Abstract
Mitogen-activated protein kinase (MAPK) signaling cascades are composed of MAPK kinase kinases (MAPKKKs), MAPK kinases (MAPKKs), and MAPKs. In this study, we characterize components of a MAPK cascade in Neurospora crassa (mik-1, MAPKKK; mek-1, MAPKK; and mak-1, MAPK) homologous to that controlling cell wall integrity in Saccharomyces cerevisiae. Growth of basal hyphae is significantly reduced in mik-1, mek-1, and mak-1 deletion mutants on solid medium. All three mutants formed short aerial hyphae and the formation of asexual macroconidia was reduced in Δmik-1 mutants and almost abolished in Δmek-1 and Δmak-1 strains. In contrast, the normally rare asexual spores, arthroconidia, were abundant in cultures of the three mutants. Δmik-1, Δmek-1, and Δmak-1 mutants were unable to form protoperithecia or perithecia when used as females in a sexual cross. The MAK-1 MAPK was not phosphorylated in Δmik-1 and Δmek-1 mutants, consistent with the involvement of MIK-1, MEK-1, and MAK-1 in the same signaling cascade. Interestingly, we observed increased levels of mRNA and protein for tyrosinase in the mutants under nitrogen starvation, a condition favoring sexual differentiation. Tyrosinase is an enzyme that catalyzes production of the secondary metabolite l-DOPA melanin. These results implicate the MAK-1 pathway in regulation of development and secondary metabolism in filamentous fungi.
Mitogen-activated protein kinase (MAPK) cascades are critical downstream components of signal transduction pathways in eukaryotic organisms. Each cascade is composed of a module of three kinases—MAPK kinase kinase (MAPKKK), MAPK kinase (MAPKK), and MAPK—that are phosphorylated in a sequential way (34). In filamentous fungi, MAPK pathways have been shown to play a crucial role in growth, development, and pathogenesis (reviewed in references 47 and 62). In particular, MAPK pathways regulating fungal sexual development, osmoregulation, and cell wall integrity are the most studied and are also well conserved among yeasts and filamentous fungi (reviewed in references 7 and 47). However, the actual functions of a given MAPK cascade in different filamentous fungi often vary, depending on the lifestyle of the species (62). In addition, functional overlaps/cross talk have been observed among different MAPK pathways in the same species, further increasing the complexity of the signaling network (4, 12, 16).
The MAPK cascade homologous to the Saccharomyces cerevisiae Slt2p pathway is the subject of increasing study in numerous fungal systems. This pathway regulates cell wall integrity and the response to oxidative stresses in S. cerevisiae (reviewed in reference 31). Components of the Slt2p MAPK cascade have been identified in publicly available genome sequences of filamentous fungi. Because the fungal cell wall is a good target for antifungal drugs, study of this MAPK pathway may yield new targets for developing disease control. In many filamentous fungi, the cascade is involved in regulating cell wall integrity, oxidative stress, vegetative growth, conidiation, hyphal morphology, and pathogenicity (5, 22, 27, 36, 38, 59). In Botrytis cinerea and Colletotrichum lagenarium, the Slt2p-related pathway regulates vegetative growth, conidiation, and pathogenicity but is not essential for maintaining cell wall integrity (26, 48). Expression of the Slt2p-like MAPK in Aspergillus fumigatus, MpkA, is induced under cell-wall-damaging conditions and the deletion of mpkA affects sensitivity to cell wall and oxidative stresses but not fungal virulence (55). Female sexual development is controlled by the Slt2p-homologous MAPK in Fusarium graminearum and Magnaporthe grisea (22, 59). In F. graminearum, this pathway controls accumulation of the deoxynivalenol toxin in plants, suggesting a role in fungal secondary metabolism (22).
The Slt2p MAPK cascade is important for the response to stress driven by antifungal drugs and cell-wall-degrading enzymes in filamentous fungi (1, 25, 27, 45). Elevated expression and activation of a MAPK in response to inhibitors of cell wall synthesis are often observed for filamentous fungi (25, 27, 39, 45). It was recently shown that both the Hog1p and Slt2p MAPK pathways can be sequentially activated in response to cell-wall-degrading enzymes in Saccharomyces cerevisiae, demonstrating an interaction between these two pathways (1).
Components of three MAPK modules homologous to the S. cerevisiae FUS3/KSS1, HOG1, and SLT2 pathways have been annotated in the genome sequence of the model filamentous fungus Neurospora crassa (3). Several kinases in these three predicted pathways have been functionally characterized (32, 60). MAK-2, a MAPK similar to S. cerevisiae Fus3p/Kss1p, regulates hyphal fusion, conidiation, and the development of female sexual structures (protoperithecia) (32, 42). Three kinases homologous to the S. cerevisiae HOG1 pathway components—OS-4, OS-5, and OS-2—are indispensable for the response to hyperosmotic stress, conidial integrity, protoperithecial development, and fungicide sensitivity (24, 60). A two-component regulatory system that includes the histidine kinase OS-1 and the response regulator RRG-1 operates upstream of the OS-2 MAPK pathway (24). It has recently been shown that this MAPK cascade is an output pathway for the Neurospora circadian clock (56).
Compared to the other two MAPK pathways, the one that includes the MAK-1 MAPK, homologous to S. cerevisiae Slt2p, is the least understood in Neurospora. In addition, there have been few investigations with filamentous fungi focused on the roles of all three kinases in the MAK-1-like or other MAPK pathways. In this study, we probe the functions of the three kinases in the MAK-1 MAPK cascade, MIK-1 (MAPKKK), MEK-1 (MAPKK), and MAK-1 (MAPK). We demonstrate an essential role for this pathway in vegetative hyphal growth, conidiation, and protoperithecial development as well as a more limited involvement in the maintenance of cell wall integrity. We also provide evidence that the MAK-1 MAPK cascade negatively regulates the expression of tyrosinase, implicating this pathway in the control of secondary metabolism in Neurospora.
MATERIALS AND METHODS
Strains and culture conditions.
Wild-type strains ORS-SL6a (FGSC 4200; mat a) and 74-OR23-IVA (FGSC 2489; mat A) were acquired from the Fungal Genetics Stock Center (FGSC, Kansas City, MO). The Δmak-2 gene replacement mutant (mat a) was generously provided by Daniel Ebbole. Δmik-1 (MAPKKK; NCU02234.3; FGSC 11326; mat A), Δmek-1 (MAPKK; NCU06419.3; FGSC 11319; mat A), and Δmak-1 (MAPK; NCU11376.3; FGSC 11320; mat A) gene replacement mutants were generated by the Neurospora Genome Project (http://www.dartmouth.edu/∼neurosporagenome). The MAPK deletion mutants were created using an hph cassette and are resistant to hygromycin (8). The region containing the mak-1 gene was previously annotated as NCU09842.2; in the current annotation, the locus has been split into two open reading frames (ORFs), with NCU11376.3 corresponding to mak-1.
The Δmak-1 mutation was complemented in trans through integration of a rescue construct at the his-3 locus (14). The mak-1 ORF was amplified by PCR using genomic DNA as a template and then inserted downstream of the ccg-1 promoter in pMF272 via XbaI and BamHI sites (14). The resulting plasmid, pGP4, was transformed into a Δmak-1 his-3 strain by electroporation as described previously (23). Transformants were selected on sorbose plates (9) lacking histidine, and homokaryotic strains were purified using a microconidiation procedure (10). The purified rescue strains were confirmed by Southern blot analysis (54).
N. crassa strains were cultured in Vogel's minimal medium (VM) (9) for vegetative growth and in synthetic crossing medium (SCM) (9) for the induction of the development of female reproductive structures (protoperithecia). All gene replacement mutants were maintained on medium supplemented with 200 μg/ml hygromycin (Calbiochem, San Diego, CA).
Phenotypic analysis.
To analyze growth of basal hyphae, the colony diameters of strains were measured after 24 h of growth on VM agar plates. The response to hyperosmotic stress was determined by analyzing colony diameters on VM plates containing 1.5 M sorbitol or 0.8 M NaCl. The growth of aerial hyphae and macroconidiation were assessed after culture of strains in 125-ml Erlenmeyer flasks containing 30 ml of VM agar for 1 week. The development of protoperithecia was examined 6 days after the inoculation of SCM plates, and perithecial formation was analyzed 4 to 7 days after the fertilization of SCM plate cultures with wild-type conidia (males) of opposite mating types (ORS-SL6a and 74-OR23-IVA).
Cell wall integrity analysis.
Cell wall integrity was tested in conidia and mycelia grown for 24 h. Conidia were harvested from 10-day-old VM agar cultures of wild-type (ORS-SL6a), Δmik-1, Δmek-1, and Δmak-1 strains. Conidia harvested from each culture were transferred to a 50-ml conical tube and washed twice with sterile water and then once with 1 M sorbitol. Washed conidia were placed in 10 ml of a solution of 1 M sorbitol containing 5 mg/ml lysing enzymes (Sigma, St. Louis, MO) at a concentration of 106/ml and then incubated at 30°C with gentle shaking. A small fraction of the sample was removed every hour and assessed for cell wall degradation (formation of protoplasts) by microscopic examination using an Olympus BX41 compound microscope (Olympus America, Lake Success, NY). To test cell wall integrity in mycelia, conidia harvested from each culture were inoculated into a 20-ml VM liquid culture at the concentration of 106/ml and then incubated with shaking at 30°C in the dark for 24 h. The mycelial mats were collected by filtration using a Masslin shop towel (Chicopee, Benson, NC) and washed with sterile water several times. After the excess water was pressed out, approximately 0.1 g of the mycelial pad was placed in a 50-ml conical tube containing 30 ml of a solution of 1 M sorbitol containing 5 mg/ml lysing enzymes and then incubated at 30°C with gentle shaking. Cell wall integrity was assessed as indicated above.
Response to oxidative stress and antifungal agents.
The extension of basal hyphae was examined in the wild type and mik-1, mek-1, and mak-1 deletion mutants in the presence of hydrogen peroxide (induces oxidative stress; Fisher Scientific, Pittsburgh, PA), aculeacin A (β −1,3-glucan synthase inhibitor; Sigma Chemical Co., St. Louis, MO), and FK506 (calcineurin inhibitor; Tecoland, Edison, NJ). An aliquot containing 1 μl of a conidial suspension was inoculated in the centers of VM plates containing one of the following agents at the indicated concentrations: hydrogen peroxide (10 mM), aculeacin A (10 μg/ml), or FK-506 (10 μg/ml). It had been predetermined that these concentrations inhibited growth but were not lethal to the wild type. Plates were photographed after incubation at 30°C in the dark for 24 h.
Assay for MAK-1 phosphorylation.
A Western blot method was developed to assay phosphorylation of the MAK-1 MAPK. Conidia were harvested from 10-day-old cultures of wild-type (ORS-SL6a), Δmik-1, Δmek-1, and Δmak-1 strains and used to inoculate VM or SCM liquid cultures (initial cell densities of 106 conidia/ml). Mycelial pads were collected from VM liquid cultures 16 h, 24 h, and 3 days after inoculation, from 3-day-old VM agar plate cultures and from SCM liquid cultures after 6 days of growth. Total protein was extracted as described previously (24). Aliquots containing 50 μg of protein were resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels which were then blotted onto nitrocellulose membranes. After being blocked in a solution containing 10 mM Tris-Cl, pH 8.0, 150 mM NaCl, and 0.05% Tween 20 (TBST) with 5% bovine serum albumin for 3 hours at room temperature, the membrane was incubated with either the p44/42 MAPK or phospho-p44/42 MAPK antibodies in the blocking solution (1:200 dilution; Cell Signaling, Danvers, MA) overnight with gentle shaking at 4°C. p44/42 and phospho-p44/42 MAPK antibodies were used for detecting the total and phosphorylated levels of MAK-1, respectively. After treatment with primary antibody, the membrane was washed three times with TBST and then incubated with anti-rabbit immunoglobulin G conjugated to horseradish peroxidase in the blocking solution (1:5,000; Bio-Rad, Hercules, CA) for 3 h at room temperature. The membrane was then washed three times with TBST, treated with SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL), and imaged using a UVP BioImaging system (UVP, Upland, CA).
The phosphorylation level of MAK-1 in submerged wild-type cultures was examined after treatment with hydrogen peroxide, aculeacin A, and FK506. Conidia from the wild type were inoculated at a concentration of 107/ml into 30 ml of liquid VM and incubated for 16 h at 30°C with shaking. Subsequently, 5-ml aliquots of the culture were placed in 25-ml flasks and brought to a final concentration of 10 mM hydrogen peroxide, 10 μg/ml aculeacin A, or 10 μg/ml FK-506. Flasks were incubated at 30°C with gentle shaking and tissue was collected after 30 min and 1 h. Extraction of total protein and Western blot analysis were performed as described above.
Mass spectrometry analysis.
Total protein was extracted from cell pads collected from 6-day-old SCM plate cultures of wild-type (ORS-SL6a), Δmik-1, Δmek-1, and Δmak-1 strains as previously described (24). Samples containing 50 μg of total proteins were subjected to electrophoresis using 12% SDS-PAGE gels. The gel was stained with Coomassie brilliant blue dye and then destained following procedures previously described (6). An area of the gel including a protein that was abundantly expressed in Δmek-1 and Δmak-1 mutants was cut into thin horizontal slices from wild-type, Δmik-1, Δmek-1, and Δmak-1 lanes. Gel slices were then digested with trypsin and processed as described previously (6). The gel digest was analyzed by a nano-liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-MS-MS) instrument with a Waters nano-Acquity UPLC/Q-TOF Premier system (Waters, Milford, MA). An LC-MS-MS survey scan method was used for analyzing all peptide precursor ions for the wild type and three mutants. The raw data-dependent acquisition from the survey scan was then processed by Protein Lynx (Waters, Milford, MA) software to generate pkl text files that were used to search the NCBI Neurospora database with the MASCOT algorithm for protein identification (6). The quantitative analysis of protein abundance for both the wild type and the mutants was carried out with LC-MS-MS experiments as described in a prior study (see reference 46).
Northern blot analysis.
Conidia were harvested from 10-day-old VM agar cultures of wild-type (ORS-SL6a), Δmik-1, Δmek-1, and Δmak-1 strains, inoculated into liquid VM or SCM to a final concentration of 106 conidia/ml, and incubated with shaking at 30°C (VM) or 25°C (SCM). VM cultures were harvested after 3 days and SCM cultures after 3 and 6 days. Total RNA was extracted from tissues with the Purescript RNA purification kit following the manufacturer's instructions (Gentra Systems, Minneapolis, MN). Samples containing 10 μg of total RNA were resolved on a denaturing agarose-formaldehyde gel as described previously (49). Blotting and hybridization were performed following standard molecular biology protocols (49). Transcript levels were quantified by densitometric analysis of bands on X-ray film with the Image J software program (version 1.38x; NIH, Bethesda, MD). All values were corrected for background and normalized to the level of rRNA.
RESULTS
mik-1, mek-1, and mak-1 encode three components of a MAPK cascade homologous to the yeast Slt2p MAPK pathway.
Three annotated genes, namely, NCU02234.3 (mik-1), NCU06419.3 (mek-1), and NCU11376.3 (mak-1), in the Neurospora genome sequence encode a MAPKKK, a MAPKK, and a MAPK homologous to Bck1p, Mkk1p and Mkk2p, and Slt2p, respectively, from S. cerevisiae (3). mik-1, mek-1, and mak-1 encode proteins of 1779, 485, and 455 amino acids, respectively. Each protein has a protein kinase domain located at the C terminus; this region comprises almost the entire protein in MAK-1 (amino acids 23 to 355; Fig. 1A). These three kinases are structurally well conserved in fungi; in particular, the protein kinase domain exhibits higher homology than any other region (data not shown). MIK-1 possesses 35 to 51% overall sequence identity with proteins in S. cerevisiae and other filamentous fungi and is most homologous to a protein in F. graminearum (51%). MEK-1 is highly homologous to MAPKKs in F. graminearum, M. grisea, and B. cinerea (73.7 to 75.1% identical) but exhibits only 36.0 to 54.2% identity to proteins in S. cerevisiae and Cryptococcus neoformans. Among the three kinases, MAK-1 displays the greatest homology with MAPKs in other fungi (from 65% identical to S. cerevisiae Slt2p to 81% identical to an F. graminearum protein).
FIG. 1.
Structure and expression of MIK-1, MEK-1, and MAK-1. (A) Coding regions and introns are indicated as boxes and lines, respectively. Black boxes represent protein kinase domains present in the three kinases. (B) Total RNA was isolated from Δmik-1, Δmek-1, Δmak-1, and wild-type (wt) strains grown in liquid VM and SCM for 3 or 6 days (3d or 6d, respectively) as indicated. Samples containing 50 μg were subjected to Northern blot analysis using radiolabeled probes corresponding to mik-1, mek-1, and mak-1. The experiment was performed three times, and a representative Northern blot is shown. (C) Expression levels of mik-1, mek-1, and mak-1 in the indicated strains cultured in VM liquid for 3 days (white bars) or in SCM liquid for 3 (black bars) or 6 (gray bars) days, according to data from panel B. The expression level is indicated by the integrated density of band intensity after normalization using rRNA as a loading control. WT, wild type.
mik-1, mek-1, and mak-1 are essential for vegetative growth and macroconidiation and also influence cell wall integrity.
We explored a possible role for the mik-1, mek-1, and mak-1 genes in Neurospora biology through analysis of deletion mutants. Gene replacement mutants for the three genes were generated by the Neurospora genome project (http://www.dartmouth.edu/∼neurosporagenome). Two independent gene replacement mutants (mating types mat A and mat a) were examined for each gene, and both strains behaved similarly in all phenotypes tested (data not shown). We also analyzed the expression of the remaining genes in each mutant under three different conditions—3-day high-nitrogen VM and 3- and 6-day nitrogen-starved SCM liquid cultures—with Northern analysis. No message for the corresponding deleted genes was observed for the mik-1, mek-1, and mak-1 knockout mutants (Fig. 1B). In the wild type, the levels of mak-1 transcript were higher in nitrogen-starved (SCM) than in vegetative (VM) cultures (Fig. 1B and C). Interestingly, the expression of mek-1 is greatly increased in SCM cultures in the absence of mak-1 (Fig. 1B and C).
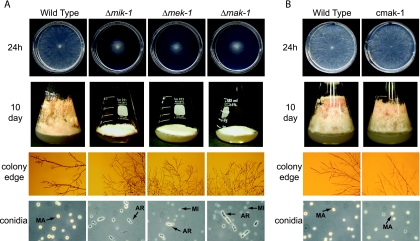
Neurospora grows vegetatively through extension and branching of basal hyphae to form the vegetative structure termed the mycelium. The fungus develops aerial hyphae from the vegetative mycelium under nutrient deprivation and desiccation and then forms budding structures at the end of aerial hyphae (conidiophores) that produce the asexual spores, macroconidia (51). Deletion mutants lacking mik-1, mek-1, or mak-1 exhibit a significant reduction in apical extension of basal hyphae compared to the wild type (Fig. 2A, top row). On VM agar plates, the mutant colonies are very flat, with diameters of only 30 to 40% of those seen for the wild type (ORS-SL6a) after 24 h of growth (Fig. 2A, top row, and Table 1). The three mutants exhibited more branching of hyphae at the colony edge than the wild type (Fig. 2A, third row). When either of two osmolytes, sorbitol (1.5 M) and NaCl (0.8 M), is added to the medium, the colony diameters of Δmik-1, Δmek-1, and Δmak-1 strains are reduced to 28 to 42% of that observed for medium without any additions (Table 1). However, a similar degree of reduction is observed in the wild type under the same conditions, indicating that mik-1, mek-1, and mak-1 are not essential for resistance to these osmotic stresses (Table 1). These results also demonstrated that in contrast to what is seen for S. cerevisiae, the growth phenotypes of mutants lacking components of the cell-wall-integrity-related MAPK pathway are not remediated by the addition of osmolytes in Neurospora.
FIG. 2.
Basal hyphal growth and macroconidiation. Basal hyphal growth and macroconidiation were examined in wild-type strain ORS-SL6a in comparison to the Δmik-1, Δmek-1, and Δmak-1 strains (A) or the mak-1 rescue strain, cmak-1 (B). The indicated strains were cultured in plates (top rows) and 125-ml flasks (second rows) containing solid VM for 24 h and 10 days, respectively. Hyphae at the colony edge (third rows) were observed using a stereomicroscope. Macroconidia (MA), microconidia (MI), and arthroconidia (AR) are indicated by arrows (bottom rows).
TABLE 1.
Apical extension on normal and hyperosmotic media
| Strain | Colony diam (cm) (% of that without addition) in VM witha:
|
||
|---|---|---|---|
| No addition | 1.5 M sorbitol | 0.8 M NaCl | |
| Wild type | 8.6 | 3.4 ± 0.21 (40) | 3.0 ± 0.46 (35) |
| Δmik-1 | 2.7 ± 0.035 | 0.99 ± 0.085 (37) | 0.82 ± 0.035 (31) |
| Δmek-1 | 2.3 ± 0.035 | 0.92 ± 0.11 (39) | 0.67 ± 0.035 (28) |
| Δmak-1 | 2.5 ± 0.035 | 1.0 ± 0.018 (42) | 0.8 (32) |
Additions to VM medium are indicated. Shown in parentheses are percentages of colony diameters obtained with VM with no addition (100% values not shown).
Examination of flask cultures showed that the Δmik-1, Δmek-1, and Δmak-1 strains have severe defects in the development of aerial hyphae and macroconidia (Fig. 2A, second row). Well-developed aerial hyphae and orange-colored macroconidia are abundant in the wild type but scarce in the three mutants (Fig. 2A, second row). Microscopic examination showed that the Δmik-1 strain produces macroconidia, but at a number significantly lower than that in the wild type (data not shown). Macroconidia were never observed in the Δmek-1 and Δmak-1 strains (data not shown). Interestingly, all three mutants produce arthroconidia, which are rare in wild-type cultures (Fig. 2A, bottom row). Arthroconidia are formed by the segmentation of vegetative hyphae in Neurospora and other fungi (17).
We complemented the Δmak-1 mutation in trans through integration of a rescue construct at the his-3 locus (see Materials and Methods). The purified complemented strains (cmak-1 and c2mak-1) behaved like the wild type for all traits tested (see below; also data not shown). Basal hyphal growth on VM agar plates for 24 h was similar for cmak-1 and wild-type strains (Fig. 2B). cmak-1 developed aerial hyphae normally and formed abundant macroconidia, similar to the wild type (Fig. 2B).
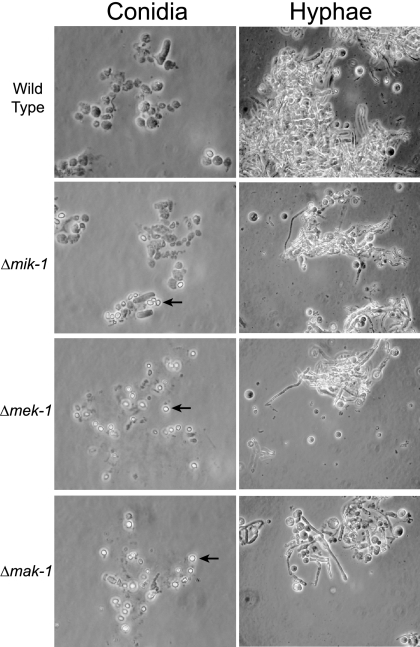
As mentioned above, the MAPK module corresponding to MIK-1/MEK-1/MAK-1 is required for cell wall integrity in S. cerevisiae (31). No hyphal lysis is observed in young vegetative cultures of Δmik-1, Δmek-1, and Δmak-1 strains (Fig. 2A, top row). However, lysis was observed for Δmek-1 and Δmak-1 agar cultures grown for more than 10 days (see Fig. 8 below). Because a subtle cell wall defect might not be obvious in intact cells of younger cultures, we also examined conidia and 24-hour vegetative cultures from the three mutants and the wild type after treatment with lysing enzymes normally used to prepare cell-wall-less protoplasts (57). After digestion with lysing enzymes for 2 h and then the addition of water, a number of macroconidia in the wild type were still intact, whereas most arthroconidia and macroconidia in the three mutants were disrupted (Fig. 3; also data not shown). Interestingly, microconidia formed by the three mutants were still intact after treatment with lysing enzymes for 2 h (Fig. 3). Vegetative mycelia from the cultures grown for 24 h released more protoplasts from Δmik-1, Δmek-1, and Δmak-1 strains than from the wild type after treatment with lysing enzymes for 3 h (Fig. 3). These results indicate that mik-1, mek-1, and mak-1 are involved in the regulation of cell wall integrity in Neurospora. However, this pathway appears to play a more minor role, particularly in younger cultures, than the corresponding Slt2p cascade in S. cerevisiae.
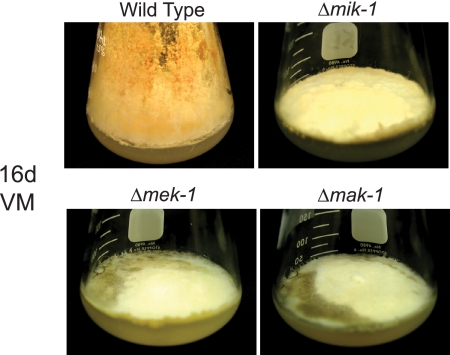
FIG. 8.
Melanization. The indicated strains were cultured in 125-ml flasks containing solid VM for 16 days (16d; 3 days at 30°C in the dark and 13 days at 25°C in the light). Melanization can be detected as the brown pigment formations in the Δmek-1 and Δmak-1 cultures.
FIG. 3.
Cell wall integrity assay. Conidia (left) and hyphae (right) from the wild type and from Δmik-1, Δmek-1, and Δmak-1 strains were treated with lysing enzymes (5 mg/ml) for 2 h (for conidia) and 3 h (for hyphae) at 30°C. Samples were observed under the microscope after the addition of water to assess cell integrity. Microconidia are indicated by arrows.
mik-1, mek-1, and mak-1 are required for female sexual development.
Neurospora is a heterothallic fungus with two mating types, mat A and mat a (37). Mating occurs between male and female cells of opposite mating types. Differentiation of female reproductive structures (protoperithecia) is induced by the growth of Neurospora under nitrogen starvation (SCM) (58). Protoperithecia further develop into perithecia after fertilization with opposite-mating-type males (usually macroconidia) (43). Mating and meiosis are followed by the formation of sexual spores (ascospores) that are ejected from the perithecium approximately 1 week after fertilization.
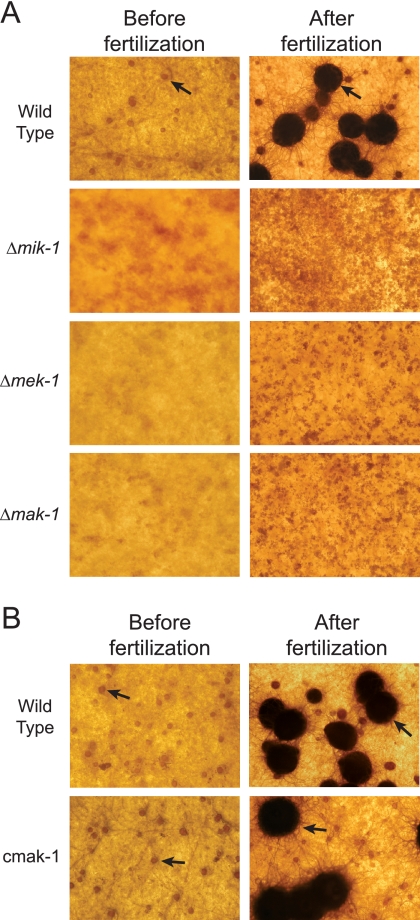
For Neurospora, it has previously been shown that the mutation of genes encoding components of the MAK-2 and OS-2 MAPK pathways blocks the development of protoperithecia (24, 32). Based on this precedent, we examined female and male sexual development and mating in the Δmik-1, Δmek-1, and Δmak-1 strains. All three mutants were male fertile (data not shown). When cultured on SCM plates for 6 days, the wild-type strain produced many protoperithecia (round brown structures in Fig. 4A); however, no protoperithecia were observed in the Δmik-1, Δmek-1, and Δmak-1 mutants. After fertilization with wild-type conidia (males) of opposite mating types, the wild type developed abundant perithecia, whereas no perithecia were ever produced in the Δmik-1, Δmek-1, and Δmak-1 cultures (Fig. 4A). Similar to what was seen for the wild type, protoperithecia were formed normally and then developed into perithecia after fertilization in the cmak-1 complemented strain (Fig. 4B). Taken together, the results indicate that the inactivation of any one of the three MAPK pathways (MAK-1, MAK-2, or OS-2) leads to a block in protoperithecial development and subsequent perithecial development in Neurospora.
FIG. 4.
Female sexual development. The Δmik-1, Δmek-1, and Δmak-1 strains (A) and the mak-1 rescue strain, cmak-1 (B), along with the wild type (ORS-SL6a) (A and B), were imaged after culture on SCM plates for 6 days under constant light (left) and incubation for 7 days after fertilization with males of opposite mating types (right). Brown round protoperithecia and black perithecia (indicated by arrows) were present in SCM plate cultures of the wild type and cmak-1.
The MAK-1 MAPK is phosphorylated in Neurospora.
Within a MAPK cascade, the environmental signal is transferred by sequential phosphorylation of the three component kinases (34). The MAPKKK phosphorylates the MAPKK and the MAPKK in turn phosphorylates the MAPK. Activation of the MAPK by phosphorylation often leads to the modulation of gene expression through the regulation of a transcription factor(s).
To investigate the existence of a pathway involving the mik-1, mek-1, and mak-1 gene products, we set up an assay system for MAK-1 in Neurospora. For S. cerevisiae, it has been reported that the phosphorylation of the homologous Slt2p MAPK can be detected using antisera to extracellular signal-regulated kinase (ERK) family MAPKs (35). Such antisera had already been shown to recognize S. cerevisiae Fus3p/Kss1p, Neurospora MAK-2, and other ERK-related MAPKs in fungi (13, 42, 61). As previously observed, we detected a phosphorylated protein of ∼43 kDa corresponding to phospho-MAK-2 in our Western blot assay using the phospho-p44/42 antibody (Fig. 5) (42). When the p44/42 antibody was used, a protein of same size was observed in the wild type but not in the mak-2 deletion mutant (data not shown). In addition to MAK-2, we also noted a protein of approximately 50 kDa, the predicted size of MAK-1, that was detected by the same antibodies in wild-type samples (Fig. 5 and data not shown). The 50-kDa protein was not detected in the mak-1 deletion mutant, consistent with its identity as the MAK-1 protein (data not shown).
FIG. 5.
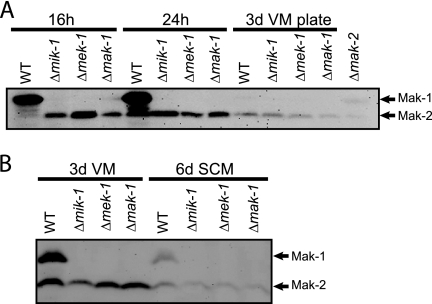
Phosphorylation of MAK-1. Western blot analysis using anti-phospho-p44/42 antibody was performed on total protein extracted from the indicated strains cultured under various conditions. (A) Strains cultured in liquid VM for 16 or 24 h or on VM agar plates for 3 days. (B) Strains cultured in liquid VM and SCM for 3 and 6 days, respectively. Protein species corresponding to MAK-1 (∼50 kDa) and MAK-2 (∼43 kDa) are indicated by arrows. 3d, 3 days; 6d, 6 days; WT, wild type.
Based on the above results, we used the phospho-p44/42 antisera to analyze the phosphorylation of MAK-1 and MAK-2 under various conditions in wild-type strains and the three mutants. Phosphorylated MAK-1 protein (50 kDa) was detected at high amounts in 16- and 24-h (Fig. 5A) and 3-day (Fig. 5B) VM-submerged hyphal cultures of the wild type. Phosphorylation of MAK-1 in the wild type was much lower in 6-day SCM liquid culture (Fig. 5B) and barely detected in 3-day VM plate culture (Fig. 5A). However, phosphorylation of MAK-1 was not observed for the mik-1 and mek-1 deletion mutants under any growth conditions, indicating that MIK-1 and MEK-1 are required for the phosphorylation of MAK-1 (Fig. 5A and B). In contrast to the results for MAK-1, the MAK-2 MAPK was phosphorylated in the wild type and the other three kinase mutants under all conditions, although the phosphorylation level was lower in nitrogen-starved SCM liquid and VM plate cultures (Fig. 5A and B).
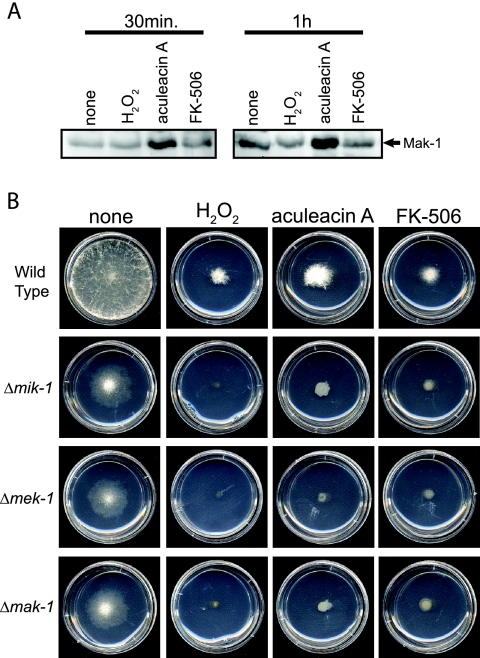
Since the Slt2p MAPK is known to be involved in stress responses in S. cerevisiae (31), we explored a role for the MAK-1 cascade under different stress conditions. We first tested the phosphorylation of MAK-1 in the wild type after treatment with hydrogen peroxide (oxidative stress; 10 mM) and two antifungal agents, aculeacin A (β-1, 3-glucan synthase inhibitor; 10 μg/ml) and FK-506 (a calcineurin inhibitor; 10 μg/ml). Pilot experiments demonstrated that these concentrations inhibited growth but were not lethal to the wild type (see below; also data not shown). Levels of MAK-1 phosphate were elevated after exposure of tissues to aculeacin A for 30 min or 1 h (Fig. 6A). We observed similar results in three independent experiments (data not shown). This result is consistent with an induction of MAK-1 phosphorylation in response to an inhibition of cell wall synthesis in Neurospora. We did not observe reproducible changes in the phosphorylation levels of MAK-1 after treatment with hydrogen peroxide or FK-506 in our studies (Fig. 6A).
FIG. 6.
Response to oxidative stress and antifungal agents. (A) Phosphorylation of MAK-1. The wild type was cultured in liquid VM at 30°C for 16 h before treatment with hydrogen peroxide (H2O2; 10 mM), aculeacin A (10 μg/ml), or FK-506 (10 μg/ml) for the indicated times. (B) Basal hyphal growth. The wild type and the mik-1, mek-1, and mak-1 deletion mutants were cultured on VM agar plates containing hydrogen peroxide (H2O2; 10 mM), aculeacin A (10 μg/ml), or FK-506 (10 μg/ml) at 30°C for 24 h.
We further tested the sensitivities of the wild type and the three mutants to hydrogen peroxide (10 mM), aculeacin A (10 μg/ml), and FK-506 (10 μg/ml) during growth on VM plates. All three agents significantly inhibited basal hyphal growth in the wild type and the three mutants after 24 h (Fig. 6B), and the reductions in growth were not significantly different among the four strains (Fig. 6B; also data not shown). This demonstrates that the loss of mik-1, mek-1, or mak-1 does not significantly affect sensitivity to hydrogen peroxide, aculeacin A, or FK-506 in Neurospora. The differences in the short-term responses to aculeacin A observed in the MAK-1 phosphorylation assays and the growth of the Δmak-1 mutant in the presence of aculeacin A may reflect compensatory mechanisms that operate during long-term exposure during growth on plates.
The MAK-1 MAPK cascade influences tyrosinase levels and melanin accumulation.
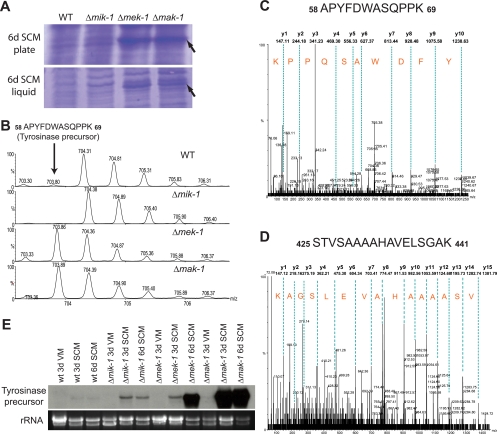
While performing control assessment by use of SDS-PAGE gels for the MAPK Western blot assays, we noted a prominent band corresponding to a protein species of 40 to 50 kDa in extracts from 6-day SCM liquid and plate cultures of Δmek-1 and Δmak-1 strains. This band was absent from the wild type and the Δmik-1 strain (Fig. 7A). In addition, this protein species was not observed in the wild type or in any of three mutant strains grown under other conditions used for the analysis of MAK-1 or MAK-2 phosphorylation (see conditions for Fig. 5A and B; also data not shown). To identify the protein(s) corresponding to the prominent band, we excised the relevant areas from gels containing proteins from the wild type and the mutants, digested the extracted proteins with trypsin, and analyzed the tryptic peptides by nano-LC-MS-MS (see Materials and Methods) using a data-dependent acquisition survey method. The LC-MS-MS spectra showed that peptide precursor ions (m/z 703.86 and 785.44 in Fig. 7C and D) were abundant in the Δmek-1 and Δmak-1 strains but undetectable in the wild type and the Δmik-1 mutant (Fig. 7B). Searches of the Neurospora genome database showed that these peptide precursor ions corresponded to the tyrosinase precursor. The protein detected in Neurospora extracts is likely to be the processed form of tyrosinase, as its molecular mass (40 to 50 kDa) is close to that of processed tyrosinase (46 kDa [28]) and much smaller than that of the precursor (75 kDa).
FIG. 7.
Tyrosinase production. (A) Accumulation of a protein in Δmek-1 and Δmak-1 tissues from 6-day (6d) SCM liquid and plate cultures. (B) An extracted ion chromatogram from LC-MS-MS experiments showing a precursor peptide ion (m/z 703.86, 2+) of tyrosinase that was abundant in Δmek-1 and Δmak-1 but not detectable in either the wild type (WT) or the Δmik-1 mutant. (C and D) LC-MS-MS spectra of precursor peptide ions of m/z 703.86, 2+ (C) and m/z 785.44, 2+ (D) that were specifically matched to Neurospora tyrosinase and spanned residues 58 to 69 and 425 to 441, respectively. Only y-series ions are labeled. (E) Expression of tyrosinase examined by Northern blotting. Samples containing 50 μg of total RNA were subjected to Northern blot analysis using a radiolabeled probe corresponding to the tyrosinase ORF (∼1.7 kb). 3d, 3 days; wt, wild type.
Earlier work with Neurospora showed that tyrosinase levels are increased during nutrient starvation (21). Similarly, the results presented above demonstrate that tyrosinase protein can be detected only in nitrogen-starved SCM tissues (grown in both liquid and solid media) of Δmek-1 and Δmak-1 strains. Based on these observations, we also tested the effect of nitrogen starvation on tyrosinase gene expression using Northern blot analysis. We compared tyrosinase mRNA levels in strains grown in liquid cultures containing either high-nitrogen VM or low-nitrogen SCM. We could detect no or only very little tyrosinase transcript in the wild-type strain grown under either condition (Fig. 7E). Similarly, no transcription of tyrosinase was observed for VM cultures of all three mutants (Fig. 7E). However, tyrosinase was produced in Δmik-1, Δmek-1, and Δmak-1 mutants grown on SCM for 3 and 6 days, with the highest expression in the Δmak-1 strain (Fig. 7E). The same result was observed for tissues from 6-day SCM plate cultures (data not shown). This indicates that nitrogen starvation influences tyrosinase levels and that mik-1, mek-1, and mak-1 are all negative regulators of tyrosinase expression, with mak-1 playing the greatest role.
In Neurospora, tyrosinase has long been known to be required for l-DOPA melanin (eumelanin) biosynthesis (30). We observed an accumulation of a dark brown pigment indicative of melanin in VM plate cultures of Δmek-1 and Δmak-1 strains incubated for 16 days (Fig. 8). This suggests that the high expression of tyrosinase observed for Δmek-1 and Δmak-1 strains is followed by melanization.
DISCUSSION
Our analysis demonstrates that the MAK-1 pathway is required for normal vegetative growth, conidiation, female sexual development, and tyrosinase expression in Neurospora. These results highlight the functional conservation and also the variations in this cascade that have been observed for yeasts and other filamentous fungi. Similar to what is the case for Neurospora, MAK-1-related MAPK pathways are also required for vegetative growth and conidiation in other filamentous fungi (5, 22, 26, 38, 48, 59). However, MGV1 and MGSLT2 in F. graminearum and Mycosphaerella graminicola, respectively, are not essential for conidiation (22, 36). Similar to what is the case for S. cerevisiae and filamentous fungi such as A. nidulans, C. neoformans, F. graminearum, Claviceps purpurea, and M. grisea, the MAK-1 MAPK cascade plays a role in the regulation of cell wall integrity in Neurospora (5, 22, 29, 38, 40, 52, 59). However, the effects due to the deletion of mik-1, mek-1, and mak-1 in Neurospora were not as great as those observed for other fungi. No hyphal autolysis was observed in young vegetative cultures of the three mutants, and the phenotypes of these strains were not rescued by the addition of osmostabilizers. In addition, microconidia that are abundant in the three mutants were resistant to cell-wall-lysing enzymes. Based on these observations, we cannot rule out the possibility that additional pathways may be involved in the regulation of cell wall integrity in Neurospora. Along these lines, recent studies show that MAK-1-homologous MAPKs have been shown to be dispensable for cell wall integrity in Botrytis cinerea (bmp3) and Colletotrichum lagenarium (MAF1) (26, 48). A recent study of Neurospora (33) shows that mak-1 deletion mutants are more sensitive to lysing enzymes than the wild type, a finding consistent with our observations.
The increased sensitivity of the mutants to cell-wall-degrading enzymes suggested that the MAK-1 MAPK pathway might play a role in generating tolerance to antifungal agents that cause cell wall damage to Neurospora. It has been demonstrated for several filamentous fungi, including C. neoformans, A. nidulans, and A. fumigatus, that the Slt2p-related pathway is activated by and functions in the tolerance to cell-wall-damaging antifungal drugs (15, 27, 55). Our tests using three antifungal agents demonstrated that only the β-1,3-glucan synthase inhibitor aculeacin A induces elevated phosphorylation of MAK-1 in wild-type Neurospora. However, the three MAK-1 MAPK cascade mutants were no more sensitive than the wild type to aculeacin A, hydrogen peroxide, or FK-506 during growth on solid medium, suggesting that the MAK-1 pathway is not essential for long-term growth in the presence of these agents. Alternatively, the already relatively severe reduction in growth observed for the three mutants on minimal medium may override any inhibitory effect of aculeacin A, hydrogen peroxide, or FK-506.
Our study shows that the MAK-1 MAPK signaling pathway regulates female sexual development and conidiation in Neurospora. Similarly, the Neurospora MAK-2 and OS-2 MAPKs, homologous to Fus3p/Kss1p and Hog1p, respectively, in S. cerevisiae, have been shown to play a crucial role in sexual development and conidiation (24, 32). Deletion of any one of the three MAPK genes, mak-1, mak-2, or os-2, abolishes the development of protoperithecia in Neurospora. Defects in protoperithecial development in the mak-1 and mak-2 mutants may be related to the diminished vegetative growth and conidiation of both strains. Although the os-2 mutant shows normal vegetative growth, it produces lysed conidia, possibly due to diminished conidial cell wall rigidity. All these observations suggest interaction and/or cooperation among the three major Neurospora MAPK signaling pathways during regulation of female sexual development and conidiation. Cross talk and coordination among distinct MAPK pathways have been observed for yeasts (4, 16, 18). In S. cerevisiae, the Slt2p MAPK is activated in response to hyperosmotic stress and by the activated Hog1p MAPK via the transcription factor Rlm1p (16, 18). Upon pheromone stimulation and activation of the Fus3p MAPK, Slt2p can also be activated in a manner that requires the upstream MAPKK (Mpk1p/Mpk2p) and Pkc1p but not the MAPKKK (Bck1p) (4). Functions governed by the MAK-1 signaling pathway, such as hyphal growth, cell wall integrity, and melanin synthesis, are major processes that occur during female sexual development and conidiation in Neurospora. It is plausible that cross talk or coordination between MAK-1 and other MAPK pathways facilitates the developmental changes that occur during sexual differentiation and conidiation in Neurospora.
Interestingly, we found that the MAK-1 pathway is involved in transcriptional control of tyrosinase, one of the enzymes catalyzing two rate-limiting steps in the biosynthetic pathway of l-DOPA melanin (eumelanin), the orthohydroxylation of monophenols and aromatic amines, and the oxidation of o-diphenols and o-aminophenols (50, 53). This finding suggests that the MAK-1 cascade plays a role in l-DOPA melanin biosynthesis through control of tyrosinase levels. Melanin is associated with fungal cell walls and is a virulence factor in many fungal pathogens (for reviews, see references 41, 44, and 50). In Neurospora, active tyrosinase is a 46-kDa monomeric protein formed via proteolytic modification of a 75-kDa protein precursor (28). The size of the protein observed in our analysis corresponds to that of the processed active form of tyrosinase (46 kDa). In our study, it appears that tyrosinase transcription is negatively regulated by the MAK-1 MAPK signaling pathway and that this regulation is very similar to that known for mammalian melanoma cells. In mammalian melanoma cells, melanin (eumelanin) levels are controlled by the cooperation of two signaling pathways, the cyclic AMP (cAMP) and MAPK pathways. cAMP positively stimulates melanization by increasing tyrosinase transcription, whereas the activation of the ERK MAPK pathway inhibits tyrosinase expression and eventual melanization (2, 11, 20). In Neurospora, a consensus sequence for a cAMP regulatory element was found in the tyrosinase gene promoter, and tyrosinase production could be induced at the transcriptional level by exogenous cAMP (28). As already shown for Neurospora, tyrosinase expression was induced by nitrogen starvation and during sexual development in our study (21). Deletion of the three kinase genes influenced the level of tyrosinase transcript only under nitrogen-starved conditions (SCM tissues). This suggests that there may be another mechanism, possibly cAMP signaling, activated by nitrogen starvation and involved in the induction of tyrosinase expression. Thus, tyrosinase expression in Neurospora is modulated by the action of two separate mechanisms: induction by unknown regulators, possibly cAMP, and inhibition by the MAK-1 pathway.
Out of the three MAPK cascade genes, the loss of the mik-1 MAPKKK gene had the least effect on tyrosinase transcription. Less severe phenotypes for the Δmik-1 mutant relative to the Δmek-1 and Δmak-1 mutants were also observed during macroconidiation, with loss of mik-1 leading to a reduction, and deletion of mek-1 and mak-1 leading to complete abolition, of macroconidiation. This suggests that MEK-1 and/or MAK-1 can be activated by additional upstream factors, such as other MAPKKKs. As discussed above, Hog1p and Slt2p MAPKs can be activated by different MAPKKKs and other kinases in response to various signals in S. cerevisiae (4).
The importance of the MAK-1 MAPK to asexual and sexual growth and development has been demonstrated for a large number of fungi. In contrast, the involvement of this pathway in cell wall integrity is not universal. Our work shows that the MAK-1 cascade also plays an important role in regulating tyrosinase expression and l-DOPA melanin biosynthesis in Neurospora. As demonstrated for many fungi, melanin can serve as a virulence factor and is also important for managing environmental stresses (for reviews, see references 19 and 41). Investigations probing the control of tyrosinase and melanin production by the MAK-1 MAPK pathway in Neurospora will be relevant to understanding the regulation of secondary metabolism and l-DOPA melanin production in fungal pathogens.
Acknowledgments
We acknowledge members of the Borkovich laboratory for many helpful discussions.
This work was supported by National Institutes of Health grants R01 GM48626 and P01 GM068087.
Footnotes
Published ahead of print on 10 October 2008.
REFERENCES
- 1.Bermejo, C., E. Rodriguez, R. Garcia, J. M. Rodriguez-Pena, M. L. Rodriguez de la Concepcion, C. Rivas, P. Arias, C. Nombela, F. Posas, and J. Arroyo. 2008. The sequential activation of the yeast HOG and SLT2 pathways is required for cell survival to cell wall stress. Mol. Biol. Cell 191113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertolotto, C., K. Bille, J. P. Ortonne, and R. Ballotti. 1996. Regulation of tyrosinase gene expression by cAMP in B16 melanoma cells involves two CATGTG motifs surrounding the TATA box: implication of the microphthalmia gene product. J. Cell Biol. 134747-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borkovich, K. A., L. A. Alex, O. Yarden, M. Freitag, G. E. Turner, N. D. Read, S. Seiler, D. Bell-Pedersen, J. Paietta, N. Plesofsky, M. Plamann, M. Goodrich-Tanrikulu, U. Schulte, G. Mannhaupt, F. E. Nargang, A. Radford, C. Selitrennikoff, J. E. Galagan, J. C. Dunlap, J. J. Loros, D. Catcheside, H. Inoue, R. Aramayo, M. Polymenis, E. U. Selker, M. S. Sachs, G. A. Marzluf, I. Paulsen, R. Davis, D. J. Ebbole, A. Zelter, E. R. Kalkman, R. O'Rourke, F. Bowring, J. Yeadon, C. Ishii, K. Suzuki, W. Sakai, and R. Pratt. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 681-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buehrer, B. M., and B. Errede. 1997. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol. Cell. Biol. 176517-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bussink, H.-J., and S. A. Osmani. 1999. A mitogen-activated protein kinase (MPKA) is involved in polarized growth in the filamentous fungus, Aspergillus nidulans. FEMS Microbiol. Lett. 173117-125. [DOI] [PubMed] [Google Scholar]
- 6.Carter, C., S. Pan, J. Zouhar, E. L. Avila, T. Girke, and N. V. Raikhel. 2004. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 163285-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, R. E., and J. Thorner. 2007. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 17731311-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colot, H. V., G. Park, G. E. Turner, C. Ringelberg, C. M. Crew, L. Litvinkova, R. L. Weiss, K. A. Borkovich, and J. C. Dunlap. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 10310352-10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, R. H., and F. J. deSerres. 1970. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 71A79-143. [Google Scholar]
- 10.Ebbole, D., and M. S. Sachs. 1990. A rapid and simple method for isolation of Neurospora crassa homokaryons using microconidia. Fungal Genet. Newsl. 3717-18. [Google Scholar]
- 11.Englaro, W., C. Bertolotto, R. Busca, A. Brunet, G. Pages, J. P. Ortonne, and R. Ballotti. 1998. Inhibition of the mitogen-activated protein kinase pathway triggers B16 melanoma cell differentiation. J. Biol. Chem. 2739966-9970. [DOI] [PubMed] [Google Scholar]
- 12.Errede, B., R. M. Cade, B. M. Yashar, Y. Kamada, D. E. Levin, K. Irie, and K. Matsumoto. 1995. Dynamics and organization of MAP kinase signal pathways. Mol. Reprod. Dev. 42477-485. [DOI] [PubMed] [Google Scholar]
- 13.Errede, B., A. Gartner, Z. Zhou, K. Nasmyth, and G. Ammerer. 1993. MAP kinase-related FUS3 from S. cerevisiae is activated by STE7 in vitro. Nature 362261-264. [DOI] [PubMed] [Google Scholar]
- 14.Freitag, M., P. C. Hickey, N. B. Raju, E. U. Selker, and N. D. Read. 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41897-910. [DOI] [PubMed] [Google Scholar]
- 15.Fujioka, T., O. Mizutani, K. Furukawa, N. Sato, A. Yoshimi, Y. Yamagata, T. Nakajima, and K. Abe. 2007. MpkA-dependent and -independent cell wall integrity signaling in Aspergillus nidulans. Eukaryot. Cell 61497-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Rodriguez, L. J., R. Valle, A. Duran, and C. Roncero. 2005. Cell integrity signaling activation in response to hyperosmotic shock in yeast. FEBS Lett. 5796186-6190. [DOI] [PubMed] [Google Scholar]
- 17.Griffin, D. H. 1994. Fungal physiology, 2nd ed. Wiley-Liss, New York, NY.
- 18.Hahn, J. S., and D. J. Thiele. 2002. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J. Biol. Chem. 27721278-21284. [DOI] [PubMed] [Google Scholar]
- 19.Halaouli, S., E. Record, L. Casalot, M. Hamdi, J.-C. Sigoillot, M. Asther, and A. Lomascolo. 2006. Cloning and characterization of a tyrosinase gene from the white-rot fungus Pycnoporus sanguineus, and overproduction of the recombinant protein in Aspergillus niger. Appl. Microbiol. Biotechnol. 70580-589. [DOI] [PubMed] [Google Scholar]
- 20.Hemesath, T. J., E. R. Price, C. Takemoto, T. Badalian, and D. E. Fisher. 1998. MAP kinase links the transcription factor microphthalmia to c-Kit signalling in melanocytes. Nature 391298-301. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch, H. M. 1954. Environmental factors influencing the differentiation of protoperithecia and their relation to tyrosinase and melanin formation in Neurospora crassa. Physiol. Plant. 772-97. [Google Scholar]
- 22.Hou, Z., C. Xue, Y. Peng, T. Katan, H. C. Kistler, and J.-R. Xu. 2002. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant-Microbe Interact. 151119-1127. [DOI] [PubMed] [Google Scholar]
- 23.Ivey, F. D., P. N. Hodge, G. E. Turner, and K. A. Borkovich. 1996. The G alpha i homologue gna-1 controls multiple differentiation pathways in Neurospora crassa. Mol. Biol. Cell 71283-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, C. A., S. E. Greer-Phillips, and K. A. Borkovich. 2007. The response regulator RRG-1 functions upstream of a mitogen-activated protein kinase pathway impacting asexual development, female fertility, osmotic stress, and fungicide resistance in Neurospora crassa. Mol. Biol. Cell 182123-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima, K., Y.-S. Bahn, and J. Heitman. 2006. Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology 152591-604. [DOI] [PubMed] [Google Scholar]
- 26.Kojima, K., T. Kikuchi, Y. Takano, E. Oshiro, and T. Okuno. 2002. The mitogen-activated protein kinase gene MAF1 is essential for the early differentiation phase of appressorium formation in Colletotrichum lagenarium. Mol. Plant-Microbe Interact. 151268-1276. [DOI] [PubMed] [Google Scholar]
- 27.Kraus, P. R., D. S. Fox, G. M. Cox, and J. Heitman. 2003. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 481377-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kupper, U., D. M. Niedermann, G. Travaglini, and K. Lerch. 1989. Isolation and characterization of the tyrosinase gene from Neurospora crassa. J. Biol. Chem. 26417250-17258. [PubMed] [Google Scholar]
- 29.Lee, K. S., K. Irie, Y. Gotoh, Y. Watanabe, H. Araki, E. Nishida, K. Matsumoto, and D. E. Levin. 1993. A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol. Cell. Biol. 133067-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerch, K. 1981. Metal ions in biological systems, vol. 13. Marcel Dekker Inc., New York, NY.
- 31.Levin, D. E. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69262-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, D., P. Bobrowicz, H. H. Wilkinson, and D. J. Ebbole. 2005. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 1701091-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maerz, S., C. Ziv, N. Vogt, K. Helmstaedt, N. Cohen, R. Gorovits, O. Yarden, and S. Seiler. 2008. The nuclear Dbf2-related kinase COT1 and the mitogen-activated protein kinases MAK1 and MAK2 genetically interact to regulate filamentous growth, hyphal fusion and sexual development in Neurospora crassa. Genetics 1791313-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall, C. J. 1994. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 482-89. [DOI] [PubMed] [Google Scholar]
- 35.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2751511-1519. [DOI] [PubMed] [Google Scholar]
- 36.Mehrabi, R., T. van der Lee, C. Waalwijk, and G. H. J. Kema. 2006. MgSlt2, a cellular integrity MAP kinase gene of the fungal wheat pathogen Mycosphaerella graminicola, is dispensable for penetration but essential for invasive growth. Mol. Plant-Microbe Interact. 19389-398. [DOI] [PubMed] [Google Scholar]
- 37.Metzenberg, R. L., and N. L. Glass. 1990. Mating type and mating strategies in Neurospora. Bioessays 1253-59. [DOI] [PubMed] [Google Scholar]
- 38.Mey, G., K. Held, J. Scheffer, K. B. Tenberge, and P. Tudzynski. 2002. CPMK2, an SLT2-homologous mitogen-activated protein (MAP) kinase, is essential for pathogenesis of Claviceps purpurea on rye: evidence for a second conserved pathogenesis-related MAP kinase cascade in phytopathogenic fungi. Mol. Microbiol. 46305-318. [DOI] [PubMed] [Google Scholar]
- 39.Meyer, V., R. A. Damveld, M. Arentshorst, U. Stahl, C. A. van den Hondel, and A. F. Ram. 2007. Survival in the presence of antifungals: genome-wide expression profiling of Aspergillus niger in response to sublethal concentrations of caspofungin and fenpropimorph. J. Biol. Chem. 28232935-32948. [DOI] [PubMed] [Google Scholar]
- 40.Navarrogarcia, F., M. Sanchez, J. Pla, and C. Nombela. 1995. Functional-characterization of the Mkc1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol. Cell. Biol. 152197-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nosanchuk, J. D., and A. Casadevall. 2003. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 5203-223. [DOI] [PubMed] [Google Scholar]
- 42.Pandey, A., M. G. Roca, N. D. Read, and N. L. Glass. 2004. Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot. Cell 3348-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raju, N. B. 1992. Genetic control of the sexual cycle in Neurospora. Mycol. Res. 96241-262. [Google Scholar]
- 44.Rast, D. M., D. Baumgartner, C. Mayer, and G. O. Hollenstein. 2003. Cell wall-associated enzymes in fungi. Phytochemistry 64339-366. [DOI] [PubMed] [Google Scholar]
- 45.Reinoso-Martin, C., C. Schuller, M. Schuetzer-Muehlbauer, and K. Kuchler. 2003. The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryot. Cell 21200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rojo, E., R. Martin, C. Carter, J. Zouhar, S. Pan, J. Plotnikova, H. Jin, M. Paneque, J. J. Sanchez-Serrano, B. Baker, F. M. Ausubel, and N. V. Raikhel. 2004. VPEgamma exhibits a caspase-like activity that contributes to defense against pathogens. Curr. Biol. 141897-1906. [DOI] [PubMed] [Google Scholar]
- 47.Roman, E., D. M. Arana, C. Nombela, R. Alonso-Monge, and J. Pla. 2007. MAP kinase pathways as regulators of fungal virulence. Trends Microbiol. 15181-190. [DOI] [PubMed] [Google Scholar]
- 48.Rui, O., and M. Hahn. 2007. The Slt2-type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization. Mol. Plant Pathol. 8173-184. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed., vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 50.Sanchez-Ferrer, A., J. N. Rodriguez-Lopez, F. Garcia-Canovas, and F. Garcia-Carmona. 1995. Tyrosinase: a comprehensive review of its mechanism. Biochim. Biophys. Acta 12471-11. [DOI] [PubMed] [Google Scholar]
- 51.Springer, M. L. 1993. Genetic control of fungal differentiation: the three sporulation pathways in Neurospora crassa. Bioessays 15365-374. [DOI] [PubMed] [Google Scholar]
- 52.Toda, T., S. Dhut, G. Superti-Furga, Y. Gotoh, E. Nishida, R. Sugiura, and T. Kuno. 1996. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 166752-6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toussaint, O., and K. Lerch. 1987. Catalytic oxidation of 2-aminophenols and ortho hydroxylation of aromatic amines by tyrosinase. Biochemistry 268567-8571. [DOI] [PubMed] [Google Scholar]
- 54.Turner, G. E., and K. A. Borkovich. 1993. Identification of a G protein alpha subunit from Neurospora crassa that is a member of the Gi family. J. Biol. Chem. 26814805-14811. [PubMed] [Google Scholar]
- 55.Valiante, V., T. Heinekamp, R. Jain, A. Hartl, and A. A. Brakhage. 2008. The mitogen-activated protein kinase MpkA of Aspergillus fumigatus regulates cell wall signaling and oxidative stress response. Fungal Genet. Biol. 45618-627. [DOI] [PubMed] [Google Scholar]
- 56.Vitalini, M. W., R. M. de Paula, C. S. Goldsmith, C. A. Jones, K. A. Borkovich, and D. Bell-Pedersen. 2007. Circadian rhythmicity mediated by temporal regulation of the activity of p38 MAPK. Proc. Natl. Acad. Sci. USA 10418223-18228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vollmer, S. J., and C. Yanofsky. 1986. Efficient cloning of genes of Neurospora crassa. Proc. Natl. Acad. Sci. USA 834869-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westergaard, M., and H. K. Mitchell. 1947. Neurospora. V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34573-577. [Google Scholar]
- 59.Xu, J.-R., C. J. Staiger, and J. E. Hamer. 1998. Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc. Natl. Acad. Sci. USA 9512713-12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, Y., R. Lamm, C. Pillonel, S. Lam, and J. R. Xu. 2002. Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 68532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao, X., Y. Kim, G. Park, and J. R. Xu. 2005. A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea. Plant Cell 171317-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao, X., R. Mehrabi, and J. R. Xu. 2007. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell 61701-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]