Abstract
Military personnel with traveler's diarrhea (n = 202) while deployed to Incirlik Air Base, Turkey, from June to September 2002 were evaluated for pathogen-specific immune responses. Serologic and fecal immunoglobulin A (IgA) titers to enterotoxigenic Escherichia coli antigens (CS6, CS3, and LT) were quite low. In contrast, subjects with Campylobacter infections had high serologic and fecal IgA responses.
Acute infectious diarrhea affects up to 60% of short-term international travelers and is one of the most common medical problems for military troops deployed abroad (21, 22, 24). Recent estimates of the diarrheal incidence in troops in Afghanistan and Iraq have been as high as 49% per month (19, 26, 30). Despite safe and effective treatment regimens (25, 31), most military personnel with diarrhea do not seek care (19, 21, 30), suggesting that primary prevention is important in mitigating the disease burden. Additionally, since widespread chemoprophylaxis is not recommended (1, 2, 9), and given the rapid development of antibiotic resistance, vaccine development is an important long-term strategy in reducing the diarrhea burden among deployed U.S. military personnel. To support vaccine development, increased understanding of immune responses in infected personnel is needed. A prospective case series study was conducted at a U.S. Air Force air base in Incirlik, Turkey, to characterize immune responses in subjects presenting with diarrheal symptoms.
A case series study was conducted at Incirlik Air Base, located in southeastern Turkey (http://www.incirlik.af.mil/). During the study period (June to September 2002), all on-base U.S. military personnel or their adult dependents reporting for medical care due to diarrhea were eligible to enroll. Following informed consent, the participants underwent a standard clinical evaluation and provided baseline blood and stool samples. The subjects returned to the clinic to provide stool and blood samples 3, 7, 14, and 28 days after enrollment.
Stools were cultured using standard procedures for the isolation and identification of common enteric bacterial species causing diarrhea. Hippurate hydrolysis was used to differentiate Campylobacter isolates into Campylobacter jejuni and non-C. jejuni (Hardy Diagnostics, Santa Maria, CA). As previously reported, the GM1 enzyme-linked immunosorbent assay (ELISA) and a competitive inhibition ELISA were utilized to identify the heat-labile (LT) and the heat-stable (ST) toxins of enterotoxigenic Escherichia coli (ETEC) (23, 29). Toxin-expressing E. coli colonies were characterized for the presence of surface colonization factors (CFs) using an immunodot blot method employing monoclonal antibodies against the CFs (5, 32). Enzyme immunoassays were used to evaluate stool samples for rotavirus (Rotaclone; Meridian Diagnostics, Inc., Cincinnati, OH), norovirus (14), Cryptosporidium, Giardia, and Entamoeba histolytica (27). Additional ovum and parasite screening was performed by light microscopy.
Immunology assays were performed on a subset of subjects based on sample availability. For Campylobacter, immunoglobulin A (IgA) and IgG levels in response to a glycine extract antigen of C. jejuni strain 81-176 were determined by ELISA (3, 4). Similarly for ETEC, serologic responses to the B subunit of the native LT and CFs CS3 and CS6 (chosen based on previous epidemiological studies) were evaluated by ELISA (10, 13, 15, 28; J. Malone, G. D. Chapman, and E. Kilbane, presented at the International Conference of Emerging Infections, Atlanta, GA, 2000). The antibody titers represented the geometric mean of duplicate determinations on different days. Reciprocal endpoint titers of <5 were assigned a value of 2.5 for computational purposes. Stool samples were aliquoted and frozen at −70°C at the U.S. Air Force hospital at Incirlik Air Base. All specimens were shipped to the Naval Medical Research Unit 3 laboratory for processing and secretory-IgA determination (16, 17). An immune response was defined as a ≥4-fold rise over the baseline titer.
Immunology data were compared using a repeated-measures analysis of variance with infection as the between-subject factor (i.e., Campylobacter, CS6 ETEC, CS3 ETEC, and LT ETEC) and sample collection time points as the repeated factor. Statistical analyses were conducted with SAS version 8.2 for Windows (SAS Institute, Inc., Cary, NC) using a two-tailed alpha of 0.05.
Initial presentation.
A total of 202 subjects met the inclusion criteria and were enrolled. The majority were male (89%) Caucasian (76%) enlisted personnel (83%) on deployment for either Operation Northern Watch (73%) or Operation Enduring Freedom (17%). Pathogens were identified in 53% (n = 108) of cases, with ETEC (n = 82) and Campylobacter (n = 25, including 5 ETEC coinfections) being the most common. The most common ETEC toxin type was ST (76%), followed by LT (13%) and LTST (11%). CS6 was the predominant CF (40%), followed by CS1CS3 (20%). Multiple ETEC phenotypes were identified from six (7%) subjects with ETEC infections. No CF was detected in 17% of the subjects.
Immune response.
Serologic responses to glycine extract of C. jejuni strain 81-176 were more common in subjects with Campylobacter infections (IgG, 56%; IgA, 72%; IgM, 72%) than in those without (IgG, 4%; IgA, 7%; IgM, 5%) (all P < 0.001). Peak responses were observed 14 days after initial presentation, and IgG persisted through day 28 (Fig. 1). Similarly, fecal-IgA (secretory-IgA) responses peaked on day 14 and were significantly higher (P < 0.001) in Campylobacter infection cases (geometric mean titer, 1,281) than in noncases (geometric mean titer, 16).
FIG. 1.
Serum IgA and IgG responses to glycine extract by initial microbiology findings.
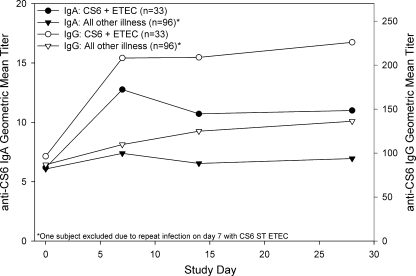
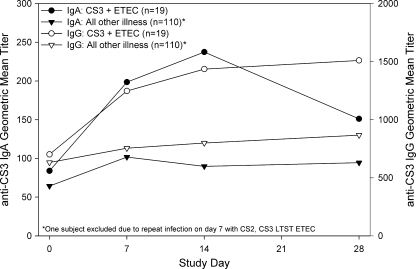
ETEC anti-CF (CS3 and CS6) serologic responses (IgA and IgG) were more common in subjects with homologous CF-expressing ETEC infections than in those without. Serologic titers (IgG and IgA) increased significantly over baseline titers (repeated measures, P < 0.001), with peak IgA titers on day 7 and IgG titers increasing through day 28 (Fig. 2 and 3). Anti-CF fecal-IgA, anti-CF serologic-IgM, and anti-LT serologic responses were not indicative of infection.
FIG. 2.
Serum IgA and IgG responses to CS6 by initial microbiology findings.
FIG. 3.
Serum IgA and IgG responses to CS3 by initial microbiology findings.
To our knowledge, this is the first study to evaluate the short-term (1-month) kinetics of the immune response in persons with traveler's diarrhea secondary to ETEC and Campylobacter infection. As reported previously, it has been confirmed that C. jejuni infections are capable of inducing robust mucosal and systemic immune responses (6, 7, 18). However, it is unknown if the magnitude of these titers would prevent subsequent infection.
Infection with CS3 and CS6 ETEC induced homologous anti-CF serologic responses. However, the maximum titers and response rates were quite low. In contrast, a recent publication of CS6 immune responses in Bangladeshi patients hospitalized with CS6 ETEC infections showed robust IgA and IgG titers (20). One fundamental difference between these two studies is the study populations. The results presented here represent a U.S. traveler population in a setting where ETEC is endemic. The study by Qadri et al. enrolled persons hospitalized with diarrhea in Dhaka, Bangladesh, a region where ETEC is hyperendemic. The robust responses in these subjects may represent a prime-and-boost effect indicative of prior infection.
This idea is supported by the results of Coster et al., who fed CS6 ETEC to a generally naïve study population (8). The authors showed serologic titers similar to those presented here. Additionally, the antibody-secreting-cell levels reported by Coster et al. were lower than those presented by Qadri et al. (anti-CS6 IgA geometric mean antibody-secreting-cells, 2/106 peripheral blood mononuclear cells and 430/106 peripheral blood mononuclear cells, respectively).
The disease severity may also have diminished the magnitude of CF-specific responses. Previous studies evaluating immune responses following Shigella infection showed a positive correlation between the disease severity and humoral responses (11, 12). Of the subjects in the study by Qadri et al., 85% were moderately or severely dehydrated and 100% were hospitalized. In contrast, none of our subjects were hospitalized and only 4% received intravenous rehydration. Additionally, early antibiotic treatment in our study may have reduced the immune response. However, the early treatment times did not result in low responses to Campylobacter glycine extract for C. jejuni-infected subjects. This may have been due to longer eradication times despite symptom resolution for Campylobacter infection or a difference in host responses to an invasive pathogen like Campylobacter versus a noninvasive pathogen, such as ETEC.
This study was limited to cases severe enough to cause an individual to seek care. This represents a subset of infected persons, potentially skewing the immunology results. Additionally, the lack of a control group in our study limits the ability to make strong inferences. Future studies should evaluate pathogen-specific immune responses in a population-based setting utilizing appropriate controls.
Acknowledgments
The study protocol was approved by the Naval Medical Research Unit 3 Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects.
This work was funded by work unit number A20019_02_NM.
We are employees of the U.S. Government. This work was prepared as part of official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government.
Footnotes
Published ahead of print on 8 October 2008.
REFERENCES
- 1.Anonymous. 1985. Travelers' diarrhea. Natl. Inst. Health Consens. Dev. Conf. Consens. Statement 5:1-7. [PubMed] [Google Scholar]
- 2.Anonymous. 1986. Travelers' diarrhea: National Institutes of Health Consensus Development Conference. Bethesda, Maryland, January 28-30, 1985. Rev. Infect. Dis. 8(Suppl. 2):S109-S233. [PubMed] [Google Scholar]
- 3.Baqar, S., A. L. Bourgeois, P. J. Schultheiss, R. I. Walker, D. M. Rollins, R. L. Haberberger, and O. R. Pavlovskis. 1995. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine 13:22-28. [DOI] [PubMed] [Google Scholar]
- 4.Baqar, S., B. Rice, L. Lee, A. L. Bourgeois, A. N. El Din, D. R. Tribble, G. P. Heresi, A. S. Mourad, and J. R. Murphy. 2001. Campylobacter jejuni enteritis. Clin. Infect. Dis. 33:901-905. [DOI] [PubMed] [Google Scholar]
- 5.Binsztein, N., M. J. Jouve, G. I. Viboud, L. Lopez Moral, M. Rivas, I. Orskov, C. Ahren, and A. M. Svennerholm. 1991. Colonization factors of enterotoxigenic Escherichia coli isolated from children with diarrhea in Argentina. J. Clin. Microbiol. 29:1893-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser, M. J., I. D. Berkowitz, F. M. LaForce, J. Cravens, L. B. Reller, and W. L. Wang. 1979. Campylobacter enteritis: clinical and epidemiologic features. Ann. Intern. Med. 91:179-185. [DOI] [PubMed] [Google Scholar]
- 7.Blaser, M. J., D. N. Taylor, and P. Echeverria. 1986. Immune response to Campylobacter jejuni in a rural community in Thailand. J. Infect. Dis. 153:249-254. [DOI] [PubMed] [Google Scholar]
- 8.Coster, T. S., M. K. Wolf, E. R. Hall, F. J. Cassels, D. N. Taylor, C. T. Liu, F. C. Trespalacios, A. DeLorimier, D. R. Angleberger, and C. E. McQueen. 2007. Immune response, ciprofloxacin activity, and gender differences after human experimental challenge by two strains of enterotoxigenic Escherichia coli. Infect. Immun. 75:252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorbach, S. L. 2005. How to hit the runs for fifty million travelers at risk. Ann. Intern. Med. 142:861-862. [DOI] [PubMed] [Google Scholar]
- 10.Hall, E. R., T. F. Wierzba, C. Ahren, M. R. Rao, S. Bassily, W. Francis, F. Y. Girgis, M. Safwat, Y. J. Lee, A. M. Svennerholm, J. D. Clemens, and S. J. Savarino. 2001. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine. Infect. Immun. 69:2853-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam, D., P. K. Bardhan, A. A. Lindberg, and B. Christensson. 1995. Shigella infection induces cellular activation of T and B cells and distinct species-related changes in peripheral blood lymphocyte subsets during the course of the disease. Infect. Immun. 63:2941-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam, D., B. Wretlind, L. Hammarstrom, B. Christensson, and A. A. Lindberg. 1996. Semiquantitative estimation of Shigella antigen-specific antibodies: correlation with disease severity during shigellosis. APMIS 104:563-574. [DOI] [PubMed] [Google Scholar]
- 13.Jertborn, M., C. Ahren, J. Holmgren, and A. M. Svennerholm. 1998. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine 16:255-260. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, X., N. Wilton, W. M. Zhong, T. Farkas, P. W. Huang, E. Barrett, M. Guerrero, G. Ruiz-Palacios, K. Y. Green, J. Green, A. D. Hale, M. K. Estes, L. K. Pickering, and D. O. Matson. 2000. Diagnosis of human caliciviruses by use of enzyme immunoassays. J. Infect. Dis. 181(Suppl. 2):S349-S359. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, Z. D., J. J. Mathewson, C. D. Ericsson, A. M. Svennerholm, C. Pulido, and H. L. DuPont. 2000. Characterization of enterotoxigenic Escherichia coli strains in patients with travelers' diarrhea acquired in Guadalajara, Mexico, 1992-1997. J. Infect. Dis. 181:779-782. [DOI] [PubMed] [Google Scholar]
- 16.Jones, F. R., S. Baqar, A. Gozalo, G. Nunez, N. Espinoza, S. M. Reyes, M. Salazar, R. Meza, C. K. Porter, and S. E. Walz. 2006. New World monkey Aotus nancymae as a model for Campylobacter jejuni infection and immunity. Infect. Immun. 74:790-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, F. R., E. R. Hall, D. Tribble, S. J. Savarino, F. J. Cassels, C. Porter, R. Meza, G. Nunez, N. Espinoza, M. Salazar, R. Luckett, and D. Scott. 2006. The New World primate, Aotus nancymae, as a model for examining the immunogenicity of a prototype enterotoxigenic Escherichia coli subunit vaccine. Vaccine 24:3786-3792. [DOI] [PubMed] [Google Scholar]
- 18.Martin, P. M., J. Mathiot, J. Ipero, M. Kirimat, A. J. Georges, and M. C. Georges-Courbot. 1989. Immune response to Campylobacter jejuni and Campylobacter coli in a cohort of children from birth to 2 years of age. Infect. Immun. 57:2542-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monteville, M. R., M. S. Riddle, U. Baht, S. D. Putnam, R. W. Frenck, K. Brooks, M. Moustafa, J. Bland, and J. W. Sanders. 2006. Incidence, etiology, and impact of diarrhea among deployed US military personnel in support of Operation Iraqi Freedom and Operation Enduring Freedom. Am. J. Trop. Med. Hyg. 75:762-767. [PubMed] [Google Scholar]
- 20.Qadri, F., T. Ahmed, F. Ahmed, M. S. Bhuiyan, M. G. Mostofa, F. J. Cassels, A. Helander, and A. M. Svennerholm. 2007. Mucosal and systemic immune responses in patients with diarrhea due to CS6-expressing enterotoxigenic Escherichia coli. Infect. Immun. 75:2269-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riddle, M. S., J. W. Sanders, S. D. Putnam, and D. R. Tribble. 2006. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am. J. Trop. Med. Hyg. 74:891-900. [PubMed] [Google Scholar]
- 22.Ryan, E. T., and K. C. Kain. 2000. Health advice and immunizations for travelers. N. Engl. J. Med. 342:1716-1725. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez, J., J. Holmgren, and A. M. Svennerholm. 1990. Recombinant fusion protein for simple detection of Escherichia coli heat-stable enterotoxin by GM1 enzyme-linked immunosorbent assay. J. Clin. Microbiol. 28:2175-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez, J. L., J. Gelnett, B. P. Petruccelli, R. F. Defraites, and D. N. Taylor. 1998. Diarrheal disease incidence and morbidity among United States military personnel during short-term missions overseas. Am. J. Trop. Med. Hyg. 58:299-304. [DOI] [PubMed] [Google Scholar]
- 25.Sanders, J. W., R. W. Frenck, S. D. Putnam, M. S. Riddle, J. R. Johnston, S. Ulukan, D. M. Rockabrand, M. R. Monteville, and D. R. Tribble. 2007. Azithromycin and loperamide is comparable to levofloxacin and loperamide for the treatment of traveler's diarrhea in U.S. military personnel in Turkey. Clin. Infect. Dis. 45:294-301. [DOI] [PubMed] [Google Scholar]
- 26.Sanders, J. W., S. D. Putnam, M. S. Riddle, D. R. Tribble, N. K. Jobanputra, J. J. Jones, D. A. Scott, and R. W. Frenck. 2004. The epidemiology of self-reported diarrhea in operations Iraqi Freedom and Enduring Freedom. Diagn. Microbiol. Infect. Dis. 50:89-93. [DOI] [PubMed] [Google Scholar]
- 27.Sharp, S. E., C. A. Suarez, Y. Duran, and R. J. Poppiti. 2001. Evaluation of the Triage Micro Parasite Panel for detection of Giardia lamblia, Entamoeba histolytica/Entamoeba dispar, and Cryptosporidium parvum in patient stool specimens. J. Clin. Microbiol. 39:332-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoll, B. J., A. M. Svennerholm, L. Gothefors, D. Barua, S. Huda, and J. Holmgren. 1986. Local and systemic antibody responses to naturally acquired enterotoxigenic Escherichia coli diarrhea in an endemic area. J. Infect. Dis. 153:527-534. [DOI] [PubMed] [Google Scholar]
- 29.Svennerholm, A. M., and G. Wiklund. 1983. Rapid GM1-enzyme-linked immunosorbent assay with visual reading for identification of Escherichia coli heat-labile enterotoxin. J. Clin. Microbiol. 17:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornton, S. A., S. S. Sherman, T. Farkas, W. Zhong, P. Torres, and X. Jiang. 2005. Gastroenteritis in US Marines during Operation Iraqi Freedom. Clin. Infect. Dis. 40:519-525. [DOI] [PubMed] [Google Scholar]
- 31.Tribble, D. R., J. W. Sanders, L. W. Pang, C. Mason, C. Pitarangsi, S. Baqar, A. Armstrong, P. Hshieh, A. Fox, E. A. Maley, C. Lebron, D. J. Faix, J. V. Lawler, G. Nayak, M. Lewis, L. Bodhidatta, and D. A. Scott. 2007. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin. Infect. Dis. 44:338-346. [DOI] [PubMed] [Google Scholar]
- 32.Viboud, G. I., N. Binsztein, and A. M. Svennerholm. 1993. Characterization of monoclonal antibodies against putative colonization factors of enterotoxigenic Escherichia coli and their use in an epidemiological study. J. Clin. Microbiol. 31:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]