Abstract
Endoplasmic reticulum (ER) stress can trigger apoptosis and necrosis in many types of mammalian cells. Previous studies in yeast found little or no cell death in response to the ER stressor tunicamycin, but a recent study suggested widespread apoptosis-like death. Here we show that wild-type laboratory Saccharomyces cerevisiae cells responding to tunicamycin die by nonapoptotic mechanisms in low-osmolyte culture media and survive for long periods of time in standard synthetic media. Survival requires calcineurin, a Ca2+/calmodulin-dependent protein phosphatase, but none of its known targets. The Ca2+/calmodulin-dependent protein kinase Cmk2 was identified as an indirect target of calcineurin that suppresses death of calcineurin-deficient cells. Death of Cmk2- and/or calcineurin-deficient S. cerevisiae cells was preceded by accumulation of reactive oxygen species but was not associated with hallmarks of apoptosis and was not dependent on Mca1, Aif1, Nuc1, or other factors implicated in apoptosis-like death. Cmk2 and calcineurin also independently suppressed the death of S. cerevisiae cells responding to dithiothreitol or miconazole, a common azole-class antifungal drug. Though inhibitors of Hsp90 have been shown to diminish calcineurin signaling in S. cerevisiae and to synergistically inhibit growth in combination with azoles, they did not stimulate death of S. cerevisiae cells in combination with miconazole or tunicamycin, and instead they prevented the death of calcineurin- and Cmk2-deficient cells. These findings reveal a novel prodeath role for Hsp90 and antideath roles for calcineurin and Cmk2 that extend the life span of S. cerevisiae cells responding to both natural and clinical antifungal compounds.
Azole-class antifungal drugs are widely employed in humans to control diverse types of fungal pathogens. Azoles target essential enzymes of the pathogen's endoplasmic reticulum (ER) that are required for biosynthesis of ergosterol (2, 51). A major limitation of these drugs is their inability to directly kill cells of most fungal pathogens (20). As a consequence, the live but nonproliferating cells may acquire drug resistance through adaptation or mutation and place additional burdens on host defenses. These disadvantages of fungistatic drugs may be avoided by the development of fungicidal alternatives or codrugs that are fungicidal in combination with fungistatic drugs.
Recently, azole-class drugs have been shown to acquire fungicidal activity when combined with FK506 or cyclosporine, both inhibitors of calcineurin (reviewed in reference 3). Such ‘fungicidal synergism’ has been observed in diverse fungal pathogens in vitro and in vivo. Additionally, inhibitors of several different essential ER enzymes appear by themselves to be fungistatic and to become potently fungicidal when combined with FK506 or cyclosporine. For example, the natural antibiotic tunicamycin, which blocks the ER enzyme UDP-N-acetyl-glucosamine-1-P transferase (Alg7) that is necessary for N glycosylation of secretory proteins and causes reversible cell cycle arrest and growth inhibition when administered alone to S. cerevisiae, Candida albicans, or Candida glabrata without appreciable cell death but causes massive cell death when used in combination with FK506 or cyclosporine (6). In S. cerevisiae, similar fungicidal synergism was observed using dithiothreitol, which blocks disulfide bond formation in the ER (6), or naturally secreted mating pheromones (71) as the arrest/death stimuli. FK506 and cyclosporine are well known as potent inhibitors of a cytoplasmic Ca2+/calmodulin-dependent protein phosphatase known as calcineurin (39), and many of the fungicidal synergisms described above have been confirmed using fungal mutants that lack calcineurin or its upstream regulators (reviewed in reference 59). Unfortunately, FK506 and cyclosporine are also potent inhibitors of human calcineurin and result in immunosuppression and other side effects that may severely limit their utility in antifungal regimens. A better understanding of the fungal antideath and prodeath pathways may lead to the identification of new compounds that promote fungicidal conversion without immunosuppressive side effects.
Though calcineurin function is not essential for growth of most fungi in laboratory conditions, several important roles have been identified in the budding yeast S. cerevisiae. Activated calcineurin dephosphorylates the Cch1 subunit of a high-affinity Ca2+ channel and inhibits Ca2+ influx by this channel during the responses to mating pheromones and tunicamycin, which triggers channel phosphorylation and activation by the mitogen-activated protein kinase Slt2 (also known as Mpk1) (5, 13). Activated calcineurin also dephosphorylates and activates the Crz1 (also known as Tcn1 and Hal8) transcription factor (45, 46, 58), which induces expression of many genes involved in diverse processes (31, 70). In response to high environmental Ca2+ levels, calcineurin induces and dephosphorylates Rcn1, a conserved regulatory protein that seems to regulate calcineurin itself by feedback (31, 34, 35). In these conditions, calcineurin also seems to limit the activity of Vcx1, a vacuolar H+/Ca2+ exchanger (15). Calcineurin may additionally regulate the Hsl1 protein kinase (47) and the Skn7 transcription modulator (65). In response to a high environmental pH, which depolarizes the cell and rapidly activates the Cch1-dependent Ca2+ channel (63), calcineurin can dephosphorylate or regulate Frt1 and Frt2 (also known as Hph1 and Hph2), two ER-resident proteins involved in tolerance to high pH (29) and possibly tolerance to azoles (14). Here we evaluate the possible involvement of these and other direct or indirect targets of calcineurin in the antideath pathway that is governed by calcineurin.
The manner by which calcineurin-deficient cells succumb and the molecular mechanisms that promote calcineurin-less death are also of great interest and significance. A recent study has proposed that apoptosis-like cell death occurs in S. cerevisiae cells treated with tunicamycin or lacking certain N-glycosylation factors (27). However, that conclusion relied on cytological methods shown previously to generate ambiguous results (66) and disagreed with previous findings that tunicamycin does not induce cell death in wild-type cells (5, 6, 13). Calcineurin-less death during the response to mating pheromones was also proposed to be apoptosis-like (57), but a subsequent study exposed additional methodological ambiguities and instead proposed a nonapoptotic manner of cell death (71) that resembles a necrosis-like cell death similar to that observed upon overexpression of human Bax in S. cerevisiae (see reference 36 and references therein). Death of wild-type cells expressing Bax or calcineurin-deficient cells responding to mating pheromones both required a functional oxidative phosphorylation system in mitochondria (i.e., coupled respiratory complexes III, IV, and V) and both involved accumulation of reactive oxygen species (ROS) (25, 67, 71). Neither condition was associated with cytological ‘hallmarks’ of apoptosis (chromatin fragmentation and phosphatidylserine externalization) or influenced by a variety of apoptotic factors (24, 52). Nevertheless, these findings have been mistaken as additional support for the hypothesis that S. cerevisiae cells can undergo apoptosis-like cell death (11, 19). Here we use improved methods to analyze several reported factors and cytological features associated with apoptosis-like death during the response of S. cerevisiae cells to tunicamycin. We confirm that tunicamycin can induce the death of wild-type cells when grown in low-osmolyte yeast-peptone-dextrose (YPD) medium (27), but in disagreement with the previous study, the observed cell death did not include an apoptotic stage. In synthetic medium containing tunicamycin, wild-type cells do not die and calcineurin-deficient cells die once again without hallmarks of apoptosis and without influence from a variety of proapoptotic factors, in agreement with our previous studies of calcineurin-less death during the response to mating pheromones (71). Calcineurin-less death therefore appears to be nonapoptotic.
We also find that Cmk2, one of two Ca2+/calmodulin-dependent protein kinases in S. cerevisiae (16, 49), is strongly induced by activation of calcineurin and Crz1 and suppresses calcineurin-less death. Calcineurin also suppresses cell death independent of Crz1, Cmk2, and many other targets, even when multiple targets have been mutated. Though Hsp90 had been proposed to promote azole resistance by stimulating calcineurin function (14), inhibitors of Hsp90 did not mimic inhibitors of calcineurin in the ability to induce calcineurin-less death. Instead, inhibitors of Hsp90 prevented calcineurin-less death. These findings suggest that calcineurin-less death is nonapoptotic and, surprisingly, dependent on clients of Hsp90.
MATERIALS AND METHODS
Strains and growth conditions.
All strains used in this study (Table 1) were derived from wild-type parent strains of W303-1A MATa ade2-1 can1-100 his3-1 leu2-3,112 trp1-1 ura3-1 (64) or BY4741 MATa his3-1 leu2-2 met15-0 ura3-0 (7) using standard gene knockout procedures (40) and/or isogenic genetic crosses. The primers used for gene knockouts are listed in Table 2. All S. cerevisiae strains were grown in synthetic complete (SC) medium or rich YPD medium containing 2% glucose. Tunicamycin was dissolved in methanol. FK506 was dissolved in dimethyl sulfoxide. Antimycin A and myxothiazol were dissolved in ethanol. Miconazole was dissolved in distilled water. All inhibitors were obtained from Sigma-Aldrich and compared to solvent controls.
TABLE 1.
Yeast strains used in this study
| Strain name | Genotype | Source |
|---|---|---|
| K1251 | MATa (wild-type BY4741) | This study |
| K601 | MATa (wild-type W303-1A) | 15 |
| K603 | MATa cnb1::LEU2 | 15 |
| W303-1AΔCOQ1 | MATa coq1::LEU2 | 22 |
| W303-1AΔCOQ2 | MATα coq2::LEU2 | 22 |
| cc304.1 | MATα cor1::HIS3 | 22 |
| NZY001 | MATa mca1::G418R | 71 |
| DDY45 | MATa nuc1::NatR | This study |
| DDY62 | MATa fis1::HIS3 | This study |
| DDY293 | MATa nma111::NatR | This study |
| DDY426 | MATa ste20::HIS3 | This study |
| DDY72 | MATa | This study |
| DDY74 | MATa cmk2::NatR | This study |
| DDY76 | MATa cnb1::LEU2 | This study |
| DDY78 | MATa crz1::G418 | This study |
| DDY80 | MATa cmk1::TRP1 | This study |
| DDY82 | MATa cmk2::NatR cnb1::LEU2 | This study |
| DDY83 | MATa cmk2::NatR crz1::G418 | This study |
| DDY85 | MATa cmk2::NatR cmk1::TRP1 | This study |
| DDY87 | MATa cmk2::NatR cnb1::LEU2 crz1::G418 | This study |
| DDY89 | MATa cmk2::NatR cnb1::LEU2 cmk1::TRP1 | This study |
| DDY91 | MATa cmk2::NatR crz1::G418 cmk1::TRP1 | This study |
| DDY93 | MATa cmk2::NatR cnb1::LEU2 crz1::G418 cmk1::TRP1 | This study |
| DDY95 | MATa cnb1::LEU2 crz1::G418 | This study |
| DDY97 | MATa cnb1::LEU2 cmk1::TRP1 | This study |
| DDY99 | MATa cnb1::LEU2 crz1::G418 cmk1::TRP1 | This study |
| DDY101 | MATa crz1::G418 cmk1::TRP1 | This study |
| DDY392 | MATa rcn1::LEU2 | This study |
| DDY400 | MATa rcn1::LEU2 crz1::G418R | This study |
| DDY408 | MATa rcn1::LEU2 cmk2::NatR | This study |
| DDY416 | MATa rcn1::LEU2 crz1::G418R cmk2::NatR | This study |
| DDY104 | MATa vcx1Δ | This study |
| DDY110 | MATa vcx1Δ crz1::G418R | This study |
| DDY109 | MATa vcx1Δ cmk2::NatR | This study |
| DDY114 | MATa vcx1Δ crz1::G418R cmk2::NatR | This study |
| K1691 | MATa frt1::HIS3 frt2::NatR | This study |
| K1693 | MATa frt1::HIS3 frt2::NatR crz1::G418R | This study |
| K1695 | MATa frt1::HIS3 frt2::NatR cmk2::TRP1 | This study |
| K1697 | MATa frt1::HIS3 frt2::NatR crz1::G418R cmk2::TRP1 | This study |
| DDY361 | MATα yap1::HIS3 skn7::TRP1 | This study |
| DDY364 | MATa yap1::HIS3 skn7::TRP1 crz1::G418R | This study |
| DDY368 | MATa yap1::HIS3 skn7::TRP1 cmk2::NatR | This study |
| DDY372 | MATa yap1::HIS3 skn7::TRP1 crz1::G418R cmk2::NatR | This study |
TABLE 2.
Oligonucleotides used in this study
| Name | Sequencea |
|---|---|
| Frt1-F1 | AAATTTTTTTTTTTTTTTGGTTTAAGTTATAGTTGATACATTAAGTGAAACGGATCCCCGGGTTAATTAA |
| Frt1-R0 | TTTATCTTTGTACGCGTACCATTTTTGTAAAGTGACCTTGTTTTGACTTTCATCGATGAATTCGAGCTCG |
| Frt2-F1 | ACACAAATTGATGGCAGTTTTTTACGTAGTCCAGTAGTTGTCCAGGTACACGGATCCCCGGGTTAATTAA |
| Frt2-R0 | TACATATGAAAAAATCACAGGATCATTTTTTGATATACAAATACTATTTTCATCGATGAATTCGAGCTCG |
| Yap1-F1 | AGTTTTTTGCCACCCAAAACGTTTAAAGAAGGAAAAGTTGTTTCTTAAACCCGGATCCCCGGGTTAATTAA |
| Yap1-R0 | ATTATAGAAAAAGTTCTTTCGGTTACCCAGTTTTCCATAAAGTTCCCGCTCATCGATGAATTCGAGCTCG |
| STE20-F1 | CCTCACACCCCATCCTAAATATCCCACAAGATCCTCGACTAATACAAGAACGGATCCCCGGGTTAATTAA |
| STE20-R0 | CAACTGTGTATATACTTTGTCGATAATAAGGTGTACCCTGCTTGCTACGTCATCGATGAATTCGAGCTCG |
| Skn7-F1 | TTCCATTATTCTTATTCCTATTTTTTCGTTGCTTACTTTTGATATCCACTCGGATCCCCGGGTTAATTAA |
| Skn7-R0 | GAATACAGAATGTCCTCTGCTAACTTAGACGCAAGGCTATTTGTAAAATTCATCGATGAATTCGAGCTCG |
| Nuc1-F0 | GATAAGAACATTTAGTTAAGTTCCCCACTTATTAGATCCCAAAGTTAAAGTTATCACATCAGGTCGACGGATCCCC |
| Nuc1-R0 | TGCTATTCTATCGTTGTGTTATGAATTAATTTATATTTACAGTTTTTCAGTACATCATCGATGAATTCGAGCTCG |
| Nma111-F1 | ATACTCTGAATACACACGTAGAGTACAGTAAAGGTTTTTTAGATCTACTACGGATCCCCGGGTTAATTAA |
| Nma111-R0 | TTGATGTATACATACATACATACATATATAAATGTTTTATCAAATCTGGCCATCGATGAATTCGAGCTCG |
| Cmk2-F1 | TCGTCACCTTTTCTTCTATCACATCGCCAATATAAATATAGACACCAAAACGGATCCCCGGGTTAATTAA |
| Cmk2-R0 | CACATTAAATATTATATACGAATTTATGTACACGAATTCAAGTCCGTAATCATCGATGAATTCGAGCTCG |
| CMK2-941F | CCCCTCGAGGGCCAGGTGATTATACCGC |
| CMK2+9R | GGGGGATCCAGACATTTTTGGTGTCTATATTT |
| CMK2K76R-F | CCACAAATGAAGATGTTGCTATAAGGATCTTATTGAAGAAGGCATTGC |
| CMK2K76R-R | GCAATGCCTTCTTCAATAAGATCCTTATAGCAACATCTTCATTTGTGG |
Underlined nucleotides are the mutated residues in the K76R point mutant.
Plasmids.
Plasmid pOO1 bearing a CMK2-lacZ reporter gene was constructed by PCR amplification of a genomic DNA segment (nucleotides −941 to +9 relative to the start codon CMK2) using primers CMK2-941F and CMK2+9R (Table 2). The product was digested with XhoI and BamHI restriction enzymes and ligated into plasmid pLGΔ178 (23) linearized with the same enzymes. High-dosage plasmids expressing CMK1 and CMK2 have been described previously (50). The latter was modified to express a ‘kinase-dead’ K76R variant of Cmk2 by using Quickchange (Stratagene) and the primers CMK2K76R-F and CMK2K76R-R. The resulting point mutation was confirmed by DNA sequencing.
Cell death assays.
Live and dead S. cerevisiae cells in cell populations were counted manually using bright-field or epifluorescence microscopy as required by the vital stains methylene blue (MB) or propidium iodide (PI). Strains were grown overnight in SC or YPD medium at 30°C. Log-phase cultures were selected and back diluted to an optical density at 600 nm (OD600) of 0.1 in fresh medium containing drugs and inhibitors. At various times, 200 μl of cells were harvested by centrifugation (13,000 rpm for 1 min), washed in 200 μl of SC medium, and resuspended in 10 μl of fresh SC medium containing 0.4 mg/ml MB or 0.4 mg/ml PI. The MB-stained cells were then spotted onto microscope slides, immediately covered by a coverslip, and counted as live (unstained) or dead (stained). The PI-stained cells were incubated at room temperature in the dark for 20 min, spotted onto slides, and immediately counted as live (nonfluorescent) or dead (fluorescent) using a Nikon Diaphot microscope fitted with a mercury arc lamp. At least 200 cells were scored for each sample.
Live and dead S. cerevisiae cells were also counted automatically using a FACSArray 96-well flow cytometer (Becton-Dickinson). In this method, cells were grown overnight in SC medium to stationary phase, diluted 100-fold into fresh medium, and grown for an additional 2 h, and then the cultures (200 μl each) were treated with drugs and inhibitors in flat-bottom 96-well dishes (Corning). At the indicated times, dishes were mixed, and 20 μl of each suspension was transferred to clean dishes containing 180 μl of phosphate-buffered saline (PBS) containing 0.6 μg/ml PI. After incubation for 15 min at room temperature, 5,000 cells from each well were counted using the yellow channel. Dead cells appeared over 2 logs more fluorescent than live cells in these conditions, which allowed simple gating to determine the percentage of cell death.
Fluorescein-isothiocyanate-valine-alanine-aspartate-fluoromethylketone (FITC-VAD-FMK) staining of S. cerevisiae cells was performed as described previously (27). Briefly, at appropriate times of incubation, 5 × 106 cells were pelleted and resuspended in 200 μl of 10 μM FITC-VAD-FMK in staining solution (CaspACE FITC-VAD-FMK in situ marker; Promega). Samples were then incubated at room temperature for 20 min. Cells were then centrifuged, washed twice in 1 ml PBS, and resuspended in 200 μl of PBS. For the double-staining experiment, cells were additionally stained with 3 μg/ml PI and incubated at room temperature for 5 min. Two-color flow cytometry was performed in a FACSCalibur flow cytometer (Becton-Dickinson). FITC-VAD-FMK fluorescence (488/525 nm; FL1) and PI fluorescence (488/620 nm; FL3) data were collected with optical spillover compensation set at 0.8%. Ten thousand cells were acquired for each sample. Data were analyzed using FCSExpress software (De Novo Software).
TUNEL assay.
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays were performed on formaldehyde-fixed cells as described previously (71), except that FITC-tagged dUTP was replaced with tetramethylrhodamine-tagged dUTP and 10,000 cells were counted in the FACSarray flow cytometer instead of manually.
ROS assays.
ROS production was visualized and scored as described previously (42), except with slight modifications. Briefly, log-phase cultures were diluted to a concentration of 3 × 106 cells/ml and exposed to tunicamycin in YPD or SC medium at 30°C. At each time point, 200 μl of cells was harvested by centrifugation, resuspended in 200 μl of fresh SC medium, and incubated with 10 μg/ml 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes) at 30°C for 10 min. Cells were concentrated by centrifugation and resuspended in 10 μl of fresh SC medium. Five microliters of cells were loaded onto slides and observed immediately under epifluorescence microscopy (excitation at 495 nm and emission at 525 nm). At least 200 cells per sample were scored manually as fluorescent or nonfluorescent.
45Ca2+ uptake assay.
Yeast cells were grown to log phase in SC medium (OD600 of 0.25 to 0.5), diluted with SC medium to an OD600 of 0.25, and then diluted with an equal volume of SC medium containing 100 cpm/nl 45CaCl2, 5 μg/ml tunicamycin, and varying concentrations of radicicol. After 2 h of incubation at 30°C (before cell death had occurred), the cells were collected onto Whatman GF/F filters by vacuum filtration, washed three times with 5 ml of ice-cold buffer A (10 mM CaCl2, 10 mM HEPES-Na, pH 7.5), dried, and processed for liquid scintillation counting as described previously (6).
RESULTS
Two manners of cell death in response to tunicamycin.
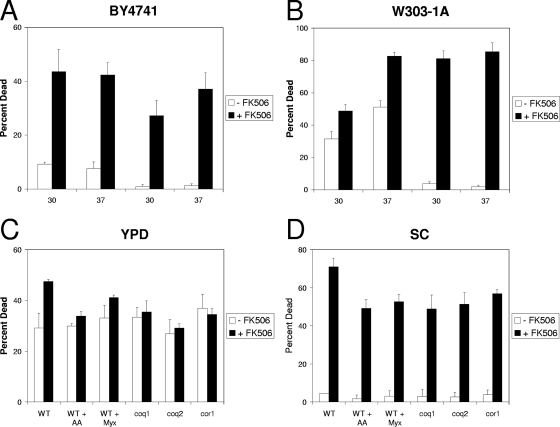
A recent study reported significant levels of apoptosis-like cell death in S. cerevisiae cultures responding to tunicamycin (27), although little cell death had been observed previously for this fungistatic compound unless calcineurin had been inactivated (5, 6). To investigate the source of the discordant findings, we systematically varied the experimental conditions (BY4741 versus W303-1A strain backgrounds, YPD versus SC growth media, and 30°C versus 37°C incubation temperatures) and the analytical methods for assaying dead or dying cells (staining with MB, PI, and FITC-VAD-FMK). In accordance with the recent report (27), tunicamycin induced significant levels of cell death in both strain backgrounds grown in YPD medium at 37°C (Fig. 1A and B). In contrast to the previous study, however, we observed little or no effect of decreasing the temperature to 30°C. In accordance with our previous findings, tunicamycin failed to induce cell death in either strain background at either temperature when grown in synthetic SC medium (Fig. 1A and B). Calcineurin has been shown to prevent tunicamycin-induced cell death in SC medium (6). Consistent with those findings, a specific inhibitor of calcineurin known as FK506 (39) strongly increased cell death in both media, temperatures, and strain backgrounds (Fig. 1A and B). Quantitatively similar results were obtained using PI or FITC-VAD-FMK staining (data not shown). Thus, the source of the discrepancy was attributed to the culture media and not the staining method, strain background, or incubation temperature.
FIG. 1.
Effects of strain background, temperature, culture medium, and respiration on the death of yeast cells treated with tunicamycin with or without FK506. Cultures of wild-type strains BY4741 (A) or W303-1A (B) were grown to log phase at either 30°C or 37°C in either SC or rich (YPD) medium as indicated and then treated with 2.5 μg/ml tunicamycin with or without 1 μM FK506. After 8 h of incubation, dead cells were stained with 0.5 μg/ml PI and counted by flow cytometry as described in Materials and Methods. Bars indicate the mean percentage of dead cells in the population (± standard deviations) of three independent cultures. Wild type (WT) and isogenic coq1, coq2, and cor1 mutants in the W303-1A background were grown to log phase at 30°C in either YPD medium (C) or SC medium (D) and then treated with 2.5 μg/ml tunicamycin, 1 μM FK506, and 1 μg/ml antimycin A (AA) or myxothiazol (Myx) as indicated to inhibit respiration. After 8 h of incubation, dead cells were stained, counted, and plotted as described above.
We have previously described two modes of nonapoptotic cell death for S. cerevisiae cells responding to mating pheromones: a ‘fast death’ at high concentrations of mating pheromones and a ‘slow death’ at lower concentrations of mating pheromones that was ordinarily prevented by calcineurin signaling (71). Although both modes of death were preceded by accumulation of ROS, inhibitors or mutations that block oxidative phosphorylation strongly delayed the slow death but did not affect the fast cell death. To test if oxidative phosphorylation is required for tunicamycin-induced death of wild-type or calcineurin-deficient cells, the effects of respiration inhibitors (antimycin A or myxothiazol) on tunicamycin-treated wild-type cells were evaluated in YPD or SC medium with or without FK506. The behaviors of mutants lacking components (coenzyme Q [coq1 and coq2 mutants] or complex III [cor1 mutants]) of the electron transport chain (ETC) were also evaluated. None of these mutations or inhibitors significantly altered the extent of cell death in tunicamycin/YPD medium (Fig. 1C). In contrast, all of these mutations and inhibitors significantly diminished the extent of death in SC-tunicamycin-FK506 medium (Fig. 1D). Therefore, similar to the effects of mating pheromones, tunicamycin appeared to induce at least two manners of cell death in S. cerevisiae: one that was partially dependent on ETC function and prevented by calcineurin signaling (hereafter termed calcineurin-less death) and another that was independent of ETC function.
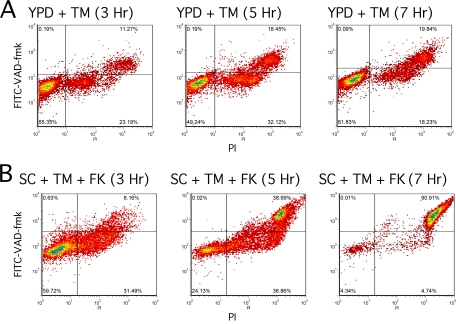
The conclusion that wild-type cells undergo apoptosis-like cell death was based primarily on staining with FITC-VAD-FMK (27), a fluorogenic sensor of active caspases in mammalian cells. However, this compound has been shown to stain dead S. cerevisiae cells nonspecifically and independent of the manner of cell death (62, 66). The recent study did not account for this background of dead cells by costaining with PI or MB (27). To determine if apoptotic cells can be detected in either manner of cell death, we sampled wild-type cultures incubated in YPD plus tunicamycin and in SC plus tunicamycin plus FK506 over a range of times, double-stained the cells with PI and FITC-VAD-FMK, and quantified the staining patterns using flow cytometry. In YPD medium supplemented with tunicamycin, the cells progressed over time from a double-negative state to a double-positive state without populating the FITC-VAD-FMK single-positive state indicative of apoptosis (Fig. 2A). A similar trend was observed for SC medium supplemented with tunicamycin plus FK506 (Fig. 2B). In neither case were apoptotic (PI-negative and FITC-VAD-FMK-positive) cells detected.
FIG. 2.
Staining of tunicamycin-treated cells with FITC-VAD-FMK, H2DCFDA, and PI. Wild-type W303-1A cells were grown to log phase in YPD medium (A) or SC medium (B) and treated with 2.5 μg/ml tunicamycin (TM) or 2.5 μg/ml tunicamycin plus 1 μM FK506 (FK) as indicated. After 3, 5, or 7 h of incubation at 30°C, cultures were double stained with 10 μM FITC-VAD-FMK and 3 μg/ml PI, and 10,000 cells were analyzed for staining intensity by flow cytometry as described in Materials and Methods.
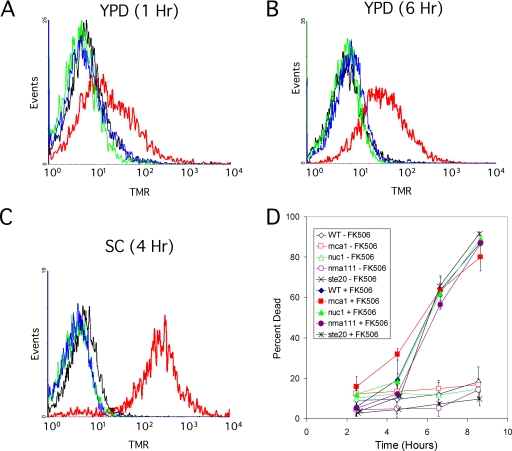
Other hallmarks of apoptosis-like cell death in S. cerevisiae include chromatin fragmentation (positive staining in a TUNEL assay) and phosphatidylserine externalization (surface binding of annexin V-GFP). Wild-type cells growing in YPD or SC-FK506 medium failed to exhibit significant binding of annexin V-GFP at any time after treatment with tunicamycin, except after cell death (costaining with PI; data not shown). Using a modified TUNEL assay that eliminates cytoplasmic background (71), no TUNEL-positive cells were detected at any time point in either condition of tunicamycin treatment (Fig. 3A to C; also data not shown). As a positive control, cells treated with 4 mM hydrogen peroxide exhibited a strong positive signal in all these conditions, although the background was noticeably higher in YPD medium than in SC medium. Therefore, important hallmarks of apoptosis were not evident during either manner of tunicamycin-induced cell death.
FIG. 3.
Chromatin fragmentation and apoptosis factors are not involved in tunicamycin-induced cell death. Wild-type yeast strains were grown in YPD medium (A, B) or SC medium (C), supplemented with nothing (black lines), 4 mM hydrogen peroxide (red lines), 2.5 μg/ml tunicamycin (blue lines), or 2.5 μg/ml tunicamycin plus 1 μg/ml FK506 (green lines) for the indicated times and then fixed with 3.7% formaldehyde and stained using a fluorescent TUNEL assay that specifically detects DNA breaks (see Materials and Methods). Fluorescence intensity of over 10,000 cells was measured by flow cytometry and plotted as histograms. (D) Mutants lacking a variety of published proapoptosis factors were grown in SC medium, treated with 2.5 μg/ml tunicamycin with and without 1 μM FK506, and stained for dead cells using 0.54 μg/ml PI at the indicated times. The average cell death in three independent cultures (± standard deviations) is plotted for each mutant and for the isogenic wild-type (WT) strain. TMR, tetramethylrhodamine fluorescence.
Finally, we tested whether the apoptosis-associated factors Mca1 (43), Nuc1 (10), Nma111 (21), or Ste20 (1) contributed to calcineurin-less death in response to tunicamycin by characterization of single-gene knockout mutants lacking each of these factors. All of the mutants died at rates and extents indistinguishable from those of the wild-type parental strain when treated with tunicamycin plus FK506 in SC medium (Fig. 3D). Thus, calcineurin-less death was not detectably associated with the factors previously reported for apoptosis-like cell death.
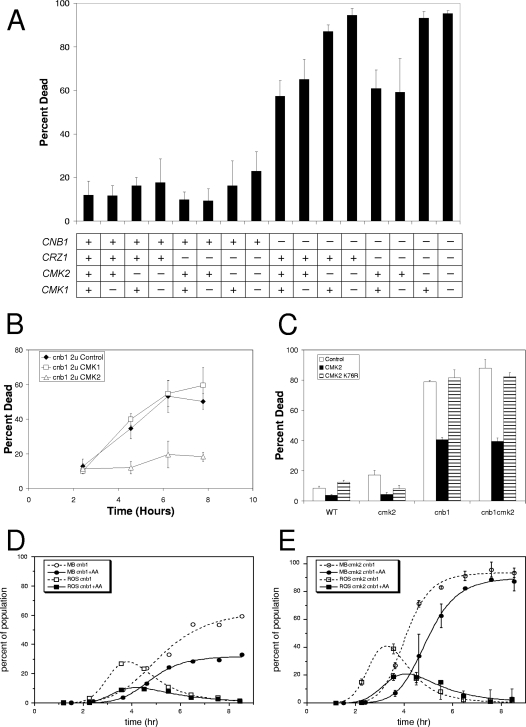
Cmk2 is an indirect target of calcineurin that regulates calcineurin-less death.
A major target of calcineurin is the transcription factor Crz1 (45, 58). Mutants lacking Crz1 behave like wild-type cells in their responses to tunicamycin and tunicamycin plus FK506 (Fig. 4A). However, a potentially significant role for Crz1 in calcineurin-less death might have been obscured if a functionally redundant pathway were also operating. Indeed, DNA microarray experiments indicate that Crz1 induces mRNAs derived from the CMK2 gene but not the CMK1 gene (31, 70), paralogous genes that encode Ca2+/calmodulin-dependent protein kinases. Furthermore, expression of a CMK2-lacZ reporter gene was induced more than 100-fold in a Crz1-dependent and FK506-sensitive fashion after 3 h of treatment with 200 mM CaCl2 (Table 3). The simultaneous loss of both Cmk2 and Cmk1, both Ca2+/calmodulin-dependent protein kinases, has been shown to elevate calcineurin-less death (6), but the expected redundancy of the calcineurin-Crz1-Cmk2 pathway with Cmk1 was not explicitly tested.
FIG. 4.
Cmk2 and calcineurin independently delay ROS accumulation and cell death. (A) A panel of yeast strains containing (+) or lacking (−) the CNB1, CRZ1, CMK2, and CMK1 genes was grown in SC medium at 30°C, treated with 2.5 μg/ml tunicamycin for 8 h, stained for dead cells with 0.4 mg/ml MB, and counted microscopically. The average cell death observed in three independent cultures (± standard deviations) is plotted for each mutant and wild-type strain. (B) cnb1 mutants transformed with 2μm plasmids overexpressing CMK2 (open triangles) or CMK1 (open squares) or no gene (filled diamonds) were grown in SC-minus-uracil medium, treated with 2.5 μg/ml tunicamycin, stained with MB at various times, and scored for cell death as in panel A. (C) Wild-type and cmk2, cnb1, and cnb1 cmk2 mutant strains transformed with 2μm plasmids overexpressing CMK2 (black bars), kinase-dead CMK2-K76R (stippled bars), or no gene (gray bars) were grown and treated with 2.5 μg/ml tunicamycin as in panel B, and then stained for dead cells using 0.54 μg/ml PI and scored by flow cytometry. Mutants lacking calcineurin (D) or both calcineurin and Cmk2 (E) were grown to log phase in SC medium and treated with 2.5 μg/ml tunicamycin in the presence (solid lines, filled symbols) and absence (dashed lines, open symbols) of 10 μg/ml antimycin A. After various periods of incubation, samples were removed and either stained for dead cells using 0.4 mg/ml MB or stained for ROS accumulation using 10 μg/ml H2DCFDA and quantified microscopically. Smooth curves were fitted to the data by nonlinear regression using the standard sigmoid function (dead cells) or the difference between two standard sigmoid functions (ROS-positive cells) as described in Materials and Methods. The curve fitting was consistent with the model that nearly all dying cells accumulate ROS for approximately 1.2 h shortly before they die.
TABLE 3.
CMK2 induction required calcineurin and Crz1
| Growth condition | β-Galactosidase activity (MMU)a
|
|
|---|---|---|
| cmk2 | cmk2 crz1 | |
| Plus nothing | 2.9 | 2.9 |
| Plus Ca | 455.3 | 10.2 |
| Plus Ca and FK506 | 2.6 | 2.4 |
Modified Miller units.
To examine the possible redundancies of Cmk1, Cmk2, and Crz1 in regulating tunicamycin-induced cell death, a panel of S. cerevisiae strains lacking these factors in all possible combinations was treated with tunicamycin in the presence and absence of Cnb1 (the regulatory subunit of calcineurin) and assayed for cell death. In all these scenarios, the loss of calcineurin greatly increased cell death (Fig. 4A), indicating that calcineurin can inhibit cell death independent of Cmk1, Cmk2, and Crz1. However, in the absence of calcineurin, all the mutants lacking Cmk2 exhibited a higher cell death than did the Cmk2-proficient strains, whereas all the mutants lacking Cmk1 or Crz1 or both were indistinguishable from the proficient strains (Fig. 4A, right half). Therefore, Cmk2 partially suppressed calcineurin-less death independent of induction by Crz1 and calcineurin, and there was no detectable activity of Cmk1 in any genetic contexts. Overexpression of Cmk2 from a high-dosage plasmid in calcineurin-deficient cnb1 mutants suppressed tunicamycin-induced cell death almost completely (Fig. 4B). On the other hand, overexpressing Cmk1 (Fig. 4B) or a ‘kinase-dead’ substitution mutant of Cmk2 (Cmk2-K76R) was without effect (Fig. 4C) relative to empty plasmid controls. These experiments identify Cmk2 as an important indirect target of calcineurin (via Crz1) whose activity can inhibit cell death independent of calcineurin, although calcineurin can also inhibit cell death independent of Cmk2.
Previously, we showed that staining with H2DCFDA, a commonly used fluorescent indicator of ROS, precedes calcineurin-less death induced by mating pheromones (71). Death of calcineurin-deficient cnb1 knockout mutants in SC medium was also preceded by the transient appearance of H2DCFDA-positive cells in the population (Fig. 4D). The transient appearance of H2DCFDA-positive cells was described very well by a mathematical model that assumes all dying cells accumulate ROS approximately 1.9 h prior to death and then become ROS negative approximately 0.8 h prior to death. The addition of antimycin A, an inhibitor of complex III of the ETC, to the cultures significantly delayed and diminished the appearance of H2DCFDA-positive cells in the population but did not radically alter the timing relative to cell death (Fig. 4D). If staining with H2DCFDA is a good indicator of ROS accumulation in yeast, these observations suggest that ROS production from both mitochondrial and nonmitochondrial sources is a feature of calcineurin-less death. A similar conclusion was reached previously using other stimuli of ER stress in yeast (28).
If Cmk2 and calcineurin divergently regulate two different manners of cell death, the kinetics of cell death may be biphasic and the appearance of H2DCFDA-positive cells may be bimodal, as observed previously for Fig. 1 (71), where calcineurin-less cell death was induced by mating pheromones. Interestingly, cmk2 cnb1 double mutants died with monophasic kinetics and unimodal appearance of H2DCFDA-positive cells (Fig. 4E). Antimycin A delayed both of these responses but did not expose biphasic or bimodal characters. Indeed, the data fit well to the same simple mathematical model used to describe the behavior of cnb1 mutants, except with more rapid kinetics. These findings suggest that Cmk2 and calcineurin may converge on factors involved in a single manner of cell death that involves accumulation of ROS for approximately 1.1 h immediately prior to cell death.
Targets of calcineurin and Cmk2 that do not strongly regulate cell death.
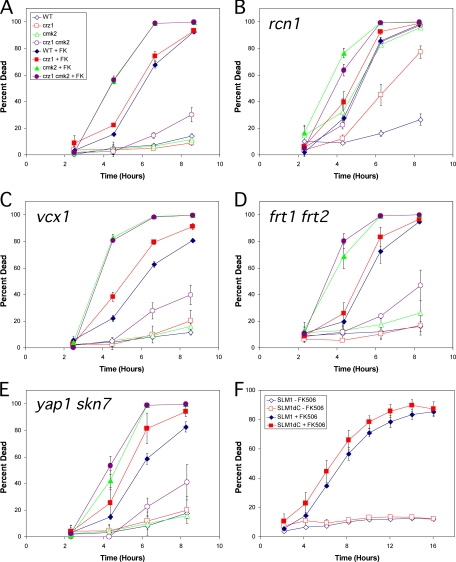
Several additional targets of activated calcineurin have been characterized. To determine if these targets play significant roles in calcineurin-less death, the genes encoding these factors were knocked out in wild-type, cmk2, crz1, and cmk2 crz1 backgrounds and the resulting panel of mutants was assayed for cell death at various times after treatment with tunicamycin in the presence or absence of FK506. The first target of calcineurin analyzed in this way was Rcn1, a direct regulator of calcineurin that is required for maximum calcineurin signaling (34). rcn1 mutants (Fig. 5B) died at faster rates than the wild type (Fig. 5A) in response to tunicamycin and at equivalent rates in response to tunicamycin plus FK506, suggesting that Rcn1 prevents cell death by increasing calcineurin signaling. The rcn1 cmk2 double mutants exhibited much higher rates of cell death than rcn1 single mutants, suggesting that Cmk2 function is very important for survival in conditions of weakened calcineurin signaling. Interestingly, rcn1 cmk2 crz1 triple mutants die at precisely the same rate as rcn1 cmk2 double mutants, whereas rcn1 crz1 double mutants die much faster than rcn1 mutants. Therefore, the induction of Cmk2 expression by Crz1 was more important in rcn1 mutants with only partial calcineurin activity than in wild-type cells with full calcineurin activity. By comparing Fig. 5A and B, it can be seen that Rcn1 had no antideath activity independent of calcineurin (i.e., in the presence of FK506). In the absence of calcineurin, however, Rcn1 is not properly induced, dephosphorylated, and stabilized (35; S.Mehta, H. Li, P. G. Hogan, and K. W. Cunningham, submitted for publication), so its role as a possible effector of calcineurin could not be fully evaluated. To avoid these problems, a nonphosphorylatable stable derivative, Rcn1-S113A, was overexpressed at high levels in cnb1 mutants from a methionine-repressible promoter and examined for effects on calcineurin-less cell death. The overexpressed Rcn1-S113A still did not alter the rate of cell death of cnb1 mutants treated with tunicamycin (data not shown). Therefore, Rcn1 increased calcineurin activity as expected from the results of previous studies, but Rcn1 seemed unable to serve as an effector of calcineurin in the control of cell death.
FIG. 5.
Known targets of calcineurin have no impact on calcineurin-less death, except for RCN1. (A) Wild-type (WT) and crz1, cmk2, and crz1 cmk2 mutant strains were grown in SC medium and treated with 2.5 μg/ml tunicamycin with or without FK506 (FK). At various times of incubation at 30°C, samples were removed, stained with 0.54 μg/ml PI, and analyzed for dead cells using flow cytometry. The averages of three replicate cultures (± standard deviations) are plotted. (B to E) Same as in panel A, except the panel of strains also contained rcn1 (B), vcx1 (C), frt1 frt2 (D), or yap1 skn7 (E) knockout mutations. (F) A slm1 slm2 double mutant transformed with plasmids bearing either full-length SLM1 (diamonds) or truncated SLM1 lacking the calcineurin-binding PxIxIT motif (squares) were grown, treated, and analyzed as in panel A.
Other targets of calcineurin include Vcx1, which functions in the vacuolar membrane as a H+/Ca2+ exchanger (15), and the redundant gene pair Frt1 and Frt2, which functions in high-pH tolerance and resistance to azole-class drugs (14, 29). Both the vcx1 mutant (Fig. 5C) and the frt1 frt2 double mutant (Fig. 5D) retained full responsiveness to FK506, Crz1 loss, and/or Cmk2 loss, and they differed from the control strains (Fig. 5A) in only two subtle ways: a slight increase in the baseline incidence of cell death at all time points and a slight shortening of the life span in all genetic contexts. Therefore, Frt1/Frt2 and Vcx1 each exhibit an extremely mild antideath activity independent of the antideath activities of calcineurin, Crz1, and Cmk2.
The stress-responsive transcription factors Yap1 and Skn7 have also been reported as targets of calcineurin in other conditions (8). The yap1 skn7 double mutant also retained full responsiveness to FK506, Crz1 loss, and Cmk2 loss (Fig. 5E), and it differed from wild-type control strains in only two subtle ways: a slightly higher baseline incidence of cell death in all strains and a slightly shorter life span in the absence of FK506 but not in the presence of FK506. Similar effects were observed in both yap1 and skn7 single-mutant backgrounds (data not shown). In the absence of Cmk2 and FK506 when their effects were most obvious, the antideath activities of Yap1 and Skn7 appeared significantly weaker than that of Crz1 and were probably insignificant. Therefore, calcineurin, Crz1, and Cmk2 inhibit cell death in these conditions independent of Yap1 and Skn7.
Finally, the plextrin homology-domain proteins Slm1 and Slm2 have been recently reported as essential targets of calcineurin involved in membrane trafficking (9, 48, 60). Because slm1 slm2 double mutants are inviable, slm1 slm2 double mutants expressing only Slm1 or Slm1ΔC lacking the calcineurin-binding PxIxIT motif (55) from plasmids were assayed for cell death in the presence of FK506 and/or tunicamycin (Fig. 5F). The Slm1ΔC mutant responded similarly to the wild-type protein, and therefore calcineurin retained antideath activity independent of Slm1 and Slm2. In summary, calcineurin retained antideath activity in the absence of Slm1/Slm2, Yap1 and Skn7, Frt1/Frt2, Vcx1, Crz1, Cmk2, and many combinations of these factors. Only Cmk2 exhibited antideath activity in the absence of calcineurin, and in rcn1 mutants, its effect was strengthened by Crz1 and calcineurin.
Relative contributions of calcineurin, Crz1, and Cmk2 vary with the conditions.
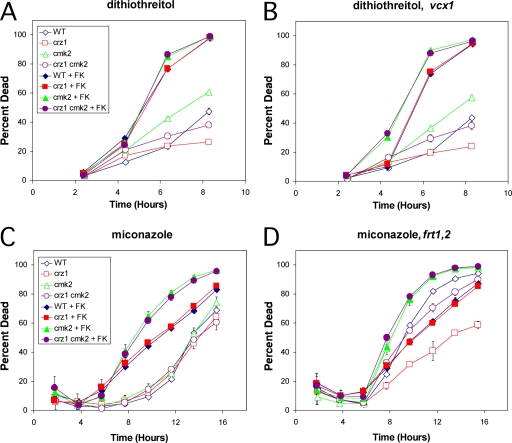
The experiments presented above suggest that calcineurin and Cmk2 have independent activities that converge on a common mechanism that prevents ROS accumulation and cell death during treatment with tunicamycin. Calcineurin and Cmk2 also prevented cell death during prolonged responses to mating pheromones (71). To test if this relationship holds for other conditions, we explored several distinct death-inducing stimuli. In response to 4 mM hydrogen peroxide, an inducer of apoptosis-like cell death (42), calcineurin, Crz1, and Cmk2 had no detectable ability to extend cell life span (data not shown). In response to 2.5 mM dithiothreitol, an inducer of ER stress that acts differently than tunicamycin, we found that calcineurin and Cmk2 independently suppressed cell death (Fig. 6A). Surprisingly, crz1 mutants and crz1 cmk2 double mutants each died significantly slower than did the wild type and the cmk2 mutants in this condition (Fig. 6A), suggesting that Crz1 possesses a net prodeath activity in response to dithiothreitol. Crz1 is known to regulate many target genes (31, 70), some of which may promote death in response to dithiothreitol but not tunicamycin or mating pheromones. The effects of dithiothreitol on mutants lacking Rcn1, Vcx1, and Frt1/Frt2 were also examined. As seen previously with tunicamycin, the rcn1 mutant background behaved like a partial deficiency of calcineurin and the frt1 frt2 double mutant background behaved indistinguishably from the wild type (data not shown). However, the vcx1 cmk2 double mutant appeared to exhibit slightly higher cell death than the cmk2 mutant in the presence of FK506 (Fig. 6B), suggesting a very mild role of Vcx1 in the process. Therefore, the change of stressor from tunicamycin to dithiothreitol slightly affected the relative contributions of Crz1 and Vcx1 but did not alter the major contributions of calcineurin and Cmk2.
FIG. 6.
Calcineurin and Cmk2 inhibit cell death induced by dithiothreitol and miconazole. The indicated strains were treated with 2.5 mM dithiothreitol (A, B) or 8 μM miconazole (C, D) instead of 2.5 μg/ml tunicamycin and analyzed for cell death using PI and flow cytometry as described in the legend for Fig. 5. WT, wild type; FK, FK506.
Inhibitors of ergosterol biosynthesis widely employed in antifungal medications have been shown in pathogenic and nonpathogenic yeasts to inhibit growth when calcineurin is functioning (20) and to induce cell death when calcineurin is inhibited or mutated (59). By treating the panel of S. cerevisiae strains described above in the presence or absence of FK506, we examined the abilities of calcineurin, Cmk2, Crz1, Rcn1, Vcx1, and Frt1/Frt2 in many combinations to suppress cell death during treatment with miconazole, an azole-class antifungal drug that directly inhibits the enzyme Erg11 (69). In these conditions, miconazole induced a slow manner of cell death in wild-type cells that was unaffected by the loss of Crz1, Cmk2, or both (Fig. 6C). FK506 significantly shortened the life span of wild-type and cmk2 mutant cells responding to miconazole. The roles of Crz1, Rcn1, and Vcx1 in the response to miconazole were very similar to those obtained in the response to dithiothreitol (data not shown). Interestingly, the frt1 frt2 double mutants (Fig. 6D) exhibited significantly higher rates of cell death than the corresponding wild-type strains in the presence or absence of FK506 and Cmk2, though the effect seemed stronger when calcineurin was functional (Fig. 6D). Addition of FK506 significantly increased the rates of cell death of frt1 frt2 double mutants. Thus, the calcineurin-dependent factors Frt1/Frt2 exhibited very mild antideath activity in response to miconazole and probably act in conjunction with other calcineurin-dependent factors and Cmk2. The mild antideath activities of Frt1/Frt2 in wild-type cells are consistent with the observation that these factors promote resistance of erg3 mutants to fluconazole, a derivative of miconazole, in growth assays (14).
Hsp90 promotes calcineurin-less death.
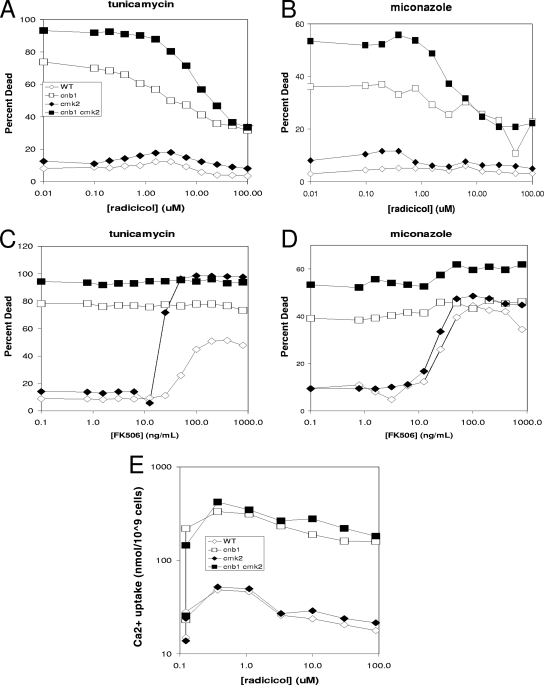
The Hsp90 chaperone has previously been shown to promote resistance of S. cerevisiae cells to fluconazole in growth assays (14). In that study, inhibitors of Hsp90 behaved like inhibitors of calcineurin in their ability to synergize with azoles. If Hsp90 promotes azole resistance by increasing calcineurin activity as proposed in the previous study, inhibitors of Hsp90 should promote death of S. cerevisiae cells treated with miconazole or tunicamycin. Contrary to expectations, increasing concentrations of the Hsp90 inhibitors radicicol (Fig. 7) or geldanamycin A (data not shown) did not increase death of wild-type or cmk2 mutant cells treated with tunicamycin or miconazole and instead prevented the death of cnb1 mutants and cmk2 cnb1 double mutants (Fig. 7A and B). In parallel experiments, FK506 increased the death of wild-type and cmk2 mutant cells (Fig. 7C to D) as expected. Similar effects of radicicol and geldanamycin A were observed using rcn1 mutants and rcn1 cmk2 double mutants that were already partially deficient in calcineurin signaling (data not shown). Though only one concentration of tunicamycin and miconazole was used in these experiments, similar results were obtained over a wide range of concentrations, including those where synergism was observed (′checkerboard' assays of cell growth and cell death; data not shown). Therefore, Hsp90 inhibitors did not mimic the calcineurin inhibitors or the calcineurin-deficient mutants; instead, they revealed an unexpected requirement for Hsp90 in calcineurin-less death.
FIG. 7.
Hsp90 inhibitors prevented calcineurin-less cell death due to tunicamycin or miconazole but did not prevent Ca2+ influx or activation of calcineurin. Wild-type (WT) and cnb1, cmk2, and cnb1 cmk2 mutant strains were grown to log phase in SC medium at 30°C, exposed to 2.5 μg/ml tunicamycin (A, C) or 8 μM miconazole (B, D) with various concentrations of the Hsp90 inhibitor radicicol (A, B) or the calcineurin inhibitor FK506 (C, D), stained with PI after 6 h of incubation, and analyzed immediately for cell death by flow cytometry as described in the legend for Fig. 5. (E) Cells were also treated as in panel A in the presence of tracer 45Ca2+ and then processed after 2 h of incubation at 30°C to determine total Ca2+ uptake. The vertical lines at 0 μM radicicol indicate the effects of omitting tunicamycin.
Because Hsp90 inhibitors synergize with tunicamycin and azoles in growth assays, it seems unlikely that Hsp90 inhibitors could somehow block the ability of the antifungal drugs to stimulate calcineurin-less death. Nevertheless, we tested this possibility directly by determining if radicicol could block the tunicamycin-induced elevation of Ca2+ uptake from the medium via the calcineurin-sensitive Cch1-Mid1 channel (5). When using 45Ca2+ in the medium as a tracer in conditions identical to those shown in Fig. 7A, tunicamycin treatment increased Ca2+ uptake approximately ninefold in cnb1 and cnb1 cmk2 double mutants relative to the level in wild-type and cmk2 single mutant cells. The addition of 0.3, 1, 3, 10, 30, or 90 μM radicicol had no significant effect on calcineurin-sensitive Ca2+ uptake (Fig. 7E). Therefore, these concentrations of radicicol did not prevent the ability of tunicamycin to stimulate the Cch1-Mid1 channel or the ability of calcineurin to inhibit the channel. The findings suggest that Hsp90 is selectively required for at least one step in the prodeath pathway induced by antifungal compounds and is not required for the activation of an antideath pathway involving Cch1-Mid1, calmodulin, Cmk2, and calcineurin.
DISCUSSION
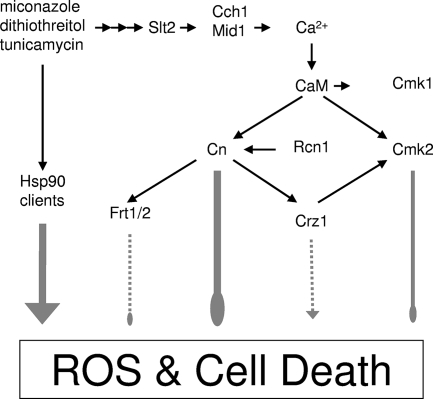
The findings presented above support a working model (Fig. 8) where certain fungistatic drugs inhibit essential targets in the ER of S. cerevisiae, generate stresses, and activate an antideath (or prosurvival) pathway that prevents a form of nonapoptotic cell death. Surprisingly, Hsp90 activity was necessary for the death of calcineurin-deficient cells in these conditions and was not necessary for the stress generation, activation of the Cch1-Mid1 Ca2+ channel, and activation of calcineurin, the latter of which has been proposed as a client of Hsp90 in yeast (32). In mammalian cells, Hsp90 has been shown to facilitate activity of a necrosis-promoting protein kinase RIP (38) and therefore to have a net effect opposite to that in yeast. S. cerevisiae lacks any recognizable orthologs of RIP but contains orthologs of many other types of serine/threonine protein kinases thought to be clients of Hsp90 (61, 72), so perhaps one of these protein kinases serves as both a client of Hsp90 and a prodeath factor opposing calcineurin in fungi. We have previously examined the possible involvement of Ire1, a protein kinase that is specifically activated in response to ER stress and is also a client of Hsp90 (44), in the response to tunicamycin and found that it was not required for either the prodeath activity of tunicamycin in calcineurin-deficient S. cerevisiae cells or the antideath activity of calcineurin in these cells (6). Interestingly, ire1 mutant cells treated with tunicamycin lose viability (as measured by CFU and other methods) much more rapidly than wild-type cells (13), yet the individual cells do not die unless calcineurin is inactivated (6). These live but nonrecovering cells are probably unable to upregulate the factors necessary to repair the damage, adapt, and proliferate, and therefore they appear to be mortally wounded when calcineurin and its upstream regulators perform their vital function. We do not yet know if Hsp90 and its relevant clients are constitutively producing toxic factors that are ameliorated by calcineurin or if they represent a prodeath signaling pathway that is governed by calcineurin. Compounds like radicicol that inhibit any of these toxic/prodeath factors may actually decrease the effectiveness of antifungal drugs because of their ability to prevent fungicidal effects. On the other hand, drugs that activate these factors or inhibit other steps in the antideath pathway may be fungicidal alone or in combination with existing fungistatic drugs without the immunosuppressive side effect of the calcineurin inhibitors.
FIG. 8.
Working model of positive and negative regulators of cell death induced by ER stressors. In SC medium, ER stressors (e.g., miconazole, dithiothreitol, and tunicamycin) activate a signaling pathway involving a mitogen-activated protein kinase (Slt2), a Ca2+ channel (Cch1-Mid1), calmodulin (CaM), Cmk2, calcineurin (Cn), Crz1, and (very weakly) Frt1/Frt2 (Frt1/2) that either inhibits a prodeath pathway or a toxic molecule that is dependent on the function of Hsp90. Direct interactions are represented by narrow black arrows, whereas indirect or undefined interactions are indicated by gray arrows, the strengths of which are proportional to the thicknesses. Pointed arrows indicate stimulatory effects, whereas blunt arrows indicate inhibitory effects.
The present study also advances our understanding of the antideath pathway in several ways. First, we identified Cmk2, but not its paralog Cmk1, as an indirect target of calcineurin that exhibits strong antideath activity independent of calcineurin. The life span of calcineurin-deficient cells responding to miconazole, tunicamycin, or dithiothreitol was shortened by the loss of Cmk2 and extended by overexpression of Cmk2. Though Cmk2 is now confirmed as a downstream effector of the Crz1 transcription factor in S. cerevisiae (Table 3) and a homologous transcription factor in C. albicans (33, 56), Crz1 was not required for Cmk2 effectiveness except in rcn1 mutants where calcineurin activity was already attenuated. As expected, Crz1 had no antideath activity in the absence of calcineurin function. In cells expressing active calcineurin, however, Crz1 function seemed to vary somewhat with the conditions. For example, Crz1 exhibited very weak antideath activity in cmk2 mutant cells responding to tunicamycin and very weak prodeath activity in cmk2 mutant cells responding to dithiothreitol or miconazole. The molecular bases of these Crz1 effects are not yet understood, but it is tempting to speculate that some of the 60 to 120 direct targets of Crz1 perform opposing or condition-specific activities. One such target of Crz1 is Rcn1, a factor that can have positive or negative effects on calcineurin signaling depending on its level of expression and phosphorylation (31, 34, 35). Calcineurin, Cmk2, and Frt1/Frt2 (very weakly) all exhibited antideath activities independent of one another and Crz1 in all of the conditions tested. Each of these independent contributions can now be studied individually in hopes of revealing their targets and modes of action, particularly those of calcineurin and Cmk2, which seem far more potent than Frt1/Frt2.
The very rapid death of S. cerevisiae cells lacking both calcineurin and Cmk2 was preceded by transient staining with H2DCFDA, a fluorogenic probe used commonly for the detection of yeast cells producing ROS. While this may indicate a convergence of calcineurin and Cmk2 onto a common target or pathway that produces ROS, many independent manners of cell death are associated with ROS accumulation. In mammalian cells, for example, ROS accumulation occurs during both apoptosis and necrosis and is thus a hallmark of neither (17). Nevertheless, it is striking that calcineurin and Cmk2 delay respiration-dependent H2DCFDA staining and cell death in several very different stress responses (mating pheromones, tunicamycin, dithiothreitol, and azoles). A similar manner of cell death in S. cerevisiae was observed upon overexpression of human Bax (24, 36, 52). Like calcineurin-less death, Bax-induced death of S. cerevisiae cells did not require apoptosis factors Mca1 or Aif1 and was not associated with typical markers of apoptosis in S. cerevisiae, including caspase activation, phosphatidylserine externalization, and chromatin fragmentation (36). Despite the fact that no homologs of Bax are evident in any other fungal genomes, the possibility that calcineurin and/or Cmk2 regulate an endogenous Bax-like factor cannot be excluded.
Our findings also suggest the existence of a second manner of cell death that occurs in wild-type cells exposed to tunicamycin in YPD medium. Unlike calcineurin-less death, this manner of cell death was insensitive to inhibitors and mutations that disrupt oxidative phosphorylation (Fig. 1). This manner of cell death also seemed distinct from apoptosis because FITC-VAD-FMK-positive and PI-negative cells were not detectable at any point after treatment with tunicamycin (Fig. 3). A previous study arrived at the opposite conclusion (27), but the analysis was flawed by the failure to distinguish live apoptotic cells from dead cells, which are known to stain nonspecifically with FITC-VAD-FMK and other fluorophores, such as DHR123 (66). Very recently, the yeast metacaspase (Mca1) was shown to be unimportant for tunicamycin-induced cell death in YPD medium (26). Therefore, little support exists for the ability of tunicamycin to induce apoptosis in wild-type S. cerevisiae in YPD medium. Recently, this manner of cell death was shown to depend on Kex1, a Golgi-localized carboxypeptidase that is also important in the death of mutant cells lacking certain N-glycosylation factors and in the death of yeast cells in response to other stresses (26). The addition of an osmotic stabilizer to the culture medium also blocked death in these conditions, suggesting that the manner of cell death may be lysis. The loss of Kex1 carboxypeptidase or the addition of sorbitol, however, had no significant effect on the tunicamycin-induced activation of Cch1-Mid1, calcineurin, or calcineurin-less death in SC medium (our unpublished observations), which further highlights the mechanistic differences between calcineurin-less and Kex1-dependent cell deaths.
Temperature-sensitive cdc48 mutants of S. cerevisiae exhibit ER stress due to the failure of ER-associated degradation and the accumulation of misfolded ER proteins (53, 68) and also exhibit several cytological hallmarks of apoptosis (41). However, some of the assays lacked controls to rule out the possibility that these occur postmortem and some of the hallmarks are now recognized as being somewhat nonspecific. For example, ROS accumulation is not a specific hallmark of apoptosis because mammalian cells undergoing necrosis and S. cerevisiae cells undergoing nonapoptotic ‘fast death’ in response to mating pheromones also stain positive for ROS when using H2DCFDA (71). Additionally, S. cerevisiae nuclei that stain positive using the TUNEL assay typically contain single-strand breaks instead of the typical double-strand breaks observed in the DNA of apoptotic cells (54), and there are indications in the literature that many TUNEL-positive S. cerevisiae cells remain viable (for example, see Fig. 1 in reference 30) and therefore are not committed to cell death in the same ways as TUNEL-positive mammalian cells. Such considerations cloud the significance of most cytological evidence for apoptosis during the response of S. cerevisiae cells to ER stresses.
To classify calcineurin-less death as a manner of either programmed or nonprogrammed cell death seems premature without additional evidence regarding the molecules and networks that govern it. Though this study identifies Hsp90 as a factor necessary for calcineurin-less death, we cannot yet conclude that a prodeath regulatory pathway operates in S. cerevisiae, because there is no evidence available to suggest that Hsp90 or its clients are activated in dying calcineurin-deficient cells. Therefore, we cannot yet determine if calcineurin acts as an inhibitor of a prodeath pathway or an activator of an essential process in stressful conditions. Nevertheless, a better understanding of the calcineurin-less death phenomenon in S. cerevisiae may lead to the development of broad-spectrum fungicidal therapies for combating fungal human pathogens such as Candida albicans (4) and Cryptococcus neoformans (18, 37) or to improved immunosuppression therapies that avoid unwanted side effects in humans (12).
Acknowledgments
We are grateful to Jeremy Thorner and Catherine Clarke for S. cerevisiae strains and plasmids and to Michael Edidin for the use of a FACSCalibur instrument. We also thank Christian Martin, Lauren Parlett, and Tovah Honor for excellent technical assistance.
This work was supported by research grants from the National Institutes of Health (GM053087 and NS057023 to K.W.C.).
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Ahn, S. H., W. L. Cheung, J. Y. Hsu, R. L. Diaz, M. M. Smith, and C. D. Allis. 2005. Sterile 20 kinase phosphorylates histone H2B at serine 10 during hydrogen peroxide-induced apoptosis in S. cerevisiae. Cell 12025-36. [DOI] [PubMed] [Google Scholar]
- 2.Ansari, S., and R. Prasad. 1993. Effect of miconazole on the structure and function of plasma membrane of Candida albicans. FEMS Microbiol. Lett. 11493-98. [DOI] [PubMed] [Google Scholar]
- 3.Blankenship, J. R., W. J. Steinbach, J. R. Perfect, and J. Heitman. 2003. Teaching old drugs new tricks: reincarnating immunosuppressants as antifungal drugs. Curr. Opin. Investig. Drugs 4192-199. [PubMed] [Google Scholar]
- 4.Blankenship, J. R., F. L. Wormley, M. K. Boyce, W. A. Schell, S. G. Filler, J. R. Perfect, and J. Heitman. 2003. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonilla, M., and K. W. Cunningham. 2003. MAP kinase stimulation of Ca2+ signaling is required for survival of endoplasmic reticulum stress in yeast. Mol. Biol. Cell 144296-4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonilla, M., K. K. Nastase, and K. W. Cunningham. 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 212343-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14115-132. [DOI] [PubMed] [Google Scholar]
- 8.Brombacher, K., B. Fischer, K. Rüfenacht, and R. Eggen. 2006. The role of Yap1p and Skn7p-mediated oxidative stress response in the defence of Saccharomyces cerevisiae against singlet oxygen. Yeast 23741-750. [DOI] [PubMed] [Google Scholar]
- 9.Bultynck, G., V. Heath, A. Majeed, J. Galan, R. Haguenauer-Tsapis, and M. Cyert. 2006. Slm1 and slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease. Mol. Cell. Biol. 264729-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Büttner, S., T. Eisenberg, D. Carmona-Gutierrez, D. Ruli, H. Knauer, C. Ruckenstuhl, C. Sigrist, S. Wissing, M. Kollroser, K. Fröhlich, S. Sigrist, and F. Madeo. 2007. Endonuclease G regulates budding yeast life and death. Mol. Cell 25233-246. [DOI] [PubMed] [Google Scholar]
- 11.Büttner, S., T. Eisenberg, E. Herker, D. Carmona-Gutierrez, G. Kroemer, and F. Madeo. 2006. Why yeast cells can undergo apoptosis: death in times of peace, love, and war. J. Cell Biol. 175521-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman, J., and B. Nankivell. 2006. Nephrotoxicity of cyclosporin A: short-term gain, long-term pain? Nephrol. Dial. Transplant. 212060-2063. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Y., D. E. Feldman, C. Deng, J. A. Brown, A. F. De Giacomo, A. F. Gaw, G. Shi, Q. T. Le, J. M. Brown, and A. C. Koong. 2005. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol. Cancer Res. 3669-677. [DOI] [PubMed] [Google Scholar]
- 14.Cowen, L., A. Carpenter, O. Matangkasombut, G. Fink, and S. Lindquist. 2006. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot. Cell 52184-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham, K. W., and G. R. Fink. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 162226-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cyert, M. S. 2001. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu. Rev. Genet. 35647-672. [DOI] [PubMed] [Google Scholar]
- 17.Danial, N. N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116205-219. [DOI] [PubMed] [Google Scholar]
- 18.Del Poeta, M., M. C. Cruz, M. E. Cardenas, J. R. Perfect, and J. Heitman. 2000. Synergistic antifungal activities of bafilomycin A1, fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenberg, T., S. Buttner, G. Kroemer, and F. Madeo. 2007. The mitochondrial pathway in yeast apoptosis. Apoptosis 121011-1023. [DOI] [PubMed] [Google Scholar]
- 20.Elewski, B. E. 1993. Mechanisms of action of systemic antifungal agents. J. Am. Acad. Dermatol. 28S28-S34. [DOI] [PubMed] [Google Scholar]
- 21.Fahrenkrog, B., U. Sauder, and U. Aebi. 2004. The S. cerevisiae HtrA-like protein Nma111p is a nuclear serine protease that mediates yeast apoptosis. J. Cell Sci. 117115-126. [DOI] [PubMed] [Google Scholar]
- 22.Gin, P., and C. F. Clarke. 2005. Genetic evidence for a multi-subunit complex in coenzyme Q biosynthesis in yeast and the role of the Coq1 hexaprenyl diphosphate synthase. J. Biol. Chem. 2802676-2681. [DOI] [PubMed] [Google Scholar]
- 23.Guarente, L., and T. Mason. 1983. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell 321279-1286. [DOI] [PubMed] [Google Scholar]
- 24.Guscetti, F., N. Nath, and N. Denko. 2005. Functional characterization of human proapoptotic molecules in yeast S. cerevisiae. FASEB J. 19464-466. [DOI] [PubMed] [Google Scholar]
- 25.Harris, M. H., M. G. Vander Heiden, S. J. Kron, and C. B. Thompson. 2000. Role of oxidative phosphorylation in Bax toxicity. Mol. Cell. Biol. 203590-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauptmann, P., and L. Lehle. 2008. Kex1 protease is involved in yeast cell death induced by defective N-glycosylation, acetic acid, and chronological aging. J. Biol. Chem. 28319151-19163. [DOI] [PubMed] [Google Scholar]
- 27.Hauptmann, P., C. Riel, L. Kunz-Schughart, K. Fröhlich, F. Madeo, and L. Lehle. 2006. Defects in N-glycosylation induce apoptosis in yeast. Mol. Microbiol. 59765-778. [DOI] [PubMed] [Google Scholar]
- 28.Haynes, C. M., E. A. Titus, and A. A. Cooper. 2004. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell 15767-776. [DOI] [PubMed] [Google Scholar]
- 29.Heath, V. L., S. L. Shaw, S. Roy, and M. S. Cyert. 2004. Hph1p and Hph2p, novel components of calcineurin-mediated stress responses in Saccharomyces cerevisiae. Eukaryot. Cell 3695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herker, E., H. Jungwirth, K. A. Lehmann, C. Maldener, K. U. Frohlich, S. Wissing, S. Buttner, M. Fehr, S. Sigrist, and F. Madeo. 2004. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 164501-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilioti, Z., D. A. Gallagher, S. T. Low-Nam, P. Ramaswamy, P. Gajer, T. J. Kingsbury, C. J. Birchwood, A. Levchenko, and K. W. Cunningham. 2004. GSK-3 kinases enhance calcineurin signaling by phosphorylation of RCNs. Genes Dev. 1835-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai, J., and I. Yahara. 2000. Role of HSP90 in salt stress tolerance via stabilization and regulation of calcineurin. Mol. Cell. Biol. 209262-9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karababa, M., E. Valentino, G. Pardini, A. T. Coste, J. Bille, and D. Sanglard. 2006. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 591429-1451. [DOI] [PubMed] [Google Scholar]
- 34.Kingsbury, T. J., and K. W. Cunningham. 2000. A conserved family of calcineurin regulators. Genes Dev. 131595-1604. [PMC free article] [PubMed] [Google Scholar]
- 35.Kishi, T., A. Ikeda, R. Nagao, and N. Koyama. 2007. The SCFCdc4 ubiquitin ligase regulates calcineurin signaling through degradation of phosphorylated Rcn1, an inhibitor of calcineurin. Proc. Natl. Acad. Sci. USA 10417418-17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kissova, I., L. T. Plamondon, L. Brisson, M. Priault, V. Renouf, J. Schaeffer, N. Camougrand, and S. Manon. 2006. Evaluation of the roles of apoptosis, autophagy, and mitophagy in the loss of plating efficiency induced by Bax expression in yeast. J. Biol. Chem. 28136187-36197. [DOI] [PubMed] [Google Scholar]
- 37.Kraus, P. R., D. S. Fox, G. M. Cox, and J. Heitman. 2003. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 481377-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis, J., A. Devin, A. Miller, Y. Lin, Y. Rodriguez, L. Neckers, and Z. G. Liu. 2000. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J. Biol. Chem. 27510519-10526. [DOI] [PubMed] [Google Scholar]
- 39.Liu, J., J. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66807-815. [DOI] [PubMed] [Google Scholar]
- 40.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14953-961. [DOI] [PubMed] [Google Scholar]
- 41.Madeo, F., E. Frohlich, and K. U. Frohlich. 1997. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 139729-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madeo, F., E. Frohlich, M. Ligr, M. Grey, S. J. Sigrist, D. H. Wolf, and K. U. Frohlich. 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madeo, F., E. Herker, C. Maldener, S. Wissing, S. Lachelt, M. Herlan, M. Fehr, K. Lauber, S. J. Sigrist, S. Wesselborg, and K. U. Frohlich. 2002. A caspase-related protease regulates apoptosis in yeast. Mol. Cell 9911-917. [DOI] [PubMed] [Google Scholar]
- 44.Marcu, M. G., M. Doyle, A. Bertolotti, D. Ron, L. Hendershot, and L. Neckers. 2002. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1α. Mol. Cell. Biol. 228506-8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 113445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendizabal, I., G. Rios, J. M. Mulet, R. Serrano, and I. F. de Larrinoa. 1998. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 425323-328. [DOI] [PubMed] [Google Scholar]
- 47.Mizunuma, M., D. Hirata, R. Miyaoka, and T. Miyakawa. 2001. GSK-3 kinase Mck1 and calcineurin coordinately mediate Hsl1 down-regulation by Ca2+ in budding yeast. EMBO J. 201074-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulet, J. M., D. E. Martin, R. Loewith, and M. N. Hall. 2006. Mutual antagonism of target of rapamycin and calcineurin signaling. J. Biol. Chem. 28133000-33007. [DOI] [PubMed] [Google Scholar]
- 49.Ohya, Y., H. Kawasaki, K. Suzuki, J. Londesborough, and Y. Anraku. 1991. Two yeast genes encoding calmodulin-dependent protein kinases. Isolation, sequencing and bacterial expressions of CMK1 and CMK2. J. Biol. Chem. 26612784-12794. [PubMed] [Google Scholar]
- 50.Pausch, M. H., D. Kaim, R. Kunisawa, A. Admon, and J. Thorner. 1991. Multiple Ca2+/calmodulin-dependent protein kinase genes in a unicellular eukaryote. EMBO J. 101511-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfaller, M., and J. Riley. 1992. Effects of fluconazole on the sterol and carbohydrate composition of four species of Candida. Eur. J. Clin. Microbiol. Infect. Dis. 11152-156. [DOI] [PubMed] [Google Scholar]
- 52.Priault, M., N. Camougrand, K. W. Kinnally, F. M. Vallette, and S. Manon. 2003. Yeast as a tool to study Bax/mitochondrial interactions in cell death. FEMS Yeast Res. 415-27. [DOI] [PubMed] [Google Scholar]
- 53.Rabinovich, E., A. Kerem, K. U. Frohlich, N. Diamant, and S. Bar-Nun. 2002. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol. Cell. Biol. 22626-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribeiro, G., M. Côrte-Real, and B. Johansson. 2006. Characterization of DNA damage in yeast apoptosis induced by hydrogen peroxide, acetic acid, and hyperosmotic shock. Mol. Biol. Cell 174584-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roy, J., H. Li, P. Hogan, and M. Cyert. 2007. A conserved docking site modulates substrate affinity for calcineurin, signaling output, and in vivo function. Mol. Cell 25889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santos, M., and I. F. de Larrinoa. 2005. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr. Genet. 4888-100. [DOI] [PubMed] [Google Scholar]
- 57.Severin, F. F., and A. A. Hyman. 2002. Pheromone induces programmed cell death in S. cerevisiae. Curr. Biol. 12R233-R235. [DOI] [PubMed] [Google Scholar]
- 58.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1 encoded transcription factor to regulate gene expression in yeast. Genes Dev. 113432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinbach, W. J., J. L. Reedy, R. A. Cramer, Jr., J. R. Perfect, and J. Heitman. 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5418-430. [DOI] [PubMed] [Google Scholar]
- 60.Tabuchi, M., A. Audhya, A. B. Parsons, C. Boone, and S. D. Emr. 2006. The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Mol. Cell. Biol. 265861-5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Truman, A., S. Millson, J. Nuttall, M. Mollapour, C. Prodromou, and P. Piper. 2007. In the yeast heat shock response, Hsf1-directed induction of Hsp90 facilitates the activation of the Slt2 (Mpk1) mitogen-activated protein kinase required for cell integrity. Eukaryot. Cell 6744-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Váchová, L., and Z. Palková. 2007. Caspases in yeast apoptosis-like death: facts and artefacts. FEMS Yeast Res. 712-21. [DOI] [PubMed] [Google Scholar]
- 63.Viladevall, L., R. Serrano, A. Ruiz, G. Domenech, J. Giraldo, A. Barcelo, and J. Arino. 2004. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J. Biol. Chem. 27943614-43624. [DOI] [PubMed] [Google Scholar]
- 64.Wallis, J. W., G. Chrebet, G. Brodsky, M. Rolfe, and R. Rothstein. 1989. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58409-419. [DOI] [PubMed] [Google Scholar]
- 65.Williams, K. E., and M. S. Cyert. 2001. The eukaryotic response regulator Skn7p regulates calcineurin signaling through stabilization of Crz1p. EMBO J. 203473-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wysocki, R., and S. J. Kron. 2004. Yeast cell death during DNA damage arrest is independent of caspase or reactive oxygen species. J. Cell Biol. 166311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu, Q., N. Ke, S. Matsuyama, and J. C. Reed. 2000. Assays for studying Bax-induced lethality in the yeast Saccharomyces cerevisiae. Methods Enzymol. 322283-296. [DOI] [PubMed] [Google Scholar]
- 68.Ye, Y., H. H. Meyer, and T. A. Rapoport. 2001. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414652-656. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida, Y., and Y. Aoyama. 1987. Interaction of azole antifungal agents with cytochrome P-45014DM purified from Saccharomyces cerevisiae microsomes. Biochem. Pharmacol. 36229-235. [DOI] [PubMed] [Google Scholar]
- 70.Yoshimoto, H., K. Saltsman, A. P. Gasch, H. X. Li, N. Ogawa, D. Botstein, P. O. Brown, and M. S. Cyert. 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 27731079-31088. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, N. N., D. D. Dudgeon, S. Paliwal, A. Levchenko, E. Grote, and K. W. Cunningham. 2006. Multiple signaling pathways regulate yeast cell death during the response to mating pheromones. Mol. Biol. Cell 173409-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao, R., and W. Houry. 2007. Molecular interaction network of the Hsp90 chaperone system. Adv. Exp. Med. Biol. 59427-36. [DOI] [PubMed] [Google Scholar]