Abstract
Efforts to develop a better diagnostic assay for bovine tuberculosis have shown that the sensitivity and specificity of an assay can be improved by the use of two or more antigens. As reported here, we developed a multiplex chemiluminescent immunoassay that can simultaneously detect antibody activity to 25 antigens in a single well in a 96-well plate array format. The chemiluminescent signal is captured with a digital imaging system and analyzed with a macro program that tracks each serum for its pattern of antibody activity for Mycobacterium bovis antigens. The comparison of sera from 522 infected and 1,489 uninfected animals showed that a sensitivity of 93.1% and a specificity of 98.4% can be achieved with a combination of antigens. The assay system is rapid and can be automated for use in a centralized laboratory.
Bovine tuberculosis (bTB) continues to be a zoonotic disease affecting multiple species, including humans (3, 9, 21, 26). The disease has been difficult to control in livestock because of the lack of an effective vaccine, the presence of wildlife reservoirs, and the lack of a diagnostic assay with sufficient sensitivity and specificity to detect animals at all stages of infection. Currently, the primary methods used for the detection of TB in humans and ruminants include the measurement of a delayed-type hypersensitivity (skin test) to purified protein derivative (PPD) and an indirect in vitro assay that measures the concentration of gamma interferon (IFN-γ) produced in response to stimulation with PPD (22, 30, 31). Although the methods have proven useful in controlling bTB, they lack sensitivity and specificity because of a cross-reactive immune response to T- and B-cell epitopes conserved on orthologous molecules present in nonpathogenic mycobacteria and Mycobacterium avium subsp. paratuberculosis (reviewed in references 8, 23, and 27). To obviate this problem, an extensive effort has been under way to identify and characterize antigens unique to Mycobacterium bovis that could be used in a diagnostic assay. To date, studies have shown that the antibody response to M. bovis is not uniform, with no evidence of a dominant persistent response to a single antigen (reviewed in references 4, 7, and 8) at any stage of infection (2, 19). This finding has suggested that some type of a multiplex assay is needed to detect animals at different stages of infection (1, 2). However, the necessity of using multiple antigens in an assay has introduced another challenge. The evaluation of the standard type of enzyme-linked immunosorbent assay (ELISA) has shown that sensitivity and specificity are reduced when multiple antigens are combined for analysis in a single well, thus limiting the way a conventional ELISA can be used (20). To address this problem, we developed a multiplex assay that can simultaneously detect and analyze the response to multiple antigens spotted in a single well in a 96-well plate array format. We demonstrate the enhanced diagnostic power of a multiplex antigen approach over that of the industry-standard methods (8).
MATERIALS AND METHODS
Serum samples.
Serum samples used in the study were obtained from several sources. Blood samples were taken into serum tubes (serum clot activator tubes; Vacuette; Greiner-Bio-One), transported at room temperature, and then stored at 2 to 8°C until processed. Following centrifugation (3,000 × g for 30 min at 2 to 8°C) the serum was removed, aliquoted, and stored at −20°C.
The TB-negative sera were obtained from the Irish Department of Agriculture from herds of animals with a known history of being free of M. bovis for at least 5 years. The TB-positive group of sera was collected from animals that were proven to be positive for M. bovis infection at the time of slaughter based on subsequent histopathological/bacteriological examination.
The third set of serum samples was from a bovine tuberculosis infectivity trial undertaken by AgResearch (New Zealand). The sera were from 8-month-old calves that were nonvaccinated but challenged via the intratracheal route with a low dose of a virulent strain of M. bovis (approximately 5,000 CFU). Sera were collected prior to challenge and then at 2, 5, 10, and 17 weeks postinfection (p.i.). A single intradermal comparative cervical tuberculin test (SICCT) was carried out prior to challenge and also during week 15 p.i. All animals in the study had lesions typical of a TB infection consisting of a series of small lung lesions (diameter, 1 to 5 mm) or pulmonary lymph node lesions ranging from 5 to 40 mm in diameter, and all animals at 17 weeks p.i. were culture positive for M. bovis (Table 1).
TABLE 1.
Summary of infectivity study samplesa
| Sample code | Lung lesion scoreb | Maximum diam (mm) of lymph node lesionc
|
Tissues confirmed M. bovis positive by culture | |||
|---|---|---|---|---|---|---|
| AM | LB | PM | RB | |||
| A | 5 | — | 15 | 15 | — | Lungs; AM, LB, and PM lymph nodes |
| B | 0 | 12 | 5 | 8 | 8 | AM, LB, PM, and RB lymph nodes |
| C | 3 | — | 8 | — | — | Lungs; AM and LB lymph nodes |
| D | 5 | 15 | 40 | 30 | — | Lungs; AM, LB, PM, and RB lymph nodes |
| E | 5 | 5 | — | — | — | Lungs; AM, LB, PM, and RB lymph nodes |
All animals were confirmed to be without infection prior to challenge by SICCT, and lesions were measured at 17 weeks p.i. Abbreviations for lymph node locations: AM, anterior mediastinal; LB, left bronchial; PM, posterior mediastinal; and RB, right bronchial.
Lung lesion scores: 0, no lesions; 1, 1 to 9 lesions; 2, 10 to 29 lesions; 2, 30 to 99 lesions; 4, 100 to 199 lesions; and 5, ≥200 lesions.
—, no lymph node lesion.
Histopathology.
Tissue sections were stained with hematoxylin and eosin for histopathological examination. A diagnosis of tuberculosis was based on the presence of a granulomatous lymphadenitis associated with areas of caseous necrosis (6).
Culture.
The decontamination of tissues with 5% oxalic acid was performed as described by Costello et al. (6). Samples were cultured on a MGIT 960 liquid culture system (Becton Dickinson, MD) and Stonebrinks medium. Isolate identification was performed by AccuProbe (Gen-Probe Inc., San Diego, CA) and GenoType MTBC (Hain Diagnostika, Nehren, Germany).
Preparation of antigens.
The genes encoding different TB proteins were expressed in Escherichia coli as N-terminal polyhistidine-tagged (6× His) fusion proteins by Fusion Antibodies Ltd. (Belfast) using the patented fusion expression technology (FET) platform. Recombinant proteins were purified and polished to near homogeneity by using the Fusion Antibodies Ltd. three-step chromatographic protocol. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis were performed on all purified and polished recombinant TB proteins to confirm that their level of purity was greater than 99%. Synthetic peptides were synthesized by Genosphere Biotechnologies (France) to a purity of >80% (by reverse-phase high-performance liquid chromatography at 220 nm). Amino acid residues were added to peptides to enhance the hydrophilicity of the peptides. Lyophilized peptides were reconstituted to 2 mg/ml in sterile phosphate-buffered saline, pH 7.4 (Sigma Aldrich, Dublin), and aliquots were prepared and stored at −20°C. Antigen quantification was performed using the micro-bicinchoninic acid protein assay kit (Pierce, Rockford, IL). See Table 2 for the list of antigens (recombinant proteins and synthetic peptides) used in this study.
TABLE 2.
Antigens used in this studya
| Protein | M. bovis locus tag |
|---|---|
| α-Crystalin_2 | Mb2057 |
| HspX | Mb2057c |
| CFP-10 | Mb3904 |
| RPFc | Mb1916c |
| Rv2878 | Mb2904c |
| ESAT-6 | Mb3905 |
| PPE68 | Mb3903 |
| Rv3616c | Mb3646c |
| Rv3879c | Mb3909c |
| MPB70 | Mb2900 |
| MPB83 | Mb2898 |
| Rv0283 | Mb0291 |
| Rv2626 | Mb2659c |
| Rv1926c | Mb1961c |
| EsxH | Mb0296 |
| PE35 | Mb3902 |
| Rv1573c | Mb1599 |
| Rv1585c | Mb1611c |
| Rv1580c | Mb1606c |
| Rv1572c | Mb1598A |
Antigens in boldface were investigated during this study but were not included in the final panel of antigens.
Multiplex antigen printing.
Antigens used in this study were printed in each well of a black polystyrene 96-well plate in a multiplex planar array format by Quansys Biosciences (Logan, UT). The optimization of the antigen printing was carried out in conjunction with Enfer Scientific (Naas, Ireland). Plates were printed by Quansys Biosciences, and the testing of plates was carried out at Enfer Scientific. Antigen-coating concentration optimization also was carried out at Enfer Scientific using a series of coating titrations printed in the optimized printing buffer. In order to optimize the spot morphology, a fluorescent marker was incorporated into all printing buffers at a concentration of 0.033 μg/ml. This allowed for visualizations of the spots using transillumination. Panels of antigens were printed using the optimized printing protocol for sample testing. All plates were shipped and stored at 2 to 8°C. Plates were allowed to warm to room temperature for 30 min prior to use.
Multiplex immunoassay.
The multiplex immunoassay method was developed in-house at Enfer Scientific. Serum samples were diluted 1:250 into sample dilution buffer (Enfer buffer A; Enfer Scientific) and mixed. A 30-μl sample dilution was added to each well. Sample dilution and plating were carried out using an automated pipettor Tecan Genesis RSP 150 (Tecan, United Kingdom). The plates were incubated at room temperature with agitation (900 rpm) for 30 min. The plates were washed with 1× Enfer wash buffer (Enfer Scientific) six times and aspirated. The detection antibody (polyclonal rabbit anti-bovine immunoglobulin horseradish peroxidase; Dako, Denmark) was prepared to a dilution of 1:3,000 in detection antibody dilution buffer (Enfer buffer B; Enfer Scientific). After the addition of 30 μl of the detection antibody to test wells, the plates were incubated at room temperature for 15 min with agitation (900 rpm). The plates were washed as described above, and 30 μl of substrate (50:50 dilution of substrate and diluent; Quansys Biosciences, Logan, UT) was added per well. Signals were captured during a 45-s exposure using a Quansys Biosciences Imaging system. Images were saved as CR2 image files. Data were extracted from the captured images as relative light units (RLU) using the Quansys Q-View software, version 2.0.
Anigen lateral-flow testing of sera.
The testing of sera was carried out according to the manufacturer;s instructions using the Anigen Rapid Bovine TB Ab lateral-flow test kit (Animal Genetics Inc., Kyonggi-do, Korea). Following warming to room temperature, 4 drops of the sera were added to the sample deposition area on the lateral-flow cassette. The test was read after 20 min. Only tests with a positive control line were considered for further evaluation. In total, 214 TB-positive sera samples and 79 TB-negative samples were tested.
Data analysis.
The data extracted from plate images with the Quansys software were compiled and analyzed with a custom-made macro in Microsoft Excel (Enfer multiplex macro, version 1.0.1.0). The macro was developed for the multifactorial comparative analysis of the sensitivity and specificity of the multiple antigens used in a single well in a 96-well format. The analysis combines the data from individual antigens and, based on a threshold for each, determines the result of the test for any serum sample. A serum sample must be positive for a minimum of any two antigens in a test well to be considered positive. This method of analysis avoids the assessment of the sensitivity and specificity of individual antigens used in the study and increases the overall accuracy of the assay.
Statistical analysis was done on the infectivity study animal sample sets with the Student's t test using SigmaStat software (Systat, San Jose, CA).
RESULTS
Development of the multiplex immunoassay.
Preliminary studies were conducted to optimize and obtain the uniform binding of each antigen using a fluorescent tag. Plates were tested and evaluated by Enfer Scientific for signal variation using a panel of serum samples. The testing of the plates with the detection antibody, with and without sera from a panel of control sera from uninfected cattle, showed that background noise in the system in areas of the surface not coated with antigens was, on average, 779.3 RLU. The optimization of the printing concentration for all antigens was carried out on a series of plates printed using sera from the TB-negative and TB-positive samples. Optimal printing concentrations were determined for all antigens and then were tested with larger sets of positive and negative sera before the subsequent testing of all samples for the determination of overall assay sensitivity and specificity. Assay optimization was carried out based on initial work done at Enfer Scientific using a single-plex ELISA on Nunc Maxisorp 96-well microtiter plates. The optimal exposure time was determined to be 45 s.
The bioinformatic analysis of pathogenic and environmental strains of mycobacteria facilitated the identification of a series of proteins that were likely to be antigenic (i.e., cell surface or secreted) and disease selective. In total, we report on 20 antigens and highlight the most important 13 that can be used in the diagnosis of M. bovis infection in cattle (Table 2).
A total of 1,489 TB-negative and 522 TB-positive sera were screened against the panel of antigens. The diagnostic value of each antigen was calculated based on its ability to correctly identify a known positive/negative sample. This allowed us to select individual thresholds (cutoffs) for each antigen that best suited their inclusion in a multiplex format. In certain instances, the sensitivity of an antigen was sacrificed by increasing the threshold in order to improve its specificity. The individual sensitivities of the antigens ranged from 5.4 to 95.0%, while individual specificities ranged from 69.1 to 99.1% (Table 3). The in-house macro was used to run a combinatorial data analysis to calculate the highest specificity and sensitivity based on the panel of antigens with the cohorts of samples tested. A two-positive-protein rule was applied to the analysis. For a sample to be considered a true positive, a response to at least two antigens must be observed above the individual thresholds for each of the antigens being tested. This rule increases the overall accuracy of the assay. The individual specificities and sensitivities for three antigens currently under study (ESAT-6, CFP-10, and MPB83) are compared to that of the multiplex assay and a commercial antigen assay in Table 3.
TABLE 3.
Cohorts of samples tested by Enfer multiplex immunoassay and the Anigen lateral-flow kit and individual responses to ESAT-6, CFP-10, and MPB83 antigens
| Test | TB-positive animals (no.) | Sensitivity (%) | TB-negative animals (no.) | Specificity (%) |
|---|---|---|---|---|
| Enfer multiplex immunoassay | 522 | 93.1 | 1,489 | 98.4 |
| ESAT-6 | 522 | 40.6a | 1,489 | 86.6a |
| CFP-10 | 522 | 82.6a | 1,489 | 69.7a |
| MPB83 | 522 | 78.5a | 1,489 | 99.1a |
| Anigen lateral flow | 214 | 83.6b | 79 | 83.0c |
Data were extracted from the multiplex assay for these individual antigens.
The sensitivity of the Anigen lateral-flow kit is 90.0% compared to that of culture-confirmed positives, and it is 85.1% compared to that of skin test-confirmed positives according to the manufacturer's instructions.
The specificity of the Anigen lateral-flow kit is 98.6% compared to that of skin test-confirmed negative animals according to the manufacturer's instructions.
Screening of infectivity study samples.
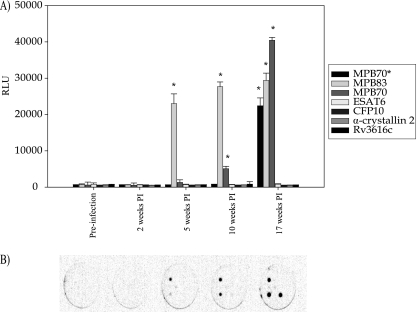
A total of five animals that were challenged with a dose of approximately 5,000 CFU M. bovis were tested on the multiplex assay. Serum samples were tested from the animals at the following time points: prechallenge (time zero) and then 2, 5, 10, and 17 weeks p.i. All animals were positive by the 10th week p.i. using the multiplex assay. Three of the five animals were detected at 5 weeks p.i. (Table 4). The time points at which each animal was determined to be positive on the Enfer multiplex immunoassay, as well as results for ESAT-6, CFP-10, and MPB83 antigens, are shown (Table 4). Figure 1A shows a representative histogram in terms of the signal (in RLU) obtained from test wells at the indicated time points for one of the infected animals (animal A), which showed a positive response by week 5. A representative image for a panel of antigens is shown in Fig. 1B.
TABLE 4.
Results for animal serum samples from the infectivity study prechallenge and at 2, 5, 10, and 17 weeks postchallenge
| Sample code | Wk | Assay result
|
|||
|---|---|---|---|---|---|
| Enfer multiplex immunoassay | ESAT-6a | CFP-10a | MPB83a | ||
| A | 0 | − | − | − | − |
| 2 | − | − | − | − | |
| 5 | + | − | − | + | |
| 10 | + | − | − | + | |
| 17 | + | − | − | + | |
| B | 0 | − | − | − | − |
| 2 | + | − | + | − | |
| 5 | − | − | − | − | |
| 10 | + | − | − | − | |
| 17 | + | − | + | + | |
| C | 0 | − | − | − | − |
| 2 | − | − | − | − | |
| 5 | − | − | − | − | |
| 10 | + | + | − | − | |
| 17 | + | + | + | + | |
| D | 0 | − | − | − | − |
| 2 | − | − | − | − | |
| 5 | + | − | − | + | |
| 10 | + | − | − | + | |
| 17 | + | − | − | + | |
| E | 0 | − | − | − | − |
| 2 | − | − | − | − | |
| 5 | + | − | − | + | |
| 10 | + | − | + | − | |
| 17 | + | − | − | + | |
Data were extracted from the Enfer multiplex immunoassay for these individual antigens.
FIG. 1.
(A) Data (in RLU) from the multiplex immunoassay obtained for animal A in the infectivity study group of animals showing representative results. Note that the pattern of response varied for each individual animal tested (*MPB70 is a peptide preparation). Significance (P < 0.05) between the prechallenge sample and the postchallenge samples are indicated by an asterisk. (B) Representative image for a panel of antigens. Each well shown represents a sample point (prechallenge and 2, 5, 10, and 17 weeks p.i.) for one animal.
Comparison of tests.
To compare the sensitivity and specificity of the multiplex assay to those of an assay based on a single antigen, we conducted a set of experiments with a commercial assay available to us, the Anigen lateral-flow assay. The Anigen lateral-flow test kit uses a recombinant MPB70 antigen for the detection of antibodies. The results with the sera selected for the comparison with the Enfer multiplex immunoassay are shown in Table 3. The analysis of results from the Enfer multiplex immunoassay showed a specificity of 98.4% and a sensitivity of 93.1% (1,489 negative and 522 positive sera). The analysis of the Anigen lateral-flow kit showed a specificity of 84.2% and a sensitivity of 84.0% (79 negative and 214 positive sera) (Table 3).
DISCUSSION
Cumulative studies have emphasized that the development of a robust rapid diagnostic assay for M. bovis infection will require the use of a multiplex system that can simultaneously detect and analyze antibody activity in response to multiple antigens in a serum sample (1, 2, 7, 19, 29). The various approaches taken to develop such an assay have included the examination of latex bead agglutination, immunochromatographic assays (2, 12, 13, 20), fluorescence polarization (15, 28), electrochemiluminescence (17, 32), and chemiluminescence (14). As reported here, the chemiluminescence-based multiplex platform developed by Quansys Biosciences for use in nonveterinary assays has proven to be an ideal platform for the development of a multiplex assay for bTB (14, 25). The initial use of the assay with a panel of expressed antigens and peptides, including some of the best-known antibody targets in M. bovis infection, has shown that the overall sensitivity and specificity is increased by using more than one antigen in the assay. A specificity of 98.4% and a sensitivity of 93.1% were obtained with a cohort of known M. bovis-positive and -negative sera using a minimum criterion of a positive reaction with any two antigens in the array. Other groups also recognized a higher level of diagnostic accuracy through the use of multiple antigens. Multiantigen print immunoassay lateral-flow developments (18, 20) and the use of fusion proteins (16) both illustrate the potential for increasing sensitivity by employing combinations of suitable antigens.
The five-animal infectivity study reported here, using the multiplex assay, demonstrates that the assay has the capability of identifying infection as early as 2 weeks p.i. Furthermore, all animals were successfully identified as positive by 10 weeks p.i. Further infectivity study trials are planned that will utilize serial samples from animals receiving different challenge doses and will be assessed for reactivity with panels of antigens on the multiplex platform.
Currently, the available tests (using SICCT and IFN-γ) have been reported to cover a range of sensitivities and specificities (6), and the combined use of these tests has the potential to increase the overall sensitivity and specificity of the testing. With these tests come some restrictions; for example, routine SICCTs can be carried out only at 60-day intervals (EU Directive 64/432, European Economic Community). The IFN-γ assay is a blood-based assay that may be influenced by the time lapse between blood sampling and conducting the assay, as reported by Gormley et al. (10, 11).
The application of the multiplex assay alone or in combination with the current SICCT and IFN-γ methods (6, 10, 11) of identifying M. bovis-infected animals could improve the detection of infected animals at earlier stages of infection. In addition, the improved ability to screen multiple M. bovis antigens on a versatile multiplex platform provides an opportunity to develop a better diagnostic test not only for cattle but also for other species. Species that could be considered in this context are other domestic ruminants and potential bTB wildlife reservoirs, such as deer and badgers. Recent reports emphasize that there is also a need for the development of a suitable ante mortem bTB test for these species (5, 24).
Acknowledgments
We thank all of the staff who participated in the project at Enfer Scientific; AgResearch staff who contributed to the infectivity study; Eamonn Costello and the staff of the Tuberculosis Laboratory Central Veterinary Research Laboratory (Backweston, Co. Kildare), where the culture and histopathology work was carried out; and Margaret Good and the staff of the Irish Department of Agriculture and Food, Dublin 2, Ireland, for sample acquisition.
This study was funded by Enfer Scientific.
Footnotes
Published ahead of print on 15 October 2008.
REFERENCES
- 1.Aagaard, C., M. Govaerts, V. Meikle, A. J. Vallecillo, J. A. Gutierrez-Pabello, F. Suarez-Güemes, J. McNair, A. Cataldi, C. Espitia, P. Andersen, and J. M. Pollock. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J. Clin. Microbiol. 44:4326-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadori, M., K. P. Lyashchenko, M. L. Gennaro, J. M. Pollock, and I. Zerbini. 2002. Use of recombinant proteins in antibody tests for bovine tuberculosis. Vet. Microbiol. 85:379-389. [DOI] [PubMed] [Google Scholar]
- 3.Amanfu, W. 2006. The situation of tuberculosis and tuberculosis control in animals of economic interest. Tuberculosis 86:330-335. [DOI] [PubMed] [Google Scholar]
- 4.Aranaz, A., L. De Juan, J. Bezos, J. Álvarez, B. Romero, F. Lozano, J. L. Paramio, J. López-Sánchez, A. Mateos, and L. Domínguez. 2006. Assessment of diagnostic tools for eradication of bovine tuberculosis in cattle co-infected with Mycobacterium bovis and M. avium subsp. paratuberculosis. Vet. Res. 37:593-606. [DOI] [PubMed] [Google Scholar]
- 5.Corner, L. A. L. 2006. The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: how to assess the risk. Vet. Microbiol. 112:303-312. [DOI] [PubMed] [Google Scholar]
- 6.Costello, E., F. Quigley, O. Flynn, A. Gigarty, J. McGuirk, A. Murphy, and L. Dolan. 1998. Laboratory examination of suspect tuberculosis lesions detected on abattoir post mortem examination of cattle from non-reactor herds. Irish Vet. J. 51:248-250. [Google Scholar]
- 7.Cousins, D. V., and N. Florisson. 2005. A review of tests available for use in the diagnosis of tuberculosis in non-bovine species. Rev. Sci. Tech. 24:1039-1059. [PubMed] [Google Scholar]
- 8.de la Rua-Domenech, R., A. T. Goodchild, H. M. Vordermeier, R. G. Hewinson, K. H. Christiansen, and R. S. Clifton-Hadley. 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci. 81:190-210. [DOI] [PubMed] [Google Scholar]
- 9.De Vos, V., R. G. Bengis, N. P. Kriek, A. Michel, D. F. Keet, J. P. Raath, and H. F. Huchzermeyer. 2001. The epidemiology of tuberculosis in free-ranging African buffalo (Syncerus caffer) in the Kruger National Park, South Africa. Onderstepoort J. Vet. Res. 68:119-130. [PubMed] [Google Scholar]
- 10.Gormley, E., M. B. Doyle, T. Fitzsimons, K. McGill, and J. D. Collins. 2006. Diagnosis of Mycobacterium bovis infection in cattle by use of the gamma-interferon (Bovigam) assay. Vet. Microbiol. 112:171-179. [DOI] [PubMed] [Google Scholar]
- 11.Gormley, E., M. B. Doyle, K. McGill, E. Costello, M. Good, and J. D. Collins. 2004. The effect of the tuberculin test and the consequences of a delay in blood culture on the sensitivity of a gamma-interferon assay for the detection of Mycobacterium bovis infection in cattle. Vet. Immunol. Immunopathol. 102:413-420. [DOI] [PubMed] [Google Scholar]
- 12.Koo, H. C., Y. H. Park, J. Ahn, W. R. Waters, M. J. Hamilton, G. M. Barrington, A. A. Mosaad, M. V. Palmer, S. Shin, and W. C. Davis. 2004. New latex bead agglutination assay for differential diagnosis of cattle infected with Mycobacterium bovis and Mycobacterium avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo, H. C., Y. H. Park, J. Ahn, W. R. Waters, M. V. Palmer, M. J. Hamilton, G. M. Barrington, A. A. Mosaad, K. T. Park, W. K. Jung, I. Y. Hwang, S.-N. Cho, S. J. Shin, and W. C. Davis. 2005. Use of rMPB70 protein and ESAT-6 peptide as antigens for comparison of the enzyme-linked immunosorbent immunochromatographic, and latex bead agglutination assays for serodiagnosis of bovine tuberculosis. J. Clin. Microbiol. 43:4498-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liew, M., M. C. Groll, J. E. Thompson, S. L. Call, J. E. Moser, J. D. Hoopes, K. Voelkerding, C. Wittwer, and R. S. Spendlove. 2007. Validating a custom multiplex ELISA against individual commercial immunoassays using clinical samples. BioTechniques 42:327-333. [DOI] [PubMed] [Google Scholar]
- 15.Lin, M., E. A. Sugden, M. E. Jolley, and K. Stilwell. 1996. Modification of the Mycobacterium bovis extracellular protein MPB70 with fluorescein for rapid detection of specific serum antibodies by fluorescence polarization. Clin. Diagn. Lab. Immunol. 3:438-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, S., S. Guo, C. Wand, M. Shao, X. Zhang, Y. Guo, and Q. Gong. 2007. A novel fusion protein-based indirect enzyme-linked immunosorbent assay for the detection of bovine tuberculosis. Tuberculosis 87:212-217. [DOI] [PubMed] [Google Scholar]
- 17.Lu, Y., W. L. Wong, and Y. G. Meng. 2006. A high throughput electrochemiluminescent cell-binding assay for therapeutic anti-CD20 antibody selection. J. Immunol. Methods 314:74-79. [DOI] [PubMed] [Google Scholar]
- 18.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, D. Greenwald, C. A. Nacy, S. Gibson, P. J. Didier, M. Washington, P. Szczerba, S. Motzel, L. Handt, J. M. Pollock, J. McNair, P. Andersen, J. A. M. Langermans, F. Verreck, S. Ervin, F. Ervin, and C. McCombs. 2007. PrimaTB STAT-PAK assay, a novel, rapid lateral-flow test for tuberculosis in nonhuman primates. Clin. Vaccine Immunol. 14:1158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyashchenko, K. P., J. M. Pollock, R. Colangeli, and M. L. Gennaro. 1998. Diversity of antigen recognition by serum antibodies in experimental bovine tuberculosis. Infect. Immun. 66:5344-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J. Immunol. Methods 242:91-100. [DOI] [PubMed] [Google Scholar]
- 21.Michel, A. L., R. G. Bengis, D. F. Keet, M. Hofmeyr, L. M. de Klerk, P. C. Cross, A. E. Jolles, D. Cooper, I. J. Whyte, P. Buss, and J. Godfroid. 2006. Wildlife tuberculosis in South African conservation areas: implications and challenges. Vet. Microbiol. 112:91-100. [DOI] [PubMed] [Google Scholar]
- 22.Monaghan, M. L., M. L. Doherty, J. D. Collins, J. F. Kazda, and P. J. Quinn. 1994. The tuberculin test. Vet. Microbiol. 40:111-124. [DOI] [PubMed] [Google Scholar]
- 23.Neill, S. D., R. A. Skuce, and J. M. Pollock. 2005. Tuberculosis—new light from an old window. J. Appl. Microbiol. 98:1261-1269. [DOI] [PubMed] [Google Scholar]
- 24.Partridge, T., D. Toolan, J. Egan, and S. More. 2008. Control of Mycobacterium bovis infection in two sika deer herds in Ireland. Irish Vet. J. 61:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickering, J. W., J. D. Hoopes, M. C. Groll, H. K. Romero, D. Wall, H. Sant, M. E. Astill, and H. R. Hill. 2007. A 22-plex chemiluminescent microarray for pneumococcal antibodies. Am. J. Clin. Pathol. 127:23-31. [DOI] [PubMed] [Google Scholar]
- 26.Pollock, J. M., and S. D. Neill. 2002. Mycobacterium bovis infection and tuberculosis in cattle. Vet. J. 163:115-127. [DOI] [PubMed] [Google Scholar]
- 27.Pollock, J. M., M. D. Welsh, and J. McNair. 2005. Immune responses in bovine tuberculosis: towards new strategies for the diagnosis and control of disease. Vet. Immunol. Immunopathol. 108:37-43. [DOI] [PubMed] [Google Scholar]
- 28.Surujballi, O. P., A. Romanowska, E. A. Sugden, C. Turcotte, and M. E. Jolley. 2002. A fluorescence polarization assay for the detection of antibodies to Mycobacterium bovis in cattle sera. Vet. Microbiol. 87:149-157. [DOI] [PubMed] [Google Scholar]
- 29.Veterinary Record. 2008. Bovine TB: EFRACom calls for a multifaceted approach using all available methods. Vet. Rec. 162:258-259. [DOI] [PubMed] [Google Scholar]
- 30.Wood, P. R., L. A. Corner, J. S. Rothel, J. L. Ripper, T. Fifis, B. S. McCormick, B. Francis, L. Melville, K. Small, K. De Witte, J. Tolson, T. J. Ryan, G. W. de Lisle, J. C. Cox, and S. L. Jones. 1992. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet. Microbiol. 31:71-79. [DOI] [PubMed] [Google Scholar]
- 31.Wood, P. R., and S. L. Jones. 2001. BOVIGAM: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis (Edinburgh) 81:147-155. [DOI] [PubMed] [Google Scholar]
- 32.Yan, G., D. Xing, S. Tan, and Q. Chen. 2004. Rapid and sensitive immunomagnetic-electrochemiluminescent detection of p53 antibodies in human serum. J. Immunol. Methods 288:47-54. [DOI] [PubMed] [Google Scholar]