Abstract
Antibodies against Saccharomyces cerevisiae mannan (ASCA) and antibodies against synthetic disaccharide fragments of glucans (ALCA) and chitin (ACCA) are biomarkers of Crohn's disease (CD). We previously showed that Candida albicans infection generates ASCA. Here, we explored ALCA and ACCA as possible biomarkers of invasive C. albicans infection (ICI). ASCA, ALCA, ACCA, and Candida mannan antigen and antibody detection tests were performed on 69 sera obtained sequentially from 18 patients with ICIs proven by blood culture, 59 sera from CD patients, 47 sera from hospitalized subjects colonized by Candida species (CZ), and 131 sera from healthy controls (HC). ASCA, ALCA, and ACCA levels in CD and ICI patients were significantly different from those in CZ and HC subjects (P < 0.0001). In ICI patients, these levels increased as infection developed. Using ASCA, ALCA, ACCA, and Platelia Candida tests, 100% of ICIs were detected, with the kinetics of the antibody response depending on the patient during the time course of infection. A large number of sera presented with more than three positive tests. This is the first evidence that the detection of antibodies against chitin and glucans has diagnostic value in fungal infections and that these tests can complement more specific tests. Future trials are necessary to assess the value of these tests in multiparametric analysis, as well as their pathophysiological relevance.
Over the past few decades, Candida albicans has become one of the leading causes of nosocomial infection (30). Basic progress has been made in our understanding of C. albicans virulence attributes, the mechanisms of saprophytic-pathogenic transition, and factors predisposing patients to infection (5). However, despite this progress and increasing expenditure on empirical/curative antifungal therapy (51), both the incidence and attributable mortality of candidemia remain high (39 to 50%) (14, 30). This situation can be explained by the difficulty in establishing a reliable and early diagnosis of invasive Candida infection (ICI), particularly when the blood cultures gave negative results (2).
Most ICIs are endogenous in origin, as revealed by genetic identity between strains isolated from the gut and blood cultures, as well as the link between gut colonization and invasive infection (31, 32, 48). Despite this link, however, little research has focused on C. albicans in its natural niche (9). Crohn's disease (CD) is an interesting topic for transversal research, since this chronic inflammatory bowel disease is generally agreed to be triggered by genetic susceptibility to gut microbiota (11). As the development of CD has also been linked to the sequential appearance of antibodies against microbial antigens (13, 23), we investigated whether anti-C. albicans antibodies were associated with this disease. Antibodies against Saccharomyces cerevisiae mannan (ASCA) are widely used as serological markers of CD (47). By using antibodies immunopurified on synthetic oligomannoses mimicking the major epitope of S. cerevisiae mannan supporting the ASCA response, we demonstrated that this epitope is overexpressed by the pathogenic phase of C. albicans. Subsequently, it was shown that ASCA are serological markers of C. albicans infections in humans and animals (16, 43).
Recently, screening of sera from patients with CD with a glycan array led to the identification of two new antiglycan antibodies as serological markers of this disease (10). The two antibodies are directed against molecular fragments corresponding to a laminaribioside (β1,3-linked glucose dimer) and chitobioside (β1,4-linked N-acetylglucosamine dimer) and have been labeled ALCA and ACCA, respectively, and complement the detection of ASCA as serological markers of CD. The combined detection of ASCA, ALCA, and ACCA was named IBDX, an acronym referring to inflammatory bowel diseases, since it improved the differential diagnosis of CD. The cumulative presence of these antibodies corresponded to complicated disease with a higher risk of surgery (13).
As well as ASCA, ALCA and ACCA could also be induced by C. albicans, since oligomers of β-d-1,3 glucose and β-1,4-linked N-acetylglucosamine are constitutive units of glucan and chitin, which are essential components of the yeast cell wall (17). The availability of the IBDX panel prompted us to investigate whether ALCA and ACCA, together with ASCA, could also be synthesized as a result of C. albicans infection. The presence of antiglucan and antichitin antibodies in patients infected by C. albicans has never been investigated, since these cell wall components were previously considered to be nonimmunogenic. We also investigated how IBDX tests complement anti-C. albicans mannan antibody and mannanemia detection tests for the diagnosis of ICI (38, 42).
(This work was presented in part at the IXth American Society for Microbiology Conference on Candida and Candidiasis, 23 to 27 March 2008, Jersey City, NJ.)
MATERIALS AND METHODS
Serum samples from patients with invasive candidiasis.
Sixty-nine serum samples were selected retrospectively between January 2005 and December 2006 from 18 patients hospitalized in Lille University Hospital and Saint Antoine University Hospital, Paris, France, who developed ICIs. The patients consisted of nine females and nine males (mean age, 48 ± 19 years). The average number of sera per patient was 3.8 ± 2.17 (Table 1). The following selection criteria were applied retrospectively: (i) fever nonresponsive to antibacterial therapy but responsive to antifungal therapy; (ii) one or several positive cultures for C. albicans from blood; (iii) availability of sera within a range of 3 weeks before to 4 weeks after positive cultures; and (iv) analysis of the medical charts of patients with special attention to risk factors.
TABLE 1.
Clinical features of patients with systemic C. albicans infection
| Patient | Sexa | Age (yr) | Hospital ward | No. of sera | Date of serum sampling in relation to blood culture (days) | Candida species |
|---|---|---|---|---|---|---|
| 1 | M | 43 | ICU | 7 | 40, 47, 75, 104, 110, 117, 154 | C. albicans |
| 2 | F | 71 | Oncology | 2 | −1, 63 | C. albicans |
| 3 | M | 57 | ICU | 2 | 7, 14 | C. albicans, C. glabrata |
| 4 | M | 18 | ICU | 4 | −14, −7, 3, 23 | C. albicans |
| 5 | F | 73 | ICU | 3 | −5, −2, 9 | C. albicans |
| 6 | F | 21 | Hematology | 5 | −14, −9, 0, 25, 33 | C. albicans |
| 7 | M | 51 | Surgery | 9 | −2, 5, 12, 19, 26, 33, 44, 55, 62 | C. albicans |
| 8 | M | 31 | Hematology | 5 | −2, 5, 22, 27, 60 | C. albicans |
| 9 | F | 75 | Surgery | 2 | −1, 2 | C. albicans |
| 10 | F | 49 | ICU | 2 | −2, 6 | C. albicans |
| 11 | M | 68 | Nephrology | 2 | −1, 1 | C. albicans |
| 12 | F | 28 | Surgery | 2 | −1, 13 | C. albicans |
| 13 | M | 15 | ICU | 2 | −1, 13 | C. albicans |
| 14 | M | 42 | ICU | 7 | −20, −13, −6, 0, 21, 28, 42 | C. albicans |
| 15 | F | 80 | ICU | 3 | −25, −16, −9 | C. albicans |
| 16 | F | 53 | Neurosurgery | 3 | −2, 21, 44 | C. albicans |
| 17 | M | 49 | Surgery | 3 | 9, 15, 29 | C. albicans |
| 18 | F | 45 | ICU | 6 | −1, 3, 6, 22, 28, 35 | C. albicans |
M, male; F, female.
Written, informed consent was obtained from all patients before serum samples were taken from the patients, and the study was approved by the institutional review board of Lille University Hospital.
Patients with Crohn's disease.
Patients were selected retrospectively from a previous study of families recruited through the Registre des Maladies Inflammatoires Chroniques de l'Intestin du Nord-Ouest de la France (EPIMAD) and the Inflammatory Bowel Disease Registry at the University Hospital, Gasthuisberg, Leuven, Belgium (46). A proband was selected from each family. The diagnosis of CD was based on the usual criteria, and phenotypes were defined according to the Montreal classification. A total of 59 CD patients (20 males and 39 females; median age, 45 years; age range, 20 to 82 years) were selected. The age at the diagnosis of CD was known for 58 patients: <17 years for 7 patients (12.1%), 17 to 40 years for 46 patients (79.3%), and ≥40 years for 5 patients (6.6%). The disease location was known for 57 patients: ileal (L1) in 10 patients (17.5%), colonic (L2) in 9 patients (15%), ileo-colonic (L3) in 37 patients (64.9%), and isolated upper disease (L4) in 1 patient (1.8%). Disease behavior was documented in 56 patients: nonstricturing, nonpenetrating (B1) in 27 patients (48.2%), stricturing (B2) in 18 patients (32.1%), penetrating (B3) in 11 patients (19.6%), and perianal in 20 patients (35.7%).
Control sera.
Control sera consisted of 47 serum samples from patients (n = 47) with one or two body sites (trachea or sputum, urine, stool specimens, etc.) colonized by Candida species and hospitalized in the intensive care unit (ICU), and 131 sera from healthy blood donors (healthy controls [HC]).
Serological tests. (i) Detection of anti-C. albicans mannan antibodies and mannanemia.
Antibodies to C. albicans mannan and mannanemia were detected using the Platelia Candida antibody (Ab) and Platelia Candida antigen (Ag) tests (Bio-Rad Laboratories, Marnes-la-Coquette, France) as described previously (41).
(ii) Detection of ASCA and antiglycan antibodies.
All sera were assayed using a panel of tests that detect novel serological markers of CD (10). This panel, named IBDX (Glycominds, Israel), comprises ASCA, ALCA, and ACCA kits involving three antigens: S. cerevisiae mannan, laminaribioside, and chitobioside, respectively. para-Nitrophenyl derivatives of laminaribioside and chitobioside and S. cerevisiae mannan were covalently bound to the surfaces of microtiter wells with a linker (oligomer of 1,8-diamino-3,6-dioxaoctan; Sigma Chemical Co., St. Louis, MO). These tests were performed according to the manufacturer's instructions. ASCA, ALCA, and ACCA results were expressed in arbitrary units, which are relative to a Glycominds laboratory (gASCA, ALCA, ACCA) calibrators that are derived from a pool of patient sera with well-characterized disease. Antibody titers for each sample are calculated by dividing the average optical density (OD) of the sample by the average OD of the calibrator, multiplied by the number of units denoted by the calibrator tube label. The cutoff values were determined using receiver operating characteristic (ROC) curves to provide 97%, 100%, and 92% specificity for ACCA, ALCA, and ASCA, respectively, for CD.
(iii) Experimental C. albicans infection in rabbits and follow-up of antiglycan antibody responses.
Three New Zealand White rabbits (2 to 3 kg) were inoculated intravenously with 500 μl of live C. albicans VW32 yeast cells (2 × 106 yeast cells/ml). Serum samples were obtained every week for 3 weeks and stored at −20°C. Antibodies against C. albicans mannan (Platelia Ab), S. cerevisiae mannan (ASCA), laminaribioside (ALCA), and chitobioside (ACCA) were detected by an enzyme-linked immunosorbent assay (ELISA), which was adapted for detection of rabbit immunoglobulin G (IgG) with classical washing and incubation steps. Sera were diluted 1:400 for Platelia Ab, ASCA, and ALCA tests and 1:200 for the ACCA test and incubated with the corresponding antigens. Antibody binding was detected using peroxidase-labeled goat anti-rabbit IgG (Zymed Laboratories Inc., San Francisco, CA) diluted 1:1,000 and tetramethylbenzidine as a substrate (Bio-Rad, Marnes-la-Coquette, France).
MAb against β-glucans.
Monoclonal antibody (MAb) 2G8, a murine IgG2b reacting with β-glucan epitopes, was provided by A. Cassone (6). Dilutions of MAb 2G8 (1:500 to 1:16,000) were prepared from a concentration of 0.6 mg/ml and tested by ELISA on ALCA microtiter plates. One hundred microliters of each dilution was added to each well and incubated for 1 h at 37°C. After the wells were washed with TNT (50 mM Tris, 150 mM NaCl, HCl [pH 7.5], 0.05% Tween 20), 100 μl of horseradish peroxidase-conjugated antibodies (goat anti-mouse IgG; Southern Biotech) diluted 1:5,000 in TNT were added for 1 h at 37°C. After the wells were washed with TNT, each well received 100 μl of tetramethylbenzidine (Bio-Rad, Hercules, CA). After 30 min, the reaction was stopped by adding 100 μl of blocking reagent,and the plate was read at 450 nm. A MAb to Candida (EB-CA1), recognizing a mannopentaose (15), was used as a control.
Statistical analysis.
As ASCA, ALCA, and ACCA were not normally distributed, the significance of differences between two independent groups was determined by the Mann-Whitney U test, and the significance of differences among more than two groups was determined by Kruskal-Wallis one-way analysis. In ICI patients, Spearman's rank correlation coefficients were calculated to estimate the interrelation between anti-yeast glycan antibodies or mannan levels and time. Antibody values were classified arbitrarily into four groups according to the time of serum sampling. The date of isolation of Candida species from blood culture was defined as day 0; group 1 included sera obtained during the period from day −25 to day −1, group 2 included sera obtained from day 0 to day +15, group 3 included sera obtained from day +16 to day +40, and group 4 included sera obtained from day +41 to day +154. Statistical analysis was performed using SPSS for Windows version 11.0 (SPSS Inc.). A P value of <0.05 was considered statistically significant.
RESULTS
ASCA, ALCA, and ACCA levels are highly significantly elevated in patients with invasive candidiasis and compared with those observed in CD patients.
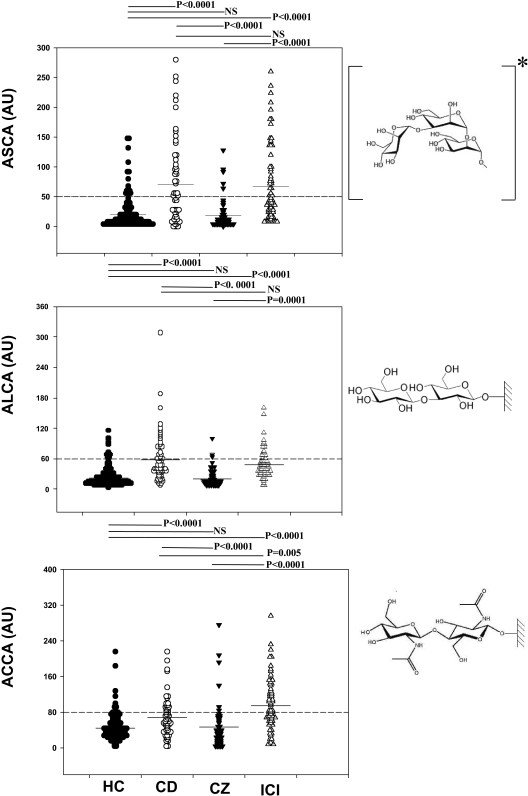
IBDX antibodies were detected in 69 sera from patients with ICI, 47 sera from hospitalized subjects colonized by Candida species (CZ patients), 131 sera from HC subjects, and 59 sera from patients with CD. Significant differences in the levels of ASCA, ALCA, and ACCA between ICI patients and CZ patients or HC subjects was observed (P < 0.0001) (Fig. 1). This was similar to the difference between CD patients and HC subjects and between CD and CZ patients. There was no significant difference in the ASCA or ALCA level in CD and ICI patients, although ACCA levels were significantly higher in ICI patients (P = 0.002).
FIG. 1.
Distribution of ASCA, ALCA, and ACCA in healthy controls, patients with Crohn's disease, ICU patients with one or two body sites colonized by Candida species, and patients with invasive candidiasis. Comparison of the values for the different groups of patients was performed using the Mann-Whitney U test (P values). ASCA, ALCA, and ACCA results were expressed in arbitrary units (AU) (see Materials and Methods). For ASCA, a highly significant difference was observed for HC versus CD patients (P < 0.0001) and HC versus ICI patients, while the difference between CD versus ICI patients was not statistically significant (NS). For ALCA, a highly significant difference was observed for HC versus CD patients (P < 0.0001) and HC versus ICI patients (P < 0.0001), while no difference was observed for CD versus ICI patients. For ACCA, similar results were observed for HC versus CD patients (P = 0.001) and HC versus ICI patients (P < 0.0001). In contrast to ASCA and ALCA, a significant difference in ACCA was observed between ICI and CD patients (P = 0.05). The same trend was observed for CZ patients, and no difference was observed for HC versus CZ patients. The chemical structures of the antigens are presented to the right of each graph; for ALCA and ACCA, synthetic oligosaccharides are coated on the wells of the ELISA plates; ASCA* antigen is a natural antigen which comprises a repertoire of oligomannose epitopes, among these we have represented the major epitope supporting the humoral response in CD patients (39, 52), since this synthetic analog was shown to specifically adsorb antibodies generated during C. albicans infection (43).
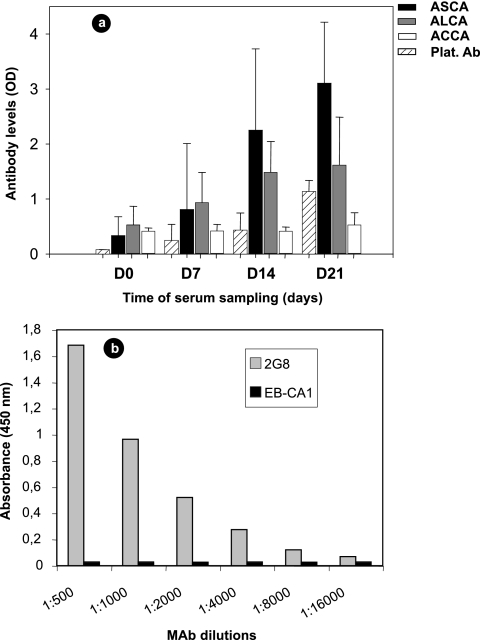
ASCA, ALCA, and ACCA are generated during experimental C. albicans infection, and the ALCA test detects antibodies against glucan epitopes protecting from C. albicans infection.
In order to assess the significance of ASCA, ALCA, and ACCA in relation to C. albicans infection, rabbits were experimentally infected with C. albicans. Figure 2 shows the results of antibody detection tests. Despite variation in background levels related to the adaptation of these tests to rabbits and differences in the antibody levels of the animals, ASCA and ALCA increased continuously as a result of C. albicans infection, as did anti-C. albicans mannan antibodies. ACCA levels were lower than the levels of ASCA and ALCA, and a delayed increase could be observed only 3 weeks after infection.
FIG. 2.
(a) Development of anti-C. albicans mannan antibodies (Platelia Ab [Plat. Ab]), ASCA, ALCA, and ACCA in New Zealand White rabbits following intravenous inoculation of live C. albicans strain VW32. Results are expressed as mean ODs plus standard errors (error bars). D0, day 0. (b) Murine MAb 2G8 (gray bars) and MAb EB-CA1 (black bars) reactivities were determined by ELISA with laminaribioside, a synthetic analog of β-1,3 glucan involved in the ALCA test.
We then investigated whether ALCA could be protective antibodies as reported for MAb 2G8 in experimental C. albicans infection. When different concentrations of MAb 2G8 were allowed to react with laminaribioside, a typical dose-dependent reactivity curve was observed, demonstrating that laminaribioside is among the epitopes recognized by this MAb (Fig. 2b). Under the same conditions, MAb EB-CA1 against Candida mannan did not exhibit any binding activity at concentrations as high as 10 μg/ml.
ASCA, ALCA, and ACCA increase in individual sera as a result of C. albicans infection as do anti-C. albicans mannan antibodies.
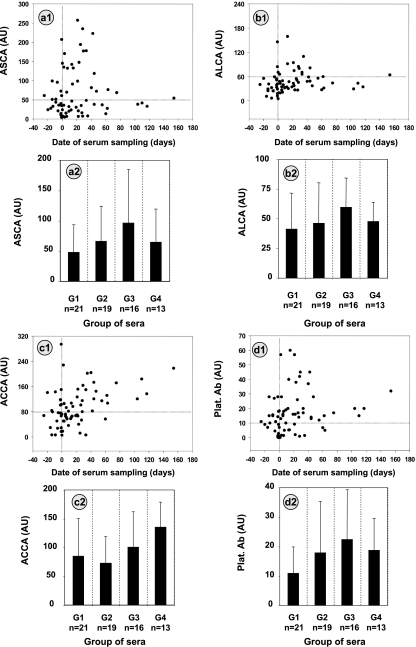
Anti-C. albicans antibody levels are known to increase during the transition of C. albicans from colonization to infection and are used as adjunct tests to blood cultures for the diagnosis of ICI. We therefore compared the Platelia Ab response to ASCA, ALCA, and ACCA levels during systemic C. albicans infection confirmed by positive blood culture. The results are shown in Fig. 3 a1, b1, c1, and d1. The correlation between antibody levels and the day when the sera were drawn was determined using Spearman's rank correlation coefficient. A correlation was observed for ACCA (P = 0.0018), ALCA (P = 0.019), and Platelia Ab (P = 0.02), demonstrating a link between C. albicans infection and an increase in these antiglycan antibodies. There was a trend toward an increase in ASCA levels during the disease, but it did not reach statistical significance. However, as shown in Fig. 3, panels 2 where sera are classified into four groups according to the date of sampling, this was related to the preferential association of ASCA with the acute phase and a more rapid decrease (Fig. 3, panel a2 versus panels b2, c3, and d2), whereas ACCA continued to increase several weeks after the onset of infection in rabbits.
FIG. 3.
Sixty-nine serum samples from 18 patients with invasive Candida infection (ICI) were screened for the presence of ASCA (a), ALCA (b), ACCA (c), and Platelia Ab (Plat. Ab) (d) as described in Materials and Methods. The antibody levels in arbitrary units (AU) (see Materials and Methods) are plotted according to the date of serum sampling (day 0 indicates the date of mycological isolation of C. albicans from blood). The horizontal line indicates the cutoff values used to define positive and negative results. The vertical line indicates day 0. Antibody values (mean titers plus standard errors [error bars]) for each test are also presented as histograms (panels a2, b2, c2, and d2) by classifying sera into four groups as follows: group 1 (G1) for sera taken during the period from day −25 to day −1, group 2 (G2) for sera taken from day 0 to day +15; group 3 for sera taken from day +16 to day +40; and group 4 for sera taken from day +41 to day +154.
ASCA, ALCA, ACCA, anti-C. albicans mannan antibodies, and C. albicans mannanemia exhibit different kinetics in individual serum samples depending on the time of serum sampling.
The contribution of IBDX and Platelia Ab and Ag tests to the diagnosis of ICI in relation to the time of serum sampling versus blood culture was investigated. After isolation of C. albicans from blood, positive antibody tests decreased: ACCA (23/48), ASCA (22/48), and ALCA (12/48), although only 1 of 48 sera was negative by both the IBDX and Platelia tests. ACCA and Platelia Ab were more frequently positive at least 1 week before the isolation of C. albicans from blood (Table 2).
TABLE 2.
Distribution of sera in relation to the date of isolation of C. albicans from blood (day 0) and number of positive results for each test and for a combination of tests
| Time of serum collection | No. of sera | No. of sera with positive results
|
No. of sera (%) with the following result by the Platelia test:
|
No. of sera with the following result for a combination of tests:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASCA | ALCA | ACCA | Platelia Ab | Platelia Ag | Positive | Negative | Negative for all tests | Positive for 1 test | Positive for 2 or 3 tests | Positive for 4 or 5 tests | ||
| >1 wk before | 8 | 2 | 0 | 4 | 5 | 1 | 6 (75) | 2 | 0 | 3 | 5 | |
| 1 wk before | 13 | 4 | 2 | 7 | 5 | 5 | 9 (69) | 4 | 3 | 2 | 7 | |
| 0−7 days after | 12 | 5 | 2 | 3 | 7 | 9 | 12 (100) | 0 | 0 | 3 | 8 | 1 |
| >7 days after | 36 | 17 | 10 | 20 | 28 | 9 | 30 (83) | 6 | 1 | 5 | 22 | 8 |
The majority of patients presented more than three positive anticarbohydrate antibody tests; in two of these tests, a lower antibody response was associated with mannanemia.
The results obtained for the 18 ICI patients are summarized in Table 3, with patients listed according to the number of sera available. ASCA, ALCA, and ACCA were detected in 13 (72%), 12 (66%), and 13 (72%) ICI patients, respectively, and only 2 patients were negative with the IBDX panel. For these two patients, Platelia tests were positive (twice for antigen and once for antimannan antibodies). Despite the limited number of sera (n = 2) available from seven patients, Table 3 indicates that 78% (14/18) of patients had at least three positive tests and 67% (12/18) had at least four positive tests. Table 3 also shows that only 4 of the 69 sera were negative by all tests. ICI was detected in all patients (100%) if at least one positive test was considered.
TABLE 3.
Results of mannanemia, antimannan antibodies, and IBDX tests in patients with invasive Candida infection
| Patienta | No. of available sera | No. of sera in which antibody was detectedb
|
No. of sera with positive result by the following test:
|
No. of sera negative for all tests | No. of tests for which patient was positive at least once (n = 5) | |||
|---|---|---|---|---|---|---|---|---|
| ASCA (50 AU) | ALCA (60 AU) | ACCA (80 AU) | Platelia Ab | Platelia Ag | ||||
| 7 | 9 | 0/9 | 2 | 6 | 8/9 | 1/9 | 0 | 4 |
| 1 | 7 | 4 | 1 | 7 | 7 | 3 | 0 | 5 |
| 14 | 7 | 4/7 | 2/7 | 2 | 5/7 | 0/7 | 1 | 4 |
| 18 | 6 | 0 | 3/6 | 6/6 | 0 | 4/6 | 0 | 4 |
| 6 | 5 | 3/5 | 1/5 | 0 | 4/5 | 3/5 | 0 | 4 |
| 8 | 5 | 0 | 0 | 0 | 3/5 | 2/5 | 0 | 2 |
| 4 | 4 | 4/4 | 0 | 1 | 4/4 | 2/4 | 0 | 4 |
| 17 | 3 | 2 | 1 | 3 | 3 | 0 | 0 | 4 |
| 15 | 3 | 2/3 | 0/3 | 1/3 | 2/3 | 1/3 | 0 | 4 |
| 5 | 3 | 0/3 | 1/3 | 0/3 | 3/3 | 3/3 | 0 | 3 |
| 16 | 3 | 2/3 | 1/3 | 2/3 | 2/3 | 0/3 | 0 | 4 |
| 10 | 2 | 2/2 | 0 | 1 | 2/2 | 1/2 | 0 | 4 |
| 2 | 2 | 1/2 | 0/2 | 1/2 | 0/2 | 0/2 | 1 | 2 |
| 9 | 2 | 2/2 | 2/2 | 2/2 | 1/2 | 1/2 | 0 | 5 |
| 11 | 2 | 0 | 0 | 0 | 0 | 1/2 | 1 | 1 |
| 12 | 2 | 1 | 1 | 0 | 1/2 | 0/2 | 1 | 3 |
| 13 | 2 | 1 | 2 | 2 | 1 | 0 | 0 | 4 |
| 3 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 2 |
Patients are listed according to the number of sera available (from the most to the least).
Number of serum samples in which antibodies at the level shown were detected. IBDX tests are ASCA, ALCA, and ACCA (the combined detection of these antibodies).
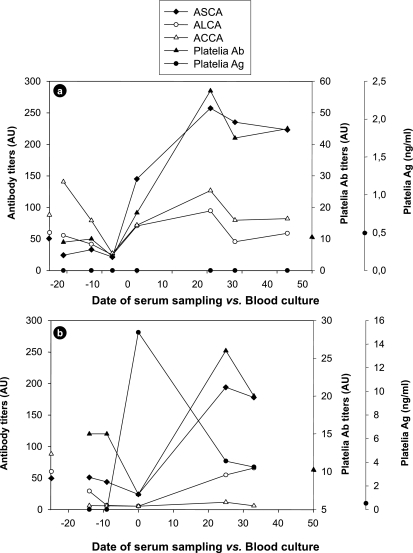
Complementation of the serological tests can also be observed when individual values are plotted in a kinetic manner during the course of C. albicans infection. Figure 4 shows two representative examples for patients 14 and 6 (see Table 3). These two patients already had two positive tests within the 2-week period before blood cultures became positive. Both showed a sharp drop in antibodies in sera taken around the time of positive blood culture. This phenomenon is associated with a massive release of fungal molecules correlating with circulation of C. albicans in the bloodstream. This was observed for patient 6 (Fig. 4b), who had very high levels of mannan detected by the Platelia Ag test, whereas for patient 14 (Fig. 4a), the epitope detected by this test was not found, suggesting the presence of other molecules interfering with the antibody detection tests. In both patients, this period was followed by a rapid increase in all anticarbohydrate antibodies, although the antibody levels to a given antigen differed in the two patients. Nevertheless, at least three different tests were simultaneously positive for both patients during this period.
FIG. 4.
Examples of the kinetics of ASCA, ALCA, ACCA, and Platelia Candida Ag and Ab tests in patients with proven invasive candidiasis. For both patients, patient 14 (a) and patient 6 (b), a gradual decrease in ASCA, ALCA, and ACCA was observed during the period preceding positive blood cultures to reach a minimum on day 0. After the candidemic episode, an overall increase could be observed for most antibody markers during the proceeding weeks. For each y axis, the symbols on the axis indicate the cutoff values for serological tests. ASCA, ALCA, ACCA, and Platelia Candida Ab titers are given in arbitrary units (AU) (see Materials and Methods).
DISCUSSION
The C. albicans cell wall consists of 80% glycans, 15% proteins, and 5% lipids (36). Glycans are distributed into 40% glucans (polymers of β-1,3- and β-1,6-glucose), 2 to 4% chitin, and 30% mannans. Mannans exist as mannoconjugates linked to proteins or lipids (22). Human sera contain anti-C. albicans mannan antibodies whose synthesis has been suggested to be due to the natural presence of C. albicans in the gut (19). In hospitalized patients, an increase in C. albicans colonization (37) slowly augments antimannan antibody levels, whereas a sharp increase is generally associated with tissue invasion (41). On this basis, regular survey of antimannan antibody levels in at risk patients has been proposed as a strategy to compensate for the poor sensitivity of blood cultures. Depending on the method used, different cutoff values have been proposed to differentiate between patients colonized by Candida species and patients infected with C. albicans (33, 42). In infected patients, a balance was observed between antimannan antibodies and mannanemia, and simultaneous screening for both markers has been recommended (42).
An important observation from this study is that C. albicans infection generates antibodies that can be detected with chitin oligomers. The presence of human antibodies against chitin was investigated only when synthetic chitobioside was discovered to be a biomarker for CD (10). ACCA react with a minimal epitope composed of two units from a linear polymer of β-1,4-d-GlcNAc from chitin. Chitin is a component of the exoskeletons of arthropods, worm cuticles, protozoan cysts, and the cell walls of some algae, yeasts, and filamentous fungi (1, 35, 49). Due to the abundance of organisms in the human environment and food, the presence of antichitin antibodies is not surprising, even if the process of antibody generation is unknown.
In our study, we found low levels of ACCA in a high percentage of the control population. In yeasts, which are present in the human diet and human gut, chitin provides cross-linking and strength to the cell wall polysaccharide scaffolding. Increased chitin synthesis is a response to cell wall weakening (20). In C. albicans, the cell wall of the invasive hyphal form contains three times more chitin than the yeast form (36). In relation to the presence of low levels of ACCA in the control population, our study clearly demonstrates that two pathogenic situations result in an increase in ACCA, namely, CD and infection by C. albicans. In this latter pathology, kinetic analysis of antibody levels during the time course of the disease clearly demonstrated a causal relationship between C. albicans infection and increase in ACCA levels. In addition, we observed that the antichitobioside antibody response is maintained at high levels many weeks after the candidemic episode.
Few studies have dealt with the interaction between chitin and innate immunity receptors that could help in our understanding of the sensing of this component of living organisms (34). In contrast, β-glucans have received much more attention due to their immunomodulatory properties (45), and significant progress in our understanding of yeast sensing has followed the identification of Dectin-1, a specific receptor interacting with Toll-like receptor 2 to trigger a pro-inflammatory response (25). In clinical circumstances, sensitive detection assays have demonstrated that the presence of circulating glucans in patients’ sera is indicative of fungal invasion (27), and studies suggest that glucan could be a valuable marker of fungal infection (50). In contrast, very little attention has been paid to the antiglucan antibody response because glucans are not immunogenic. Recently, laminarin, an analog of fungal glucans synthesized by algae, coupled to proteins was shown to be an effective vaccine for generating antibodies that protect against C. albicans experimental infection (44).
In this study, we demonstrated that ALCA are also generated in some patients during C. albicans infection and in rabbits with experimental ICI. This is not surprising, since C. albicans cell wall glucans are linked to proteins (17) and are therefore able to induce an antibody response (3, 12). Until now, it has not been possible to detect human antibodies against glucans due to the lack of a reproducible test. The ALCA test provides a positive response to this obstacle.
Recent studies have shown that, as established for mannanemia (40), the levels of circulating serum β-1,3 glucans fluctuate during the course of the disease (26) so that biweekly examination of patients is recommended (29). Such fluctuations could be due to interaction of β-glucans with specific or scavenger receptors or with complement fraction 3 leading to activation of the alternative pathway (4). Our study suggests an additional mechanism based on the presence of high levels of anti-β-glucan antibodies that could accelerate glucan clearance through immune complexes. The sharp drop in anticarbohydrate antibody response observed around the time of blood culture suggests that the balance between mannanemia and antimannan antibodies observed previously in ICI patients (42) could also apply to glucanemia and antiglucan antibodies. Taking into account the fluctuations in ALCA response revealed in this study and fluctuations in glucanemia reported elsewhere (18), it would be worthwhile to investigate whether combined detection of serum glucans and ALCA could improve the sensitivity of diagnosis. Unfortunately, it was not possible to address the question of glucanemia in the present study because we could not guarantee the sterility of all serum samples.
A striking observation was that the nature of the glycan biomarkers can vary from patient to patient and during the time course of the disease. This raises the question of the pathophysiological significance of these antibodies and their role in protection or facilitation of infection (8).
With regard to ALCA, we observed that laminaribioside reacts with a MAb that has been shown to be protective in animal models of vaginal and systemic candidiasis. As far as ASCA are concerned, these antibodies are prominent markers of severe inflammation in CD, and recent evidence shows that they can be generated in mice after oral administration of C. albicans against an inflammatory background (16). This association with inflammation was also observed in the current study since, in most patients, ASCA presented a peak associated with the acute phase of the infection (Fig. 4) and a rapid decrease which contrasted with the more sustained generation of ACCA. The diagnostic value and clinical significance of these qualitative markers need to be validated in a large prospective study including more controls and other categories of at risk patients.
Pathophysiologically, mannans, glucans, and chitin may be synthesized individually by various microbes which have adapted to the human gut. However, yeasts are the only organisms known to synthesize large quantities of each of these glycans in a single envelope. The present demonstration that ASCA, ALCA, and ACAA can also be induced by C. albicans reinforces the serological observations, suggesting a link between this yeast and immune alterations observed in CD. In this respect, it is interesting to note that recent studies on the interleukin-23 Th17 pathway have revealed its major role in inflammatory bowel diseases (21), as well as in anti-C. albicans defense mechanisms (28), where triggering molecules include yeast cell wall glycans (24).
Whatever the relationship between C. albicans and CD, the availability of these tests complements the current panel of tests designed specifically for the diagnosis of candidiasis in providing an earlier and more specific diagnosis. Thus, the future of serological diagnosis appears to be based on the use of multiple antigens and currently available technologies (7). In the case of fungal cell wall carbohydrates, the ubiquitous distribution of chitin and glucans suggests that ALCA and ACCA tests may also contribute to the diagnosis of other invasive mycoses.
Acknowledgments
We thank Nadine François, Laurence Richard, and Nawal el Messaoudi for their technical assistance and Val Hopwood for editing the manuscript.
This work was supported by funds from the Institut National de la Santé et de la Recherche Médicale (INSERM), Glycominds Ltd., and the network “INTESTINFO.”
N.D. and A.D. are employees of Glycominds Ltd., Israel. There are no other conflicts of interest.
Footnotes
Published ahead of print on 29 October 2008.
REFERENCES
- 1.Avron, B., R. M. Deutsch, and D. Mirelman. 1982. Chitin synthesis inhibitors prevent cyst formation by Entamoeba trophozoites. Biochem. Biophys. Res. Commun. 108:815-821. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, P. D., and K. A. Marr. 2007. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br. J. Haematol. 139:519-531. [DOI] [PubMed] [Google Scholar]
- 3.Breinig, F., K. Schleinkofer, and M. J. Schmitt. 2004. Yeast Kre1p is GPI-anchored and involved in both cell wall assembly and architecture. Microbiology 150:3209-3218. [DOI] [PubMed] [Google Scholar]
- 4.Brown, G. D., and S. Gordon. 2003. Fungal beta-glucans and mammalian immunity. Immunity 19:311-315. [DOI] [PubMed] [Google Scholar]
- 5.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 6.Cassone, A. 2006. Protective anti-glycan antibodies with preferences for beta-1,3-glucans. Italian patent WO/2006/030, 318.
- 7.Clancy, C. J., M. L. Nguyen, S. Cheng, H. Huang, G. Fan, R. A. Jaber, J. R. Wingard, C. Cline, and M. H. Nguyen. 2008. Immunoglobulin G responses to a panel of Candida albicans antigens as accurate and early markers for the presence of systemic candidiasis. J. Clin. Microbiol. 46:1647-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler, J. E., G. S. Deepe, Jr., and B. S. Klein. 2007. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 5:13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Luca, A., C. Montagnoli, T. Zelante, P. Bonifazi, S. Bozza, S. Moretti, C. D'Angelo, C. Vacca, L. Boon, F. Bistoni, P. Puccetti, F. Fallarino, and L. Romani. 2007. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 179:5999-6008. [DOI] [PubMed] [Google Scholar]
- 10.Dotan, I., S. Fishman, Y. Dgani, M. Schwartz, A. Karban, A. Lerner, O. Weishauss, L. Spector, A. Shtevi, R. T. Altstock, N. Dotan, and Z. Halpern. 2006. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn's disease. Gastroenterology 131:366-378. [DOI] [PubMed] [Google Scholar]
- 11.Eckburg, P. B., and D. A. Relman. 2007. The role of microbes in Crohn's disease. Clin. Infect. Dis. 44:256-262. [DOI] [PubMed] [Google Scholar]
- 12.Ecker, M., R. Deutzmann, L. Lehle, V. Mrsa, and W. Tanner. 2006. Pir proteins of Saccharomyces cerevisiae are attached to beta-1,3-glucan by a new protein-carbohydrate linkage. J. Biol. Chem. 281:11523-11529. [DOI] [PubMed] [Google Scholar]
- 13.Ferrante, M., L. Henckaerts, M. Joossens, M. Pierik, S. Joossens, N. Dotan, G. L. Norman, R. T. Altstock, K. Van Steen, P. Rutgeerts, G. Van Assche, and S. Vermeire. 2007. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut 56:1394-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudlaugsson, O., S. Gillespie, K. Lee, J. Vande Berg, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172-1177. [DOI] [PubMed] [Google Scholar]
- 15.Jacquinot, P. M., Y. Plancke, B. Sendid, G. Strecker, and D. Poulain. 1998. Nature of Candida albicans-derived carbohydrate antigen recognized by a monoclonal antibody in patient sera and distribution over Candida species. FEMS Microbiol. Lett. 169:131-138. [DOI] [PubMed] [Google Scholar]
- 16.Jawhara, S., X. Thuru, A. Standaert-Vitse, T. Jouault, S. Mordon, B. Sendid, P. Desreumaux, and D. Poulain. 2008. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 197:972-980. [DOI] [PubMed] [Google Scholar]
- 17.Klis, F. M., P. Mol, K. Hellingwerf, and S. Brul. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239-256. [DOI] [PubMed] [Google Scholar]
- 18.Kondori, N., L. Edebo, and I. Mattsby-Baltzer. 2004. Circulating β (1-3) glucan and immunoglobulin G subclass antibodies to Candida albicans cell wall antigens in patients with systemic candidiasis. Clin. Diagn. Lab. Immunol. 11:344-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozel, T. R., R. S. MacGill, A. Percival, and Q. Zhou. 2004. Biological activities of naturally occurring antibodies reactive with Candida albicans mannan. Infect. Immun. 72:209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latge, J. P. 2007. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol. 66:279-290. [DOI] [PubMed] [Google Scholar]
- 21.McGovern, D., and F. Powrie. 2007. The IL23 axis plays a key role in the pathogenesis of IBD. Gut 56:1333-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mille, C., P. Bobrowicz, P. A. Trinel, H. Li, E. Maes, Y. Guerardel, C. Fradin, M. Martinez-Esparza, R. C. Davidson, G. Janbon, D. Poulain, and S. Wildt. 2008. Identification of a new family of genes involved in beta-1,2-mannosylation of glycans in Pichia pastoris and Candida albicans. J. Biol. Chem. 283:9724-9736. [DOI] [PubMed] [Google Scholar]
- 23.Mow, W. S., E. A. Vasiliauskas, Y. C. Lin, P. R. Fleshner, K. A. Papadakis, K. D. Taylor, C. J. Landers, M. T. Abreu-Martin, J. I. Rotter, H. Yang, and S. R. Targan. 2004. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology 126:414-424. [DOI] [PubMed] [Google Scholar]
- 24.Netea, M. G., G. D. Brown, B. J. Kullberg, and N. A. Gow. 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6:67-78. [DOI] [PubMed] [Google Scholar]
- 25.Netea, M. G., N. A. Gow, C. A. Munro, S. Bates, C. Collins, G. Ferwerda, R. P. Hobson, G. Bertram, H. B. Hughes, T. Jansen, L. Jacobs, E. T. Buurman, K. Gijzen, D. L. Williams, R. Torensma, A. McKinnon, D. M. MacCallum, F. C. Odds, J. W. Van der Meer, A. J. Brown, and B. J. Kullberg. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 116:1642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odabasi, Z., G. Mattiuzzi, E. Estey, H. Kantarjian, F. Saeki, R. J. Ridge, P. A. Ketchum, M. A. Finkelman, J. H. Rex, and L. Ostrosky-Zeichner. 2004. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199-205. [DOI] [PubMed] [Google Scholar]
- 27.Ostrosky-Zeichner, L., B. D. Alexander, D. H. Kett, J. Vazquez, P. G. Pappas, F. Saeki, P. A. Ketchum, J. Wingard, R. Schiff, H. Tamura, M. A. Finkelman, and J. H. Rex. 2005. Multicenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654-659. [DOI] [PubMed] [Google Scholar]
- 28.Palm, N. W., and R. Medzhitov. 2007. Antifungal defense turns 17. Nat. Immunol. 8:549-551. [DOI] [PubMed] [Google Scholar]
- 29.Pazos, C., M. D. Moragues, G. Quindos, J. Ponton, and A. del Palacio. 2006. Diagnostic potential of (1,3)-beta-D-glucan and anti-Candida albicans germ tube antibodies for the diagnosis and therapeutic monitoring of invasive candidiasis in neutropenic adult patients. Rev. Iberoam. Micol. 23:209-215. [DOI] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piarroux, R., F. Grenouillet, P. Balvay, V. Tran, G. Blasco, L. Millon, and A. Boillot. 2004. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit. Care Med. 32:2443-2449. [DOI] [PubMed] [Google Scholar]
- 32.Pittet, D., M. Monod, P. M. Suter, E. Frenk, and R. Auckenthaler. 1994. Candida colonization and subsequent infections in critically ill surgical patients. Ann. Surg. 220:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prella, M., J. Bille, M. Pugnale, B. Duvoisin, M. Cavassini, T. Calandra, and O. Marchetti. 2005. Early diagnosis of invasive candidiasis with mannan antigenemia and antimannan antibodies. Diagn. Microbiol. Infect. Dis. 51:95-101. [DOI] [PubMed] [Google Scholar]
- 34.Reese, T. A., H. E. Liang, A. M. Tager, A. D. Luster, N. Van Rooijen, D. Voehringer, and R. M. Locksley. 2007. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447:92-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiss, E. 1986. Molecular immunology of mycotic and actinomycotic infections. Elsevier Science Publishing, Inc., New York, NY.
- 36.Ruiz-Herrera, J., M. V. Elorza, E. Valentin, and R. Sentandreu. 2006. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 6:14-29. [DOI] [PubMed] [Google Scholar]
- 37.Samonis, G., A. Gikas, E. J. Anaissie, G. Vrenzos, S. Maraki, Y. Tselentis, and G. P. Bodey. 1993. Prospective evaluation of effects of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans. Antimicrob. Agents Chemother. 37:51-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sendid, B., D. Caillot, B. Baccouch-Humbert, L. Klingspor, M. Grandjean, A. Bonnin, and D. Poulain. 2003. Contribution of the Platelia Candida-specific antibody and antigen tests to early diagnosis of systemic Candida tropicalis infection in neutropenic adults. J. Clin. Microbiol. 41:4551-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sendid, B., J. F. Colombel, P. M. Jacquinot, C. Faille, J. Fruit, A. Cortot, D. Lucidarme, D. Camus, and D. Poulain. 1996. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin. Diagn. Lab. Immunol. 3:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sendid, B., T. Jouault, R. Coudriau, D. Camus, F. Odds, M. Tabouret, and D. Poulain. 2004. Increased sensitivity of mannanemia detection tests by joint detection of alpha- and beta-linked oligomannosides during experimental and human systemic candidiasis. J. Clin. Microbiol. 42:164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sendid, B., J. L. Poirot, M. Tabouret, A. Bonnin, D. Caillot, D. Camus, and D. Poulain. 2002. Combined detection of mannanaemia and antimannan antibodies as a strategy for the diagnosis of systemic infection caused by pathogenic Candida species. J. Med. Microbiol. 51:433-442. [DOI] [PubMed] [Google Scholar]
- 42.Sendid, B., M. Tabouret, J. L. Poirot, D. Mathieu, J. Fruit, and D. Poulain. 1999. New enzyme immunoassays for sensitive detection of circulating Candida albicans mannan and antimannan antibodies: useful combined test for diagnosis of systemic candidiasis. J. Clin. Microbiol. 37:1510-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Standaert-Vitse, A., T. Jouault, P. Vandewalle, C. Mille, M. Seddik, B. Sendid, J. M. Mallet, J. F. Colombel, and D. Poulain. 2006. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn's disease. Gastroenterology 130:1764-1775. [DOI] [PubMed] [Google Scholar]
- 44.Torosantucci, A., C. Bromuro, P. Chiani, F. De Bernardis, F. Berti, C. Galli, F. Norelli, C. Bellucci, L. Polonelli, P. Costantino, R. Rappuoli, and A. Cassone. 2005. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 202:597-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tzianabos, A. O. 2000. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin. Microbiol. Rev. 13:523-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Kruiningen, H. J., M. Joossens, S. Vermeire, S. Joossens, S. Debeugny, C. Gower-Rousseau, A. Cortot, J. F. Colombel, P. Rutgeerts, and R. Vlietinck. 2007. Familial Crohn's disease in Belgium: pedigrees, temporal relationships among cases, and family histories. J. Clin. Gastroenterol. 41:583-590. [DOI] [PubMed] [Google Scholar]
- 47.Vernier, G., B. Sendid, D. Poulain, and J. F. Colombel. 2004. Relevance of serologic studies in inflammatory bowel disease. Curr. Gastroenterol. Rep. 6:482-487. [DOI] [PubMed] [Google Scholar]
- 48.Voss, A., R. J. Hollis, M. A. Pfaller, R. P. Wenzel, and B. N. Doebbeling. 1994. Investigation of the sequence of colonization and candidemia in nonneutropenic patients. J. Clin. Microbiol. 32:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward, H. D., J. Alroy, B. I. Lev, G. T. Keusch, and M. E. Pereira. 1985. Identification of chitin as a structural component of Giardia cysts. Infect. Immun. 49:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wingard, J. R. 2007. New approaches to invasive fungal infections in acute leukemia and hematopoietic stem cell transplant patients. Best Pract. Res. Clin. Haematol. 20:99-107. [DOI] [PubMed] [Google Scholar]
- 51.Wingard, J. R., H. L. Leather, C. A. Wood, W. C. Gerth, R. J. Lupinacci, M. L. Berger, and E. C. Mansley. 2007. Pharmacoeconomic analysis of caspofungin versus liposomal amphotericin B as empirical antifungal therapy for neutropenic fever. Am. J. Health Syst. Pharm. 64:637-643. [DOI] [PubMed] [Google Scholar]
- 52.Young, M., M. J. Davies, D. Bailey, M. J. Gradwell, B. Smestad-Paulsen, J. K. Wold, R. M. Barnes, and E. F. Hounsell. 1998. Characterization of oligosaccharides from an antigenic mannan of Saccharomyces cerevisiae. Glycoconj. J. 15:815-822. [DOI] [PubMed] [Google Scholar]