/50); relative specificity, 99.1% (973/982); relative agreement, 97.1% (1,008/1,038).
DISCUSSION
Since the CDC's designation of LD as a nationally reportable disease in 1991, requests for serology tests have grown considerably and now exceed several million per year (61). Not surprisingly, the concerns over appropriate use and performance of such tests have spawned numerous research and clinical studies and have been the frequent topic of scientific conferences (6, 7, 57). The development of optimal immunoassays, however, is complicated by the complexity of the various genospecies of B. burgdorferi sensu lato and their numerous antigenic constituents, particularly glycolipids, as well as the chronological presentation of antibodies to these antigens over the course of infection (2). Most early-generation assays (some of which are still in the marketplace) employed whole-cell extracts of B31 which included homologues of immunodominant bacterial heat shock proteins (66, 60, and 58 kDa) (21, 32) and flagellar antigens (41 kDa) expressed in other spirochetal (B. hermsii, responsible for tick-borne relapsing fever, and Treponema pallidum, the causative agent for syphilis) (34, 49) and bacterial infections (18, 33). Nonspecific detection of antibodies to these cross-reactive antigens has contributed to false-positive laboratory results and overdiagnosis of LD. In contrast, false-negative results for subjects with weak or absent immune responses have been reported, and these may in part be due to tests performed too early in the course of the immune response or on samples from individuals who fail to seroconvert after prompt and effective antibiotic therapy.
Thus far, no measurable antibody response to a single antigen that displays adequate sensitivity and specificity to eliminate the need for two-tiered testing has been identified. There are, however, a growing number of assays (mostly in-house) that have used combinations of recombinant or synthetic peptides to increase early sensitivity (2, 5, 39, 50). The DiaSorin assay represents a third-generation immunoassay that uses recombinant Borrelia VlsE antigens derived from two (B. burgdorferi sensu stricto and B. garinii) of the three predominant LD-causing genospecies to improve diagnostic performance. As B. garinii infection has been confirmed only in LD patients with exposures in Europe and Asia (not in North America), the advantage of this addition for the testing of North American patients is not intuitive. Empirical data for North American patients, however, indicate increased test sensitivity without a loss in specificity. Although we don't have clinical histories for either the healthy-donor sera or the prospective LD sera, we do not believe that European genospecies infections are major confounders in these cases. Unpublished data provided by DiaSorin revealed that, among 39 samples in a well-characterized CDC panel from LD patients, 5 samples from patients with North American exposure histories gave results of negative (2) or equivocal (3) when tested with recombinant VlsE from B. burgdorferi sensu stricto strain B31 alone. Upon addition of recombinant VlsE from B. garinii strain PBi to strain B31, four of the five samples became positive, while one of the negative samples became equivocal. Thus, increased sensitivity using VlsE antigens from both B. burgdorferi sensu stricto strain B31 and B. garinii strain PBi over that achieved using the B31 antigen alone was seen. The basis for increased sensitivity is unknown but may involve subtle differences in presentation of both variable and invariable regions of the proteins during bacterial infection and antigen processing. Further, detection of antibodies against specific epitopes may vary as a result of differential folding of the recombinant proteins within the environment of the in vitro assay (42).
We found that the operation of the Liaison analyzer was straightforward and flexible and that the analyzer was capable of handling large loads. The assay requires minimal sample volume, making it ideal for pediatric samples, research samples of limited volume, and samples that must be split for processing. Within-run, between-run, and total CVs were acceptable at all levels studied, owing in part to the automated sample and reagent pipetting of the analyzer. The manufacturer's cutoff points revealed good discrimination between positive and negative samples. Interestingly, the positive prevalence was three times greater among healthy donors tested with the C6 ELISA (9/600) than among those tested with the CLIA VlsE (3/600). Although this difference was not significant, these findings may indicate a slight advantage in terms of specificity for the VlsE-based test. Additional analysis may substantiate this finding. None of these sera were positive when tested by WB.
Although persons of all ages and both sexes are susceptible to LD, our prospective testing of suspected LD patients revealed a bimodal distribution, with the highest incidence occurring among children under 10 years of age and adults between 60 and 69 years of age. These findings are consistent with national data from the CDC, which reveals a peak incidence among boys and girls aged 5 to 9 years, followed by a gradual decline through the second decade of life and a subsequent peak in the sixth decade (http://www.cdc.gov/ncidod/dvbid/lyme/ld_MeanAnnualIncidence.htm, CDC, May 2006, posting date).
Overall specificity determined for 600 healthy donors and 196 sera from patients with other diseases was excellent for the recombinant VlsE test (98.0%) and the peptide C6 assay (97.8%). As mentioned above, cross-reactivity issues plague many existing assays, particularly those which contain whole-cell antigen preparations whose epitopes cross-react with antibodies to antigens produced by other spirochetes (T. pallidum and B. hermsii), infectious agents (e.g., EBV, CMV, and HIV), or autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis (3, 52). Assay specificity for the VlsE and the C6 test was >90% for most of the non-LD sera. The most problematic sera that we encountered for either test were those from patients with tick-borne relapsing fever. This is not too surprising given that the vls system in B. burgdorferi has been previously shown to resemble a genetic system that encodes surface-exposed variable major proteins in B. hermsii (8). Patients with tick-borne relapsing fever have been shown to have elevated antibody titers to B. burgdorferi by IFA, and these cross-reactive antibodies were confirmed by WB analysis (49). We also observed interference from subjects who had anti-mouse antibodies. Although interference from heterophilic antibodies in immunometric assays has been widely reported (25), there are scant data for LD serologic assays. The prevalence of heterophilic antibodies, however, is thought to be greater than reported because problematic samples are detected only when the test result is at odds with the clinical picture. Patients who are receiving monoclonal antibodies for therapy frequently generate an immune response to mouse IgG; thus, clinical judgment remains an important component when interpreting serology results.
As anticipated, none of the 11 sera from vaccinated subjects tested positive with either the C6 or VlsE assay. False-positive serologies have been associated with recombinant OspA-vaccinated subjects. Since OspA is one of the antigens present in traditional whole-cell ELISAs, these tests cannot discriminate between naturally acquired infection and vaccinated individuals; thus, WB must be performed (1, 13). Although the LYMErix vaccine is no longer marketed in the United States (there are efforts to create a second-generation vaccine based on engineered fragments of OspA [27]), more than 1.4 million doses were distributed to an estimated 0.5 million recipients in the United States (40). In a study of WB methods applied to 152 vaccine recipients, Fawcett and coworkers reported interference in up to 25% of individuals and noted that the interference persisted longer than 6 years in some study subjects (16). They observed multiple (>6) discrete bands as well as graying in high-molecular-mass regions, which required additional technical expertise for interpretation. Both the VlsE and C6 assays circumvent these challenges due to use of recombinant protein- and peptide-based technologies which eliminate reactivity to anti-OspA antibodies.
Assays based on VlsE have shown sensitivity comparable to that of recombinant protein-based OspC ELISAs during early LD (2). Although the binding of IgM antibodies in sera from patients with EM reportedly occurs more frequently in VlsE-based assays than in assays based on the C6 peptide (15), published reports indicate that IgG anti-C6 antibodies appear to be present during EM (2).
For CDC panel I, we observed a sensitivity of 68.4% for the VlsE assay among patients with early localized disease. This value is slightly higher than those that we observed for the C6 ELISA (57.9%) and the Marblot WB assay (47.4%) and is comparable to that reported by Lawrenz et al. (63%), who used an in-house polyvalent recombinant VlsE assay to test a similar patient group (28). Whether the slightly increased sensitivity of VlsE is due to more antibodies being detected by additional epitopes not present in the IR of the C6 peptide is unclear. All three test sensitivities increased for early disease patients with evidence of dissemination (range, 73 to 84% positive), a finding and level consistent with many reports (1, 5, 22, 26, 28, 31, 41). Although another report (39) determined higher sensitivity using a mixture of whole-cell antigens and recombinant VlsE, the specificity of this approach was sufficiently low that WB was still required.
For CDC panel II, we observed sensitivities ranging from 61.5 to 100% for VlsE and 76.9 to 100% for C6 among patients who presented with early and late stage LD, respectively. The C6 assay results are consistent with those of Liang et al. (31), who used the same CDC panel and an in-house C6 ELISA. However, the sensitivity disparity between the VlsE and C6 assays on samples from early disease patients in this panel was initially unexpected in view of performance findings for early disease patients in CDC panel I. Patients with sera in both panels presented with EM and similar signs and symptoms, and all received prompt and appropriate antibiotic therapy. A notable sample caveat, however, was the difference in timing of test sample collection. Panel I samples from early disease patients were collected between the day of presentation and 3 weeks postpresentation (mean of 7.8 days). In contrast, panel II samples from early disease patients were collected between 21 days and 150 days postonset (mean of 85.9 days). All discrepant samples (n = 4) were detected as positive by C6 and negative by VlsE and were collected between 80 and 141 days postonset. All four of these patients experienced complete clinical recovery within 8 weeks of antibiotic therapy. These findings may suggest that VlsE titers as measured by the DiaSorin assay decline more fully over the measured time interval than those measured by the C6 Immunetics assay.
For the remaining nine VlsE-negative sera (seven were negative by CDC Western blot criteria), most of the patients were treated at presentation (<10 days from symptom onset) and sera were collected more than 30 days after initial disease onset. It is widely recognized that antibiotics given to patients can interfere with the immune response to some antigens (1, 5, 46). In published studies using either C6 or VlsE as the antigen source, patients who received treatment soon after infection were more likely to have undetectable antibody levels than patients who were treated after the infection had disseminated (38, 48).
The sensitivity performance of the DiaSorin VlsE test in the current study differs slightly from other published findings which utilized similar VlsE preparations (5, 15). These differences are likely due to many variables, including the physical parameters of the ELISAs (CLIA and kinetic and standard ELISAs) themselves, the combination of Borrelia burgdorferi sensu stricto and B. garinii VlsE proteins in the current assay versus only one source in the other studies, the antibody classes being measured, and the clinical samples being tested. In addition, establishment of negative- and positive-cutoff set points and the equivocal ranges was based on differing algorithms in the three studies. In the DiaSorin assay, these parameters are determined by reactivity differences between negative (healthy) and positive (LD) control samples whereas the Bacon et al. study utilized a targeted 99% specificity goal with a large set of healthy and alternate-disease (non-LD) negative controls to set a cutoff (5). In contrast, the Embers et al. study set cutoff points on the basis of 3 standard deviations above a mean reactivity for five negative (healthy) controls (15). With these caveats in mind, the LD samples evaluated by the respective VlsE assays yielded sensitivities of 73% for the current and Bacon et al. studies compared to 62% in the Embers et al. study. These compare to C6 sensitivities of 77%, 66%, and 78% for the current, Bacon et al., and Embers et al. studies, respectively. Thus, the current and Bacon et al. studies found no statistically significant sensitivity differences between the VlsE and C6 assays whereas the Embers et al. study found much higher sensitivity with the C6 assay than with the VlsE assay. The Embers et al. study also described antibody binding that was masked within the native VlsE molecule and made accessible only when smaller portions of the full-length protein were tested. The authors concluded that full-length VlsE assay reactivity is largely due to detection of antibody binding to conformational and/or VR epitopes, while the C6-based reactivity measures more binding to linear- and to constant-region epitopes. We agree with these conclusions. The Embers et al. sample set was a small subset of samples in the current study. Comparative sensitivities with the common samples were 73% versus 62% for VlsE-based assays and 84% versus 78% for C6-based assays in the current and Embers et al. studies, respectively. Thus, increased sensitivity is observed for both assays in the current study compared to that of Embers et al., and while the C6 sensitivity (29/37 [78%]) in the Embers et al. study is higher than that of VlsE (27/37 [73%]) in the current study, these differences are statistically insignificant. We believe that further comparative test performance will be best assessed through direct side-by-side comparison.
Intertwined with the performance characteristics of first-tier serology tests are those of second-tier WB. A specific but insensitive WB test may invalidate a sensitive and specific first-tier test (43). Moreover, the persistence of IgM and IgG antibodies to some B. burgdorferi antigens months to years after successful therapy may complicate the use of serologic testing when a patient is evaluated for a new infection (17). Understandably, these issues complicate the use and interpretation of WB for diagnosis of both acute and chronic LD. To help clinicians determine the predictive value of the serologic diagnosis of LD, the American College of Physicians has published clinical guidelines (4). These guidelines stress that serology testing should be reserved for patients with objective clinical signs and whose pretest probability for LD is between 0.20 and 0.80. However, since the incidence of LD and competent tick infection rates vary significantly in different geographic areas, determining pretest probability will remain a difficult task (51).
This study reveals that serodiagnosis of LD using a recombinant VlsE-based antigen in an automated CLIA system is a sensitive, specific, and reliable alternative to diagnosis that currently relies on enzyme immunoassays. Comparative data from the method comparison study reveals a high level of concordance to results from an existing FDA-cleared device that employs a synthetic C6 peptide. The improved specificity afforded by recombinant technology over traditional whole-cell bacterial antigen preparations coupled with the automated sample processing of the Liaison analyzer should reduce the need for expensive and time-consuming WB analysis.
Acknowledgments
We thank Wendy Y. Craig and Gavin Welch for their technical assistance and DiaSorin, Inc., for providing the reagents and supplies to perform the analysis.
Footnotes
Published ahead of print on 22 October 2008.
REFERENCES
- 1.Aguero-Rosenfeld, M. E., J. Nowakowski, S. Bittker, D. Cooper, R. B. Nadelman, and G. P. Wormser. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J. Clin. Microbiol. 34:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguero-Rosenfeld, M. E., G. Wang, I. Schwartz, and G. P. Wormser. 2005. Diagnosis of Lyme borreliosis. Clin. Microbiol. Rev. 18:484-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akin, E., G. L. McHugh, R. A. Flavell, E. Fikrig, and A. C. Steere. 1999. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 67:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Physicians. 1997. Guidelines for laboratory evaluation in the diagnosis of Lyme disease. Ann. Intern. Med. 127:1106-1108. [DOI] [PubMed] [Google Scholar]
- 5.Bacon, R. M., B. J. Biggerstaff, M. E. Schriefer, R. D. Gilmore, Jr., M. T. Philipp, A. C. Steere, G. P. Wormser, A. R. Marques, and B. J. Johnson. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakken, L. L., S. M. Callister, P. J. Wand, and R. F. Schell. 1997. Interlaboratory comparison of test results for detection of Lyme disease by 516 participants in the Wisconsin State Laboratory of Hygiene/College of American Pathologists Proficiency Testing Program. J. Clin. Microbiol. 35:537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakken, L. L., K. L. Case, S. M. Callister, N. J. Bourdeau, and R. F. Schell. 1992. Performance of 45 laboratories participating in a proficiency testing program for Lyme disease serology. JAMA 268:891-895. [PubMed] [Google Scholar]
- 8.Barbour, A. G. 1990. Antigenic variation of a relapsing fever Borrelia species. Annu. Rev. Microbiol. 44:155-171. [DOI] [PubMed] [Google Scholar]
- 9.Bergstrom, S., A. Sjostedt, L. Dotevall, B. Kaijser, B. Ekstrand-Hammarstrom, C. Wallberg, G. Skogman, and A. G. Barbour. 1991. Diagnosis of Lyme borreliosis by an enzyme immunoassay detecting immunoglobulin G reactive to purified Borrelia burgdorferi cell components. Eur. J. Clin. Microbiol. Infect. Dis. 10:422-427. [DOI] [PubMed] [Google Scholar]
- 10.CDC. 1997. Lyme disease—United States, 1996. MMWR Morb. Mortal. Wkly. Rep. 46:531-535. [PubMed] [Google Scholar]
- 11.CDC. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 12.CLSI. 2005. User verification of performance for precision and trueness. Approved guideline EP15-A2, 2nd ed. CLSI, Wayne, PA.
- 13.DePietropaolo, D. L., J. H. Powers, J. M. Gill, and A. J. Foy. 2005. Diagnosis of Lyme disease. Am. Fam. Physician 72:297-304. [PubMed] [Google Scholar]
- 14.Eicken, C., V. Sharma, T. Klabunde, M. B. Lawrenz, J. M. Hardham, S. J. Norris, and J. C. Sacchettini. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J. Biol. Chem. 277:21691-21696. [DOI] [PubMed] [Google Scholar]
- 15.Embers, M. E., G. P. Wormser, I. Schwartz, D. S. Martin, and M. T. Philipp. 2007. Borrelia burgdorferi spirochetes that harbor only a portion of the lp28-1 plasmid elicit antibody responses detectable with the C6 test for Lyme disease. Clin. Vaccine Immunol. 14:90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fawcett, P. T., C. D. Rose, and V. Maduskuie. 2004. Long-term effects of immunization with recombinant lipoprotein outer surface protein A on serologic test for Lyme disease. Clin. Diagn. Lab. Immunol. 11:808-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feder, H. M., Jr., M. A. Gerber, S. W. Luger, and R. W. Ryan. 1992. Persistence of serum antibodies to Borrelia burgdorferi in patients treated for Lyme disease. Clin. Infect. Dis. 15:788-793. [DOI] [PubMed] [Google Scholar]
- 18.Gassmann, G. S., E. Jacobs, R. Deutzmann, and U. B. Gobel. 1991. Analysis of the Borrelia burgdorferi GeHo fla gene and antigenic characterization of its gene product. J. Bacteriol. 173:1452-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gern, L., C. M. Hu, E. Kocianova, V. Vyrostekova, and J. Rehacek. 1999. Genetic diversity of Borrelia burgdorferi sensu lato isolates obtained from Ixodes ricinus ticks collected in Slovakia. Eur. J. Epidemiol. 15:665-669. [DOI] [PubMed] [Google Scholar]
- 20.Goettner, G., U. Schulte-Spechtel, R. Hillermann, G. Liegl, B. Wilske, and V. Fingerle. 2005. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J. Clin. Microbiol. 43:3602-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen, K., J. M. Bangsborg, H. Fjordvang, N. S. Pedersen, and P. Hindersson. 1988. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect. Immun. 56:2047-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen, K., K. Pii, and A. M. Lebech. 1991. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a μ-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J. Clin. Microbiol. 29:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heikkila, T., I. Seppala, H. Saxen, J. Panelius, H. Yrjanainen, and P. Lahdenne. 2002. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 40:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jouda, F., M. Crippa, J. L. Perret, and L. Gern. 2003. Distribution and prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks of canton Ticino (Switzerland). Eur. J. Epidemiol. 18:907-912. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan, I. V., and S. S. Levinson. 1999. When is a heterophile antibody not a heterophile antibody? When it is an antibody against a specific immunogen. Clin. Chem. 45:616-618. [PubMed] [Google Scholar]
- 26.Karlsson, M., I. Mollegard, G. Stiernstedt, and B. Wretlind. 1989. Comparison of Western blot and enzyme-linked immunosorbent assay for diagnosis of Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 8:871-877. [DOI] [PubMed] [Google Scholar]
- 27.Koide, S., X. Yang, X. Huang, J. J. Dunn, and B. J. Luft. 2005. Structure-based design of a second-generation Lyme disease vaccine based on a C-terminal fragment of Borrelia burgdorferi OspA. J. Mol. Biol. 350:290-299. [DOI] [PubMed] [Google Scholar]
- 28.Lawrenz, M. B., J. M. Hardham, R. T. Owens, J. Nowakowski, A. C. Steere, G. P. Wormser, and S. J. Norris. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J. Clin. Microbiol. 37:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledue, T. B., M. F. Collins, and W. Y. Craig. 1996. New laboratory guidelines for serologic diagnosis of Lyme disease: evaluation of the two-test protocol. J. Clin. Microbiol. 34:2343-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 163:5566-5573. [PubMed] [Google Scholar]
- 31.Liang, F. T., A. C. Steere, A. R. Marques, B. J. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luft, B. J., P. D. Gorevic, W. Jiang, P. Munoz, and R. J. Dattwyler. 1991. Immunologic and structural characterization of the dominant 66- to 73-kDa antigens of Borrelia burgdorferi. J. Immunol. 146:2776-2782. [PubMed] [Google Scholar]
- 33.Ma, B., B. Christen, D. Leung, and C. Vigo-Pelfrey. 1992. Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J. Clin. Microbiol. 30:370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnarelli, L. A., J. F. Anderson, and R. C. Johnson. 1987. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J. Infect. Dis. 156:183-188. [DOI] [PubMed] [Google Scholar]
- 35.Magnarelli, L. A., E. Fikrig, S. J. Padula, J. F. Anderson, and R. A. Flavell. 1996. Use of recombinant antigens of Borrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J. Clin. Microbiol. 34:237-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magnarelli, L. A., J. W. Ijdo, S. J. Padula, R. A. Flavell, and E. Fikrig. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magnarelli, L. A., J. M. Meegan, J. F. Anderson, and W. A. Chappell. 1984. Comparison of an indirect fluorescent-antibody test with an enzyme-linked immunosorbent assay for serological studies of Lyme disease. J. Clin. Microbiol. 20:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marangoni, A., V. Sambri, S. Accardo, F. Cavrini, V. Mondardini, A. Moroni, E. Storni, and R. Cevenini. 2006. A decrease in the immunoglobulin G antibody response against the VlsE protein of Borrelia burgdorferi sensu lato correlates with the resolution of clinical signs in antibiotic-treated patients with early Lyme disease. Clin. Vaccine Immunol. 13:525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marangoni, A., M. Sparacino, V. Mondardini, F. Cavrini, E. Storni, M. Donati, R. Cevenini, and V. Sambri. 2005. Comparative evaluation of two enzyme linked immunosorbent assay methods and three Western blot methods for the diagnosis of culture-confirmed early Lyme borreliosis in Italy. New Microbiol. 28:37-43. [PubMed] [Google Scholar]
- 40.Marques, A. R., D. S. Martin, and M. T. Philipp. 2002. Evaluation of the C6 peptide enzyme-linked immunosorbent assay for individuals vaccinated with the recombinant OspA vaccine. J. Clin. Microbiol. 40:2591-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massarotti, E. M., S. W. Luger, D. W. Rahn, R. P. Messner, J. B. Wong, R. C. Johnson, and A. C. Steere. 1992. Treatment of early Lyme disease. Am. J. Med. 92:396-403. [DOI] [PubMed] [Google Scholar]
- 42.McDowell, J. V., S. Y. Sung, L. T. Hu, and R. T. Marconi. 2002. Evidence that the variable regions of the central domain of VlsE are antigenic during infection with Lyme disease spirochetes. Infect. Immun. 70:4196-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mogilyansky, E., C. C. Loa, M. E. Adelson, E. Mordechai, and R. C. Tilton. 2004. Comparison of Western immunoblotting and the C6 Lyme antibody test for laboratory detection of Lyme disease. Clin. Diagn. Lab. Immunol. 11:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molloy, P. J., D. H. Persing, and V. P. Berardi. 2001. False-positive results of PCR testing for Lyme disease. Clin. Infect. Dis. 33:412-413. [DOI] [PubMed] [Google Scholar]
- 45.Nadelman, R. B., and G. P. Wormser. 1998. Lyme borreliosis. Lancet 352:557-565. [DOI] [PubMed] [Google Scholar]
- 46.Peltomaa, M., G. McHugh, and A. C. Steere. 2003. Persistence of the antibody response to the VlsE sixth invariant region (IR6) peptide of Borrelia burgdorferi after successful antibiotic treatment of Lyme disease. J. Infect. Dis. 187:1178-1186. [DOI] [PubMed] [Google Scholar]
- 47.Philipp, M. T., L. C. Bowers, P. T. Fawcett, M. B. Jacobs, F. T. Liang, A. R. Marques, P. D. Mitchell, J. E. Purcell, M. S. Ratterree, and R. K. Straubinger. 2001. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J. Infect. Dis. 184:870-878. [DOI] [PubMed] [Google Scholar]
- 48.Philipp, M. T., G. P. Wormser, A. R. Marques, S. Bittker, D. S. Martin, J. Nowakowski, and L. G. Dally. 2005. A decline in C6 antibody titer occurs in successfully treated patients with culture-confirmed early localized or early disseminated Lyme borreliosis. Clin. Diagn. Lab. Immunol. 12:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rath, P. M., F. J. Fehrenbach, G. Rogler, H. D. Pohle, and A. Schonberg. 1992. Relapsing fever and its serological discrimination from Lyme borreliosis. Infection 20:283-286. [DOI] [PubMed] [Google Scholar]
- 50.Rauer, S., N. Spohn, C. Rasiah, U. Neubert, and A. Vogt. 1998. Enzyme-linked immunosorbent assay using recombinant OspC and the internal 14-kDa flagellin fragment for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 36:857-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed, K. D. 2002. Laboratory testing for Lyme disease: possibilities and practicalities. J. Clin. Microbiol. 40:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sayahtaheri-Altaie, S., F. A. Meier, and H. P. Dalton. 1993. Identification of species-specific, non-cross-reactive proteins of Borrelia burgdorferi. Diagn. Microbiol. Infect. Dis. 16:43-51. [DOI] [PubMed] [Google Scholar]
- 53.Smith, R. P. 2005. Current diagnosis and treatment of Lyme disease. Compr. Ther. 31:284-290. [DOI] [PubMed] [Google Scholar]
- 54.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 55.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 57.Steere, A. C., E. Taylor, G. L. McHugh, and E. L. Logigian. 1993. The overdiagnosis of Lyme disease. JAMA 269:1812-1816. [PubMed] [Google Scholar]
- 58.Strle, F., and M. Stantic-Pavlinic. 1996. Lyme disease in Europe. N. Engl. J. Med. 334:803. [DOI] [PubMed] [Google Scholar]
- 59.Takada, N., T. Masuzawa, F. Ishiguro, H. Fujita, M. Kudeken, H. Mitani, M. Fukunaga, K. Tsuchiya, Y. Yano, and X. H. Ma. 2001. Lyme disease Borrelia spp. in ticks and rodents from northwestern China. Appl. Environ. Microbiol. 67:5161-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trevejo, R. T., P. J. Krause, M. E. Schriefer, and D. T. Dennis. 2001. Evaluation of a two-test serodiagnostic method for community assessment of Lyme disease in an endemic area. Am. J. Trop. Med. Hyg. 65:563-566. [DOI] [PubMed] [Google Scholar]
- 61.Tugwell, P., D. T. Dennis, A. Weinstein, G. Wells, B. Shea, G. Nichol, R. Hayward, R. Lightfoot, P. Baker, and A. C. Steere. 1997. Laboratory evaluation in the diagnosis of Lyme disease. Ann. Intern. Med. 127:1109-1123. [DOI] [PubMed] [Google Scholar]
- 62.van Dam, A. P., H. Kuiper, K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaard, A. C. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708-717. [DOI] [PubMed] [Google Scholar]
- 63.Wang, G., A. P. van Dam, I. Schwartz, and J. Dankert. 1999. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 12:633-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilske, B., G. Schierz, V. Preac-Mursic, K. Weber, H. W. Pfister, and K. Einhaupl. 1984. Serological diagnosis of erythema migrans disease and related disorders. Infection 12:331-337. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, Z. F., K. L. Wan, and J. S. Zhang. 1997. Studies on epidemiology and etiology of Lyme disease in China. Zhonghua Liu Xing Bing Xue Za Zhi. 18:8-11. [In Chinese.] [PubMed] [Google Scholar]