Abstract
It has been suggested that a defective adaptive immune response contributes to septic immunosuppression. Here, the response of monocytes to CD40 ligand (CD40L) for patients with sepsis due to infection with gram-negative organisms has been analyzed. Compared to cells from controls, monocytes from septic patients showed significantly reduced production of tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-12 and were unable to acquire high levels of CD80 and CD86 molecules. These alterations were observed at the onset of sepsis and persisted at day 7. However, the ability of monocytes to respond to CD40L stimulation was partially but significantly restored in cells from patients who recovered from sepsis. In addition, costimulation of autologous CD4+ T lymphocytes by CD40L-activated monocytes from septic patients failed to induce cell proliferation and gamma interferon production. Finally, the ability of CD40L to rescue monocytes from apoptosis was severely impaired. We conclude that downregulation of the CD40L response may be an appropriate model for the monocyte alteration observed during septic immunosuppression and may help in the development of novel therapeutic strategies.
It has recently been appreciated that patients with sepsis suffer from altered immune responses, known as “immune paralysis” (10, 51). This explains their difficulty in fighting their primary bacterial infection and their propensity to develop superinfections. Innate immunity functions are profoundly affected during sepsis. Circulating phagocytes show a marked decrease in their capacity to mount a proinflammatory reaction in response to microorganisms (9, 20, 35). Monocytes express low levels of major histocompatibility class II molecules (16). Adaptive responses are also markedly impaired during sepsis. This is highlighted by the development of opportunistic infections usually seen only in immunocompromised patients and the reactivation of dormant viruses, such as cytomegalovirus (21, 52). Reduced T-lymphocyte proliferation and massive T- and B-lymphocyte apoptosis have been reported during sepsis and are responsible, at least in part, for the impairment of adaptive responses (7, 22).
CD40 is a 50-kDa molecule expressed on different cell types, including monocyte-macrophages (1). The human CD40 ligand (CD40L) is a type II integral membrane protein expressed primarily on activated CD4+ T cells (2, 17). Upon CD40 engagement, monocytic cells secrete a vast array of cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-12, which are important in promoting and maintaining Th1 and proinflammatory responses during bacterial infection (1, 27, 41). CD40L activation of macrophages also results in the upregulation of surface molecules, such as CD80 and CD86, which play a critical role in T-cell activation (5). Thus, CD40-CD40L interaction is an essential step for triggering the adaptive immune response (19) and is very likely to play a prominent role during sepsis, as demonstrated by the increased mortality observed in septic animals with mutations of the CD40L gene (39).
Our research group has recently characterized lipopolysaccharide (LPS)-exposed monocytes in vitro in terms of their capacity to respond to CD40L stimulation (47). Our findings demonstrated that tolerance in such cells involved a distinct functional state of activation and/or differentiation which is not restricted to LPS tachyphylaxis. Indeed, pretreatment with LPS substantially reduced the response of monocytic cells to CD40L in terms of both cytokine production and the expression of costimulatory molecules. Here, we have analyzed the response of monocytes to CD40L for patients with sepsis caused by gram-negative organisms. Compared to cells from healthy subjects, monocytes from septic patients showed significantly reduced production of TNF-α, IL-1β, and IL-12. In addition, monocytes from septic patients were unable to acquire high levels of the costimulatory molecules CD80 and CD86. Accordingly, costimulation of autologous CD4+ T lymphocytes by CD40L-activated monocytes from septic patients failed to induce cell proliferation and gamma interferon (IFN-γ) production. Finally, the ability of CD40L to rescue monocytes from apoptosis induced by serum withdrawal was severely impaired during sepsis.
MATERIALS AND METHODS
Subjects.
This study was approved by the Ethics Committee of the University of Rome Policlinico Tor Vergata hospital. Informed consent was obtained from all subjects. Sixteen patients were admitted to the Medical Intensive Care Unit, and 10 healthy, age- and sex-matched control subjects were enrolled. In the 24 h before entry, each patient met the following criteria: an identifiable site of infection and two or more systemic inflammatory response syndrome criteria, including a temperature of more than 38°C or less than 36°C, heart rate of more than 90 beats per minute, respiratory rate of more than 20 breaths per minute, and white blood cell count of more than 12,000 or less than 3,000 mm3. Patients were excluded from participation if they were less than 18 years of age; had an active malignancy, human immunodeficiency virus disease, end-stage renal disease, or end-stage hepatic disease; required chemotherapy or ongoing immunosuppressive therapy; had received corticosteroids within 4 weeks before entry; or if pregnancy was not excluded. APACHE II and SOFA scores were calculated on admission to the study.
Compounds.
Immunex (Seattle, WA) provided soluble trimeric recombinant CD40L. Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) containing 5.4 × 106 chronic myelogenous leukemia units per milligram of glycoprotein was obtained from Sandoz Research Institute (East Hanover, NJ). [3H]thymidine, with a specific activity of 80 mCi/mmol, was purchased from Amersham, Little Chalfont, United Kingdom.
Limulus amebocyte lysate test.
All the compounds and media used in this study were analyzed for endotoxin contamination by using a limulus amebocyte lysate test (QCL-1000; BioWhittaker, Inc., Walkersville, MD). All the samples analyzed were found to be free of endotoxin contamination (less than 0.1 endotoxin unit/ml).
Antibodies.
For fluorescence-activated cell sorter (FACS) analysis, the following monoclonal antibodies were used: anti-CD14, anti-CD40, anti-CD86, anti-CD80, anti-TNF-α, anti-IL-1β, anti-IL-12 (this antibody reacts with human IL-12 p40 monomer and p70 heterodimer, but not p35 monomer), anti-IL-2, and anti-IFN-γ (all from PharMingen, San Diego, CA). Staining was performed with fluorescein isothiocyanate-, phycoerythrin-, and Cy-chrome-coupled antibodies.
Cell stimulation.
Peripheral blood from controls or patients was enriched for peripheral blood mononuclear cells (PBMC) by centrifugation over Ficoll-Hypaque. The cells were cultured in RPMI 1640 medium supplemented with 20% heat-inactivated fetal calf serum, 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, referred to as complete medium. The cells were kept at 37°C in a humidified atmosphere of 5% CO2 in air in 96-well V-bottom plates (Corning, Incorporated, Corning, NY) at a concentration of 5 × 105 cells/well in 250 μl medium.
For the determination of intracellular cytokine production by FACS analysis, PBMC from patients and controls were cultured at a concentration of 5 × 105 cells/well in 250 μl medium for 18 h in 96-well V-bottom plates (Corning, Incorporated, Corning, NY), using complete medium in the presence or in the absence of 500 ng/ml CD40L. Thirty minutes after stimulation, 1 μg/ml of the protein transport inhibitor brefeldin A (Sigma Chemical Co., St. Louis, MO) was added. At the end of the incubation period, the cells were analyzed by FACS for intracellular cytokine production.
To evaluate the ability of CD40L and GM-CSF to upregulate the expression of CD40, CD80, and CD86, PBMC from patients and controls were cultured for 72 h in complete medium supplemented with either 500 ng/ml CD40L or 100 U/ml GM-CSF. At the end of the incubation period, the cells were collected and analyzed by FACS for CD80, CD86, and CD40 expression.
The experiments to evaluate the costimulatory ability of monocytes were carried out as follows. PBMC were cultured for 72 h in complete medium supplemented with either 500 ng/ml CD40L or 100 U/ml GM-CSF. At the end of the incubation period, the cells were washed, refed with fresh medium, and plated at a concentration of 2 × 105/ml in a flat-bottom 96-well plate coated with anti-CD3 (10 μg/ml) (Becton Dickinson, San Jose, CA) in the presence or in the absence of 1 μg/ml soluble anti-CD28 (Becton Dickinson). For the evaluation of cell proliferation, 0.25 mCi/well of [3H]thymidine was added for the last 18 h of culture. The cultures were then harvested by using a multichannel harvester. The amount of [3H]thymidine incorporated was determined by liquid scintillation spectroscopy (β-counter; Canberra Packard Ltd., Pangbourne, United Kingdom). For intracellular cytokine detection, 30 min after plating in anti-CD3-coated wells, 1 μg/ml brefeldin A was added. At the end of the incubation period (18 h), the cells were analyzed by FACS for intracellular cytokine production.
Assessment of hypodiploid DNA formation.
Hypodiploid DNA formation was assessed by propidium iodide assay as previously described (13, 38). In brief, PBMC (5 × 105) were incubated in RPMI 1640 alone for 72 h with or without 500 ng/ml CD40L or 100 U/ml GM-CSF. At the end of the incubation period, the cells were washed twice in phosphate-buffered saline (PBS), detached by gentle scraping, and collected by low-speed centrifugation. After centrifugation, the supernatant was removed and the cell pellet was resuspended in 1 ml of 0.1% Triton X-100 solution in PBS containing 5 mg/ml bovine serum albumin. This step was repeated once for a total of two rinses. Then, the supernatant was removed and the cell pellet was resuspended in 1.5 ml propidium iodide solution (freshly diluted to 5 μg/ml in PBS) containing 250 μg of DNase-free RNase A. Finally, the cells were incubated at room temperature for 30 min in the dark and then the propidium iodide fluorescence of individual nuclei was measured by FACS as described by Nicoletti et al. (38). The red fluorescence due to propidium iodide staining of the DNA was registered on a logarithmic scale at >620 nm. The forward and side scatter of particles were measured simultaneously. Cell debris was excluded from analysis by appropriately raising the forward-scatter threshold. The residual cell debris had a very low DNA fluorescence emission and a low side-scatter signal. At least 104 cells of each sample were analyzed.
Surface marker and intracellular cytokine staining.
After incubation, the cells were washed and stained for surface markers. Cells were then either analyzed by FACS to determine cell surface antigen expression or permeabilized in Cytofix/Cytoperm solution (Pharmingen), stained for intracellular cytokines, and then analyzed by FACS.
FACS analysis.
Flow cytometry was performed by using a FACScan flow cytometer and analyzed with CellQuest software (Becton Dickinson). For each analysis, 104 events were gated on CD14 expression and a light scatter gate designed to include only viable cells. Isotype-matched negative-control antibodies (PharMingen) were used to verify the staining specificity.
Statistics.
The normality of variable distribution was assessed by the Kolmogorov-Smirnov goodness-of-fit test. Comparison of the distribution of two variables for a single group was performed by using either Student's paired t test or the Mann-Whitney U test, as appropriate. All P values are two-tailed. P values of <0.05 were considered significant. Statistical analyses were performed by using the SPSS (version 10.0; SPSS, Inc., Chicago, IL) statistical package.
RESULTS
Patients.
The clinical characteristics of patients and the sites of infection and strains diagnosed at the onset of sepsis are reported in Tables 1 and 2.
TABLE 1.
Clinical characteristics of patients
| Characteristica | Value |
|---|---|
| Male/female | 6/10 |
| Age (yr)b | 63.7 ± 16 (24-83) |
| Neutrophils (109/liter)b | 13.3 ± 12.6 (3.7-59) |
| PaO2/FIO2b | 275.88 ± 99.4 (73-490) |
| PCTb | 6.16 ± 8 (0.07-33) |
| CRPb | 148.3 ± 109.7 (11-360) |
| APACHE II scoreb | 24.5 ± 6.5 (9-36) |
| SOFA scoreb | 9.28 ± 3.8 (3-20) |
| Deathc | 6 (37.5) |
PCT, procalcitonin; CRP, C-reactive protein.
Values are means ± standard deviations. Values in parentheses are ranges.
Value in parentheses is a percentage.
TABLE 2.
Sites of infection and strains diagnosed at the onset of sepsis
| Variable | No. of patients |
|---|---|
| Positive microbial documentation of infection | 5 |
| Positive blood culture result | 11 |
| Site of infection | |
| Abdomen | 6 |
| Genitourinary tract | 3 |
| Respiratory tract | 2 |
| Other | 3 |
| Primary infecting microorganism | |
| Escherichia coli | 6 |
| Klebsiella | 2 |
| Pseudomonas aeruginosa | 2 |
| Proteus species | 2 |
| Other gram-negative species | 4 |
Cytokine production by monocytes during sepsis.
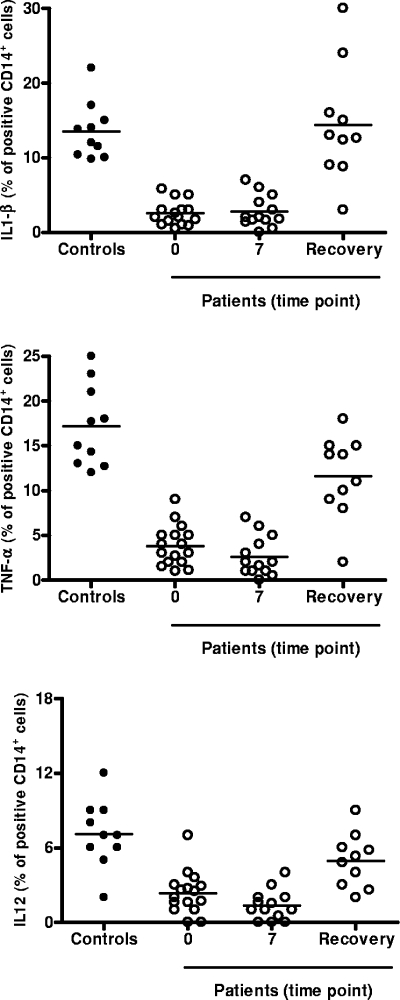
The CD40L-induced cytokine response of monocytes was analyzed at different time points during sepsis: day 0 (within 24 h of meeting enrollment criteria), day 7, and at the point of clinical recovery (defined as the time when the survivor was afebrile, hemodynamically stable, and without evidence of organ dysfunction attributable to infection); the average and range of time until recovery were 20.2 and 10 to 32 days, respectively. As shown in Fig. 1, the cytokine response to CD40L stimulation for patients was severely impaired compared with that for controls. In particular, suppression of cytokine production was observed at the onset of sepsis and persisted at day 7 (for levels for controls versus levels for patients at day 0 and day 7, the P value was <0.05 for TNF-α, IL-1β, and IL-12). The cytokine response of monocytes to CD40L was partially but significantly restored for patients who recovered from sepsis (for levels on day 0 and day 7 versus levels at recovery, the P value was <0.05 for TNF-α, IL-1β, and IL-12).
FIG. 1.
CD40L-induced cytokine production at different time points during sepsis. The numbers of donors used for the data points shown were as follows: controls, n = 10; patients on day 0, n = 16; on day 7, n = 13; and at recovery, n = 10. Intracellular cytokine production was assessed by FACS. Less than 1% TNF-α, IL-1β, and IL-12 production was found in unstimulated cells. Appropriate control experiments with isotype-matched irrelevant monoclonal antibodies were carried out and consistently showed <1% positive cells. Horizontal bars show the means of the results.
Effect of sepsis on the upregulation of surface molecules induced by CD40L.
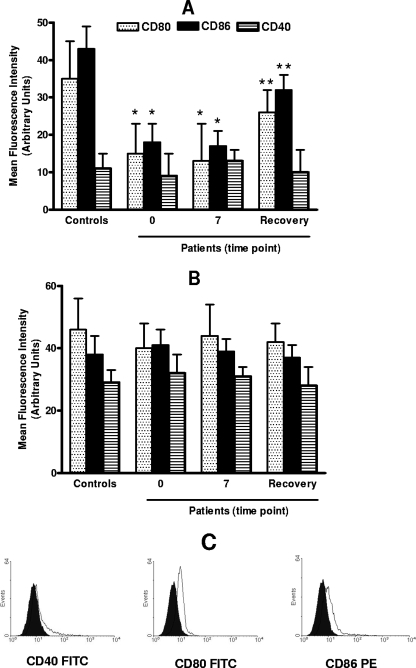
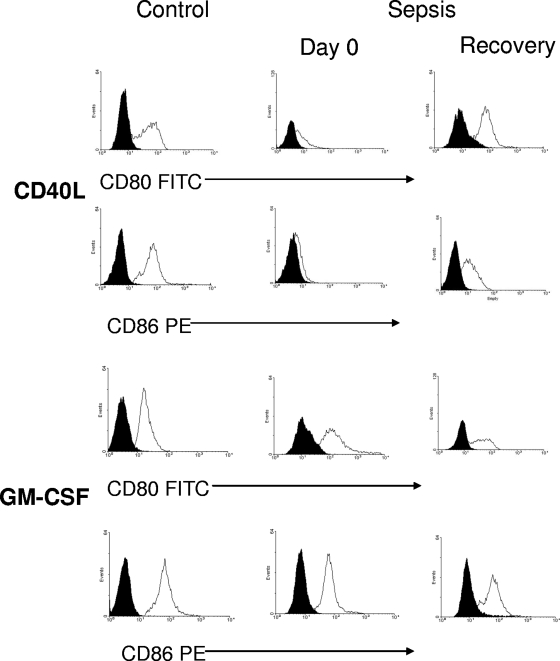
Data on the CD40L-induced expression of surface molecules for septic patients and controls are summarized in Fig. 2A. The results of the analysis of surface markers showed that for healthy controls, 3 days of CD40 ligation resulted in the expression of high levels of CD80, CD86, and CD40. The upregulation of CD40 expression to the levels reached in cells from controls was also observed in monocytes from septic patients after CD40L stimulation. In contrast, only a suboptimal response could be elicited in these cells with respect to CD80 and CD86 expression at both day 0 and 7. Again, the inhibitory effect of sepsis on monocyte susceptibility to CD40L almost disappeared after clinical recovery. The results in Fig. 2B show that the effect of GM-CSF activation on CD80 and CD86 expression for septic patients was not abrogated in comparison to its effect for controls, thus suggesting that the interference with CD40L activity that takes place during sepsis is rather selective. Shown in Fig. 3 are representative FACS plots illustrating the effect of sepsis on CD40L-induced CD80, CD86, and CD40 expression.
FIG. 2.
Effect of sepsis on the upregulation of surface molecules induced by CD40L and GM-CSF. The numbers of donors used for the data points shown were as follows: controls, n = 10; patients on day 0, n = 16; on day 7, n = 13; and at recovery, n = 10. (A and B) PBMC were cultured for 72 h in the presence of 500 ng/ml CD40L (A) or 100 U/ml GM-CSF (B). The expression of CD80, CD86, and CD40 on CD14+-gated cells was analyzed by FACS. Appropriate control experiments with isotype-matched irrelevant monoclonal antibodies were carried out and consistently showed <1% positive cells. The data represent the means ± standard deviations (error bars) of the results. (A) A single asterisk indicates a P value of <0.05 with respect to results for controls; a double asterisk indicates a P value of <0.05 with respect to results from day 0 and 7. No statistically significant differences (P > 0.05) were found between controls and patients with respect to CD40 expression at each time point. (B) No statistically significant differences (P > 0.05) were found between controls and patients with respect to CD80, CD86, and CD40 expression at each time point. (C) FACS measurement of CD40, CD80, and CD86 expression (mean channel fluorescence) on freshly explanted monocytes, illustrated by a representative FACS plot. Mean fluorescence intensities were 4.6, 14.9, and 18.1, respectively, for CD40, CD80, and CD86. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
FIG. 3.
FACS measurement of surface molecule expression, illustrated by exemplary FACS plots. Monocytes from a representative patient and control subject were cultured for 72 h in the presence of 500 ng/ml CD40L or 100 U/ml GM-CSF. The expression of CD80 and CD86 on CD14+ cells was analyzed by FACS. Black histograms, isotype-matched irrelevant monoclonal antibodies; white histograms, anti-CD80-fluorescein isothiocyanate (FITC) or anti-CD86-phycoerythrin (PE) monoclonal antibody.
Sepsis interferes with the ability of CD40L to induce costimulatory functions in monocytes.
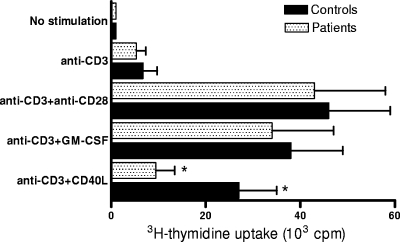
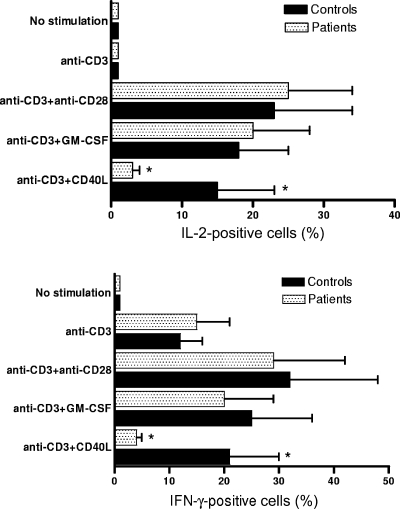
The finding of a reduction of CD40L-induced costimulatory surface molecule expression in monocytes from septic patients prompted us to compare the T-cell-stimulatory potential of these cells with that of cells from healthy controls. PBMC from sepsis patients and controls were cultured for 72 h in the presence or in the absence of CD40L or GM-CSF and then stimulated by immobilized anti-CD3 antibody. The proliferative response was assessed by measuring [3H]thymidine uptake (Fig. 4), and cytokine production by CD3+ T lymphocytes was evaluated by FACS (Fig. 5). For both patients and controls, limited [3H]thymidine uptake, no IL-2 production, and low levels of IFN-γ production were detected in the absence of CD40L or GM-CSF. Both cell proliferation and cytokine expression in PBMC from control subjects were significantly increased by treatment with CD40L or GM-CSF in comparison with their levels in untreated cells. However, only GM-CSF proved able to induce costimulatory activity in monocytes from septic patients. Indeed, in PBMC from these subjects, CD40L treatment resulted in cell proliferation and cytokine expression that were statistically not different from that detected in untreated cells. Finally, costimulation using anti-CD28 antibody (1 μg/ml) induced similar levels of cell proliferation and cytokine production for both patients and controls, thus indicating that the reduced T-cell response observed in CD40L-activated PBMC from septic patients was not due to selective defects of T lymphocytes.
FIG. 4.
CD40L fails to increase cell proliferation in anti-CD3-stimulated PBMC from septic patients. PBMC from septic patients (n = 13) were obtained at day 7. The proliferative response was assessed by pulsing the cultures with [3H]thymidine for the last 18 h of culture. No statistically significant differences were found between results for patients and controls in unstimulated samples, cells stimulated by anti-CD3 alone, cells stimulated by anti-CD3 and anti-CD28, or cells stimulated by anti-CD3 and GM-CSF. An asterisk indicates that the amount of [3H]thymidine uptake was significantly lower (P < 0.05) for patients than for controls in cells stimulated by anti-CD3 and CD40L. The data represent the means ± standard deviations (error bars).
FIG. 5.
CD40L fails to increase IFN-γ and IL-2 production in CD3+ lymphocytes from septic patients. PBMC from septic patients (n = 13) were obtained at day 7. Production of IFN-γ and IL-2 was measured by FACS. The error bars represent the standard deviations. No statistically significant differences were found between results for patients and controls in unstimulated samples, cells stimulated by anti-CD3 alone, cells stimulated by anti-CD3 and anti-CD28, or cells stimulated by anti-CD3 and GM-CSF. An asterisk indicates that the amount of both IL-2 and IFN-γ was significantly lower (P < 0.05) for patients than for controls in cells stimulated by anti-CD3 and CD40L. The data represent the means ± standard deviations (error bars).
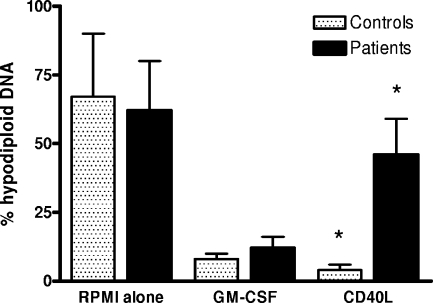
CD40L-mediated rescue of monocytes from apoptosis is reduced during sepsis.
In the absence of an appropriate stimulus, such as CD40L or GM-CSF, cultured peripheral blood monocytes rapidly undergo apoptosis (39, 49). In this set of experiments, we tested the effect of sepsis on the ability of CD40L and GM-CSF to rescue cultured monocytes from apoptosis. The percentage of hypodiploid DNA (a reliable marker of apoptosis) was quantified by flow cytometry after staining with propidium iodide (Fig. 6). After 72 h of culture in the absence of growth factors, the percentage of hypodiploid DNA was readily detectable for cells from both patients and controls. CD40L prevented much of this hypodiploid DNA formation in cells from control subjects but was significantly less active in monocytes from septic patients. However, GM-CSF proved able to prevent hypodiploid DNA formation in monocytes from both controls and sepsis patients.
FIG. 6.
Reduced ability of CD40L to rescue monocytes from apoptosis during sepsis. PBMC from septic patients (n = 13) were obtained at day 7. The percentage of hypodiploid DNA was quantified by flow cytometry following staining of the cells with propidium iodide. No statistically significant differences were found between results for patients and controls in unstimulated samples, cells stimulated by anti-CD3 alone, cells stimulated by anti-CD3 and anti-CD28, or cells stimulated by anti-CD3 and GM-CSF. An asterisk indicates that the percentage of hypodiploid DNA was significantly lower (P < 0.05) for patients than for controls in cells stimulated by anti-CD3 and CD40L. The data represent the means ± standard deviations (error bars).
DISCUSSION
It is widely recognized that sepsis patients are at risk for nosocomial infections, as well as for opportunistic infections usually seen only in immunocompromised patients (21, 52). The results of clinical and experimental studies have suggested that this might be explained by a biphasic immunological pattern during sepsis: an early hyperinflammatory phase followed by a hypoinflammatory state, the so-called compensatory anti-inflammatory response syndrome (25, 29, 40, 51). The initial studies of the pathophysiologic events occurring during the hypoinflammatory state of sepsis concentrated on monocyte hyporesponsiveness due to endotoxin tolerance. Endotoxin tolerance consists of a reprogrammed monocyte response to a repeated LPS challenge with respect to the release of proinflammatory cytokines. Monocytes from septic patients have been reported to have a diminished capacity to release TNF-α, IL-1α, IL-1β, IL-6, and IL-12 (9, 35), whereas unaltered or even enhanced production of anti-inflammatory factors, such as IL-10 and tumor growth factor-β, has been reported (46, 50). Based on the results of these studies, attempts have been made to restore the systemic proinflammatory cytokine response to endotoxin and/or to neutralize the immunosuppressive effects of anti-inflammatory factors. Both GM-CSF and IFN-γ proved able to restore the LPS responsiveness of monocytes from septic patients (36, 42). In addition, the ability of human septic plasma to induce tolerance was significantly reduced by anti-IL-10 antibodies (45). However, none of these therapies were associated with increased bacterial clearance or decreased long-term mortality (36, 42). Moreover, the results of recent investigations with infectious models using either Cryptococcus neoformans (44) or Salmonella enterica (28) established that LPS-tolerant mice had an increased resistance to fungal or bacterial infection that was associated with a reduced burden of pathogens within the tissues. It is therefore difficult to assume that endotoxin tolerance per se is directly linked to the increased susceptibility of septic patients to nosocomial infections.
In the quest for immunological alterations that may help explain the impaired immune responses during sepsis, we have explored the possible role of monocyte hyporesponsiveness to CD40L stimulation. We found that for patients with sepsis caused by infection with gram-negative organisms, the ability of monocytes to produce proinflammatory and immunoregulatory cytokines, to act as costimulatory cells, and to avoid spontaneous apoptosis in response to CD40L stimulation is markedly reduced.
The role of TNF-α in combating infections has recently been underscored by the finding that sepsis and other infectious complications developed in patients with rheumatoid arthritis who were treated with TNF-α antagonists (24). Moreover, in clinical trials, immunotherapy against TNF-α significantly increased mortality (12). Similarly, IL-1β plays an important role in the activation of innate, as well as adaptive, immunity. IL-1β induces neutrophil recruitment and plays a critical host defense role against Staphylococcus aureus-caused brain abscesses, septic arthritis, and systemic infections (11, 26). In addition, this cytokine has been shown to influence the growth and differentiation of immunocompetent lymphocytes (30). Finally, IL-12 is an immunoregulatory cytokine that is critical to the orchestration of cell-mediated immune responses in both the innate and adaptive immune system. IL-12 augments the production of IFN-γ and other cytokines from natural killer and T cells (6). Furthermore, IL-12 appears to be a vital component of the host defense against gram-negative bacterial organisms, as evidenced by the heightened host resistance conferred by IL-12 administration in several bacterial infection models (18, 33). Therefore, the likely relevance of sepsis-related dysregulation of TNF-α, IL-1β, and IL-12 production to the increased risk of bacterial superinfection in survivors of sepsis is of considerable interest. On the other hand, it should be noted that mortality during sepsis may result from the development of multisystem organ failure, which is associated with the increased production of proinflammatory cytokines (4, 34). The production of proinflammatory cytokines in the early phases of sepsis is controlled in part by the innate immune response. However, CD40L-dependent or -independent monocyte CD40 activation has also been suggested to play a role. In particular, it has been reported that CD40 knockout mice had delayed death and improved survival after cecal ligation and puncture. The improvements in survival were associated with reduced serum levels of proinflammatory cytokines and IL-12 (15, 39). At variance with those results, we found that the CD40-mediated production of both proinflammatory cytokines and IL-12 is severely reduced in monocytes from septic patients from the early phases of the disease. This suggests a likelihood that the activation of monocytes by CD40 may not share a central role in the development of the early hyperinflammatory stages of sepsis.
In addition to reduced production of TNF-α, IL-1β, and IL-12, CD40L-activated monocytes from septic patients also showed a profoundly affected ability to upregulate CD80 and CD86 and to induce T-cell proliferation and cytokine secretion. A number of different cell types, including monocytes, perform antigen-presenting cell functions. However, to become competent antigen-presenting cells, monocytes first require activation by CD40L in order to upregulate the expression of CD80 and CD86 (53). CD28/CD80-CD86 interactions play a central role in providing costimulatory signals to T cells (23). The ligation of CD28 by CD80 and/or CD86 has been shown to induce T-cell production of growth factors and T-cell proliferation (8). Thus, it is possible that the impaired costimulatory ability of CD40L-activated monocytes from septic patients was due to reduced surface expression of these molecules. In agreement with these findings, the results of recent studies indicate that the macrophage antigen-presenting capacity appears to become dysfunctional by 24 h after sepsis is induced (3) and remains at subnormal levels for up to 14 days (14). In contrast with the reduced ability to upregulate the expression of CD80 and CD86, CD40L-activated monocytes from septic patients proved able to optimally increase the expression of CD40. This indicates that the downmodulating effects of sepsis on CD40L-stimulated monocytes are not simply due to suboptimal CD40 expression. These data disagree with the results from our recent in vitro study in which LPS was found to interfere with CD40L-directed CD40 upregulation. The differences between the results of the in vitro and in vivo studies may be due to the presence in vivo of additional mediators able to affect the expression of coreceptors. Alternatively, it should be noted that in our in vitro experimental system the effect on CD40 expression was observed at LPS concentrations (>100 ng/ml) that are not usually detected during sepsis. In accord with the data presented here, several authors have reported that CD40 expression is unaltered or even enhanced on monocytes during sepsis (37, 48).
The results of recent studies of the means by which monocyte apoptosis is regulated have demonstrated that LPS activation or the treatment of monocytes with IL-1β or TNF-α protects monocytes from apoptosis (32). Interestingly, cytokines involved in the recruitment of monocytes to sites of inflammation (e.g., monocyte chemoattractant protein 1 and transforming growth factor-β) have no such effect (31). This suggests that monocytes may be recruited to a site of inflammation but will undergo apoptosis unless they receive further stimuli allowing for survival through activation. Signaling through CD40 has also been shown to regulate monocyte homeostasis during immune responses by counteracting apoptosis (41). As we show here, monocytes from septic patients proved to be poorly responsive to the antiapoptotic effect of CD40 activation. The reduced ability of CD40L to rescue monocytes from apoptosis may increase the rate of monocyte apoptosis induced by antigen presentation (43) and contribute to the decrease of the effectiveness of the immune response observed during sepsis.
Sepsis is a very complex syndrome due to the marked biologic differences of the etiologic determinants, heterogeneity of patients, and mostly unknown physiopathologic mechanisms. Thus, no single restricted patient population can be considered representative for this syndrome. Due to the very probable, literature-supported role of LPS in the pathogenesis of sepsis and, in particular, of monocyte dysfunction during sepsis, for this study we selected a cohort of patients with sepsis of documented gram-negative-organism etiology. With this background in mind, we wish to stress that any specific physiopathologic or therapeutic inference from our data should be made only with great caution at this time. However, a substantial share of the morbidity and mortality observed during sepsis is due to the consequences of sepsis-associated immunosuppression. Patients with severe sepsis may therefore benefit from treatments aimed at stimulating innate and adaptive immune responses. Monocyte hyporesponsiveness to CD40L may contribute substantially to the impairment of adaptive immune response during sepsis, and therapeutic strategies based on “boosting” the response of monocytes to CD40L may prove useful in ameliorating the clinical outcome in sepsis patients.
Footnotes
Published ahead of print on 22 October 2008.
REFERENCES
- 1.Alderson, M. R., R. J. Armitage, T. W. Tough, L. Strockbine, W. C. Fanslow, and M. K. Spriggs. 1993. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J. Exp. Med. 178:669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage, R. J., W. C. Fanslow, L. Strockbine, T. A. Sato, K. N. Clifford, B. M. Macduff, D. M. Anderson, S. D. Gimpel, T. Davis-Smith, C. R. Maliszewski, E. A. Clark, C. A. Smith, K. H. Grabstein, D. Cosman, and M. K. Spriggs. 1992. Molecular and biological characterization of a murine ligand for CD40. Nature 357:80-82. [DOI] [PubMed] [Google Scholar]
- 3.Ayala, A., M. A. Urbanich, C. D. Herdon, and I. H. Chaudry. 1996. Is sepsis-induced apoptosis associated with macrophage dysfunction? J. Trauma 40:568-573. [DOI] [PubMed] [Google Scholar]
- 4.Bone, R. C., C. J. Grodzin, and R. A. Balk. 1997. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest 112:235-243. [DOI] [PubMed] [Google Scholar]
- 5.Brossart, P., F. Grunebach, G. Stuhler, V. L. Reichardt, R. Möhle, L. Kanz, and W. Brugger. 1998. Generation of functional human dendritic cells from adherent peripheral blood monocytes by CD40 ligation in the absence of granulocyte-macrophage colony-stimulating factor. Blood 11:4238-4247. [PubMed] [Google Scholar]
- 6.Brunda, M. 1994. Interleukin-12. J. Leukoc. Biol. 55:280-288. [DOI] [PubMed] [Google Scholar]
- 7.Choudhry, M. A., S. Ahamad, K. D. Thompson, and M. M. Sayeed. 1994. T-lymphocyte Ca2+ signaling and proliferative responses during sepsis. Shock 1:267-272. [DOI] [PubMed] [Google Scholar]
- 8.Damle, N. K., K. Klussman, P. S. Linsley, and A. Aruffo. 1992. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J. Immunol. 148:1985-1992. [PubMed] [Google Scholar]
- 9.Ertel, W., J. Kremer, U. Steckholzer, D. Jarrar, O. Trentz, and F. W. Schildberg. 1995. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 85:1341-1347. [PubMed] [Google Scholar]
- 10.Faist, E., C. Schinkel, and S. Zimmer. 1996. Update on the mechanisms of immune suppression of injury and immune modulation. World J. Surg. 20:454-459. [DOI] [PubMed] [Google Scholar]
- 11.Fearon, D. T., and R. M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50-53. [DOI] [PubMed] [Google Scholar]
- 12.Fisher, C. J., Jr., J. M. Agosti, S. M. Opal, S. F. Lowry, R. A. Balk, J. C. Sadoff, E. Abraham, R. M. Schein, E. Benjamin, et al. 1996. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N. Engl. J. Med. 334:1697-1702. [DOI] [PubMed] [Google Scholar]
- 13.Fried, J., A. G. Perez, and B. D. Clarkson. 1978. Rapid hypotonic method for flow cytofluorometry of monolayer cell cultures. Some pitfalls in staining and data analysis. J. Hystochem. Cytochem. 26:921-933. [DOI] [PubMed] [Google Scholar]
- 14.Gallinaro, R. N., W. Naziri, K. M. McMasters, J. C. Peyton, and W. G. Cheadle. 1994. Alteration of mononuclear cell immune-associated antigen expression, interleukin-1 expression, and antigen presentation during intra-abdominal infection. Shock 1:130-134. [DOI] [PubMed] [Google Scholar]
- 15.Gold, J. A., M. Parsey, Y. Hoshino, S. Hoshino, A. Nolan, H. Yee, D. B. Tse, and M. D. Weiden. 2003. CD40 contributes to lethality in acute sepsis: in vivo role for CD40 in innate immunity. Infect. Immun. 71:3521-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González-Roldán, N., E. Ferat-Osorio, R. Aduna-Vicente, I. Wong-Baeza, N. Esquivel-Callejas, H. Astudillo-de la Vega, P. Sánchez-Fernández, L. Arriaga-Pizano, M. A. Villasís-Keever, C. López-Macías, and A. Isibasi. 2005. Expression of triggering receptor on myeloid cell 1 and histocompatibility complex molecules in sepsis and major abdominal surgery. World J. Gastroenterol. 11:7473-7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graf, D., U. Korthauer, H. W. Mages, G. Senger, and R. A. Kroczek. 1992. Cloning of TRAP, a ligand for CD40 on human T cells. Eur. J. Immunol. 22:3191-3194. [DOI] [PubMed] [Google Scholar]
- 18.Greenberger, M. J., S. L. Kunkel, R. M. Strieter, N. W. Lukacs, J. Bramson, J. Gauldie, F. L. Graham, M. Hitt, J. M. Danforth, and T. J. Standiford. 1996. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J. Immunol. 157:3006-3012. [PubMed] [Google Scholar]
- 19.Grewal, I. S., and R. A. Flavell. 1998. CD40 and CD154 in cell mediated immunity. Annu. Rev. Immunol. 16:111-135. [DOI] [PubMed] [Google Scholar]
- 20.Haas, J. G., P. A. Baeuerle, G. Riethmuller, and H. W. L. Ziegler-Heitbrock. 1990. Molecular mechanisms in down-regulation of tumor necrosis factor expression. Proc. Natl. Acad. Sci. USA 87:9563-9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartemink, K. J., M. A. Paul, J. J. Spijkstra, A. R. J. Girbes, and K. H. Polderman. 2003. Immunoparalysis as a cause for invasive aspergillosis? Intensive Care Med. 29:2068-2071. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss, R. S., K. W. Tinsley, P. E. Swanson, R. E. Schmieg, Jr., J. J. Hui, K. C. Chang, D. F. Osborne, B. D. Freeman, J. P. Cobb, T. G. Buchman, and I. E. Karl. 2001. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans J. Immunol. 166:6952-6963. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins, M. K. 1994. The ups and downs of T cell costimulation. Immunity 1:443-446. [DOI] [PubMed] [Google Scholar]
- 24.Keane, J., S. Gershon, R. P. Wise, E. Mirabile-Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 25.Keel, M., and O. Trentz. 2005. Pathophysiology of polytrauma. Injury 36:691-709. [DOI] [PubMed] [Google Scholar]
- 26.Kielian, T., E. D. Bearden, A. C. Baldwin, and N. Esen. 2004. IL-1 and TNF-α play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J. Neuropathol. Exp. Neurol. 63:381-396. [DOI] [PubMed] [Google Scholar]
- 27.Kiener, P. A., P. Moran-Davis, B. M. Rankin, A. F. Wahl, A. Aruffo, and D. Hollenbaugh. 1995. Stimulation of CD40 with purified soluble gp 39 induces proinflammatory responses in human monocytes. J. Immunol. 155:4917-4925. [PubMed] [Google Scholar]
- 28.Lehner, M. D., J. Ittner, D. S. Bundschuh, N. van Rooijen, A. Wendel, and T. Hartung. 2001. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar Typhimurium infection despite attenuated cytokine response. Infect. Immun. 69:463-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy, M. M., M. P. Fink, J. C. Marshall, E. Abraham, D. Angus, D. Cook, J. Cohen, S. M. Opal Vincent, and G. Ramsay. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31:1250-1256. [DOI] [PubMed] [Google Scholar]
- 30.Lichtman, A. H., J. Chin, J. A. Schmidt, and A. K. Abbas. 1988. Role of interleukin 1 in the activation of T lymphocytes. Proc. Natl. Acad. Sci. USA 85:9699-9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangan, D. F., and S. Wahl. 1991. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J. Immunol. 147:3408-3412. [PubMed] [Google Scholar]
- 32.Mangan, D. F., G. Welch, and S. Walml. 1991. Lipopolysaccharide, tumor necrosis factor-α, and IL-1 prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J. Immunol. 146:1541-1546. [PubMed] [Google Scholar]
- 33.Metzger, D. W., R. Raeder, V. H. Van Cleave, and M. D. Boyle. 1995. Protection of mice from group A streptococcal skin infection by interleukin-12. J. Infect. Dis. 171:1643-1645. [DOI] [PubMed] [Google Scholar]
- 34.Morrison, D. C., and J. L. Ryan. 1987. Endotoxins and disease mechanisms. Annu. Rev. 38:417-432. [DOI] [PubMed] [Google Scholar]
- 35.Munoz, C., B. Misset, C. Fitting, J. P. Blériot, J. Carlet, and J. M. Cavaillon. 1991. Dissociation between plasma and monocyte-associated cytokines during sepsis. Eur. J. Immunol. 21:2177-2184. [DOI] [PubMed] [Google Scholar]
- 36.Murphey, E. D., and E. R. Sherwood. 2006. Bacterial clearance and mortality are not improved by a combination of IL-10 neutralization and IFN-gamma administration in a murine model of post-CLP immunosuppression. Shock 26:417-424. [DOI] [PubMed] [Google Scholar]
- 37.Newton, S., Y. Ding, C. S. Chung, Y. Chen, J. L. Lomas-Neira, and A. Ayala. 2004. Sepsis-induced changes in macrophage co-stimulatory molecule expression: CD86 as a regulator of anti-inflammatory IL-10 response. Surg. Infect. 5:375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring tymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 39.Nolan, A., M. D. Weiden, Y. Hoshino, and J. A. Gold. 2004. CD40 but not CD154 knockout mice have reduced inflammatory response in polymicrobial sepsis: a potential role for Escherichia coli heat shock protein 70 in CD40-mediated inflammation in vivo. Shock 22:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberholzer, A., C. Oberholzer, and L. L. Moldawer. 2001. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16:83-96. [DOI] [PubMed] [Google Scholar]
- 41.O'Sullivan, B., and R. Thomas. 2003. CD40 and dendritic cell function. Crit. Rev. Immunol. 23:83-107. [DOI] [PubMed] [Google Scholar]
- 42.Presneill, J. J., T. Harris, A. G. Stewart, J. F. Cade, and J. W. Wilson. 2002. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am. J. Respir. Crit. Care Med. 166:138-143. [DOI] [PubMed] [Google Scholar]
- 43.Pryjma, J., M. Zembala, J. Baran, M. Ernst, and H.-D. Flad. 1995. Elimination of monocytes from cultures activated with recall antigens. Immunol. Lett. 46:229-235. [DOI] [PubMed] [Google Scholar]
- 44.Rayhane, N., C. Fitting, O. Lortholary, F. Dromer, and J. M. Cavaillon. 2000. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infect. Immun. 68:3748-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ronco, C., A. Brendolan, G. Lonnemann, R. Bellomo, P. Piccinni, A. Digito, M. Dan, M. Irone, G. La Greca, P. Inguaggiato, U. Maggiore, C. De Nitti, M. L. Wratten, Z. Ricci, and C. Tetta. 2002. A pilot study of coupled plasma filtration with adsorption in septic shock. Crit. Care Med. 30:1250-1255. [DOI] [PubMed] [Google Scholar]
- 46.Sfeir, T., D. C. Saha, M. Astiz, and E. C. Rackow. 2001. Role of interleukin-10 in monocyte hyporesponsiveness associated with septic shock. Crit. Care Med. 1:129-133. [DOI] [PubMed] [Google Scholar]
- 47.Sinistro, A., C. Ciaprini, S. Natoli, E. Sussarello, F. Calò Carducci, C. Almerighi, M. Capozzi, F. Bolacchi, G. Rocchi, and A. Bergamini. 2007. Lipopolysaccharide desensitizes monocytes-macrophages to CD40L stimulation. Immunology 122:362-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugimoto, K., C. Galle, J.-C. Preiser, J. Creteur, J.-L. Vincent, and O. Pradier. 2003. Monocyte CD40 expression in severe sepsis. Shock 19:24-27. [DOI] [PubMed] [Google Scholar]
- 49.Tushinski, R. J., I. T. Oliver, L. J. Guilbert, W. Tynan, J. R. Warner, and E. R. Stanley. 1982. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 28:71-81. [DOI] [PubMed] [Google Scholar]
- 50.van Deuren, M., J. van der Ven-Jongekrijg, P. N. Demacker, A. K. Bartelink, R. van Dalen, R. W. Sauerwein, H. Gallati, J. L. Vannice, and J. W. van der Meer. 1994. Differential expression of proinflammatory cytokines and their inhibitors during the course of meningococcal infections. J. Infect. Dis. 169:157-161. [DOI] [PubMed] [Google Scholar]
- 51.Volk, H. D., P. Reinke, and W. D. Docke. 2000. Clinical aspects: from systemic inflammation to “immunoparalysis.” Chem. Immunol. 74:162-177. [DOI] [PubMed] [Google Scholar]
- 52.von Müller, L., A. Klemm, M. Weiss, M. Schneider, H. Suger-Wiedeck, N. Durmus, W. Hampl, and T. Mertens. 2006. Active cytomegalovirus infection in patients with septic shock. Emerg. Infect. Dis. 12:1517-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Würtzen, P. A., M. H. Nissen, and M. H. Claesson. 2001. Maturation of dendritic cells by recombinant human CD40L-trimer leads to a homogeneous cell population with enhanced surface marker expression and increased cytokine production. Scand. J. Immunol. 53:579-587. [DOI] [PubMed] [Google Scholar]