Abstract
The combined stresses of moderate heat shock (45°C) and analog-induced glucose deprivation constitute a lethal stress for Neurospora crassa. We found that this cell death requires fatty acid synthesis and the cofactor biotin. In the absence of the cofactor, the stressed cells are particularly sensitive to exogenous ceramide, which is lethal at low concentrations. When we extracted endogenous sphingolipids, we found that unique ceramides were induced (i) by the inhibitory glucose analog 2-deoxyglucose and (ii) by combined heat shock and 2-deoxyglucose. We determined that the former is a 2-deoxyglucose-modified ceramide. By structural analysis, we identified the latter, induced by dual stress, as C18(OH)-phytoceramide. We also identified C24(OH)-phytoceramide as a constitutive ceramide that continues to be produced during the combined stresses. The unusual C18(OH)-phytoceramide is not made by germinating asexual spores subjected to the same heat and carbon stress. Since these spores, unlike growing cells, do not die from the stresses, this suggests a possible connection between synthesis of the dual-stress-induced ceramide and cell death. This connection is supported by the finding that a (dihydro)ceramide synthase inhibitor, australifungin, renders cells resistant to death from these stresses. The OS-2 mitogen-activated protein kinase, homologous to mammalian p38, may be involved in the cell death signaling pathway. Strains lacking OS-2 survived the combined stresses better than the wild type, and phosphorylated OS-2 increased in wild-type cells in response to heat shock and combined heat and carbon stress.
The ability of organisms to tolerate and survive environmental stresses, such as high temperature, depends in part upon synthesis of proteins that prevent or assist recovery from cellular damage (55). The mycelial fungus Neurospora crassa, which normally grows at 30°C, produces heat shock proteins maximally at the nonlethal temperature of 45°C. These proteins allow cells to survive the otherwise lethal temperature of 50°C (58). However, cellular adaptation may fail in the presence of multiple stresses. N. crassa is unable to survive concurrent exposure to 45°C and carbohydrate deprivation (59). Cellular resistance to heat stress is energy intensive, both for stress-induced gene expression and for the ATP-requiring chaperone functions of heat shock proteins (23). Disruption of glucose metabolism and energy generation may impair the cellular response to heat stress, establishing an additional stress that becomes lethal.
We are interested in learning how cells detect this lethal stress and, if the resulting death is regulated, how this response is signaled. Early in this study we found that the death of cells subjected to a high temperature under glucose deprivation was likely a regulated event, since it was prevented when lipid biosynthesis was blocked by genetic mutation or by withholding the vitamin biotin, required for lipid synthesis. These observations suggested to us that a lipid molecule might be required as a signal for this dual-stress response. When we supplemented the biotin-lacking growth medium with various types of lipids, we found that low concentrations of sphingolipids were extremely lethal to these stressed cells.
Complex sphingolipids are important structural and regulatory components of cell membranes, where they aggregate into lipid rafts and associate with glycosyl-phosphatidylinositol (GPI)-anchored proteins (46). The simpler sphingolipids, such as sphingosine and ceramide and their phosphorylated derivatives, are known to act as signaling molecules that participate in such divergent processes as cell proliferation and apoptosis (22, 67). Ceramide differs structurally from sphingosine in having an amide link to a fatty acid, rather than a free amino group. It has been suggested that mammalian cells maintain a balance between sphingosine-1-phosphate and ceramide, with the former promoting cell proliferation and the latter signaling apoptosis (14). Although phytoceramide and phytosphingosine are the predominant sphingolipids in fungi, most fungi can also produce the mammalian types of ceramide and sphingosine, and their genomes encode the desaturase enzyme involved in their synthesis; an exception to this is Saccharomyces cerevisiae (72).
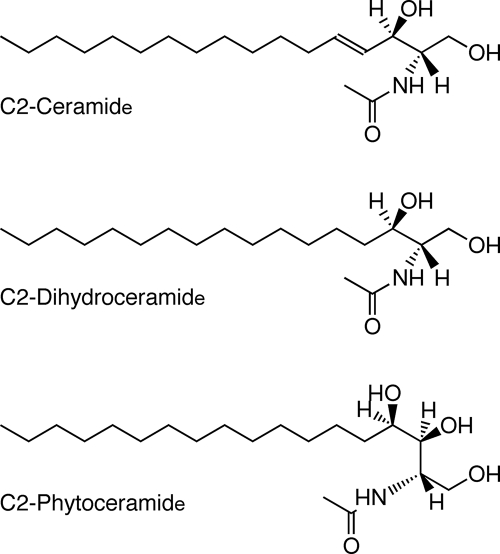
In fungi, there are two predominant pools of sphingolipids that have been characterized (17, 36, 75). The glycosylinositol-phosphorylceramides, which associate with GPI-anchored proteins, are modified phytoceramides. Phytoceramides differ from the common mammalian ceramides by having a hydroxyl group attached to C-4 of the sphingoid base, whereas the mammalian ceramides are desaturated at the sphingoid C-4 (50) (Fig. 1). In ascomycetous fungi, such as Neurospora, phytoceramides contain hydroxylated fatty acids of 24 or 26 carbons (referred to as very-long-chain fatty acids). The second sphingolipid pool in fungi consists of monohexose-modified ceramides that are methylated at C-9 and desaturated at C-8 as well as C-4 (78). These ceramides typically contain hydroxylated fatty acids of 16 to 18 carbons (long-chain fatty acids).
FIG. 1.
Structures of ceramides employed in experiments shown in Table 2.
In light of the strong effect of exogenous sphingolipids upon stressed Neurospora cells, we analyzed the profile of sphingolipids induced in cells by the stresses, applied individually or together. In analyzing cell extracts, we found that the profile was, indeed, altered by these stresses. Most notably, carbohydrate deprivation, induced by adding the inhibitory glucose analog 2-deoxyglucose (2-DG) to cells, produced a novel sphingolipid. A second novel sphingolipid was induced in 2-DG-treated cells that were transferred from the normal temperature of 30°C to the heat shock temperature of 45°C. We have identified the dual-stress-induced sphingolipid as a long-chain phytoceramide, and we propose that this molecule could signal cell death. We have also identified a constitutive sphingolipid that continues to be made actively during stress as a very-long-chain phytoceramide.
MATERIALS AND METHODS
Cell survival measurements.
For stress assays on solid medium, conidiospores were grown for 2 h at 30°C in liquid Vogel's medium (77) containing 0.05% glucose. Aliquots of spore suspensions were diluted 5,000-fold in 10% Vogel's medium (without glucose), and 50 μl was spread onto a plate with 25 ml solidified Vogel's medium containing 0.05% glucose and 0.015% 2-DG, which was placed in a protective chamber in a 45°C incubator. A minimum of three plates was used for each treatment. Sphingolipids were added from a 20 mM stock solution in ethanol. After ∼44 h at 45°C surviving colonies were counted. Where colonies were minuscule, due to growth inhibition, they were counted again after 24 h at 26°C. In the control treatment, Vogel's medium contained 0.05% glucose, 0.05% fructose, and 1% sorbose, and the plates were placed at 26°C until colonies were counted. For measuring survival in liquid medium, the original cultures were grown for 5 h at 30°C, at which time 2-DG was added to 0.015%, and they were transferred to a 45°C water bath with shaking. At hourly intervals, spore suspension aliquots were withdrawn and mixed with an equal volume of 0.4% trypan blue in Vogel's medium (without glucose). After 20 min, the number of spores that had accumulated trypan blue, relative to the total number of spores (>300) in the microscopic fields, was determined. Accumulation of the vital dye occurs in dead or compromised cells (25). All experiments were repeated two or more times, and the data cited are typical results.
Mutant strains, chemicals, and inhibitors.
The N. crassa mutant cel-1 (24), suc (4), and os-2 (81) strains were derived by traditional mutagenesis techniques and provided by the Fungal Genetics Stock Center (48). The Δos-2 (NCU07024, FGSC 17933), Δgcs (NCU01116, FGSC 13794), and Δdes (NCU08927, FGSC 15707) strains were gene deletion knockout strains, constructed by the Neurospora Genome Project (13) and provided by the Fungal Genetics Stock Center. Sphingolipids were from Sigma-Aldrich, Avanti Polar Lipids, and Matreya. Myriocin was from Sigma-Aldrich, and australifungin was kindly provided by Merck.
Radiolabeling and lipid extraction of cells.
[3H]palmitic acid (Amersham), [3H]2-DG (MP Biochemicals), and [3H]glucose (ARC) were added (1 μC/ml) to liquid cultures at 30°C or 15 min after their transfer to 45°C, and the cultures were harvested by filtration after an additional 15 min. [3H]serine (Amersham), at 1 μC/ml, was present during the first 60 min of stress. Lipids were extracted according to the protocol of Sullards and Merrill (70), with modifications. Washed cells were extracted twice in methanol-chloroform (2:1, vol/vol) by vortexing with 0.1-mm glass beads. The combined supernatants were alkalized with methanolic potassium hydroxide (0.1 M) and incubated at 37°C to hydrolyze glyceride ester bonds. The supernatant was neutralized with acetic acid and extracted with water-chloroform (2:1). The organic layer was dried and resuspended in a small volume of chloroform-methanol (3:1), which was stored at −20°C until use.
Fractionation of lipids by solid phase extraction.
Liquid cultures were radiolabeled, as described above, or subjected to treatment for 1 to 2 h without radiolabel. To optimize for ceramides, the washed, harvested mycelia were extracted with hexane-ethanol (95:5) by vortexing twice with glass beads (21). The combined supernatants were dried, and solids were dissolved in chloroform and applied to a preconditioned Sep-Pak (Waters) solid-phase extraction (SPE) silica column (63). After a chloroform wash, ceramides were eluted with chloroform-methanol (98:2), followed by chloroform-methanol (95:5), and glycolipids were eluted with chloroform-acetone (1:1). The identified phytoceramides eluted in the second (95:5) ceramide subfraction.
Thin-layer chromatography (TLC).
The lipid samples in chloroform-methanol (3:1) and sphingolipid standards were applied to silica gel GHL plates (Analtech), which were developed with chloroform-methanol-2 N ammonium hydroxide (40:10:1) solvent (7). The separated sphingolipid standards or cellular lipids were visualized under long-wave UV light by spraying with a solution of 0.1% primuline (Sigma-Aldrich) in 80% acetone; radioactive lipids were sprayed with the fluorography reagent En3Hance (Perkin-Elmer) for film development.
Isolation of lipids.
UV-fluorescing spots, corresponding to separated lipids, were scraped from the TLC plate, and the silica gel was extracted once with chloroform-methanol (2:1) and three times with chloroform-methanol (1:1) in glass tubes. Extraction was performed in a sonicating water bath combined with vortexing. The supernatants, containing the lipids, were dried with nitrogen, and the dried material was stored at −20°C prior to analysis.
+ESI-IT-MS of underivatized lipids.
Positive-ion-mode electrospray-ionization mass spectrometry (+ESI-IT-MS1 and -MS2) spectra of lipid fractions were acquired on a linear ion trap mass spectrometer (LTQ; ThermoFinnigan). Samples were introduced by direct infusion in methanol, both with and without addition of LiI, as described previously (1, 26, 38). Nominal, monoisotopic m/z values are used in the labeling and description of +ESI-MS results. Interpretation of spectra derived from [M+Na]+ and [M+Li]+ adducts of phytoceramides was essentially as described previously for the ceramide moieties of fungal glycosylceramides and glycosylinositol phosphorylceramides (38, 74).
Fatty acid and sphingoid component analysis by GC-MS.
Following acid-catalyzed methanolysis of native samples, ceramide-derived sphingoid bases and 2-hydroxy fatty acids were detected as their N-acetyl-per-O-trimethylsilyl and 2-O-trimethylsilyl methyl ester derivatives, respectively. All derivatives were prepared and analyzed by gas chromatography (GC)-MS according to protocols described previously (37). The instrument used was a GCQ (Finnigan MAT) operated in electron ionization mode.
Gel electrophoresis and Western blotting.
Collected cells were vortexed with glass beads in double-strength Laemmli sample buffer (35) and heated to 95°C for 5 min; the pellet was washed with sample buffer and vortexed again. After storage at −20°C, the combined supernatants were heated, cleared by centrifugation, and applied to a sodium dodecyl sulfate-10% polyacrylamide gel (44). After protein separation, the gel was rinsed three times in transfer buffer, and proteins were transferred electrophoretically to a pre-equilibrated nitrocellulose membrane (Protran) in Tris-glycine buffer (pH 8.3) with 15% methanol (76). After drying, the membrane was blocked with 5% dry milk in Tris-buffered saline-Tween 20 (TBST) and reacted 1:1,000 with anti-phospho-p38 primary antibody (Cell Signaling) in 5% bovine serum albumin-TBST at 4°C overnight, followed by 1:15,000 horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch) in 5% milk-TBST. Detection was with SuperSignal (Pierce) and X-ray film. Protein concentration was assayed by the Bio-Rad RC DC assay, and blotted proteins were visualized by amido black stain.
RESULTS
Cell survival assays.
Neurospora spores germinate and grow into a confluent mycelial mat on agar medium. However, if cell wall extension is partially inhibited by a nonmetabolizable glucose analog such as l-sorbose (9) or, transiently, by a high temperature, the individual spores develop into discrete, compact colonies that can be counted. Earlier, we quantified cell survival at high temperature with and without the addition of sorbose (58). We found that 90% of cells survived long-term exposure (40 h) to 45°C, but sorbose addition (0.5%) reduced survival two- to threefold (59). Even more dramatic is the effect of adding small amounts of 2-DG (0.015%), an alternative glucose analog that strongly inhibits glycolysis; only 0 to 5% of cells survived extended exposure to 45°C (Table 1). These findings indicated that the dual stress of high, sublethal temperature combined with restricted carbohydrate availability becomes lethal to cells.
TABLE 1.
Effects of biotin deprivation upon the survival of dual-stressed cells
| Strain or mutation | Percent survival (no. of colonies) in mediuma:
|
No. of colonies in controlb | |
|---|---|---|---|
| With biotin | Without biotin | ||
| Wild type | 1 (1 ± 1) | 83 (55 ± 2.1) | 66 ± 9.2 |
| cel-1 | 59 (18 ± 2.4) | 55 (16 ± 2.5) | 30 ± 5.9 |
| succ | 10 (9 ± 3) | 43 (36 ± 8) | 84 ± 15.3 (wt) |
| os-2 | 49 (22 ± 6.1) | 62 (27 ± 3.8) | 44 ± 5.1 |
| Δos-2 | 80 (43 ± 10.3) | ND | 54 ± 6.3 |
| Δdes | 0 (0) | ND | 32 ± 2.1 |
Number of colonies in treatment/number of colonies in control × 100. ND, not determined.
Number of colonies that developed at 26°C.
The glucose analog was 0.75% sorbose. wt, wild type.
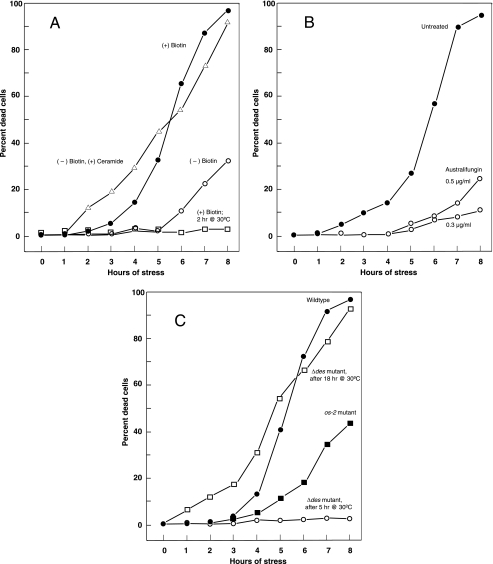
To understand this phenomenon better, we adjusted various components of the growth medium, to test whether they might enhance or diminish survival during this dual stress. When the one essential vitamin, biotin, was omitted from the medium (77), approximately 80% of cells on solid medium survived long-term exposure to the dual stress (Table 1), suggesting that biotin was required for cell death. Since long-term assays on solid medium can be affected by the rate of mycelial growth, we also assayed the effect of dual stress on cells over a shorter period in liquid culture, using a vital dye to report inviability. Whereas almost all cells in complete medium were dead (96%) after 8 h of dual stress, only 33% of cells in biotin-deficient medium were dead after 8 h (Fig. 2A).
FIG. 2.
Kinetics of cell death at 45°C in liquid minimal medium containing 0.015% 2-DG. The retention of the vital stain trypan blue was interpreted as an indication of death. (A) Cell death in complete minimal medium, compared with death when biotin was omitted from the medium. Addition of 10 μM ceramide to biotin-deficient medium restores the steep death curve. The high rate of survival of activated spores (2 h) in liquid medium, when subjected to the same conditions of stress, contrasts with the death of 5-h growing cells. (B) Protective effect on stressed cells of adding australifungin, an inhibitor of (dihydro)ceramide synthase, to the medium. (C) Response of mutant os-2 and Δdes strains to stress conditions, compared with the wild type. The os-2 strain is more resistant to the stresses than the wild type. The Δdes strain (18 h) responds like the wild type, but since it is slow growing, 5-h cultures appear comparable to 2-h cultures of the wild type (compare panel A).
Biotin has multiple biochemical roles in cells, functioning as a cofactor for enzymes involved in carboxyl group transfer (52). Two major roles are (1) regeneration of tricarboxylic acid cycle intermediates through pyruvate carboxylase and propionyl coenzyme A (propionyl-CoA) carboxylase and (2) fatty acid synthesis through acetyl-CoA carboxylase. To differentiate between these two possible roles for biotin in stress-induced death, we tested survival of two mutant strains of Neurospora; one of these strains is defective in pyruvate carboxylase and the other in fatty acid synthase. When exposed to high temperatures in the presence of sorbose, the suc strain, defective in pyruvate carboxylase (4), survived at a fourfold-higher level in biotin-deficient medium (43%) than in complete medium (10%), thus displaying the same biotin-dependent death as the wild type (Table 1). In this case, the wild-type strain was used as the 26°C control, since the suc strain did not grow at 26°C without addition of succinate as an energy source. Furthermore, when succinate, a tricarboxylic acid cycle intermediate, was added to wild-type cells during dual stress in the absence of biotin, it did not reduce the cells' enhanced survival (data not shown). These results make it highly unlikely that biotin acts to promote death as a cofactor to either pyruvate carboxylase or propionyl-CoA carboxylase, which is in the pathway of succinate synthesis.
A fatty acid synthase α subunit cel-1 mutant (24) was similarly tested for stress survival. When cells were exposed to the dual stress of high temperature and a glucose analog, this strain survived at rates of 59% in complete medium and 55% in biotin-deficient medium (Table 1). Therefore, unlike the wild-type and suc strains, the cel-1 strain displayed stress resistance that did not vary with biotin addition. This result suggests that it is the role of biotin in fatty acid synthesis that is crucial for the stress-induced cell death.
To determine which types of lipid might be crucial for cell death, we added various fatty acids and sphingolipids to biotin-deficient solid medium, since these classes of lipids have been reported to be toxic to mammalian cells or involved in apoptosis (34, 41). We decided to focus our analysis on sphingolipids (Fig. 1), which killed stressed cells at very low concentrations, ranging from 5 to 20 μM. As shown in Table 2, the most striking effects were produced by C2-ceramide and sphingosine, which were lethal at 5 μM and 10 μM, respectively. C2-phytoceramide was lethal at 20 μM, while 10 μM reduced colony size and decreased survival of stressed cells to 45%. C2-dihydroceramide, phytosphingosine, and dihydrosphingosine, at these concentrations, had little or no effect upon survival of these cells. These results indicate that exogenous ceramide is the most toxic of these simple sphingolipids for stressed cells. Furthermore, ceramide and phytoceramide were approximately twice as potent as their counterpart sphingosines. These low concentrations of ceramides had minimal effects under normal growth conditions in these solid-medium assays (data not shown).
TABLE 2.
Effects of sphingolipids upon the survival of dual-stressed cells in the absence of biotin
| Lipid | Percent survival (no. of colonies) with sphingolipid concn (μM)a
|
No. of control coloniesb | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 10 | 20 | ||
| C2-ceramide | 89 (71 ± 3.2) | 72 (58 ± 7) | 0 (0) | 0 (0) | 0 (0) | 80 ± 4.9 |
| C2-phytoceramide | 72 (64 ± 14.6) | 81 (72 ± 8) | 70 (62 ± 7.5) | 45 (40 ± 7.5) | 0 (0) | 89 ± 8.9 |
| C2-dihydroceramide | 72 (64 ± 14.6) | ND | 82 (73 ± 10.7) | 85 (76 ± 9.6) | ND | 89 ± 8.9 |
| Sphingosine | 84 (67 ± 9.1) | ND | 44 (35 ± 11.5) | 0 (0) | ND | 79 ± 3.2 |
| Phytosphingosine | 81 (63 ± 5.1) | ND | ND | 82 (64 ± 9.1) | 60 (47 ± 12) | 78 ± 1.5 |
| Dihydrosphingosine | 84 (67 ± 9.1) | ND | 79 (63 ± 9.3) | 93 (74 ± 5.5) | 58 (46 ± 3.5) | 79 ± 3.2 |
Number of colonies in treatment/number of colonies in control × 100. ND, not determined.
Number of colonies that developed at 26°C.
Cells stressed in liquid medium by heat shock and 2-DG are also extremely sensitive to C2-ceramide (Fig. 2A). Whereas only one-third of the biotin-deprived cells had died by 8 h of stress, 91% of the cells supplemented with 10 μM C2-ceramide had died, a response similar to that of cells supplied with biotin in complete medium. Supplementation with 20 μM C2-ceramide led to 96% cell death at 4 h of stress (data not shown).
Radiolabeling and analysis of endogenous lipids.
The lethality of exogenous ceramide suggested that a related endogenous ceramide might be essential to stress-induced death. To detect endogenous sphingolipids, we added [3H]palmitic acid to cells for a 15-min interval and prepared the radiolabeled lipid fractions, optimized for sphingolipids, from cells exposed to four different conditions: (i) normal temperature of 30°C, (ii) 30°C plus 2-DG, (iii) heat shock temperature of 45°C, and (iv) 45°C plus 2-DG. Therefore, cells were exposed either to no stress, to one of the two stresses, or to two concurrent stresses. The lipids in these extracts were separated by TLC and visualized by fluorography.
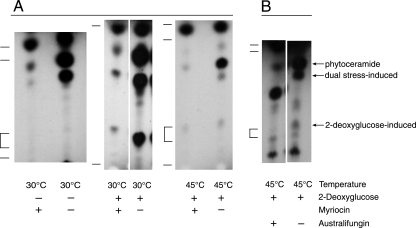
The lipids produced by cells exposed to either a single or double stress are shown in Fig. 3. The five lipids from 30°C cells were detected by their incorporation of both [3H]palmitate and [3H]serine, the two substrates of serine palmitoyl transferase, the committed step of sphingolipid biosynthesis. One of these lipids, comigrating with cerebrosides (glycosylated ceramides), was seen only when 2-DG is added to the cells (Fig. 4A). Heat-shocked cells exposed to 2-DG produce a novel lipid that is not made by cells experiencing only temperature or carbon stress. To gauge whether these extracted lipids were indeed sphingolipids, we added myriocin, an inhibitor of serine palmitoyl transferase (51), to fungal cultures under normal and stress conditions. Figure 4A shows that cells grown at 30°C or exposed to 45°C appear to produce the same lipids (although in different relative amounts) and that two of the three constitutive lipids shown are inhibitable by myriocin and therefore likely de novo-synthesized sphingolipids. The novel lipids induced at 30°C and 45°C by adding 2-DG are also inhibited by myriocin (Fig. 4A).
FIG. 3.
Lipids extracted from growing cells exposed to either heat shock (45°C) or 2-DG or to both stresses together, with 30°C being the normal growth temperature. The lipids were separated by TLC, and index marks to the left of each fluorogram denote migration positions of sphingolipid standards in descending order: C18-ceramide, C18-phytoceramide, human glucocerebrosides, and sphingosine. [3H]palmitate (1 μCi/ml) was added at 60 min of stress, and the cells were harvested for extraction after an additional 15 min. [3H]serine (1 μCi/ml) was added at the time of stress, and the cells were harvested after an additional 60 min.
FIG. 4.
(A) Addition of myriocin, which inhibits sphingolipid synthesis; (B) addition of australifungin, which inhibits ceramide synthesis. Myriocin (50 nM) was added 10 min prior to stress treatment, and australifungin (0.5 μg/ml) or methanol solvent was added 30 min prior to stress. The lipids were separated by TLC, and index marks to the left of each fluorogram denote migration positions of sphingolipid standards in descending order: C18-ceramide, C18-phytoceramide, human glucocerebrosides, and sphingosine. Cellular lipids were radiolabeled for 15 min with [3H]palmitate under normal conditions or at 15 min after single stress (2-DG) or dual stress (45°C and 2-DG). This supports the identification of three lipids as ceramides. The identity of the lipid increased upon australifungin addition is not known.
Australifungin is an inhibitor of dihydroceramide synthase (45), which N-links fatty acids from acyl-CoA to sphingoid bases; it thereby blocks synthesis of all ceramides but not that of sphingoid base precursors. To further characterize the sphingolipids we detected, we tested whether they were produced in the presence of australifungin (Fig. 4B); we found that [3H]palmitate incorporation was conspicuously reduced in three of the sphingolipids that were also affected by myriocin, including the novel lipids induced by 2-DG alone and by 2-DG coupled with heat shock (dual stress). This indicates that the induced sphingolipids are de novo-synthesized ceramides.
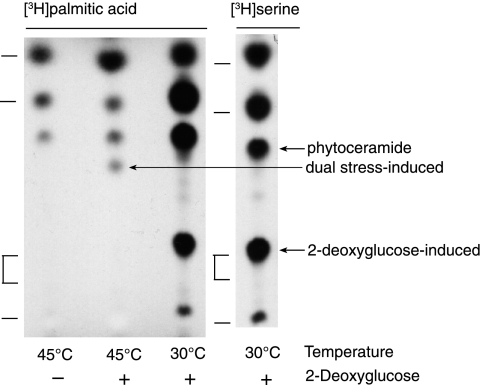
Since 2-DG induces synthesis of a sphingolipid that comigrates with cerebroside, we provided cells with [3H]2-DG, added before or in the absence of unlabeled 2-DG to avoid competition (Fig. 5). The [3H]2-DG was incorporated into two lipids; the lower comigrates with the 2-DG-induced sphingolipid that incorporates [3H]palmitate, suggesting that it is a 2-DG-modified ceramide, and the upper has been structurally identified as ergosterol-deoxyglucoside (described below). Interestingly, incorporation of [3H]glucose was blocked by coadministration of 2-DG, but in its absence, heat-shocked cells incorporated [3H]glucose into a lipid that migrated slightly below the 2-DG-modified ceramide (Fig. 5). Although positive identification of the 2-DG-modified ceramide awaits its isolation and structural analysis, we found that it was absent or strongly reduced, according to [3H]palmitate labeling, in the Δgcs mutant strain, which lacks the putative glucosyl-ceramide synthase (36) (Fig. 5). In vivo substitution of 2-DG for glucose in sphingolipids was reported earlier for inositol-containing sphingolipids (68). These experiments together suggest that glucose and 2-DG may modify the same or related sphingolipids, but these modifications may not occur or may be extremely transient, due to further modifications, under nonstress conditions.
FIG. 5.
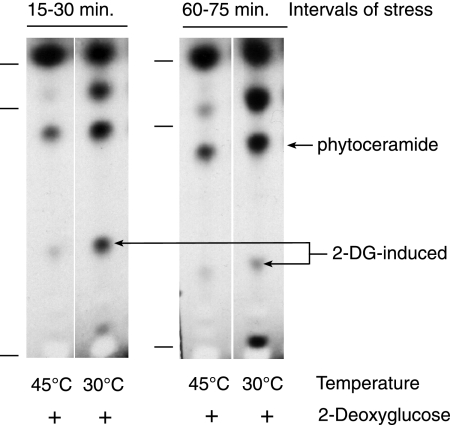
Glucose and 2-DG modification of lipids in stressed cells. [3H]glucose (1 μCi/ml) or [3H]2-DG (1 μCi/ml) was provided to cells between 15 and 30 min after transfer to 45°C. Wild-type and Δgcs cells were radiolabeled with [3H]palmitic acid for 15 min, either at 30°C or beginning 15 min after transfer to 45°C; 2-DG was added 15 min prior to radiolabel. Little or none of the 2-DG-modified ceramide is made by Δgcs cells, but there is strong synthesis of the dual-stress-induced C18(OH)-phytoceramide. Lipids were separated by TLC, and C18-ceramide, C18-phytoceramide, cerebrosides, and sphingosine (descending order) were standards (indicated by index marks). For alignment, all the sample lanes were run together on a single TLC plate, but the left panel ([3H]glucose) was substituted from a similar fluorogram.
The most interesting finding is that cells exposed concurrently to heat shock and 2-DG incorporate [3H]palmitate into a unique ceramide that is not evident under normal or nonlethal stress conditions. This lipid begins to be produced during an early radiolabeling interval, 15 to 30 min of dual stress (Fig. 4), and it continues to be synthesized during the second hour of stress (Fig. 3), when synthesis of the 2-DG-modified ceramide is discontinued by heat-shocked cells.
Synthesis of stress-induced ceramide and cell death.
Of particular interest is the correlation between induction of this particular ceramide by dual stress and the lethality of dual stress. Concurrent exposure to heat shock and 2-DG, by itself, does not cause all cells to produce this ceramide, since synthesis is influenced by the developmental stage of a fungal culture. The cells we assayed are derived from spores 5 h after their activation, and they experience 96% death after an additional 8 h of stress in liquid culture (Fig. 2A). In contrast, spores that have been activated for only 2 h but are susceptible to lethal heat stress (58) do not die from exposure to dual stress. Even after 8 h of exposure to 45°C and 2-DG, they showed no death (Fig. 2A). These resistant 2-h-activated spores have an induced lipid profile at 45°C (Fig. 6) that resembles that of cells at 30°C (Fig. 3 and 4); they do not incorporate [3H]palmitate into the dual-stress-induced ceramide, nor do they discontinue making the 2-DG-induced ceramide in the second hour of stress (60 to 75 min).
FIG. 6.
Lipids made by activated 2-h spores incubated at 30°C or 45°C in the presence of 2-DG. Radiolabeling with [3H]palmitate was either between 15 and 30 min after the applied stresses or between 60 and 75 min. Lipids were separated by TLC, and sphingolipid standards (descending) were C18-ceramide, C18-phytoceramide, and sphingosine. Lipid synthesis by dual-stressed spores is similar in the early and late labeling intervals, in contrast to that by stressed 5-h cells.
Why do cells that are vulnerable to dual stress synthesize this unique ceramide? One possibility is that the ceramide may be a signaling component in a cell death pathway. Alternatively, the ceramide may be an early, aberrant by-product of the combined stresses that is irrelevant to cell death. To help differentiate between these possibilities, we added australifungin to 5-h cells exposed to the high temperature and 2-DG, to inhibit ceramide synthesis (45). If a newly synthesized, unique ceramide promotes cell death, the addition of australifungin should help cells survive. Remarkably, a low dose of australifungin (0.3 μg/ml) slowed the kinetics of stress-induced death, so that only 10% of the cells had died by 8 h of stress, whereas 94% of the uninhibited cells had died by this time (Fig. 2B). A slightly higher dose of australifungin (0.5 μg/ml) had a similar but less dramatic effect of 26% death at 8 h, possibly due to general detrimental effects of blocked ceramide synthesis. This experiment was repeated twice with similar results. Therefore, moderately inhibiting ceramide synthesis appears to be optimal for rescuing stressed cells, suggesting that a newly synthesized ceramide contributes to cell death.
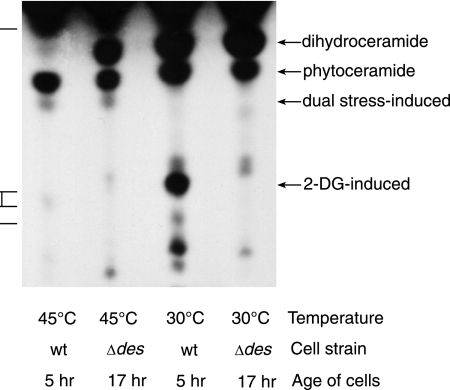
Most fungi, including Neurospora, have the same classes of ceramides (72), based on sphingoid composition, as do other eukaryotes. Dihydroceramide is a precursor to ceramide, by desaturation at C-4 and other possible modifications, and to phytoceramide, by hydroxylation at C-4 (Fig. 1). The strikingly lethal effect of exogenous ceramide suggested that the stress-induced type of ceramide would likely be a desaturated ceramide. To test this possibility we employed the mutant Neurospora Δdes strain (13), which has a deletion of the dihydroceramide delta(4)-desaturase gene. This strain should make dihydroceramide and phytoceramide but not desaturated ceramide. When we added [3H]palmitate to cells of the Δdes strain under dual-stress conditions, we found that they produced the 2-DG-modified ceramide, but the dual-stress-induced lipid was not evident (data not shown). Since the Δdes strain grows very slowly and synthesis of the stress-induced ceramide in the wild type depends on sufficient prior growth, we grew the Δdes cells for a longer period of time, 17 h, during which they formed long unbranched germ tubes. When these older Δdes cells were subjected to high temperature and 2-DG, they incorporated [3H]palmitate into the dual-stress-induced ceramide (Fig. 7), which indicates that this ceramide could not be a delta(4)-desaturated ceramide.
FIG. 7.
Lipids made by stressed wild-type or Δdes cells, radiolabeled with [3H]palmitate between 15 and 30 min of stress. 2-DG was added to all cells. Wild-type cells were grown for 5 h before treatment, whereas the slow-growing Δdes cells were grown for 17 h before stress. Although the C18(OH)-phytoceramide was not made by 5-h dual-stressed Δdes cells (not shown), it was made by 17 h dual-stressed Δdes cells. Standards (descending order) are C18-phytoceramide, glucocerebrosides, and sphingosine.
If an endogenous desaturated ceramide did contribute to Neurospora cell death, we would expect enhanced survival of the Δdes strain under dual stress. Therefore, we measured survival of Δdes cells that were stressed at earlier and later times in development (Fig. 2C). We found that the younger (5-h) cells do not die after being exposed to 45°C and 2-DG for an additional 8 h; this resembles the response of 2-h wild-type cells (Fig. 2A). However, after growing for 18 h, more than 90% of Δdes cells were dead after 8 h of stress, confirming solid plate assays (Table 1), showing that a desaturated ceramide is not required for cell death.
Identification of induced and constitutive ceramides made by stressed cells.
Our aim was to identify the dual-stress-induced ceramide. Since it is present in cell extracts at low levels, not being constitutively made, we also isolated chromatographically adjacent lipids, which could be contaminants in the induced ceramide fraction. The faster-migrating adjacent lipid (Fig. 3) is a ceramide that is constitutively made and continues to be strongly produced by dual-stressed cells. The slower-migrating lipid stained strongly with primuline but did not incorporate [3H]palmitate. A lipid extract of stressed cells was fractionated by silica SPE columns, the relevant ceramide-containing fraction was further fractionated by silica TLC, and the desired bands were excised from the plate. Lipids in the three fractions were eluted from the silica, dried under nitrogen, and subjected to MS-MS and GC-MS analysis.
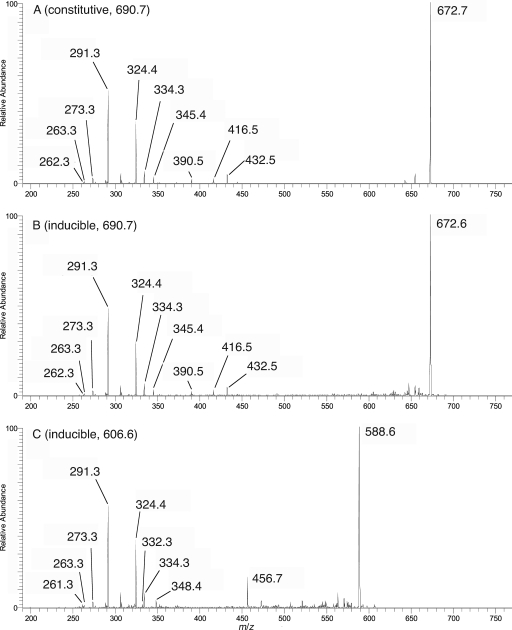
The +ESI-MS1 profile spectrum of the constitutive ceramide fraction, subjected to lithiation (Fig. 8A), exhibited a major molecular adduct at m/z 690, consistent with a phytoceramide composed of either h24:0 fatty acid and t18:0 phytosphingosine or h22:0 fatty acid and t20:0 phytosphingosine. One of the minor ions, m/z 618, is consistent with a lithiated ceramide composed of h18:1 fatty acid and d19:2 sphingosine, which is generally found in glucosylceramide of N. crassa (54) and many other fungi. The +ESI-IT-MS1 profile spectrum of the untreated sample (not shown) exhibited an essentially identical profile, with all molecular adducts shifted m/z 16 higher (the difference in mass between Na+ and Li+). To analyze these variants further, +ESI-IT-MS2 spectra were acquired from all significant precursors in the lithium adduct profile, since Li+ ion facilitates fragmentation. The MS2 spectrum of the major m/z 690 adduct (Fig. 9A) yielded, along with a major dehydration product m/z 672, characteristic fragments (38) at m/z 291, 306, 324, and 334, consistent with t18:0 sphingoid exclusively. By extension, this must be linked to an h24:0 fatty acid, consistent with the appearance of a W fragment (= acyl C2-Cω) at m/z 345. Similar MS2 analyses of the m/z 618 ion (data not shown) confirmed its identity as h18:1/d19:2 ceramide
FIG. 8.
ESI-linear-ion-trap-MS profile spectra of lithium-adducted ceramide-containing lipid fractions. (A) Constitutive ceramide fraction. The major ion is at m/z 690, and minor ions (3 to 9% relative abundance) were observed at m/z 618 and at m/z 662, 676, 704, and 718, which are consistent with phytoceramides differing from the major molecular species by the number of CH2 units. Odd m/z adduct ions are most likely diacylglycerol lipid components. (B) Inducible ceramide fraction. Even m/z values indicate ceramide MLi+ adduct ions confirmed by collision-induced-dissociation-MS2 analysis; odd m/z values indicate confirmed nonceramide (probably phospholipid) MLi+ adduct ions. The asterisk indicates a residual MNa+ adduct from the major ceramide component (m/z 16 increment from m/z 690).
FIG. 9.
ESI-linear-ion-trap-MS2 product ion spectra of lithium-adducted ceramide precursors selected from profiles in Fig. 8. Similar MS2 analyses of minor ions yielded phytoceramides (data not shown).
The +ESI-MS1 profile spectrum of the inducible ceramide (Fig. 8B) was noisy; however, after addition of lithium ion, two low-level signals consistent with Li+-adducted phytoceramides appeared, one at m/z 690 and the other at m/z 606. An MS2 spectrum of the former (Fig. 9B) was virtually identical to that obtained previously for the constitutive ceramide of the same m/z, again indicating an h24:0/t18:0 ceramide composition. Interestingly, the m/z 606 Li+ adduct yielded an MS2 product spectrum (Fig. 9C) that was almost identical with respect to the major fragments in the lower m/z range; however, among the minor fragments, a number of differences were observed, e.g., the absence of the W ion at m/z 345. This is consistent with the same sphingoid, t18:0, linked instead to an h18:0 fatty acid. A W ion consistent with this fatty acid can be observed in the spectrum at m/z 261 (decrement of m/z 84, corresponding to six CH2 units). Some other products appearing in the m/z 690 product spectrum (Fig. 9B) appeared to be either absent or decremented by m/z 84 in the m/z 606 spectrum, e.g., m/z 672 → 588, m/z 432 → 348, m/z 416 → 332, and m/z 390 → 306 (isobaric with another product appearing in both spectra). These ions have not yet been characterized, but they must include the h18:0 fatty-N-acyl group. An ion appearing at m/z 456, for which no correlate was observed in the h24:0/t18:0 ceramide spectrum, has not been assigned.
Confirmation of these results was provided by composition analysis, using GC-MS, after methanolysis of the lipid samples and separate derivatization of their sphingoid and fatty-N-acyl components (data not shown). The identities of all detected components were confirmed by both their GC retention times and their electron ionization-MS spectra, compared with authentic standards. Both the constitutive and inducible ceramide samples yielded t18:0 phytosphingosine (as its N-acetyl, tri-O-trimethylsilyl derivative) as the only detectable sphingoid. In the fatty acid analysis, the constitutive ceramide sample yielded mainly h24:0 fatty acid (89%, as its methyl ester); small amounts of h26:0, h23:0, h22:0, h18:0, and h16:0 fatty acids were also detected (2%, 5%, 3%, 2%, and <2%, respectively). In the fatty acid analysis of the inducible ceramide sample, fatty acid methyl esters were detected mainly for h24:0 and h18:0, in an approximately 1:1 ratio (49% and 48%, respectively); traces (<1%) of h23:0, h22:0, and h16:0 fatty acids were also detected. These results confirm the identity of the constitutive ceramide as C24(OH)-phytoceramide (h24:0/t18:0) and the identity of the dual-stress-induced ceramide as C18(OH)-phytoceramide (h18:0/t18:0).
The C24(OH)-phytoceramide is a common fungal ceramide (72), but a C18(OH)-phytoceramide has not, to our knowledge, been detected in ascomycetous fungi. Typically, a hydroxylated C18 fatty acid would be incorporated into the desaturated ceramide; in fungi, this is additionally modified by C-8 desaturation and C-9 methylation of the sphingoid, variable C-3 desaturation of the fatty acid, and glucosylation (36, 54). A trace of residual h18:1/d19:2 ceramide was detected in the constitutive lipid fraction.
Characterization of adjacent nonceramide lipid.
In a subsidiary analysis, the +ESI-MS1 profile spectrum (data not shown) of nonceramide lipid from dual-stressed cells exhibited a major Na+ adduct ion, m/z 565, consistent with a sterol glycoside composed of ergosterol linked to a deoxyhexose. Key fragments produced in an MS2 spectrum of the m/z 565 parent included (i) a pair of products, m/z 187 (base peak) and 169, consistent with cleavages on either side of the glycosidic oxygen, producing the sodium adduct of a deoxyhexose and its corresponding dehydrated fragment, respectively; (ii) a product ion at m/z 379, consistent with loss of sodiated deoxyhexose from ergosterol, with back transfer of a proton; and (iii) m/z 253, consistent with neutral loss of the C20-28 side chain from the deglycosylated ergosterol nucleus (m/z 379 − C9H18). The production of unmetalated sterol product ions from sodium-adducted sterol glycoside parents has been reported previously (5). Following addition of LiI, a corresponding Li+ adduct ion was observed for this fraction in the +ESI-MS1 profile at m/z 549, and a corresponding deoxyhexose product ion was observed in the MS2 spectrum from the m/z 549 parent at m/z 171 (data not shown). The putative ergosterol product ions were observed unshifted at m/z 379 and 253.
Ergosterol-β-glucoside was previously identified as a metabolic product of N. crassa, the expression of which increased in defensin-resistant mutant strains (54) and is reported to be induced in fungi by stress (65). Since the cells in the present study were deprived of glucose and treated with 2-DG, it seemed likely that the nonceramide lipid could be ergosterol-2-deoxyglucoside. This was confirmed by acquisition of a 1-D 1H nuclear magnetic resonance spectrum (dimethyl sulfoxide-d6-2% D2O; 25°C). Compared with data previously obtained for ergosterol-β-glucoside from N. crassa (54), resonances corresponding to ergosterol were observed at essentially identical chemical shifts and with identical coupling patterns. In contrast, resonances corresponding to the monosaccharide residue appeared with coupling patterns characteristic of a β-2-deoxyglucoside spin system, shifted as expected from comparisons with published data (6), allowing for the influence of different solvent systems. These resonances included a downfield-shifted H-1 at 4.61 ppm (3J1,2eq ≈ 2 Hz; 3J1,2ax ≈ 10 Hz), which appears at 4.25 ppm (3J1,2a ≈ 8.0 Hz) in the spectrum of ergosterol-β-glucoside. This indicates that the nonceramide lipid is ergosterol-β-2-deoxyglucoside.
Involvement of a stress MAP kinase.
The stress-related mitogen-activated protein (MAP) kinases of mammals, JNK and p38, are known to contribute to cell death signaling. Of the four p38 isotypes (3), the α form particularly is reported to participate in apoptotic signaling and in autophagy, two types of programmed death (60, 71). Depending on inducing conditions and cell type, p38 may have either pro- or antiapoptotic effects (2, 71). In addition, p38 MAP kinase is activated by free fatty acids or low glucose concentrations to stimulate gluconeogenesis and inhibit lipogenesis (12, 80). Given the involvement of p38 in both programmed cell death and glucose sensing, we asked if its homolog in Neurospora might influence the dual-stress-induced death we observed. OS-2 is the Neurospora ortholog of S. cerevisiae Hog1 and mammalian p38 MAP kinase (81). Like hog1 mutants, os-2 mutant strains are particularly sensitive to osmotic stress, and OS-2 is activated by high salt concentrations (28). When we assayed the dual-stress resistance of an os-2 mutant that produces a truncated, nonfunctional protein, we found that this strain survived exposure to high temperature and 2-DG much better than the wild type, with 49% of cells surviving on solid medium, compared with 1% survival of the wild type (Table 1). The gene deletion mutant Δos-2 showed even stronger resistance to dual stress, with an 80% survival rate (Table 1). In growth medium that lacked biotin, the os-2 strain showed no advantage over the wild type. The enhanced stress resistance of the os-2 strain was also evident in liquid medium, where only 42% of the os-2 cells had died by 8 h of stress, compared with 95% of the wild type (Fig. 2C). These results indicate that the stress MAP kinase OS-2 may contribute to the cell death we have been investigating.
Given that the absence of OS-2 or OS-2 function enhances cell resistance to dual stress, we asked if OS-2 might become activated by the stresses of high temperature and 2-DG. We probed Western blots with heterologous polyclonal antibodies against yeast Hog1 and against a conserved phosphorylated epitope of p38 MAP kinase. These antibodies bound to a protein of the expected size in wild-type cell extracts that was absent in extracts from os-2 cells (Fig. 10). No difference in the amount of OS-2 was seen among the three treatments of 30°C, 45°C, and 45°C plus 2-DG. However, heat shock alone led to increased phosphorylation of OS-2, and the addition of 2-DG during heat shock further increased phosphorylated OS-2 (Fig. 10). These results demonstrate that OS-2 becomes activated in response to heat shock and the dual stress.
FIG. 10.
Effect of heat stress and 2-DG addition on phosphorylated OS-2 level. Shown is a Western blot of SDS-polyacrylamide gel electrophoresis-separated cellular proteins from 5-h cells which was probed with phosphospecific p38 MAP kinase antibody. The same blot (adjacent lanes) was probed with anti-Hog1 antibody to compare total OS-2 levels. All lanes contained equal amounts of protein. The amido black-stained blot is shown at the bottom, and the 45-kDa marker is indicated on the left. Phosphorylation of OS-2 increases after 10 min exposure of cells to 45°C; there is an additional increase in phosphorylation when 2-DG is present during this heat shock. This experiment was performed three times with similar results.
DISCUSSION
The stratagems employed by cells to withstand damaging physical and chemical stresses, such as the heat shock response (55), sometimes prove inadequate. When this occurs, a characteristic response to cell damage is the regulated death of targeted cells (43). Of the various types of programmed cell death that have been characterized (32), apoptosis and autophagy are the best studied in eukaryotes (29, 43), but necrosis has also been found to have regulated components (18).
We found that N. crassa undergoes cell death in response to moderately high temperature when it is combined with carbohydrate deprivation. This deprivation consists of supplying cells with a small amount of glucose (0.05%) and a smaller amount of the competitive inhibitor 2-DG (0.015%). The asexual spores of N. crassa require glucose as a germination signal, although they contain stored carbohydrate and lipids for initial energy requirements (8). It may be these reserve energy stores, in fact, that make the 2-h germinating spores refractory to death by heat shock and 2-DG, in contrast to 5-h cells. Unlike other inhibitory glucose analogs, 2-DG can be phosphorylated by hexokinase in the initial step of glycolysis. In this way it depletes the cells of ATP, as well as blocking glycolysis and other steps in glucose metabolism (30). Glycolysis and heat shock-induced glycolytic enzymes appear to be especially important for energy generation at high temperature (56, 57). Although phosphorylated 2-DG has been reported to act as a high-glucose signal, e.g., in activating Akt kinase (20, 61), the chief effect of 2-DG in our assays likely stems from its inhibition of glucose metabolism, since another glucose analog, l-sorbose, that is not phosphorylated (66) also leads to cell death, albeit less dramatically and at higher concentrations. We are aware that glucose has other cellular functions with which 2-DG could interfere, such as the modification of proteins and of sphingolipids, and we have not ruled out the possibility that their inhibition may contribute to death at high temperatures.
We believe that this death is regulated, based on our findings that (i) it requires fatty acid synthesis, as indicated by its dependence on biotin and a functioning fatty acid synthase, (ii) it specifically requires ceramide synthesis that is inhibitable by australifungin, and (iii) the stress MAP kinase OS-2 contributes to cell death.
In mammalian cells, ceramide is a potent activator of apoptosis (34). By stimulating protein phosphatase 2A, ceramide activates proapoptotic Bax (79), while it inactivates antiapoptotic Bcl2 (64). Green plants may also utilize ceramide for programmed cell death. An Arabidopsis mutant that accumulates high levels of ceramide, due to a defective ceramide kinase, underwent excessive cell death when infected with the bacterial pathogen Pseudomonas syringae (40). Glycosylceramides are likely involved in the lethal vegetative incompatibility reaction of the filamentous fungus Podospora anserina (47). In Aspergillus nidulans, sphingosines, rather than ceramides, were reported to be detrimental, since exogenous phytosphingosine and dihydrosphingosine proved to induce apoptosis without increasing intracellular ceramide levels (11). Conversely, these sphingosines protected S. cerevisiae during heat shock, allowing continued growth (27). Without sphingosine synthesis, yeast cells were deficient in translating mRNA at high temperatures, resulting in a lack of heat shock protein synthesis (49). However, sphingosines likely have pleiotropic effects in yeast through their activation of Pkh1/2 protein kinases (15).
In our assays with stressed Neurospora cells, exogenous C2-ceramides were more toxic than sphingosines, and the desaturated ceramide was more lethal than phytoceramide. These results suggested that an endogenous desaturated ceramide might promote death in Neurospora, similar to its role in mammalian cells. We found instead that a novel phytoceramide was produced in vivo that was made only under the experimental dual-stress conditions. This ceramide was not detected in younger cells, which survive dual stress, opening the possibility that a unique ceramide is involved in Neurospora death.
We isolated this ceramide by SPE chromatography and TLC and structurally identified it by GC, ESI-MS, and MS-MS. This lipid, induced by combined heat shock and carbohydrate stress, was identified as an uncommon phytoceramide that contains a hydroxylated C18 fatty acid. Also isolated were the lipids flanking it on the TLC plate, which were present in much greater abundance, to ensure that we were not identifying nearby contaminants as the induced lipid. We identified the faster-migrating adjacent ceramide as phytoceramide containing a hydroxylated C24 fatty acid. This phytoceramide is made constitutively, but continues to be produced during dual stress in greater amounts than other sphingolipids. The slower-migrating lipid was identified as ergosterol-2-deoxyglucoside, a modification of the ergosterol-glucoside that is reported to be induced in fungi by stress (65).
Fungi typically produce two types of complex ceramides, the inositolphosphorylceramides and the glycosylceramides (17). The inositolphosphorylceramides are modified phytoceramides that contain very-long-chain hydroxylated C24 or C26 fatty acids. Further modified by mannose and other sugars, these localize to the plasma membrane and, in association with sterols and GPI-anchored proteins, contribute to lipid rafts. The C-4-desaturated ceramide that is characteristic of mammals is also made by green plants and most fungi, where it typically contains long-chain hydroxylated C16 or C18 fatty acids, and it is further modified by C-8 desaturation, C-9 methylation, and monoglycosylation. These distinct modifications of ceramides containing long-chain versus very-long-chain fatty acids suggest the existence of two separate intracellular pools of ceramides (36, 75). Although the specific functions of monoglucosylated ceramide are not known, experiments with several fungi suggest that defensins of green plants bind these ceramides, thereby permeabilizing fungal membranes and inhibiting growth (73). A mutant strain of Neurospora, selected for its resistance to defensin, was found to have a glucosylceramide with a shortened fatty acid compared to that of the wild type (54).
The Neurospora phytoceramide that is induced by combined high temperature and glucose deprivation is unusual in containing, rather than a very-long-chain fatty acid, a long-chain C18 fatty acid; a fatty acid of this length is usually linked to sphingosine, rather than to phytosphingosine. This uniqueness may render the induced phytoceramide an ideal signaling molecule. Its formation may depend upon and reflect the inhibition of pathway enzymes due to severe stress. For example, the dihydroceramide desaturase may be slowed, relative to ceramide hydroxylase, encouraging formation of the induced phytoceramide. Indeed, animals have bifunctional ceramide delta(4)-desaturases with C-4-hydroxylating activity (72). Alternatively, since fungi have two distinct classes of (dihydro)ceramide synthase, they may have different substrate specificities. The encoded ceramide synthases of Neurospora fall into the same two classes as those of Aspergillus, LagA and BarA (39). In contrast, the two S. cerevisiae enzymes, Lag1 and Lac1, are in the same class as LagA (39) and have redundant activities (33). Interestingly, this absence of a BarA-related ceramide synthase in S. cerevisiae parallels its lack of a dihydroceramide desaturase and monoglucosylated ceramide. In mammalian cells, Lag1 family members show specificity for transferring fatty acids of different length to sphingoid bases (62). If the two ceramide synthases in Neurospora have distinct substrate preferences, differential inhibition of these synthases might account for the unusual coupling of C18 fatty acid with phytosphingosine.
The 2-DG-modified ceramide observed in our experiments is likely either dihydroceramide or phytoceramide, since it is produced by 5-h cells of the Δdes strain (data not shown); surprisingly, it is not made by 17-h cells of this strain (Fig. 7). Modification of phytoceramide by glucosylceramide synthase, while unusual, has been observed under experimental conditions (36). Although wild-type cells maintained at 30°C and heat-shocked germinating spores continue to produce the 2-DG-modified ceramide during the second hour of stress, it ceases to be synthesized by stressed 5-h cells. Why the modified ceramide ceases to be synthesized by these cells is unknown, although there appears to be an inverse relationship between its synthesis and that of C18-phytoceramide. It is worth noting that glycosylation can be a mechanism for inactivating ceramide and reducing its toxicity in mammalian cells (42). Further experiments are needed to understand the genesis of the C18-phytoceramide and its relationship, if any, to the 2-DG-modified ceramide.
The unique ceramide that we have identified is induced by heat shock combined with an inhibitory glucose analog, but only when these stresses are lethal. The production of this ceramide early in stress, along with the evident contribution of ceramide synthesis to cell death, strongly suggests that it is involved in a death-signaling pathway. Another possible participant in this pathway appears to be the OS-2 MAP kinase, whose activation increases in Neurospora in response to heat shock, as reported previously (53), and increases further when 2-DG is present during heat stress. We found that mutant cells lacking OS-2 become resistant to dual stress. Not only is its mammalian homolog, p38, associated with regulated cell death, but also its interactions with ceramide have been described. Administration of C2-ceramide led to a rapid increase in p38 phosphorylation in neuronal cells, and the apoptosis induced by ceramide was reduced by addition of an inhibitor of p38 (69). p38 is known to phosphorylate Bcl-2 family members with proapoptotic effects (16, 19, 31). Homologs of Bcl-2 are not known in fungi, but proteins with analogous functions may be similarly regulated, as suggested by the conservation in plants and fungi of a Bax inhibitor protein (10), despite the absence of Bax itself. We do not currently know if a common pathway connects ceramide with OS-2 MAP kinase in Neurospora. This is a question we intend to explore, beginning with the effect of ceramide addition on OS-2 activation.
The atypical C18(OH)-phytoceramide that we have identified may be a product of the specific stresses in these experiments and related particularly to 2-DG exposure. Nevertheless, we have observed that unusual ceramides also result from administering hydrogen peroxide, a common inducer of apoptosis, at concentrations leading to Neurospora death (data not shown). These examples suggest that ceramide synthesis may be readily altered by damaging environmental stresses, and the resulting ceramides may constitute ideal signaling molecules for death pathways in fungi.
Acknowledgments
We thank John Obenchain and May Godoy of Merck & Co., Inc., Rahway, NJ, for generously providing us with australifungin.
This work was partially supported by a Grant-in-Aid of Research from the Office of the Dean of the Graduate School at the University of Minnesota.
Footnotes
Published ahead of print on 24 October 2008.
REFERENCES
- 1.Adams, J., and Q. Ann. 1993. Structure determination of sphingolipids by mass spectrometry. Mass Spectrom. Rev. 1251-85. [Google Scholar]
- 2.Alvarado-Kristensson, M., and T. Andersson. 2005. Protein phosphatase 2A regulates apoptosis in neutrophils by dephosphorylating both p38 MAPK and its substrate caspase 3. J. Biol. Chem. 2806238-6244. [DOI] [PubMed] [Google Scholar]
- 3.Askari, N., R. Diskin, M. Avitzour, R. Capone, O. Livnah, and D. Engelberg. 2007. Hyperactive variants of p38α induce, whereas hyperactive variants of p38γ suppress, activating protein 1-mediated transcription. J. Biol. Chem. 28291-99. [DOI] [PubMed] [Google Scholar]
- 4.Beever, R. E. 1973. Pyruvate carboxylase and N. crassa suc mutants. Neurospora Newsl. 2015-16. [Google Scholar]
- 5.Bhandari, P., N. Kumar, B. Singh, and V. K. Kaul. 2006. Bacosterol glycoside, a new 13,14-seco-steroid glycoside from Bacopa monnieri. Chem. Pharm. Bull. (Tokyo) 54240-241. [DOI] [PubMed] [Google Scholar]
- 6.Bock, K., and H. Thogersen. 1982. Nuclear magnetic resonance spectroscopy in the study of mono- and oligosaccharides. Annu. Rep. NMR Spectrosc. 131-57. [Google Scholar]
- 7.Bodennec, J., G. Brichon, G. Zwingelstein, and J. Portoukalian. 2000. Purification of sphingolipid classes by solid-phase extraction with aminopropyl and weak cation exchanger cartridges. Methods Enzymol. 312101-114. [DOI] [PubMed] [Google Scholar]
- 8.Bonnen, A., and R. Brambl. 1983. Germination physiology of Neurospora crassa conidia. Exp. Mycol. 7197-207. [Google Scholar]
- 9.Brockman, H. E., and F. J. deSerres. 1963. “Sorbose toxicity” in Neurospora. Am. J. Bot. 50709-714. [Google Scholar]
- 10.Chae, H.-J., N. Ke, H.-R. Kim, S. Chen, A. Godzik, M. Dickman, and J. C. Reed. 2003. Evolutionarily conserved cytoprotection provided by Bax Inhibitor-1 homologs from animals, plants, and yeast. Gene 323101-113. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, J., T.-S. Park, L.-C. Chio, A. S. Fischl, and X. S. Ye. 2003. Induction of apoptosis by sphingoid long-chain bases in Aspergillus nidulans. Mol. Cell. Biol. 23163-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins, Q. F., Y. Xiong, E. G. Lupo, Jr., H.-Y. Liu, and W. Cao. 2006. p38 mitogen-activated protein kinase mediates free fatty acid-induced gluconeogenesis in hepatocytes. J. Biol. Chem. 28124336-24344. [DOI] [PubMed] [Google Scholar]
- 13.Colot, H. V., G. Park, G. E. Turner, C. Ringelberg, C. M. Crew, L. Litvinkova, R. L. Weiss, K. A. Borkovich, and J. C. Dunlap. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 10310352-10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuvillier, O., G. Pirianov, B. Kleuser, P. G. Vanek, O. A. Coso, J. S. Gutkind, and S. Spiegel. 1996. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381800-803. [DOI] [PubMed] [Google Scholar]
- 15.Daquinag, A., M. Fadri, S. Y. Jung, J. Qin, and J. Kunz. 2007. The yeast PH domain proteins Slm1 and Slm2 are targets of sphingolipid signaling during the response to heat stress. Mol. Cell. Biol. 27633-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Chiara, G., M. E. Marcocci, M. Torcia, M. Lucibello, P. Rosini, P. Bonini, Y. Higashimoto, G. Damonte, A. Armirotti, S. Amodei, A. T. Palamara, T. Russo, E. Garaci, and F. Cozzolino. 2006. Bcl-2 phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J. Biol. Chem. 28121353-21361. [DOI] [PubMed] [Google Scholar]
- 17.Dickson, R. C., and R. L. Lester. 1999. Yeast sphingolipids. Biochim. Biophys. Acta 1426347-357. [DOI] [PubMed] [Google Scholar]
- 18.Dinnen, R. D., L. Drew, D. P. Petrylak, Y. Mao, N. Cassai, J. Szmulewicz, P. Brandt-Rauf, and R. L. Fine. 2007. Activation of targeted necrosis by a p53 peptide: a novel death pathway that circumvents apoptotic resistance. J. Biol. Chem. 28226675-26686. [DOI] [PubMed] [Google Scholar]
- 19.Farley, N., G. Pedraza-Alva, D. Serrano-Gomez, V. Nagaleekar, A. Aronshtam, T. Krahl, T. Thornton, and M. Rincón. 2006. p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells. Mol. Cell. Biol. 262118-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlob, K., N. Majewski, S. Kennedy, E. Kandel, R. B. Robey, and N. Hay. 2001. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 151406-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haak, D., K. Gable, T. Beeler, and T. Dunn. 1997. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 27229704-29710. [DOI] [PubMed] [Google Scholar]
- 22.Hannun, Y. A., and L. M. Obeid. 2002. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 27725847-25850. [DOI] [PubMed] [Google Scholar]
- 23.Hendrick, J. P., and F.-U. Hartl. 1993. Molecular chaperone functions of heat-shock proteins. Annu. Rev. Biochem. 62349-384. [DOI] [PubMed] [Google Scholar]
- 24.Henry, S. A., and A. D. Keith. 1971. Saturated fatty acid requirer of Neurospora crassa. J. Bacteriol. 106174-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hockenbery, D. M., Z. N. Oltvai, X.-M. Yin, C. L. Milliman, and S. J. Korsmeyer. 1993. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75241-251. [DOI] [PubMed] [Google Scholar]
- 26.Hsu, F.-F., and J. Turk. 2001. Structural determination of glycosphingolipids as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisional-activated dissociation on a triple stage quadrupole instrument. J. Am. Soc. Mass Spectrom. 1261-79. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins, G. M., A. Richards, T. Wahl, C. Mao, L. Obeid, and Y. Hannun. 1997. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J. Biol. Chem. 5132566-32572. [DOI] [PubMed] [Google Scholar]
- 28.Jones, C. A., S. E. Greer-Phillips, and K. A. Borkovich. 2007. The response regulator RRG-1 functions upstream of a mitogen-activated protein kinase pathway impacting asexual development, female fertility, osmotic stress, and fungicide resistance in Neurospora crassa. Mol. Biol. Cell 182123-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang, C., Y.-J. You, and L. Avery. 2007. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 212161-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang, H. T., and E. S. Hwang. 2006. 2-Deoxyglucose: an anticancer and antiviral therapeutic, but not any more a low glucose mimetic. Life Sci. 781392-1399. [DOI] [PubMed] [Google Scholar]
- 31.Kim, B.-J., S.-W. Ryu, and B.-J. Song. 2006. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 28121256-21265. [DOI] [PubMed] [Google Scholar]
- 32.Kissová, I., L.-T. Plamondon, L. Brisson, M. Priault, V. Renouf, J. Schaeffer, N. Camougrand, and S. Manon. 2006. Evaluation of the roles of apoptosis, autophagy, and mitophagy in the loss of plating efficiency induced by Bax expression in yeast. J. Biol. Chem. 28136187-36197. [DOI] [PubMed] [Google Scholar]
- 33.Kolaczkowski, M., A. Kolaczkowska, B. Gaigg, R. Schneiter, and W. S. Moye-Rowley. 2004. Differential regulation of ceramide synthase components LAC1 and LAG1 in Saccharomyces cerevisiae. Eukaryot. Cell 3880-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroesen, B.-J., S. Jacobs, B. J. Pettus, H. Sietsma, J. W. Kok, Y. A. Hannun, and L. F. M. H. de Leij. 2003. BcR-induced apoptosis involves differential regulation of C16 and C24-ceramide formation and sphingolipid-dependent activation of the proteasome. J. Biol. Chem. 27814723-14731. [DOI] [PubMed] [Google Scholar]
- 35.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 36.Leipelt, M., D. Warnecke, U. Zähringer, C. Ott, F. Müller, B. Hube, and E. Heinz. 2001. Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. J. Biol. Chem. 27633621-33629. [DOI] [PubMed] [Google Scholar]
- 37.Levery, S. B., M. S. Toledo, A. H. Straus, and H. K. Takahashi. 1998. Structure elucidation of sphingolipids from the mycopathogen Paracoccidioides brasiliensis: an immunodominant beta-galactofuranose residue is carried by a novel glycosylinositol phosphorylceramide antigen. Biochemistry 378764-8775. [DOI] [PubMed] [Google Scholar]
- 38.Levery, S. B., M. S. Toledo, A. H. Straus, and H. K. Takahashi. 2001. Comparative analysis of glycosylinositol phosphorylceramides from fungi by electrospray tandem mass spectrometry with low-energy collision-induced dissociation of Li+ adduct ions. Rapid Commun. Mass Spectrom. 152240-2258. [DOI] [PubMed] [Google Scholar]
- 39.Li, S., L. Du, G. Yuen, and S. D. Harris. 2006. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 171218-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang, H., N. Yao, J. T. Song, S. Luo, H. Lu, and J. T. Greenberg. 2003. Ceramides modulate programmed cell death in plants. Genes Dev. 172636-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Listenberger, L. L., D. S. Ory, and J. E. Schaffer. 2001. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 27614890-14895. [DOI] [PubMed] [Google Scholar]
- 42.Liu, Y.-Y., T.-Y. Han, A. E. Giuliano, and M. C. Cabot. 1999. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J. Biol. Chem. 2741140-1146. [DOI] [PubMed] [Google Scholar]
- 43.Maiuri, M. C., E. Zalckvar, A. Kimchi, and G. Kroemer. 2007. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8741-752. [DOI] [PubMed] [Google Scholar]
- 44.Maizel, J. V. 1971. Polyacrylamide gel electrophoresis of viral proteins. Methods Virol. 5179-246. [Google Scholar]
- 45.Mandala, S. M., R. A. Thornton, B. R. Frommer, J. E. Curotto, W. Rozdilsky, M. B. Kurtz, R. A. Giacobbe, G. F. Bills, M. A. Cabello, and I. Martin. 1995. The discovery of australifungin, a novel inhibitor of sphinganine N-acyltransferase from Sporormiella australis: producing organism, fermentation, isolation, and biological activity. J. Antibiot. (Tokyo) 48349-356. [DOI] [PubMed] [Google Scholar]
- 46.Masserini, M., and D. Ravasi. 2001. Role of sphingolipids in the biogenesis of membrane domains. Biochim. Biophys. Acta 1532149-161. [DOI] [PubMed] [Google Scholar]
- 47.Mattjus, P., B. Turcq, H. M. Pike, J. G. Molotkovsky, and R. E. Brown. 2003. Glycolipid intermembrane transfer is accelerated by HET-C2, a filamentous fungus gene product involved in the cell-cell incompatibility response. Biochemistry 42535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCluskey, K. 2003. The Fungal Genetics Stock Center: from molds to molecules. Adv. Appl. Microbiol. 52245-262. [DOI] [PubMed] [Google Scholar]
- 49.Meier, K. D., O. Deloche, K. Kajiwara, K. Funato, and H. Riezman. 2006. Sphingoid base is required for translation initiation during heat stress in Saccharomyces cerevisiae. Mol. Biol. Cell 171164-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merrill, A. H., Jr. 2002. De novo sphingolipid biosynthesis: a necessary, but dangerous pathway. J. Biol. Chem. 27725843-25846. [DOI] [PubMed] [Google Scholar]
- 51.Miyake, Y., Y. Kozutsumi, S. Nakamura, T. Fujita, and T. Kawasaki. 1995. Serine palmitoyltransferase in the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Biophys. Res. Commun. 211396-403. [DOI] [PubMed] [Google Scholar]
- 52.Mock, D. M. 1999. Biotin, p. 459-466. In M. E. Shils, J. A. Olson, M. Shike, and A. C. Ross (ed.), Modern nutrition in health and disease, 9th ed. Williams & Wilkins, Baltimore, MD.
- 53.Noguchi, R., S. Banno, R. Ichikawa, F. Fukumori, A. Ichiishi, M. Kimura, I. Yamaguchi, and M. Fujimura. 2007. Identification of OS-2 MAP kinase-dependent genes induced in response to osmotic stress, antifungal agent fludioxonil, and heat shock in Neurospora crassa. Fungal Genet. Biol. 44208-218. [DOI] [PubMed] [Google Scholar]
- 54.Park, C., B. Bennion, I. E. J. A. François, K. K. A. Ferket, B. P. A. Cammue, K. Thevissen, and S. B. Levery. 2005. Neutral glycolipids of the filamentous fungus Neurospora crassa: altered expression in plant defensin-resistant mutants. J. Lipid Res. 46759-768. [DOI] [PubMed] [Google Scholar]
- 55.Parsell, D. A., and S. Lindquist. 1993. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 27437-496. [DOI] [PubMed] [Google Scholar]
- 56.Plesofsky, N., and R. Brambl. 1999. Glucose metabolism in Neurospora is altered by heat shock and by disruption of HSP30. Biochim. Biophys. Acta 144973-82. [DOI] [PubMed] [Google Scholar]
- 57.Plesofsky, N. 2004. Heat shock proteins and the stress response, p. 143-173. In R. Brambl and G. A. Marzluf (ed.), The mycota III, 2nd ed. Springer-Verlag, Berlin, Germany.
- 58.Plesofsky-Vig, N., and R. Brambl. 1985. Heat shock response of Neurospora crassa: protein synthesis and induced thermotolerance. J. Bacteriol. 1621083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plesofsky-Vig, N., and R. Brambl. 1995. Disruption of the gene for hsp30, an α-crystallin-related heat shock protein of Neurospora crassa, causes defects in thermotolerance. Proc. Natl. Acad. Sci. USA 925032-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prick, T., M. Thumm, K. Köhrer, D. Häussinger, and S. vom Dahl. 2006. In yeast, loss of Hog1 leads to osmosensitivity of autophagy. Biochem. J. 394153-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rathmell, J. C., C. J. Fox, D. R. Plas, P. S. Hammerman, R. M. Cinalli, and C. B. Thompson. 2003. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell. Biol. 237315-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riebeling, C., J. C. Allegood, E. Wang, A. H. Merrill, Jr., and A. H. Futerman. 2003. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J. Biol. Chem. 27843452-43459. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz-Gutierrez, V., and M. C. Perez-Camino. 2000. Update on solid-phase extraction for the analysis of lipid classes and related compounds. J. Chromatogr. A 885321-341. [DOI] [PubMed] [Google Scholar]
- 64.Ruvolo, P. P., X. Deng, T. Ito, B. K. Carr, and W. S. May. 1999. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J. Biol. Chem. 27420296-20300. [DOI] [PubMed] [Google Scholar]
- 65.Sakaki, T., U. Zähringer, D. C. Warnecke, A. Fahl, W. Knogge, and E. Heinz. 2001. Sterol glycosides and cerebrosides accumulate in Pichia pastoris, Rhynchosporium secalis and other fungi under normal conditions or under heat shock and ethanol stress. Yeast 18679-695. [DOI] [PubMed] [Google Scholar]
- 66.Scarborough, G. A. 1970. Sugar transport in Neurospora crassa. J. Biol. Chem. 2451694-1698. [PubMed] [Google Scholar]
- 67.Spiegel, S., and S. Milstien. 2002. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 27725851-25854. [DOI] [PubMed] [Google Scholar]
- 68.Steiner, S., and R. L. Lester. 1972. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J. Bacteriol. 10981-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stoica, B. A., V. A. Movsesyan, S. M. Knoblach, and A. I. Faden. 2005. Ceramide induces neuronal apoptosis through mitogen-activated protein kinases and causes release of multiple mitochondrial proteins. Mol. Cell. Neurosci. 29355-371. [DOI] [PubMed] [Google Scholar]
- 70.Sullards, M. C., and A. H. Merrill, Jr. 2001. Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Sci. STKE 671-11. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka, Y., M. V. Gavrielides, Y. Mitsuuchi, T. Fujii, and M. G. Kazanietz. 2003. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J. Biol. Chem. 27833753-33762. [DOI] [PubMed] [Google Scholar]
- 72.Ternes, P., S. Franke, U. Zähringer, P. Sperling, and E. Heinz. 2002. Identification and characterization of a sphingolipid Δ4-desaturase family. J. Biol. Chem. 27725512-25518. [DOI] [PubMed] [Google Scholar]
- 73.Thevissen, K., D. C. Warnecke, I. E. J. A. François, M. Leipelt, E. Heinz, C. Ott, U. Zähringer, B. P. H. J. Thomma, K. K. A. Ferket, and B. P. A. Cammue. 2004. Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 2793900-3905. [DOI] [PubMed] [Google Scholar]
- 74.Toledo, M. S., S. B. Levery, B. Bennion, L. L. Guimaraes, S. A. Castle, R. Lindsey, M. Momany, C. Park, A. H. Straus, and H. K. Takahashi. 2007. Analysis of glycosylinositol phosphorylceramides expressed by the opportunistic mycopathogen Aspergillus fumigatus. J. Lipid Res. 481801-1824. [DOI] [PubMed] [Google Scholar]
- 75.Toledo, M. S., S. B. Levery, A. H. Straus, E. Suzuki, M. Momany, J. Glushka, J. M. Moulton, and H. K. Takahashi. 1999. Characterization of sphingolipids from mycopathogens: factors correlating with expression of 2-hydroxy fatty acyl (E)-Δ3-unsaturation in cerebrosides of Paracoccidioides brasiliensis and Aspergillus fumigatus. Biochemistry 387294-7306. [DOI] [PubMed] [Google Scholar]
- 76.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vogel, H. J. 1956. A convenient growth medium for Neurospora. Microb. Genet. Bull. 1342. [Google Scholar]
- 78.Warnecke, D., and E. Heinz. 2003. Recently discovered functions of glucosylceramides in plants and fungi. Cell. Mol. Life Sci. 60919-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xin, M., and X. Deng. 2006. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J. Biol. Chem. 28118859-18867. [DOI] [PubMed] [Google Scholar]
- 80.Xiong, Y., Q. F. Collins, J. An, E. Lupo, Jr., H.-Y. Liu, D. Liu, J. Robidoux, Z. Liu, and W. Cao. 2007. p38 mitogen-activated protein kinase plays an inhibitory role in hepatic lipogenesis. J. Biol. Chem. 2824975-4982. [DOI] [PubMed] [Google Scholar]
- 81.Zhang, Y., R. Lamm, C. Pillonel, S. Lam, and J.-R. Xu. 2002. Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 68532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]