Abstract
Bdellovibrio bacteriovorus bacteria are predatory organisms that attack other gram-negative bacteria. Here, we report that Bd0714 is a Nudix dGTPase from B. bacteriovorus HD100 with a substrate specificity similar to that of Escherichia coli MutT and complements an E. coli mutT-deficient strain. We observed different transcription levels of the gene throughout the predator life cycle.
Bdellovibrio bacteriovorus bacteria are gram-negative Deltaproteobacteria and obligate predators of a variety of gram-negative bacteria, including some pathogens (22). They have a biphasic life cycle consisting of an extracellular attack phase and a replicative phase that occurs within an intact prey cell (Fig. 1) (14, 17, 23). Despite the fascinating predatory life cycle that involves two major lytic events, prey cell invasion and release of the progeny by lysis of the prey cell, the numerous hydrolytic enzymes present in their genome have not been characterized (14).
FIG. 1.
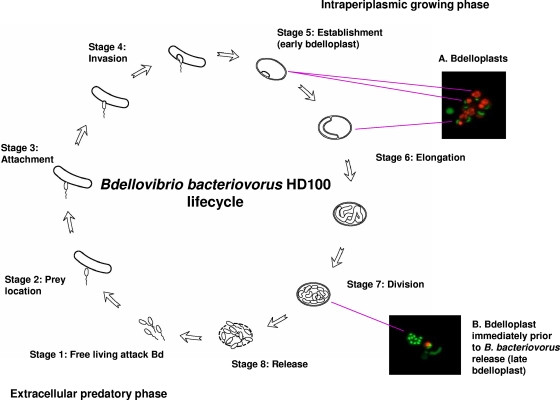
Bdellovibrio bacteriovorus life cycle depicted in eight stages. In the extracellular predatory phase (stage 1, free-living attack Bdellovibrio [Bd]), the predators search for (stage 2, prey location) and attach to (stage 3, attachment) prey cells. They enter the prey cell wall initiating the intraperiplasmic or replicative phase (stage 4, invasion) (24-26, 28). In the periplasm, they feed upon the cytoplasmic contents of the prey (stage 5, establishment or early bdelloplast) (10) and form what is called the bdelloplast. They elongate (stage 6, elongation or bdelloplast) and segmentate into approximately 6 to 10 smaller components (stage 7, division or late bdelloplast) (1, 15, 16), finally releasing the progeny by lysis of the prey (stage 8, release) (14, 20). Interestingly, some strains of Bdellovibrio can generate host-independent mutants that grow axenically (18). The inserts show fluorescence staining of bdelloplasts that distinguishes live cells from dead cells; BacLight-stained live bacteria with intact membranes are green (Bdellovibrio), and dead cells with disrupted membranes are red (prey, E. coli) in the indicated examples.
Nudix hydrolases are a superfamily of phosphoanhydrases that catalyze cation-dependent hydrolysis of a wide range of nucleoside diphosphates linked to another moiety X (Nudix) to yield NMP plus P-X (2). These enzymes are characterized by the presence of a highly conserved sequence, GX5EX7REUXEEXGU (where U is Ile, Leu, or Val) (2, 13). Nudix substrates include nucleoside triphosphates, such as 8-oxo-dGTP (11), biosynthetic metabolites such as dihydroneopterin triphosphate (7), 5′-triphosphate-RNA (5), capped mRNA (19), and other nucleotide diphosphate compounds. Since the concentrations of these substrate molecules must be stringently regulated, Nudix hydrolases can be described as “house-cleaning” enzymes (2, 12).
In silico sequence analysis of the B. bacteriovorus HD100 genome showed the presence of six open reading frames containing the Nudix consensus sequence. One of them, Bd0714, has the highest sequence identity with Escherichia coli MutT (3). Here, we report the characterization of Bd0714 by genetic analysis and complementation assays, as well as with the in vitro kinetic analysis of its product, indicating that it can function as the mutator enzyme in B. bacteriovorus HD100.
Nudix sequence identification.
Amino acid sequence searches for a functional homologue of MutT in Bdellovibrio bacteriovorus HD100 (gi:42494925) identified five putative Nudix hydrolases with canonical Nudix signature sequence (NP_967680, NP_969942, NP_969058, NP_967241, and NP_969548) and one with a modified signature sequence (NP_967621) (Fig. 2). One of these sequences, NP_967680 (Bd0714), shares 43 identical residues of the total 153 (28.1% identity) with E. coli MutT.
FIG. 2.
Nudix sequences of the Bdellovibrio bacteriovorus genome. Bd0714 (NP_967680), Bd3179 (NP_969942), Bd2220 (NP_969058), Bd0236 (NP_967241), Bd2755 (NP_969548), and one modified signature sequence in Bd0654 (NP_967621). The black background indicates identity in a proline residue and in the Nudix signature sequence. Bd0654, the open reading frame with the modified signature sequence, has a lysine residue instead of the typical first glutamate in GX5EX7REUXEEXGU. The boxes show homologous residues. The alignment was done with ClustalW and the figure with ESPript (8).
Substrate specificities and kinetics of Bd0714 and Bd0714-E70Q.
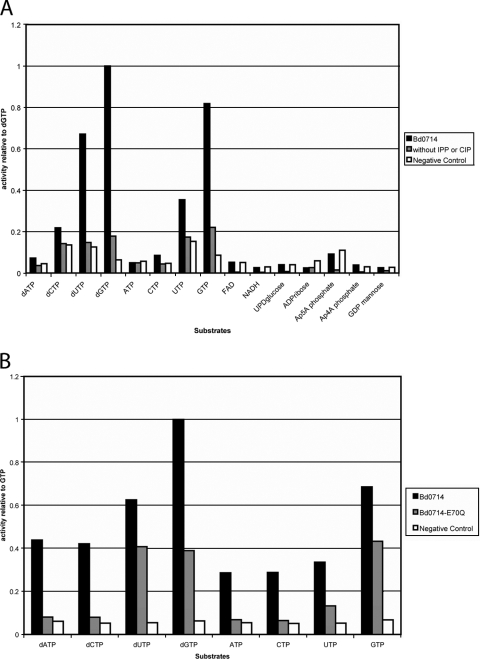
The open reading frame corresponding to Bd0714 was cloned into pET24a (pBd0714; bacterial strains and plasmids are listed in Table S1 in the supplemental material). The Bd0714 protein that was overexpressed and purified to a >95% homogeneity (see the supplemental material) was shown to be a dimer by gel filtration chromatography. Purified enzyme hydrolyzed the eight canonical nucleoside triphosphates tested with a strong preference for dGTP (Fig. 3). The wild-type enzyme was optimally active with Mg2+ as the divalent cation and less or nonactive with Mn2+, depending on the substrate tested (data not shown). Bd0714 hydrolyzes pyrophosphates but lacks (exo) phosphatase activity, since no release of phosphate is observed when inorganic pyrophosphatase is left out of the reaction (Fig. 3A). This indicates that, as in the case of MutT, Bd0714 cleaves the α-β pyrophosphate bond of dGTP.
FIG. 3.
(A) Relative activity of the Bd0714 for possible nucleotide substrates; dGTPase activity was normalized to 100%. Black bars correspond to the colorimetric assay with added enzyme, gray bars correspond to all components of the assay except for inorganic pyrophosphatase (IPP) or calf intestinal phosphatase enzyme (CIP), and white bars correspond to the reaction carried out without the Bd0714 enzyme. (B) Relative activity of Bd0714 versus Bd0714-E70Q for deoxynucleoside triphosphates, with dGTP normalized to 100% activity.
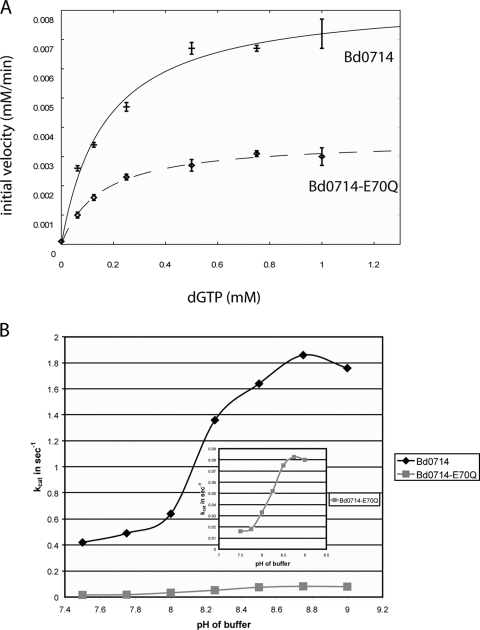
The Km value of wild-type Bd0714 for dGTP is 268 μM, and the kcat value is 6.03 s−1 (Fig. 4A and 4B). The kcat and Km values for dGTP are very close to those determined for E. coli MutT. As with MutT from E. coli, the Bd0714 protein prefers dGTP but is able to cleave other deoxynucleoside triphosphate and nucleoside triphosphate substrates to some degree, in particular dUTP (4) (Fig. 3). This biochemical evidence points to the identification of Bd0714 as a MutT equivalent.
FIG. 4.
Kinetic data for Bd0714 and Bd0714-E70Q. (A) Initial velocities as a function of dGTP concentration. The continuous curves correspond to nonlinear least-square fits of the data to the Michaelis-Menten equation. (B) Plot of the kcat values of pH in the range of 7.5 to 9 for Bd0714 and Bd0714-E70Q.
In some Nudix hydrolases, such as E. coli ADPRase, the glutamate after the arginine in the Nudix signature sequence is a bifurcated ligand of the required catalytic metal (6). Mutational analysis showed that this residue is the catalytic base in E. coli MutT (9). Mutation of this residue (E70Q) results in a reduction of the catalytic activity in Bd0714-E70Q (Fig. 3B and 4B). However, whereas in MutT this mutation leads to a 50,000-fold drop in kcat (Fig. 4A) (9), in B. bacteriovorus it results in only a 13.5-fold drop in kcat with no effect on Km. Furthermore, in contrast to its effect in MutT, this mutation does not flatten or shift the pH profile of the enzyme, indicating that Glu70 may not be the catalytic base, but only a ligand of the catalytic metal (Fig. 4B). Nevertheless, the kinetic evidence still points to Bd0714 as having MutT type activity, but likely with an altered mechanism in which Glu70 is not the catalytic base. This may suggest that Bd0714 has an additional role in B. bacteriovorus in addition to its role as a mutator enzyme.
Complementation of the mutator phenotype.
To obtain direct evidence that the Bd0714 enzyme is a functional homologue of MutT, the Bd0714 gene was expressed in an E. coli strain in which the mutT gene was inactivated (SB3 mutT mutant) (see Table S1 in the supplemental material) (3). The SB3 mutT mutant was transformed with pTrc99a, pTrc99mutT, or pTrc99Bd0714 (see Table S1 in the supplemental material) and challenged with media supplemented with either streptomycin or nalidixic acid, and the mutation frequencies per 109 cells were evaluated. The SB3 mutant harboring pTrc99Bd0714 had a much lower mutation frequency than did the SB3 mutant harboring pTrc99A in both media (Table 1). This rate is even lower than that observed in the SB3 mutant transformed with pTrc99mutT, which had a threefold-higher mutational frequency. This analysis showed that Bd0714 can efficiently complement MutT function in E. coli SB3. The comparable kinetic and biochemical profiles, along with the complementation analysis, indicate that Bd0714 can function as a mutator enzyme in B. bacteriovorus.
TABLE 1.
Mutation frequencies of the E.coli SB3 mutT mutants transformed with different plasmids
| Plasmid | No. of indicated mutants per 109 cells
|
|
|---|---|---|
| Streptomycin resistant | Nalidixic acid resistant | |
| pTrc99A | 491 ± 206 | 1,123 ± 257 |
| pTrc99mutT | 50 ± 26 | 79 ± 75 |
| pTrc99Bd0714 | 7 ± 2 | 23 ± 2 |
B. bacteriovorus HD100 Bd0714 deletion mutant.
A deficiency of the mutT gene in E. coli has been shown to increase the level of spontaneous mutations compared to that in wild-type E. coli (27). To evaluate the functional role of Bd0714 in B. bacteriovorus, an in-frame deletion mutant was constructed by allelic exchange (21). An extremely low frequency of knockout mutants was found during the construction, compared with the results of previous work (21); only one deletion excisant was recovered on a total of 123 plaques screened by PCR.
Both the B. bacteriovorus wild-type and ΔBd0714 deletion strains were grown in liquid coculture along with E. coli SB3 prey and plated with E. coli SB3Nal prey in media supplemented with nalidixic acid to determine the difference in the number of spontaneous mutants obtained between the wild-type and Bd0714 mutant strains. No mutants were found for either strain. Similar phenotypic analysis of the mutant strain could not be performed using streptomycin since the wild-type B. bacteriovorus is already streptomycin resistant (21). The B. bacteriovorus wild-type and ΔBd0714 strains were synchronically cultured and monitored by microscopy to determine the effect of the deletion on their life cycle; no substantial difference was found.
Transcription of the Bd0714 gene during the B. bacteriovorus life cycle.
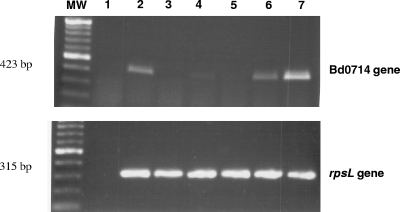
To better understand the role of Bd0714 during the B. bacteriovorus life cycle, transcription of the Bd0714 gene at various stages in the life cycle was monitored by reverse transcription-PCR (RT-PCR). RNA was extracted from B. bacteriovorus at stages 1, 5, 6, and 7 of the life cycle (Fig. 1). RNA was also obtained from cultures of the attack phase (stage 1) of the B. bacteriovorus HD100 ΔBd0714 mutant and from the host-independent B. bacteriovorus HI100. Transcription of the Bd0714 gene was observed during the free-swimming attack phase, but barely perceptible in stage 5 (establishment or early bdelloplast) (Fig. 5). However, increasing transcription was seen throughout the bdelloplast stages, reaching a maximum in stage 7 (late bdelloplast or prior to release). Similar levels of transcription were found in the host-independent strain and wild-type B. bacteriovorus in stage 6 (elongation). As further confirmation of the deletion, RT-PCR product was not observed in the extracellular attack phase for the HD100 ΔBd0714 deletion mutant, even though product was observed for the wild-type strain in the same stage in the life cycle.
FIG. 5.
RT-PCR analysis of the Bd0714 gene in B. bacteriovorus HD100, the ΔBd0714 mutant, and host-independent B. bacteriovorus. Total RNA was extracted from E. coli ML35 prey (lane 1), host-independent B. bacteriovorus (lane 2), the attack-phase ΔBd0714 mutant (lane 3), and wild-type B. bacteriovorus HD100 (lane 4, free-living attack phase; lane 5, early bdelloplast; lane 6, bdelloplast; and lane 7, late bdelloplast) throughout the life cycle and used in RT-PCRs with primers specific for the Bd0714 gene. The rpsL gene was used as a load control for B. bacteriovorus RNA. The absence of an RT-PCR product in lane 1 indicates that the product seen for both the Bd0714 and rpsL genes corresponds to B. bacteriovorus RNA rather than to any possible contaminating E. coli RNA. MW, molecular weight marker.
Biochemical and molecular evidence indicates that Bd0714 is a mutator enzyme in B. bacteriovorus. However, while most of the evidence points to Bd0714 as an E. coli MutT orthologue, kinetic analysis of the Glu70 mutant enzyme indicates that, in contrast to MutT, in Bd0714, this residue is not the catalytic base. These results suggest that Bd0714 and MutT may use different catalytic mechanisms, possibly reflecting the fact that Bd0714 may have additional physiological functions.
Supplementary Material
Acknowledgments
This work was supported by departmental start-up funds to S.A.P. Support was provided by NIGMS grant GM066895 to L.M.A.
Special thanks to Maurice J. Bessman for introducing us to the Nudix enzymes and for the donation of the E. coli SB3 strains. We thank Marcelo H. Amador for the rendition of Fig. 1.
Footnotes
Published ahead of print on 17 October 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Araki, Y., and E. G. Ruby. 1988. A soluble enzyme activity that attaches free diaminopimelic acid to bdelloplast peptidoglycan. Biochemistry 272624-2629. [DOI] [PubMed] [Google Scholar]
- 2.Bessman, M. J., D. N. Frick, and S. F. O'Handley. 1996. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 27125059-25062. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar, S. K., and M. J. Bessman. 1988. Studies on the mutator gene, mutT of Escherichia coli. Molecular cloning of the gene, purification of the gene product, and identification of a novel nucleoside triphosphatase. J. Biol. Chem. 2638953-8957. [PubMed] [Google Scholar]
- 4.Bhatnagar, S. K., L. C. Bullions, and M. J. Bessman. 1991. Characterization of the mutT nucleoside triphosphatase of Escherichia coli. J. Biol. Chem. 2669050-9054. [PubMed] [Google Scholar]
- 5.Deana, A., H. Celesnik, and J. G. Belasco. 2008. The bacterial enzyme RppH triggers messenger RNA degradation by 5′ pyrophosphate removal. Nature 451355-358. [DOI] [PubMed] [Google Scholar]
- 6.Gabelli, S. B., M. A. Bianchet, M. J. Bessman, and L. M. Amzel. 2001. The structure of ADP-ribose pyrophosphatase reveals the structural basis for the versatility of the Nudix family. Nat. Struct. Biol. 8467-472. [DOI] [PubMed] [Google Scholar]
- 7.Gabelli, S. B., M. A. Bianchet, W. Xu, C. A. Dunn, Z. D. Niu, L. M. Amzel, and M. J. Bessman. 2007. Structure and function of the E. coli dihydroneopterin triphosphate pyrophosphatase: a Nudix enzyme involved in folate biosynthesis. Structure 151014-1022. [DOI] [PubMed] [Google Scholar]
- 8.Gouet, P., E. Courcelle, D. I. Stuart, and F. Metoz. 1999. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15305-308. [DOI] [PubMed] [Google Scholar]
- 9.Harris, T. K., G. Wu, M. A. Massiah, and A. S. Mildvan. 2000. Mutational, kinetic, and NMR studies of the roles of conserved glutamate residues and of lysine-39 in the mechanism of the MutT pyrophosphohydrolase. Biochemistry 391655-1674. [DOI] [PubMed] [Google Scholar]
- 10.Hespell, R. B., R. A. Rosson, M. F. Thomashow, and S. C. Rittenberg. 1973. Respiration of Bdellovibrio bacteriovorus strain 109J and its energy substrates for intraperiplasmic growth. J. Bacteriol. 1131280-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maki, H., and M. Sekiguchi. 1992. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 355273-275. [DOI] [PubMed] [Google Scholar]
- 12.McLennan, A. G. 2006. The Nudix hydrolase superfamily. Cell. Mol. Life Sci. 63123-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mildvan, A. S., Z. Xia, H. F. Azurmendi, V. Saraswat, P. M. Legler, M. A. Massiah, S. B. Gabelli, M. A. Bianchet, L. W. Kang, and L. M. Amzel. 2005. Structures and mechanisms of Nudix hydrolases. Arch. Biochem. Biophys. 433129-143. [DOI] [PubMed] [Google Scholar]
- 14.Rendulic, S., P. Jagtap, A. Rosinus, M. Eppinger, C. Baar, C. Lanz, H. Keller, C. Lambert, K. J. Evans, A. Goesmann, F. Meyer, R. E. Sockett, and S. C. Schuster. 2004. A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303689-692. [DOI] [PubMed] [Google Scholar]
- 15.Romo, A. J., E. G. Ruby, and M. H. Saier, Jr. 1992. Effect of Bdellovibrio bacteriovorus infection on the phosphoenolpyruvate:sugar phosphotransferase system in Escherichia coli: evidence for activation of cytoplasmic proteolysis. Res. Microbiol. 1435-14. [DOI] [PubMed] [Google Scholar]
- 16.Ruby, E. G., and J. B. McCabe. 1988. Metabolism of periplasmic membrane-derived oligosaccharides by the predatory bacterium Bdellovibrio bacteriovorus 109J. J. Bacteriol. 170646-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidler, R. J., and M. P. Starr. 1969. Factors affecting the intracellular parasitic growth of Bdellovibrio bacteriovorus developing within Escherichia coli. J. Bacteriol. 97912-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidler, R. J., and M. P. Starr. 1969. Isolation and characterization of host-independent Bdellovibrios. J. Bacteriol. 100769-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.She, M., C. J. Decker, N. Chen, S. Tumati, R. Parker, and H. Song. 2006. Crystal structure and functional analysis of Dcp2p from Schizosaccharomyces pombe. Nat. Struct. Mol. Biol. 1363-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starr, M. P., and N. L. Baigent. 1966. Parasitic interaction of Bdellovibrio bacteriovorus with other bacteria. J. Bacteriol. 912006-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steyert, S. R., and S. A. Pineiro. 2007. Development of a novel genetic system to create markerless deletion mutants of Bdellovibrio bacteriovorus. Appl. Environ. Microbiol. 734717-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolp, H., and H. Petzold. 1962. Untersuchungen uber einen obligat parasitischen mikroorganismus mit lytischer aktivita tfur Pseudomonas bakterien. Phytopathology 45364-390. [Google Scholar]
- 23.Strauch, E., D. Schwudke, and M. Linscheid. 2007. Predatory mechanisms of Bdellovibrio and like organisms. Future Microbiol. 263-73. [DOI] [PubMed] [Google Scholar]
- 24.Thomashow, M. F., and S. C. Rittenberg. 1978. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: attachment of long-chain fatty acids to Escherichia coli peptidoglycan. J. Bacteriol. 1351015-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomashow, M. F., and S. C. Rittenberg. 1978. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: N-deacetylation of Escherichia coli peptidoglycan amino sugars. J. Bacteriol. 1351008-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomashow, M. F., and S. C. Rittenberg. 1978. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J. Bacteriol. 135998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treffers, H. P., V. Spinelli, and N. O. Belser. 1954. A factor (or mutator gene) influencing mutation rates in Escherichia coli. Proc. Natl. Acad. Sci. USA 401064-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tudor, J., M. McCann, and I. Acrich. 1990. A new model for the penetration of prey cells by bdellovibrios. J. Bacteriol. 1722421-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.