Abstract
In most species, chromatin remodeling mediates critical biological processes ranging from development to disease states. In fungi within the genus Aspergillus, chromatin remodeling may regulate expression of metabolic gene clusters, but other processes regulated by chromatin structure remain to be elucidated. In many eukaryotic species, methylation of lysine 9 of histone 3 (H3K9) is a hallmark of heterochromatin formation and subsequent gene silencing. The sole H3K9 methyltransferase in Schizosaccharomyces pombe is Clr4. We report that disruption of the Clr4 homolog in the pathogenic mold Aspergillus fumigatus (ClrD), which is involved in both mono- and trimethylation of H3K9, results in several growth abnormalities. Developmental defects in ΔAfclrD include reduction in radial growth, reduction in conidial production, and delayed conidiation after developmental competence mediated by delayed expression of brlA, the master regulator of conidiophore development. Sensitivity of ΔAfclrD to 6-azauracil suggests that ClrD influences transcriptional processing in A. fumigatus. Despite growth abnormalities, macrophage assays suggest ClrD may be dispensable for host interactions.
In eukaryotes, gene expression is greatly influenced by chromatin structure. Heterochromatin exists in either a constitutively transcriptionally silent state, as at centromeres and telomeres, or as facultative heterochromatin capable of switching between transcriptionally active and silent states. The expression of developmentally critical genetic loci present in facultative heterochromatin can be regulated by this shift. Classical examples include X-chromosome inactivation in females (9), derepression of mating-type loci in fission yeast (15), and position effect variegation in Drosophila melanogaster (13).
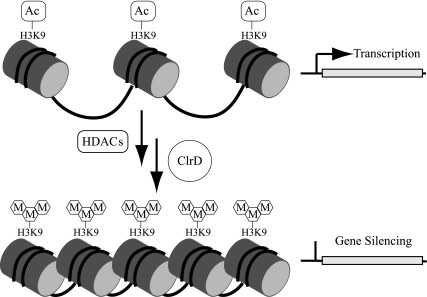
The “histone code” influences nucleosome positioning and chromatin compaction in facultative heterochromatin (Fig. 1). This code consists of specific patterns of posttranslational modifications of key amino acids on histone tails (reviewed in references 18, 20, 22, and 44). Modifications may include acetylation, ubiquitination, phosphorylation, and/or methylation. Such modifications recruit regulatory binding proteins that direct processes such as chromatin compaction (which dictates steric accessibility of DNA to transcriptional activators and repressors) or stabilization of transcriptional machinery.
FIG. 1.
Diagram illustrating the putative role of ClrD in chromatin remodeling. Histone tail modifications have been proposed to alter chromatin structure, thereby regulating access of transcriptional machinery to DNA. H3K9 has consistently been identified as a residue that can be either acetylated or methylated and modifications of this residue are associated with different chromatin arrangements; in general acetylation is associated with transcriptionally activated regions and trimethylation with silenced regions. In the oversimplified model depicted here, histone deacetylases (HDACs) remove acetyl groups from transcriptionally active regions, followed by subsequent methylation by ClrD, which leads to inactivation of loci regulated by this mechanism. Under this model, heterochromatic silenced regions can be activated by removal of methyl groups from H3K9 followed by subsequent acetylation.
There is limited information available about chromatin remodeling proteins and chromatin-level regulation of gene expression in filamentous fungi of the genus Aspergillus. Three histone arginine methyltransferases (RmtA to RmtC) have been analyzed biochemically in the model species A. nidulans; however, detailed analysis of deletion strain phenotypes was not reported (50). An A. nidulans histone deacetylase, HdaA, is required for normal growth under oxidative stress (49) and is involved in regulating telomere-proximal secondary metabolite gene clusters (42). However, there is a near-absence of studies on chromatin regulation in the opportunistic pathogen A. fumigatus. The degree to which such processes impact growth, development, and pathogenesis in this species is largely unknown.
A key histone modification influencing heterochromatin formation and transcriptional activation state is mono-, di-, or trimethylation at lysine 9 of histone H3 (H3K9). Trimethylation at H3K9 recruits heterochromatin protein 1 [encoded by HP1 in mammals, swi6 in fission yeast, and su(var)205 in Drosophila], leading to heterochromatin spread (18) (Fig. 1). H3K9 methylation frequently demarks a transcriptionally silent state; however, a recent study found that H3K9 trimethylation was associated with active expression in a variety of human cancer cell lines (53). H3K9 methylation regulates developmental processes in many species. Examples include the regulation of stem cell pluripotency via expression of the HOXA cluster (5), regulation of KIR gene expression in natural killer cells (37), vulval development in Caenorhabditis elegans (4), and pleiotropic regulation of Drosophila development (52).
The major enzyme responsible for histone H3K9 methylation is SU(VAR)3-9 in Drosophila (38), Clr4 in Schizosaccharomyces pombe (32), and DIM-5 in Neurospora crassa (47). Mammals have a homologous H3K9 methyltransferase, SUV39H1; however, there are several other mammalian enzymes capable of methylating H3K9 (34). A Clr4 null mutant in S. pombe shows derepression of silent mating-type loci (15), increased chromosome loss (2), improper chromosomal segregation, diminished growth, and increased susceptibility to microtubule-destabilizing agents (14). Disruption of DIM-5 in N. crassa causes loss of H3K9 trimethylation and results in decreased DNA methylation and growth abnormalities (47).
We investigated the role of the Clr4 homolog in the pathogenic mold Aspergillus fumigatus (AfclrD). We report that AfClrD methylates H3K9, mediates 6-azauracil (6AU) sensitivity, and is required for normal growth and development but does not affect in vitro conidial uptake by macrophages.
MATERIALS AND METHODS
Culturing conditions and DNA isolations.
Fungal strains used in this study are listed in Table 1. All growth assays were conducted with prototrophic strains at 37°C on glucose minimal medium (GMM) as described in reference 41 unless noted otherwise. When appropriate, medium was supplemented with 5 mM of uridine and uracil for pyrG auxotrophs and 5 mM arginine for argB auxotrophs. Radial growth was assayed by inoculation of approximately 500 spores in the center of appropriate media and measurement of colony diameters every 24 h, and data are presented as the means of four replicates. Conidial production was quantified from two inoculation methods; point-inoculated cultures were inoculated as per the radial growth experiment, and overlay-inoculated cultures were set up by pipetting 1 × 106 conidia/ml into 0.75% GMM molten agar that was subsequently poured over 1.5% GMM solid agar petri dishes. One agar plug was removed from the center of point-inoculated plates and three agar plugs were removed from the overlay plates; subsequent spore suspensions were counted with a hemocytometer. All conidial quantification experiments were performed in triplicate. Graphs and statistical analysis were done using Prism 5.0 software. Genomic DNA was isolated from fungi and Escherichia coli using standard procedures (36, 41), and all primers used are listed in Table 2.
TABLE 1.
Fungal strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| AF293 | Aspergillus fumigatus wild type | 54 |
| AF293.6 | argB1 pyrG1 | 54 |
| TJW55.1 | pyrG1; A. parasiticus pyrG | 8 |
| TRMP1.60 | ΔAfclrD::A. nidulans argB argB1 pyrG1 | This study |
| TJMP1.11 | ΔAfclrD::A. nidulans argB A. parasiticus pyrG argB1 pyrG1 | This study |
| TRMP4.18 | ΔAfclrD::A. nidulans argB A. fumigatus clrD::A. parasiticus pyrG argB1 pyrG1 | This study |
| TRMP4.25 | ΔAfclrD::A. nidulans argB A. fumigatus clrD::A. parasiticus pyrG argB1 pyrG1 | This study |
| TRMP4.40 | ΔAfclrD::A. nidulans argB A. fumigatus clrD::A. parasiticus pyrG argB1 pyrG1 | This study |
TABLE 2.
Primers used in this study
| Primer | Sequence (5′ to 3′)a | Purpose |
|---|---|---|
| RMP10 | TTAATCTAGAGCCTGATTATGACTGGGTTTATTC | Forward for AfclrD 5′ flank |
| RMP15 | AATTAGCGGCCGCTATTTTGGGTTAGCCGTGTCT | Reverse for AfclrD 5′ flank |
| RMP14 | TTAAGGGCCCCATGGATTTTCAGGGCATGT | Forward for AfclrD 3′ flank |
| RMP13 | AATTGGGCCCCGCACCTTACTTCATCGTCA | Reverse for AfclrD 3′ flank |
| RMP17 | TTAACTCGAGAACGCTGTGTAAAGCGGAGT | Forward for A. nidulans ArgB |
| RMP18 | AATTCTCGAGCCATTGCGAAACCTCAGAAG | Reverse for A. nidulans ArgB |
| RMP23 | TTCCCTTTAGCATTGGAACG | Forward for AfclrD internal probe |
| RMP24 | CTTCTCCCCACAGAGACAGC | Reverse for AfclrD internal probe |
| RMP21 | TTAAGAACTAGTCAGGCAGGCTTAATCTCAGG | Forward for AfclrD complementation |
| RMP22 | TTAAGAGAGCTCCGAGTTACAATGTTGCTGC | Reverse for AfclrD complementation |
| JP Act F | TGACAATGTTACCGTAGAGATCC | Forward for actin probe |
| JP Act R | GGAGAAGATCTGGCATCACA | Reverse for actin probe |
| JP SSM1 F | CGACTACGGAATGTCCCAC | Forward for SSM1 probe |
| JP SSM1 R | GCTCCTCAAGGCTGCGAATC | Reverse for SSM1 probe |
| JP IMD2 F | GTCACATCCAGAGATATCCAG | Forward for IMD2 probe |
| JP IMD2 R | TTGACGCCGTCGTGGAGCTC | Reverse for IMD2 probe |
| JP PPR2 F | GATGCGAAGGAAATCGAGCTC | Forward for DST1 probe |
| JP PPR2 R | CCTCCACGATTTACCACAGTTC | Reverse for DST1 probe |
| JP RTF1 F | TGATGCTGGGTCTCACCATT | Forward for RTF1 probe |
| JP RTF1 R | GGCGACATACTCTTGATCAA | Reverse for RTF1 probe |
| JP SPT3 F | AGCTTGCACGAAGCACGATG | Forward for SPT3 probe |
| JP SPT3 R | CTATCGTAGTATAGCTCCGC | Reverse for SPT3 probe |
| JP POP2 F | CATAGGATACGGAGTTCCC | Forward for POP2 probe |
| JP POP2 R | CACCAGGAGTTAGTGCACC | Reverse for POP2 probe |
Restriction sites are underlined.
Generation of transformation cassettes and genetic manipulations.
A plasmid (pRMPargB/Topo2) bearing the A. nidulans argB cassette (AN4409.3) was generated using primers RMP17 and RMP18 to amplify a 1.9-kb fragment beginning 0.4 kb upstream of the argB translational start codon and ending 0.35 kb downstream of the translational stop codon. Following the manufacturers' protocols, the product was amplified from genomic DNA of A. nidulans FGSC4 using TripleMaster polymerase (Eppendorf), blunt ended with PfuUltra (Stratagene), and cloned into pCR-Blunt-II Topo (Invitrogen). Flanking sequences of the A. fumigatus clrD construct (Afu1g11090) were amplified from A. fumigatus AF293 genomic DNA. Primers RMP10 and RMP15 were used to amplify an 1.1-kb fragment upstream of the AfclrD open reading frame. The resulting PCR product was blunt ended with PfuUltra and the fragment was cloned into pCR-Blunt-II. A downstream flanking fragment 0.9 kb in length was generated using primers RMP14 and RMP13 and was cloned into this pCR-Blunt-II/5′ clrD intermediate construct. Finally, a 1.9-kb XhoI fragment from pRMPargB/Topo2 containing the A. nidulans argB gene was cloned in between the flanking fragments of the intermediate construct to create the final AfclrD disruption cassette pRMP7.
The AfclrD complementation cassette was amplified from AF293 genomic DNA using primers RMP21 and RMP22, yielding a 3.1-kb fragment composed of approximately 1 kb upstream and 0.5 kb downstream of the putative open reading frame. Product was generated using TripleMaster polymerase, blunt ended with PfuUltra polymerase, and ligated with pCR-Blunt-II vector to create pRMPclrD/comp#1. The EcoRI fragment of pRMPclrD/comp#1 was ligated with pJW24 (10) to create the A. fumigatus clrD complementation construct pRMP10.
Strain AF293.6 was transformed with the AfclrD disruption fragment by amplification of pRMP7 with primers RMP10 and RMP13 to generate TRMP1.60 (ΔAfclrD pyrG1). Fungal transformation was done essentially as described previously (29), with the modification of embedding the protoplasts in top agar (0.75%) as described above. The transformant TRMP1.60 was analyzed by PCR and Southern analysis, confirming the disruption of AfclrD. This strain was then transformed with A. parasiticus pyrG(pJW24) to generate TJMP1.11 and with the A. fumigatus clrD complementation cassette pRMP10 to generate TRMP4.18, TRMP4.25, and TRMP4.40. All strains were confirmed by preliminary PCR screens and secondarily by Southern analysis.
Western blot assays to detect global histone H3K9 modifications.
Nuclei were prepared from TJW55.1, TJMP1.11, and TRMP4.40 strains. Cultures (500 ml) of GMM were inoculated with 1 × 106 conidia/ml and incubated at 37°C for 48 h. Mycelia were collected by vacuum filtration, frozen in liquid nitrogen, and ground to powder under liquid nitrogen. Ground mycelial powder was suspended in 200 ml nuclei isolation buffer (1 M sorbitol, 10 mM Tris-HCl, pH 7.5, 0.15 mM spermine, 0.5 mM spermidine, 10 mM EDTA, 2.5 mM phenylmethylsulfonyl fluoride) on ice. Samples were centrifuged in a GSA rotor (1,000 × g) at 4°C for 10 min, and supernatant was filtered through two layers of Miracloth. The filtrate was centrifuged at 10,000 × g for 15 min at 4°C. The pellet was then resuspended in 15 ml resuspension buffer (identical to nuclei isolation buffer but containing 1 mM EDTA) and then centrifuged in an SS-35 rotor (9,700 × g) for 15 min at 4°C. Supernatant was removed, and crude nuclei were resuspended in 0.6 ml of ST buffer (1 M sorbitol, 10 mM Tris-HCl, pH 7.5, protease inhibitor cocktail for fungi [Sigma]). Debris was pelleted by centrifugation (1,500 × g) in a microcentrifuge for 30 s. The protein concentration was determined in a Bradford assay in triplicate. Approximately 50 μg protein per sample was subjected to electrophoresis on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane. Membranes were blocked for 30 min at room temperature on a rocking platform using 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T). Samples were incubated with primary antibodies for 1 h in TBS-T. Antibodies were anti-histone H3 (catalog no. 07-690; Upstate Biotechnology) used at 1:5,000, anti-H3K9 mono-methyl (catalog no. 07-450; Upstate) used at 1:100, anti-H3K9 di-methyl (catalog no. ab7312; AbCam) used at 1:20, and anti-H3K9 tri-methyl (catalog no. 07-442; Upstate) used at 1:2,000. Blots were washed three times for 10 min each in TBS-T. Secondary antibodies (goat anti-rabbit-horseradish peroxidase conjugate; 1:20,000; Pierce) were applied in TBS-T for 1 h at room temperature. Blots were washed and used for chemiluminescence detection.
Developmental competence.
Developmental competence (synchronous asexual development) was assayed as described elsewhere (27). A follow-up experiment was performed identically, with the exception that liquid shake cultures were incubated for 24 h before switching to solid medium. Briefly, 100 ml of liquid medium (GMM plus 0.1% yeast extract) was inoculated with 1 × 106 conidia/ml and incubated in a shaking culture at 250 rpm at 37°C for 18 or 24 h. Mycelia were collected on sterile filter paper, transferred to solid medium, and further incubated at 37°C. Cultures were examined microscopically, and total RNA was extracted using Trizol (Invitrogen) at 0, 3, 6, 9, 12, and 24 h. Northern analysis was conducted using standard techniques (36), and probes were generated with the following PCR primers: AfclrD with RMP23 and RMP24, AfbrlA with OJH81 and OJH82 (27), AfwetA with OJH25 and OJH29 (27), AfvosA with oMN164 and oMN165 (33), AffluG with OJH91 and OJH92 (27), and actin with JP Act F and JP Act R.
Expression analysis upon challenge with 6-azauracil.
Liquid GMM was inoculated with 1 × 106 spores/ml and grown for 24 h at 250 rpm and 37°C. 6AU (100 μg/ml) was added to the liquid shake cultures, and total RNA was extracted at 0, 3, 6, 12, and 24 h. A BLASTp search of the A. fumigatus genome identified putative homologs of the yeast genes DST1 (Afu3g07660), POP2 (Afu5g07370), RTF1 (Afu2g01900), SPT3 (Afu1g14030), IMD2 (Afu2g03610), and SDT1 (Afu2g13470). Probes for Northern analysis were constructed with the following PCR primer sets: Afdst1 with JP PPR2 F and JP PPR2 R, Afpop2 with JP POP2 F and JP POP2 R, Afrtf1 with JP RTF1 F and JP RTF1 R, Afspt3 with JP SPT3 F and JP SPT3 R, Afimd2 with JP IMD2 F and JP IMD2 R, and Afsdt1 with JP SSM1 F and JP SSM1 R.
Macrophage phagocytosis assay.
The RAW 264.7 and MH-S cell lines were purchased from ATCC (Manassas, VA) and maintained in RPMI 1640 supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and gentamicin (Sigma, St. Louis, MO). RAW cells (5 × 104/well) were plated onto 16-well chamber slides (Nunc, Rochester, NY) and incubated at 37°C, 5% CO2. After overnight incubation (18 h), freshly harvested conidia (2.5 × 105 conidia/well) were added to the macrophages for 1 hour, wells were washed twice with RPMI medium, and macrophages were allowed to incubate for an additional 2 hours to allow complete uptake of conidia. Macrophages were fixed in 2% formaldehyde, washed three times with phosphate-buffered saline, and stained with calcofluor white (Sigma) to label extracellular conidia, which were excluded from analysis. The percent uptake (number of macrophages with conidia per total number of macrophages × 100) and the average number of conidia per macrophage (of macrophages containing conidia) were determined. These experiments were conducted in triplicate.
RESULTS
Identification of Aspergillus fumigatus clrD, creation of AfclrD null mutant, and subsequent complementation.
We identified AfclrD by a BLASTp search (3) of the A. fumigatus genomic sequence (40) at the Aspergillus Comparative Database (www.broad.harvard.edu/annotation/genome/aspergillus_group/) with the amino acid sequence of Clr4 from S. pombe (CAA07709). Locus Afu1g11090 was identified with an e value of 0.0 despite only 39% identity overall. Further in silico analysis using the Conserved Domain Database indicated the presence of the conserved methyltransferase PRE-SET and SET domains (28).
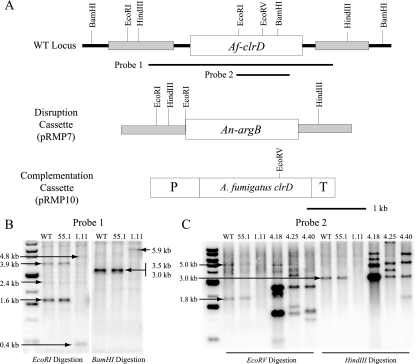
The AfclrD locus was replaced by double homologous recombination with the A. nidulans argB marker gene by transforming A. fumigatus strain AF293.6 (54) with the gene replacement construct pRMP7. Gene replacement was confirmed by PCR of over 100 transformants and Southern blot analysis of a subset of transformants. Several strains showing obvious growth defects proved to be ΔAfclrD transformants (Fig. 2B). To confirm that the ΔAfclrD phenotype was due solely to loss of AfclrD, one ΔAfclrD transformant (TRMP1.60) was transformed with the AfclrD complementation construct pRMP10. Resulting transformants were screened by PCR and Southern blot analysis (Fig. 2C), and TRMP4.18, TRMP4.25, and TRMP4.40 were chosen for further analysis. TRMP1.60 was transformed with pJW24 harboring the A. parasiticus pyrG gene to generate prototrophic ΔAfclrD strain TJMP1.11 (data not shown).
FIG. 2.
Schematic overview and confirmation of the constructs used to disrupt and complement AfclrD. (A) AfclrD was replaced with the A. nidulans argB gene as a selectable marker in A. fumigatus AF293.6 (argB1 pyrG1) and complemented with pRMP10 containing a 3.1-kb fragment composed of 1 kb upstream of the putative start codon (P) and 0.5 kb downstream of the putative stop codon (T) of the AfclrD open reading frame. This fragment was coupled with the A. parasiticus pyrG marker gene for selection. (B) Resulting transformants were confirmed by Southern digestion of genomic DNA. With EcoRI digestion, a 3.1-kb probe (probe 1) was expected to hybridize to 3.9-kb, 2.4-kb, and 1.6-kb bands for the wild type and 4.8-kb, 2.4-kb, and 0.4-kb bands for the ΔAfclrD strain. Additionally, BamHI digestion was expected to show 3.5-kb and 3.0-kb bands for the wild type and a single 5.9-kb band for ΔAfclrD. (C) Ectopic integration of at least two copies of the complementation cassette was confirmed by Southern analysis for all three complementation strains (TRMP4.18, TRMP4.25, and TRMP4.40) with an internal probe (probe 2). All schematics are drawn to scale.
AfClrD methylates lysine 9 of histone 3 in A. fumigatus.
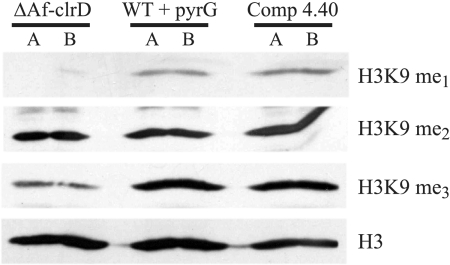
Functionality of AfClrD was examined by Western analysis of nuclear protein extracts from the wild type with pyrG (TJW55.1), ΔAfclrD (TJMP1.11), and complemented ΔAfclrD (TRMP4.40) with antibodies to H3K9-me1, H3K9-me2, and H3K9-me3. Figure 3 shows that AfclrD is primarily involved in trimethylation and monomethylation of H3K9. No substantial difference in dimethylation between the strains was observed under the conditions tested.
FIG. 3.
Immunoblot detection of H3K9 methylation patterns in nuclear protein extracts of selected strains. Nuclear protein extracts of ΔAfclrD (TJMP1.11), the wild type with pyrG (TJW55.1), and a complemented control strain (TRMP4.40) were assayed via Western blotting with antibodies to monomethylated (me1), dimethylated (me2), and trimethylated (me3) H3K9. Immunoblot analysis was conducted in duplicate for each strain, corresponding to lanes A and B. The faint upper band seen in the dimethylation panel is nonspecific background.
AfClrD is required for normal growth and BrlA-mediated conidiophore development.
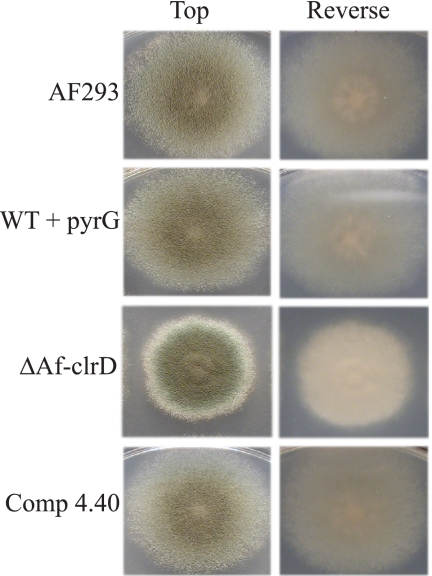
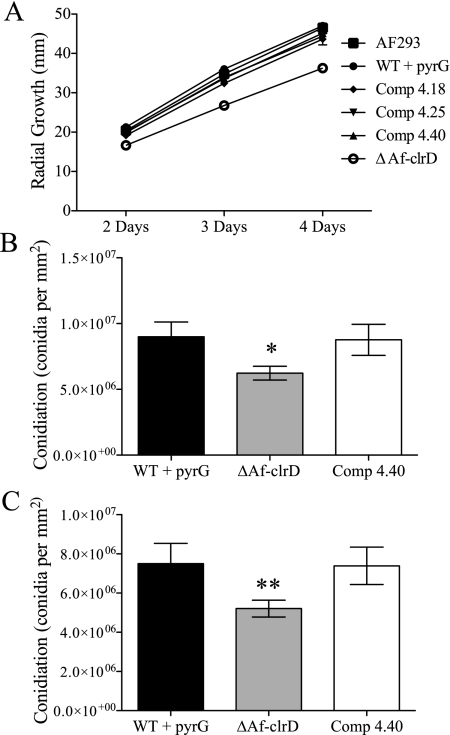
The ΔAfclrD mutant had reduced pigmentation on the reverse side of the colony, an increased white colony margin, and increased colony crenulation on GMM at 37°C (Fig. 4). The deletion strain showed a significant reduction in radial growth when cultures were grown on GMM at 37°C (Fig. 5A). Furthermore, conidial production was comparatively decreased in the ΔAfclrD mutant after 5 days of growth on GMM using both an overlay inoculation method and a point-inoculation method (Fig. 5B and C). No significant differences in germination rates from liquid stationary culture were observed (data not shown).
FIG. 4.
Phenotype of ΔAfclrD, control, and wild-type strains on glucose minimal medium plates. Gross phenotypic differences observed for the ΔAfclrD mutant include reduced radial growth, reduced conidiation, presence of a white colony margin, and colony crenulation after growth for 3 days at 37°C on GMM. Strain numbers correspond to the following genotypes: WT, AF293; WT with pyrG, TJW55.1; ΔAfclrD, TJMP1.11; Comp 4.40, TRMP4.40.
FIG. 5.
Quantification of colony diameter and conidial production in ΔAfclrD, complemented control, and wild-type strains. Standard physiological experiments demonstrated growth abnormalities in the ΔAfclrD strain. (A) Radial growth of ΔAfclrD on GMM at 37°C was significantly reduced compared to the wild type (AF293; P < 0.001) and complemented control strains were not significantly different than wild type (AF293). (B and C) Conidial production is reduced after 5 days incubation at 37°C in ΔAfclrD (TJMP1.11) compared to wild type with pyrG (TJW55.1) and an AfclrD-complemented control strain (TRMP4.40) (*, P = 0.026; **, P = 0.028). Error bars indicate 1 standard deviation.
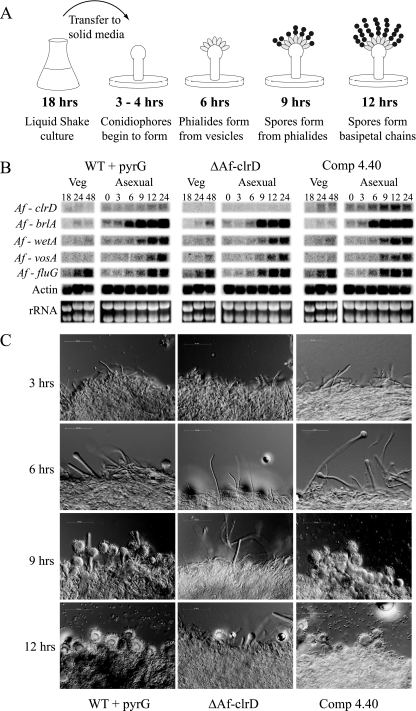
To further examine the mechanism underlying the delayed conidiogenesis, developmental competence assays were performed as per the methods of Mah and Yu (27). These assays distinguish between the ability to initiate conidiophore formation (e.g., competence) versus a delay in conidiophore formation once development has started (Fig. 6A). Liquid shake cultures were grown for either 18 or 24 h and then shifted to solid medium. Conidiophore formation was temporally identical in both regimens after the shift, indicating that ΔAfclrD is delayed in conidiogenesis after reaching competence. ΔAfclrD was delayed in conidiophore formation by 3 to 4 h (Fig. 6C).
FIG. 6.
Developmental competence assays to compare ΔAfclrD, control, and wild-type strains. (A) Diagram of the developmental competence assay and relative timing of conidiophore development in wild-type A. fumigatus. (B) Northern analysis to determine expression of key genes in the conidiogenesis pathway. Expression of a critical activator, AfbrlA, is delayed in ΔAfclrD. (C) Microscopic analysis of tissue from a developmental competence assay. The ΔAfclrD mutant (TJMP1.11) does not start to produce phialides until ∼9 h after reaching competence, while wild type with pyrG (TJW55.1) and the complemented control (TRMP4.40) strains produced phialides by the 6-hour time point.
Conidiophore development is under strict control of several genes in Aspergillus spp. (1). Northern analysis of four conidiogenesis genes (AfbrlA, AfwetA, AffluG, and AfvosA [27, 33]) indicated a clear delay in expression of the transcriptional activator AfbrlA and possibly a slight delay in AfwetA, but not AffluG or AfvosA (Fig. 6B).
ΔAfclrD is sensitive to 6-azauracil.
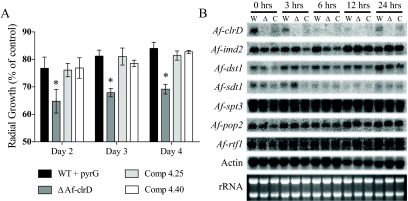
Histone methyltransferase mutants are frequently sensitive to chromatin-targeting inhibitors. Mutants generated in this study were screened for sensitivity to thiabendazole (a microtubule-destabilizing agent), hydroxyurea (an inhibitor of ribonucleotide reductase and indicator of DNA replication efficiency), and 6AU (an inhibitor of guanine nucleotide synthesis and indicator of transcriptional defects). The ΔAfclrD strain did not show differential sensitivity to thiabendazole, and there was only a slight trend toward higher susceptibility to hydroxyurea than the wild type (data not shown). However, the mutant did show increased sensitivity to 6AU at all concentrations tested (25 μg/ml, 50 μg/ml, 100 μg/ml, and 300 μg/ml) in a radial growth assay (data not shown). Additionally, ΔAfclrD remained sensitive to 6AU (100 μg/ml) over time (Fig. 7A).
FIG. 7.
ΔAfclrD is more sensitive to 6AU than wild-type or complemented control strains. (A) The ΔAfclrD mutant (TJMP1.11) remained sensitive over 3 days of exposure to 100 μg/ml 6AU compared to the wild type with pyrG (TJW55.1) and two complemented control strains (TRMP4.25 and TRMP4.40). Error bars indicate 1 standard deviation (*, P < 0.001). (B) Northern analysis of mycelia challenged with 6AU resulted in induction of Afimd2 and Afsdt1 to similar levels in all three strains. In addition, no detectable difference in expression between strains was seen for genes involved in IMP deyhydrogenase-independent 6AU sensitivity in yeast (Afdst1, Afspt3, Afpop2, and Afrtf1). Abbreviations: W, TJW55.1; Δ, TJMP1.11; C, TRMP4.40.
To explore in more detail the mechanism of the 6AU response, we analyzed expression of IMP dehydrogenase (Afimd2) and pyrimidine nucleotidase (Afsdt1). Neither Afimd2 nor Afsdt1 was differentially induced in ΔAfclrD compared to control strains upon challenge with 100 μg/ml 6AU (Fig. 7B). Additionally, genes involved in 6AU sensitivity that are independent of IMP dehydrogenase activity in yeast (Afdst1, Afspt3, Afpop2, and Afrtf1) were examined but were not differentially expressed in the ΔAfclrD mutant compared to control strains (Fig. 7B).
Conidial uptake by macrophages is unaltered in ΔAfclrD.
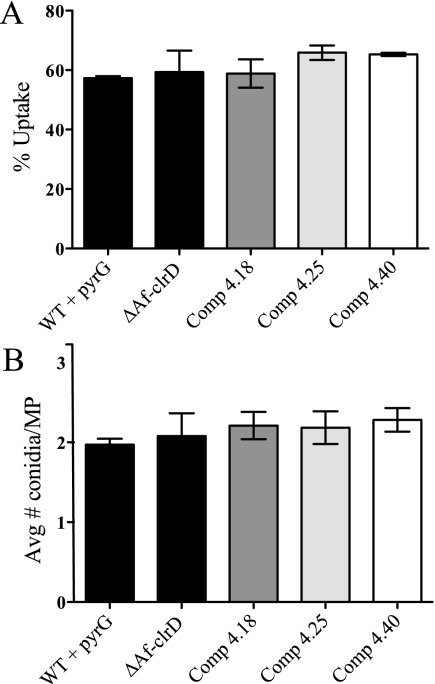
Alveolar macrophages represent the first line of host defense to inhaled Aspergillus conidia. To determine whether AfclrD contributes to A. fumigatus pathogenesis, we conducted macrophage uptake experiments using the RAW 264.7 and MH-S cell lines. We have observed that both cell lines phagocytose Aspergillus conidia similarly to primary murine alveolar macrophages (unpublished results). Neither the number of macrophages containing conidia nor the average number of conidia per macrophage was significantly different among strains (Fig. 8 and data not shown). Additionally, ΔAfclrD conidia displayed similar susceptibility as wild-type conidia to hydrogen peroxide, an indicator of reaction to host reactive oxygen species (data not shown). These experiments suggest that AfclrD may be dispensable for defense against host alveolar macrophages.
FIG. 8.
Conidial uptake by macrophages is not altered in ΔAfclrD. RAW 264.7 macrophages were coincubated with freshly harvested conidia of the wild-type, ΔAfclrD, and three complemented controls for 1 hour at 37°C, washed, and incubated an additional 2 hours to permit conidial phagocytosis. Cultures were fixed and stained with calcofluor white to label extracellular conidia. The percent uptake (percentage of macrophages containing one or more conidia) (A) and the average number of conidia per macrophage (of those containing conidia) (B) were calculated from triplicate wells per strain. At least 100 macrophages were counted per well. Means are presented, and error bars indicate 1 standard deviation. Data represent at least three independent experiments.
DISCUSSION
Chromatin remodeling affects a range of developmental processes in a variety of organisms, from mitotic defects in fission yeast (2, 14) to clinical diseases in humans (reviewed in references 7 and 19). These studies highlight the impact of H3K9 methylation on normal growth and development. Here, we provide evidence that AfClrD is a lysine-9 histone-3 methyltransferase required for normal growth and asexual development in the opportunistic pathogen A. fumigatus. Our data suggest that the growth abnormalities associated with loss of methylation at H3K9 could be due to transcriptional impairment, possibly associated with defects in transcriptional elongation machinery as evidenced by 6AU sensitivity of the ΔAfclrD strain. Furthermore, AfClrD does not appear to be critical for host interactions with A. fumigatus, as sensitivity to hydrogen peroxide and phagocytosis of conidia by macrophages were unaffected in the ΔAfclrD strain compared to wild-type and complemented control strains. Other A. fumigatus mutants harboring growth defects have similarly shown little to no effect on pathogenicity (12, 39).
Immunoblot analysis of nuclear extracts from ΔAfclrD and complemented control strains indicates strong involvement of AfClrD in mono- and trimethylation of H3K9, but not in dimethylation. Several studies from model organisms suggest that different methyltransferases are involved in differential methylation of H3K9 at distinct chromosomal regions. For example, mammalian H3K9 trimethylation by SUV39H1 is associated with pericentric heterochromatin, but centromeric regions contain dimethylated H3K9 independent of SUV39H1 activity (25). Additionally, the human G9a histone methyltransferase is associated with euchromatic gene activation by mono- and dimethylation of H3K9 (24, 45). Our work implies the existence of at least one other H3K9 methyltransferase in A. fumigatus. Of 13 SET domain-containing proteins we have identified in the A. fumigatus genome, at least 5 have very low homology to other described methyltransferases (data not shown). A possible A. fumigatus homolog of Drosophila Ash1 is a plausible candidate, because Ash1 has been shown to methylate H3K4, H3K9, and H4K20 (6).
Growth abnormalities have been reported for mutants lacking H3K9 trimethylation. Tamaru and Selker (47) reported that dim-5 null mutants of N. crassa had reduced apical growth and altered asexual development reminiscent of the decreased radial growth and delayed conidiation observed in ΔAfclrD. The authors suggested that trimethylation at H3K9 was necessary for DNA methylation by DIM-2 (47, 48). However, it is important to note that DNA methylation is minimal in Aspergillus spp. (23, 46) and therefore is not likely to play a key role in epigenetic regulation in this genus. Moreover, N. crassa DIM-2 null mutants do not show the same growth abnormalities as dim-5 mutants, suggesting that trimethylation at H3K9 in N. crassa affects growth and development independently of DNA methylation (11). Contrary to the methylation patterns of H3K9 observed in A. fumigatus, mono- and dimethylation of H3K9 were not detected in wild-type N. crassa (11, 48). We propose that despite similarities in developmental phenotypes of the N. crassa dim-5 and A. fumigatus clrD mutants, the mechanisms leading to these aberrations may take place through alternative routes.
Significantly reduced radial growth, a decrease in conidial production, and a temporal shift in conidiophore maturation were observed in the ΔAfclrD strain, but competence (the ability to form conidiophores) was not affected. Decreased conidial production and delay in maturation may be a function of delayed AfbrlA expression. BrlA is an essential transcription factor coordinating asexual development, and null mutants of BrlA in both A. nidulans and A. fumigatus exhibit completely suppressed conidial production (27). Aberrations in brlA expression are commonly associated with delays or reduction in conidiophore formation (43, 51). BrlA is also a primary regulator of development-specific gene expression during conidiation, including wetA. Accordingly, the slight delay in AfwetA expression observed in ΔAfclrD may be the result of delayed AfbrlA expression.
Recently, a novel regulator of sporogenesis, VosA, has been described. VosA contributes to asexual development, specifically to spore viability (33). We did not detect a delay in AfvosA expression, suggesting that once formed, conidia were fully functional in ΔAfclrD. Interestingly, Mah and Yu (27) reported that AffluG was not required for conidiation in A. fumigatus, contrary to its role in A. nidulans. However, AfbrlA expression was delayed in the ΔAfclrD mutant. AffluG expression was unaltered in the ΔAfclrD mutant background compared to wild type, indicating that AfClrD affects conidiation through an AfFluG-independent pathway and functions upstream of AfBrlA. Since the ΔAfclrD phenotype was pleiotropic, we investigated whether normal cellular metabolism was altered. Although fission yeast Clr4 null mutants were susceptible to the microtubule-destabilizing agent thiabendazole (14), we did not observe a reduction in radial growth on thiabendazole compared to control plates for the ΔAfclrD mutant (data not shown). ΔAfclrD mutants were slightly more susceptible to hydroxyurea treatment, but this trend was not statistically significant (data not shown). From these experiments, we concluded that ΔAfclrD does not have a serious mitotic defect.
In contrast, the ΔAfclrD strain showed increased sensitivity to 6AU, an indicator of defects in transcriptional elongation via inhibition of guanine nucleotide biosynthesis (17). 6AU has been used to identify mutants, including histone methyltransferases of Saccharomyces cerevisiae, which are defective in transcriptional elongation (30, 40). Our results are similar to those of other histone methyltransferase mutants (21, 26) and could imply a defect in GMP synthesis and/or impairment in transcriptional activity. 6AU inhibits IMP dehydrogenase (IMD2/PUR5), an enzyme catalyzing the first step of the GMP synthesis pathway (16). In S. cerevisiae, exposure to 6AU results in increased expression of IMD2/PUR5 as well as SDT1/SSM1, a pyrimidine nucleotidase required for detoxification of 6AU (31). S. cerevisiae mutants defective in transcriptional elongation pathways fail to induce IMP dehydrogenase after being challenged with 6AU. However, differences in IMP dehydrogenase (Afimd2) or pyrimidine nucleotidase (Afsdt1) transcript levels were not observed in ΔAfclrD, wild-type, or complemented control strains.
A large-scale screen of single-gene disruption mutants in S. cerevisiae identified several mutants susceptible to 6AU that did not differ in their ability to transcribe IMD2, suggesting alternative mechanisms of 6AU sensitivity (35). We examined expression patterns of genes known to be sensitive to 6AU in an IMD-independent manner in S. cerevisiae (35). However, no differences were observed in transcriptional patterns between the wild type and ΔAfclrD for transcriptional elongation factor S-II (Afdst1), a member of the PAF complex (Afrtf1), a member of the SAGA histone acetyltransferase complex (Afspt3), or a transcription factor associated with transcriptional processes (Afpop2). While a defect in transcriptional machinery could explain the pleiotropic phenotype of ΔAfclrD, understanding the mechanism of AfClrD involvement in 6AU sensitivity requires further analysis.
Acknowledgments
This material is based upon work supported in part by the U.S. Department of Agriculture, under agreement no. 59-0790-3-081. This is a cooperative project with the U.S. Wheat & Barley Scab Initiative. This research was also funded in part by NSF MCB-0236393 and NIH R01 AI065728-01A1 to N.P.K.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 10 October 2008.
REFERENCES
- 1.Adams, T. H., J. K. Wieser, and J. H. Yu. 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 6235-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allshire, R. C., E. R. Nimmo, K. Ekwall, J. P. Javerzat, and G. Cranston. 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9218-233. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen, E. C., and H. R. Horvitz. 2007. Two C. elegans histone methyltransferases repress lin-3 EGF transcription to inhibit vulval development. Development 1342991-2999. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson, S. P., C. M. Koch, G. K. Clelland, S. Willcox, J. C. Fowler, R. Stewart, M. Lako, I. Dunham, and L. Armstrong. 2008. Epigenetic marking prepares the human HOXA cluster for activation during differentiation of pluripotent cells. Stem Cells 261174-1185. [DOI] [PubMed] [Google Scholar]
- 6.Beisel, C., A. Imhof, J. Greene, E. Kremmer, and F. Sauer. 2002. Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature 419857-862. [DOI] [PubMed] [Google Scholar]
- 7.Bhaumik, S. R., E. Smith, and A. Shilatifard. 2007. Covalent modifications of histones during development and disease pathogenesis. Nat. Struct. Mol. Biol. 141008-1016. [DOI] [PubMed] [Google Scholar]
- 8.Bok, J. W., S. A. Balajee, K. A. Marr, D. Andes, K. F. Nielsen, J. C. Frisvad, and N. P. Keller. 2005. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot. Cell 41574-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boumil, R. M., and J. T. Lee. 2001. Forty years of decoding the silence in X-chromosome inactivation. Hum. Mol. Genet. 102225-2232. [DOI] [PubMed] [Google Scholar]
- 10.Calvo, A. M., J. W. Bok, W. Brooks, and N. P. Keller. 2004. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol. 704733-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins, R. E., M. Tachibana, H. Tamaru, K. M. Smith, D. Jia, X. Zhang, E. U. Selker, Y. Shinkai, and X. Cheng. 2005. In vitro and in vivo analyses of a Phe/Tyr switch controlling product specificity of histone lysine methyltransferases. J. Biol. Chem. 2805563-5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Gouvêa, P. F., F. M. Soriani, I. Malavazi, M. Savoldi, M. H. Goldman, O. Loss, E. Bignell, M. E. da Silva Ferreira, and G. H. Goldman. 2008. Functional characterization of the Aspergillus fumigatus PHO80 homologue. Fungal Genet. Biol. 451135-1146. [DOI] [PubMed] [Google Scholar]
- 13.Ebert, A., G. Schotta, S. Lein, S. Kubicek, V. Krauss, T. Jenuwein, and G. Reuter. 2004. su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 182973-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekwall, K., E. R. Nimmo, J. P. Javerzat, B. Borgstrøm, R. Egel, G. Cranston, and R. Allshire. 1996. Mutations in the fission yeast silencing factors clr4+ and rik1+ disrupt the localisation of the chromo domain protein Swi6p and impair centromere function. J. Cell Sci. 1092637-2648. [DOI] [PubMed] [Google Scholar]
- 15.Ekwall, K., and T. Ruusala. 1994. Mutations in rik1, clr2, clr3 and clr4 genes asymmetrically derepress the silent mating-type loci in fission yeast. Genetics 13653-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar-Henriques, M., and B. Daignan-Fornier. 2001. Transcriptional regulation of the yeast GMP synthesis pathway by its end products. J. Biol. Chem. 2761523-1530. [DOI] [PubMed] [Google Scholar]
- 17.Exinger, F., and F. Lacroute. 1992. 6-Azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr. Genet. 229-11. [DOI] [PubMed] [Google Scholar]
- 18.Grewal, S. I., and S. Jia. 2007. Heterochromatin revisited. Nat. Rev. Genet. 835-46. [DOI] [PubMed] [Google Scholar]
- 19.Hatchwell, E., and J. M. Greally. 2007. The potential role of epigenomic dysregulation in complex human disease. Trends Genet. 23588-595. [DOI] [PubMed] [Google Scholar]
- 20.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 2931074-1080. [DOI] [PubMed] [Google Scholar]
- 21.Krogan, N. J., M. Kim, A. Tong, A. Golshani, G. Cagney, V. Canadien, D. P. Richards, B. K. Beattie, A. Emili, C. Boone, A. Shilatifard, S. Buratowski, and J. Greenblatt. 2003. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 234207-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachner, M., R. J. O'Sullivan, and T. Jenuwein. 2003. An epigenetic road map for histone lysine methylation. J. Cell Sci. 1162117-2124. [DOI] [PubMed] [Google Scholar]
- 23.Lee, D. W., M. Freitag, E. U. Selker, and R. Aramayo. 2008. A cytosine methyltransferase homologue is essential for sexual development in Aspergillus nidulans. PLoS ONE 3e2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, D. Y., J. P. Northrop, M. H. Kuo, and M. R. Stallcup. 2006. Histone H3 lysine 9 methyltransferase G9a is a transcriptional coactivator for nuclear receptors. J. Biol. Chem. 2818476-8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehnertz, B., Y. Ueda, A. A. Derijck, U. Braunschweig, L. Perez-Burgos, S. Kubicek, T. Chen, E. Li, T. Jenuwein, and A. H. Peters. 2003. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr. Biol. 131192-1200. [DOI] [PubMed] [Google Scholar]
- 26.Li, B., L. Howe, S. Anderson, J. R. Yates, and J. L. Workman. 2003. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J. Biol. Chem. 2788897-8903. [DOI] [PubMed] [Google Scholar]
- 27.Mah, J. H., and J. H. Yu. 2006. Upstream and downstream regulation of asexual development in Aspergillus fumigatus. Eukaryot. Cell 51585-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchler-Bauer, A., J. B. Anderson, M. K. Derbyshire, C. DeWeese-Scott, N. R. Gonzales, M. Gwadz, L. Hao, S. He, D. I. Hurwitz, J. D. Jackson, Z. Ke, D. Krylov, C. J. Lanczycki, C. A. Liebert, C. Liu, F. Lu, S. Lu, G. H. Marchler, M. Mullokandov, J. S. Song, N. Thanki, R. A. Yamashita, J. J. Yin, D. Zhang, and S. H. Bryant. 2007. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 35D237-D240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, B. L., K. Y. Miller, and W. E. Timberlake. 1985. Direct and indirect gene replacements in Aspergillus nidulans. Mol. Cell. Biol. 51714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakanishi, T., A. Nakano, K. Nomura, K. Sekimizu, and S. Natori. 1992. Purification, gene cloning, and gene disruption of the transcription elongation factor S-II in Saccharomyces cerevisiae. J. Biol. Chem. 26713200-13204. [PubMed] [Google Scholar]
- 31.Nakanishi, T., and K. Sekimizu. 2002. SDT1/SSM1, a multicopy suppressor of S-II null mutant, encodes a novel pyrimidine 5′-nucleotidase. J. Biol. Chem. 27722103-22106. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama, J., J. C. Rice, B. D. Strahl, C. D. Allis, and S. I. Grewal. 2001. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292110-113. [DOI] [PubMed] [Google Scholar]
- 33.Ni, M., and J. H. Yu. 2007. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS ONE 2e970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun, M. Schmid, S. Opravil, K. Mechtler, C. P. Ponting, C. D. Allis, and T. Jenuwein. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406593-599. [DOI] [PubMed] [Google Scholar]
- 35.Riles, L., R. J. Shaw, M. Johnston, and D. Reines. 2004. Large-scale screening of yeast mutants for sensitivity to the IMP dehydrogenase inhibitor 6-azauracil. Yeast 21241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Santourlidis, S., N. Graffmann, J. Christ, and M. Uhrberg. 2008. Lineage-specific transition of histone signatures in the killer cell Ig-like receptor locus from hematopoietic progenitor to NK cells. J. Immunol. 180418-425. [DOI] [PubMed] [Google Scholar]
- 38.Schotta, G., A. Ebert, V. Krauss, A. Fischer, J. Hoffmann, S. Rea, T. Jenuwein, R. Dorn, and G. Reuter. 2002. Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. EMBO J. 211121-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrettl, M., H. S. Kim, M. Eisendle, C. Kragl, W. C. Nierman, T. Heinekamp, E. R. Werner, I. Jacobsen, P. Illmer, H. Yi, A. A. Brakhage, and H. Haas. 2008. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 7027-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw, R. J., and D. Reines. 2000. Saccharomyces cerevisiae transcription elongation mutants are defective in PUR5 induction in response to nucleotide depletion. Mol. Cell. Biol. 207427-7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu, K., and N. P. Keller. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shwab, E. K., J. W. Bok, M. Tribus, J. Galehr, S. Graessle, and N. P. Keller. 2007. Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot. Cell 61656-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soriani, F. M., I. Malavazi, M. E. da Silva Ferreira, M. Savoldi, M. R. Von Zeska Kress, M. H. de Souza Goldman, O. Loss, E. Bignell, and G. H. Goldman. 2008. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol. Microbiol. 671274-1291. [DOI] [PubMed] [Google Scholar]
- 44.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 40341-45. [DOI] [PubMed] [Google Scholar]
- 45.Tachibana, M., K. Sugimoto, T. Fukushima, and Y. Shinkai. 2001. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J. Biol. Chem. 27625309-25317. [DOI] [PubMed] [Google Scholar]
- 46.Tamame, M., F. Antequera, J. R. Villanueva, and T. Santos. 1983. High-frequency conversion to a “fluffy” developmental phenotype in Aspergillus spp. by 5-azacytidine treatment: evidence for involvement of a single nuclear gene. Mol. Cell. Biol. 32287-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamaru, H., and E. U. Selker. 2001. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414277-283. [DOI] [PubMed] [Google Scholar]
- 48.Tamaru, H., X. Zhang, D. McMillen, P. B. Singh, J. Nakayama, S. I. Grewal, C. D. Allis, X. Cheng, and E. U. Selker. 2003. Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat. Genet. 3475-79. [DOI] [PubMed] [Google Scholar]
- 49.Tribus, M., J. Galehr, P. Trojer, G. Brosch, P. Loidl, F. Marx, H. Haas, and S. Graessle. 2005. HdaA, a major class 2 histone deacetylase of Aspergillus nidulans, affects growth under conditions of oxidative stress. Eukaryot. Cell 41736-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trojer, P., M. Dangl, I. Bauer, S. Graessle, P. Loidl, and G. Brosch. 2004. Histone methyltransferases in Aspergillus nidulans: evidence for a novel enzyme with a unique substrate specificity. Biochemistry 4310834-10843. [DOI] [PubMed] [Google Scholar]
- 51.Tsitsigiannis, D. I., T. M. Kowieski, R. Zarnowski, and N. P. Keller. 2005. Three putative oxylipin biosynthetic genes integrate sexual and asexual development in Aspergillus nidulans. Microbiology 1511809-1821. [DOI] [PubMed] [Google Scholar]
- 52.Tzeng, T. Y., C. H. Lee, L. W. Chan, and C. K. Shen. 2007. Epigenetic regulation of the Drosophila chromosome 4 by the histone H3K9 methyltransferase dSETDB1. Proc. Natl. Acad. Sci. USA 10412691-12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiencke, J. K., S. Zheng, Z. Morrison, and R. F. Yeh. 2008. Differentially expressed genes are marked by histone 3 lysine 9 trimethylation in human cancer cells. Oncogene 272412-2421. [DOI] [PubMed] [Google Scholar]
- 54.Xue, T., C. K. Nguyen, A. Romans, D. P. Kontoyiannis, and G. S. May. 2004. Isogenic auxotrophic mutant strains in the Aspergillus fumigatus genome reference strain AF293. Arch. Microbiol. 182346-353. [DOI] [PubMed] [Google Scholar]