Abstract
Queuosine (Q) and archaeosine (G+) are hypermodified ribonucleosides found in tRNA. Q is present in the anticodon region of tRNAGUN in Eukarya and Bacteria, while G+ is found at position 15 in the D-loop of archaeal tRNA. Prokaryotes produce these 7-deazaguanosine derivatives de novo from GTP through the 7-cyano-7-deazaguanine (pre-Q0) intermediate, but mammals import the free base, queuine, obtained from the diet or the intestinal flora. By combining the results of comparative genomic analysis with those of genetic studies, we show that the first enzyme of the folate pathway, GTP cyclohydrolase I (GCYH-I), encoded in Escherichia coli by folE, is also the first enzyme of pre-Q0 biosynthesis in both prokaryotic kingdoms. Indeed, tRNA extracted from an E. coli ΔfolE strain is devoid of Q and the deficiency is complemented by expressing GCYH-I-encoding genes from different bacterial or archaeal origins. In a similar fashion, tRNA extracted from a Haloferax volcanii strain carrying a deletion of the GCYH-I-encoding gene contains only traces of G+. These results link the production of a tRNA-modified base to primary metabolism and further clarify the biosynthetic pathway for these complex modified nucleosides.
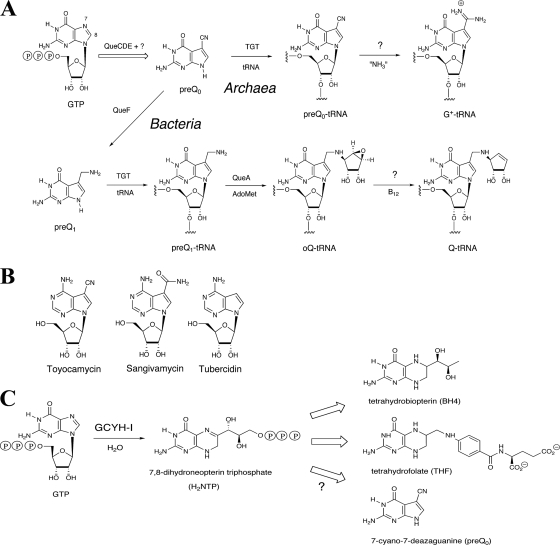
It is well established that GTP is not only a molecule central to energy conservation and a precursor to the synthesis of RNA and DNA but also the precursor to a number of essential metabolites. These include riboflavin and the pterin-related coenzymes tetrahydropterin (BH4), tetrahydrofolate (THF), methanopterin, and molybdopterin. It is less well known that GTP is also the precursor of the 7-deazapurine derivatives found in tRNA (queuosine [Q] and archaeosine [G+]) (Fig. 1A) and in secondary metabolites such as toyocamacin (Fig. 1B) (26, 48).
FIG. 1.
(A) Biosynthesis of Q and G+. (B) Structure of toyacamacin. (C) The role of GCYH-I in the biosynthesis of BH4, THF, and pre-Q0.
Due to the essential role of BH4 and THF as cofactors in many metabolic reactions and their links to inborn diseases (29, 49), their biosynthetic pathways have been extensively studied and fully characterized at the molecular level in several model organisms (8, 16). The first enzyme of the THF pathway, GTP cyclohydrolase I (GCYH-IA; encoded in Escherichia coli by the folE gene) (19) catalyzes the conversion of GTP to 7,8-dihydroneopterin triphosphate (Fig. 1C), a complex reaction that begins with hydrolytic ring opening of the purine ring at C-8 to generate an N-formyl intermediate, followed by deformylation and subsequent rearrangement and cyclization of the ribosyl moiety to generate the pterin ring (34, 58) (Fig. 1C). A homologous GCYH-I is found in mammals, where it catalyzes the first step of the BH4 pathway (Fig. 1C) (49). Recently, a distinct class of GCYH-I enzymes, GCYH-IB (encoded by the folE2 gene), was discovered in prokaryotes (12, 17).
Q and G+ are among the most-complex modified nucleosides found in tRNA. Q is ubiquitous in prokaryotes and eukaryotes and is located in the wobble position of a subset of tRNAs possessing a GUN anticodon, while G+ is found at position 15 in most archaeal tRNAs. Although Q is present in both mammals and bacteria, only bacteria are capable of de novo Q biosynthesis. Eukaryotes acquire Q from the diet and/or from the intestinal flora (21) and insert queuine, the free base of Q, directly into the appropriate tRNAs (44). The pathway for deazapurine biosynthesis is not as well characterized as are the THF and BH4 pathways, but the results of early radiolabeling experiments established that GTP is the probable primary precursor of 7-deazapurines (26, 47, 48). In Salmonella enterica serovar Typhimurium, incorporation into Q was observed with [2-14C]guanine, but not with [8-14C]guanine, suggesting a loss of carbon-8 in a process analogous to that observed in the early step of pteridines and folic acid (25). In Streptomyces, a nonconstitutive GCYH was implicated in toyocamycin biosynthesis (10, 11). It was recently shown that a GCYH-I gene was indeed involved in deazapurine biosynthesis (31).
The first established intermediate in the Q pathway is 7-cyano-7-deazaguanine (pre-Q0) (37) (Fig. 1A). In bacteria, pre-Q0 undergoes reduction to 7-aminomethyl-7-deazaguanine (pre-Q1) in a reaction catalyzed by the NADPH-dependent pre-Q0 oxidoreductase (QueF; EC 1.7.1.13). Interestingly, while it catalyzes disparate chemistry, QueF is homologous to GCYH-IA (50). Pre-Q1 is subsequently inserted into tRNA by the enzyme tRNA-guanine transglycosylase (TGT; EC 2.4.2.29) (36, 37), which is encoded in E. coli by the tgt gene (35). The rest of the biosynthesis of Q occurs at the level of the tRNA and involves the formation of epoxyqueuosine by S-adenosylmethionine:tRNA ribosyltransferase-isomerase (QueA; EC 2.4.2.-) (22, 45, 46), followed by reduction of the epoxide in epoxyqueuosine by an as-yet-unidentified enzyme to give Q (13).
Pre-Q0 is also the precursor of G+ (51) and is inserted into tRNA by an archaeal TGT enzyme (5). The formation of G+ then occurs through the formal addition of ammonia to the nitrile of pre-Q0, but the enzyme responsible for this reaction is not known. Three other protein families essential for the formation of Q have been identified: the ATPase family member QueC (COG0603), the 6-pyruvoyl-tetrahydropterin synthase-like enzyme QueD (COG0720), and the radical-S-adenosylmethionine family member QueE (COG0602) (14, 40). Mutants with the corresponding genes deleted lack Q in tRNA, but the role of the corresponding proteins remains to be determined. However, the fact that these three families are found in both the Archaea and Bacteria (40) implicates them in the early steps of the pathway prior to pre-Q0 formation, as this metabolite is the last common intermediate in the pathways to Q and G+ (Fig. 1A).
Using a combination of comparative genomics and experimental validation, we demonstrate that GCYH-I is not only the first enzyme of the THF and BH4 pathways, but also the first enzyme of Q and G+ biosynthesis.
MATERIALS AND METHODS
Bioinformatics.
Analysis of the Q and G+ subsystem was performed in the SEED database (38). Results are available in the “Queuosine-Archeosine Biosynthesis” subsystem on the public SEED server (http://theseed.uchicago.edu/FIG/subsys.cgi). We also used the BLAST tools and resources at NCBI (2). Annotations for paralog families were made using physical clustering on the chromosome when possible, by building phylogenetic trees using the ClustalW tool (7) integrated in SEED or by deriving specific protein motifs. The Holoferax volcanii genome sequence was accessed through the UCSC archaeal genome browser (43).
Strains, media, and growth conditions.
The strains used in this study are listed in Table 1. E. coli derivatives were routinely grown in LB medium (BD Diagnostic System) at 37°C. The growth media were solidified with 15 g/liter agar (BD Diagnostic System) for the preparation of plates. Transformations of E. coli were performed by following standard procedures (33, 42). Thymidine (dT; 300 μM), ampicillin (Amp; 100 μg/ml), kanamycin (Kan; 50 μg/ml), isopropyl-beta-d-thiogalactopyranoside (IPTG; 1 mM), and l-arabinose (0.02 to 0.2%) were added as required. H. volcanii derivatives were grown at 45°C in Hv-rich medium (20) (125 g NaCl, 50 g MgCl 6H2O, 2.5 g KSO4, 0.134 g CaCl 2H2O, 5 g tryptone, and 5 g yeast extract) supplemented when needed with 40 μg/ml dT.
TABLE 1.
Strains and plasmids
| Plasmid or strain | Description | Reference or source |
|---|---|---|
| Plasmids | ||
| pBAD24 | AmprcolE1 | 18 |
| pCN24pfolEEc | CmrcolE1 folEEc | 3 |
| pBY143.1 | pBAD24 folE2Bs | 12 |
| pBY144.1 | pBAD24 folESs | This work |
| pBY151.3 | pBAD24 folE2Mm | This work |
| Strains | ||
| E. coli | ||
| TOPO 10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara leu) 7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| W6 | K12 F+ SmrrelA1 spoT1 metB1 rrnB-2 creC510 | 27 |
| VDC5211 | W6 ΔfolE::Kanr | 23 |
| VDC5190 | W6 ΔfolE::Kanr pBAD24 | 12 |
| VDC7049 | W6ΔfolE::Kanr pfolEEc | 12 |
| VDC5196 | W6 ΔfolE::Kanr pfolE2Bs | 12 |
| VDC5200 | W6 ΔfolE::Kanr pfolESs | This work |
| VDC5198 | W6 ΔfolE::Kanr pfolE2Mm | This work |
| H. volcanii | ||
| H26 | ΔpyrE2 | 1 |
| VDC3235 | ΔpyrE2 ΔfolE2 | El Yacoubi et al., submitted |
aEc, E. coli; Bs, B. subtilis; Ss, S. solfataricus; Mm, M. maripaludis.
Plasmid constructions.
The Methanococcus maripaludis S2 folE2 (MMP0034) and the Sulfolobus solfataricus P2 folE1 (SSO0364) genes were PCR amplified from the respective genomic DNAs as previously described (12). The following primers, corresponding to the 5′ and 3′ regions of the genes, were used: MmfolE2 sense primer (5′-ATTCCATGGAATGCAATGACGTTCAGGCAA-3′), MmfolE2 antisense primer (5′-ATTCTGCAGTTAATTTGTTAAAGTTTTAAGTTC-3′), SsfolE1 sense primer (5′-ATTCCATGGAAAAGACAGAATTAGA-3′) and SsfolE1 antisense primer (5′-AATAAGCTTTCATAATAAGTTTACCTTATTTG-3′). PCR products were purified as described in reference 12, digested with NcoI/PstI for MmfolE2 and NcoI/SalI for SsfolE1, ligated into pBAD24 (18) previously digested with the appropriate endonucleases, and transformed into TOPO 10 cells (Invitrogen, Carlsbad, CA). The resulting constructs, pBY151.3 and pBY144.1, respectively, were confirmed by sequencing. To confirm the presence of the ΔfolE::Kanr allele and of the correct pBAD24 derivative, colonies from the different strains were analyzed by PCR using oligonucleotides located upstream and downstream of the folE gene (ChkDfolE-ol1 [5′-CTCCTTGTTGTGTTGTTTGCAA-3′] and ChkDfolE-ol2 [5′-GGGGCAGCAACATTTGCAGG-3′]) or upstream and downstream of the polylinker in the pBAD24 derivatives (pBADrev2 [5′-TTCTGATTTATTCTGTATCAGGC-3′] and pBADol5 [5′AAGATTAGCGGATCCTACCTG-3′]).
Purification of bulk tRNA.
For E. coli derivatives, two liters of cultures were grown overnight at 37°C in multiple Fernbach flasks of LB medium supplemented with Amp (50 μg/ml), dT, arabinose, or IPTG when required; harvested; and then stored at −20°C. The cell pellets were defrosted and resuspended in 20 ml of buffer (10 mM Tris, 10 mM MgCl2, pH 7.4) and extracted with an equal volume of buffer-saturated phenol, pH 4.3 (Sigma). For H. volcanii derivatives, two liters of cell cultures in Hv-rich medium were grown until late log phase at 45°C in 3-liter Fernbach flasks, harvested, and then stored at −20°C. The cell pellets were defrosted and resuspended in 20 ml of buffer (10 mM Tris, 10 mM MgCl2, pH 7.4) and disrupted with an HC-8000 Microfluidizer processor (Microfluidics Corp., Newton, MA). The homogenate was treated with buffer-saturated phenol, pH 7.4 (Sigma). For both E. coli and H. volcanii tRNA preparations, the aqueous layer was collected after the phenol extraction step, and the RNA was precipitated with ethanol. The bulk tRNA was then purified on Nucleobond AX-400 columns (Clontech, Palo Alto, CA) (according to the manufacturer's protocol) and precipitated with isopropanol.
HPLC separation and electrospray MS-MS analysis of digested bulk tRNA.
To compare the nucleoside constituents of the bulk tRNA purified from the different strains, samples of each purified bulk tRNA preparation were enzymatically hydrolyzed as described by P. Crain (9). Approximately 100 μg of bulk tRNA was digested with 10 units of nuclease P1 (Sigma), 0.01 units of phosphodiesterase I (Sigma), and 3 μl of E. coli alkaline phosphatase (Sigma P4252) in a total volume of 113 μl. The digested extracts were lyophilized and resuspended in 20 microliters of water. The resuspended tRNA degradation extracts were injected into a liquid chromatography-tandem mass spectrometry (LC-MS-MS) system for separation and identification of the nucleosides. A C18-RP (Gemini 5μ, 250 by 4.6 mm; Phenomenex, Torrance, CA) analytical column was used, with a C18 cartridge to prolong its lifetime. The mobile phase consisted of two solvents: 250 mM of ammonium acetate, pH 6.0, for solvent A and 40% acetonitrile for solvent B. The gradient used for nucleoside separation was previously described in Pomerantz and McCloskey (39). It consisted of an elution gradient of several steps: 0 to 5% B in 7 min, 5 to 10% B in 15 min, 25 to 50% B in 5 min, 50 to 75% B in 4 min and stay at 75% B for 3 min, then 75 to 100% B in 8 min. The column was then washed at 100% B for 7 min and reequilibrated at 100% A for 10 min. The flow rate used was 1 ml·min−1. Chromatography was performed at room temperature. The elution of nucleosides was followed by UV detection at 254 nm. The high-performance liquid chromatography (HPLC) system was coupled to a hybrid triple-quadrupole ion trap (4000 Q-Trap, Applied Biosystems, Foster City, CA) MS equipped with a TurboIonSpray (TIS) interface operated in the positive ion mode. The electrospray ionization parameters were curtain gas at 30 lb/in2, ion source at 5,000 V, nebulizer gas at 45 lb/in2, TIS gas at 45 lb/in2, and TIS probe temperature at 300°C. The information-dependent acquisition mode of operation was employed, in which a survey scan from 100 to 600 m/z was acquired, followed by collision-induced dissociation of the two most-intense ions. Survey and MS-MS spectra for each information-dependent acquisition cycle were accumulated for 1 and 3 s, respectively. To compare tRNA concentrations, we compared the ratio of the levels of the Ψ-modified base (245 m/z) with the levels of Q (410 m/z) and G+ (325 m/z) in each sample by integrating the peak area from the extraction ion chromatograms. The MS-MS fragmentation data was also used to confirm the presence or absence of nucleosides Q and G+. All tRNA extractions and the analyses were performed at least twice independently.
RESULTS
Clustering of folE1 and folE2 with Q biosynthesis genes.
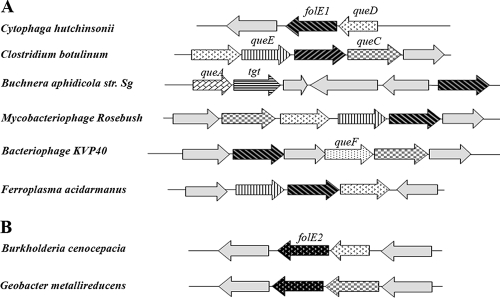
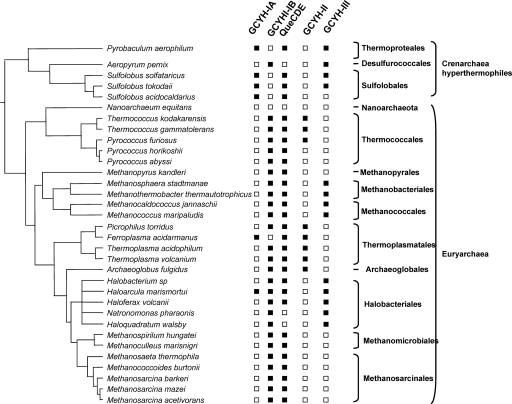
While QueF and GCYH-I are similar in sequence, they catalyze markedly different reactions (50). However, genes of this family are still annotated as GCYH-I or as “related to GCYH-I” in half of the genomes in GenBank. Previous structure-based alignment of GCYH-I and QueF led to the identification of a QueF signature motif, E78(S/L)K(S/A)XK(L/Y) (Y/F/W)85 (residue numbers are those of the Bacillus subtilis QueF ortholog YkvM) (50). The presence of this motif was used to propagate the QueF annotations in the SEED database (38): all homologs that contained the QueF signature motif where named “NADPH-dependent pre-Q0 reductase” so that both QueF and FolE orthologs were correctly annotated in all genomes. Taking advantage of the SEED subsystem clustering tool that will identify the genomes in which genes of a given subsystem are physically clustered by a coloring scheme, we added the folE gene in the “Queuosine-Archeosine Biosynthesis” subsystem that had previously been encoded (38) and observed that folE genes clustered with known Q biosynthesis genes (tgt, queA, and QueCDEF) in several bacteria and in one archaeal organism (Fig. 2A). This physical clustering evidence, combined with the results of early labeling studies implicating GCYH-I activity in Q biosynthesis, leads to the natural hypothesis that GCYH-I is the first enzyme of both the THF and pre-Q0 pathways. However, phylogenetic distribution analysis revealed that not all organisms predicted to contain a pre-Q0 pathway (because of the presence of the queCDE genes) contain a folE homolog (see archaeal examples in Fig. 3). This apparent discrepancy was reconciled by the discovery of the folE2 gene family (12, 17). FolE2 genes also cluster with Q biosynthesis genes (Fig. 2B), and nearly all genomes containing queCDE gene orthologs contain a folE or a folE2 gene (see Fig. 3 for archaea and the “Queuosine-Archaeosine Biosynthesis” subsystem in the SEED database for bacteria).
FIG. 2.
Clustering of folE1 (A) and folE2 (B) genes with predicted Q and G+ genes in prokaryotes. Only a subset of genomes where clustering is observed are given for illustration (abbreviations for the genes are given in the text).
FIG. 3.
Phylogenetic distribution of genes encoding GCYH-I, -II, and -III in members of the Archaea. Open squares denote absence, and closed squares denote presence. The data were derived from the “Queuosine-Archeosine Biosynthesis” and “Riboflavin Biosynthesis” subsystems in the SEED database.
GCYH-I is involved in Q biosynthesis.
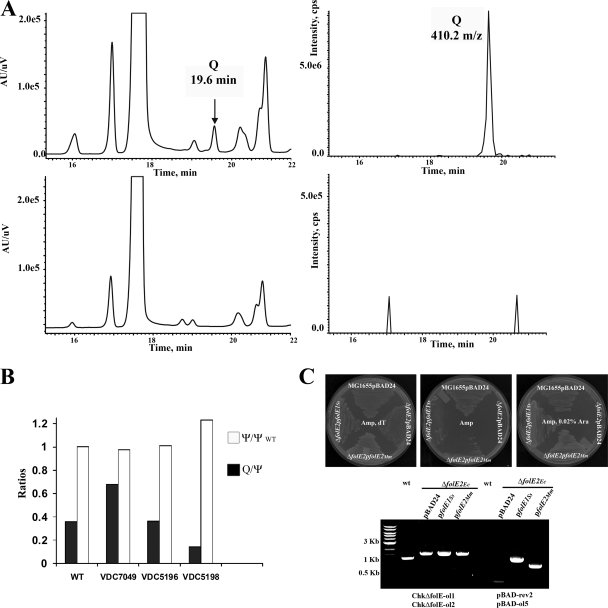
If GCYH-I catalyzes the first step of Q synthesis, then tRNA purified from a ΔfolE strain of E. coli should lack the Q ribonucleoside. To examine the nucleoside constituents, bulk tRNA was purified from cultures of the E. coli W6 (wild type [WT]) and ΔfolE::Kmr strains, enzymatically hydrolyzed, and dephosphorylated, and the mononucleosides analyzed by LC-MS-MS as described in Materials and Methods. As shown in Fig. 4A, the 410 m/z ion which corresponds to the protonated molecular weight (MH+) of Q was detected by MS at 19.6 min for the WT, while no 410 m/z ion was detected in the ΔfolE (VDC5211) strain. The peak detected by the MS is usually delayed by a few seconds from the peak detected by the UV detector, which is the time for the compound to travel from the HPLC column into the MS. As the growth of the ΔfolE strain is poor even in the presence of dT (most certainly because of the lack of formylation of initiator tRNA), the yield of tRNA was lower, with a Ψ ratio of 1.8 between the yield of the WT and that of the mutant strain. We then transformed the ΔfolE strain with a plasmid expressing E. coli folE (VDC7049). LC-MS-MS analysis of bulk tRNA showed that complementation of the Q− phenotype had occurred (Fig. 4B), with an actual increase in the level of Q when the E. coli folE gene was overexpressed (∼1.9-fold increase). Complementation also occurred with a plasmid expressing the folE2 gene from B. subtilis (VDC5196) (Fig. 4B).
FIG. 4.
(A) Results of LC-MS-MS analysis of E. coli tRNA extracted from different strains. Results for WT (W6) are in upper panels; results for VDC5211 (ΔfolE::Kanr) are in lower panels; the UV traces (left) at 254 nm and the extraction ion chromatograms (right) for 410 m/z are shown. (B) Complementation of the Q− phenotype analyzed by LC-MS-MS. The ratios of Q/Ψ in tRNA extracted from the E. coli ΔfolE::Kanr strains complemented with E. coli folE (VDC7049), B. subtilis folE2 (VDC5196), or M. maripaludis folE2 (VDC5198) are shown by the filled bars. To control for the amount of tRNA, the Ψ content in the complemented strains was compared to the Ψ content in the WT control (white bars). The result of a typical experiment is presented. (C) Upper panel shows results for complementation of the ΔfolE::Kanr strain dT auxotrophy by the archaeal folE genes on LB supplemented with Amp and dT (left plate), LB supplemented with Amp (middle plate), and LB supplemented with Amp and 0.02% arabinose (right plate). The control is ΔfolE::Kanr transformed by pBAD24. The plates were incubated at 37°C for 48 h. Lower panel shows results of PCR amplifications to check for the presence of the ΔfolE::Kanr allele and to check for the presence of an insert of the expected size in the pBAD derivatives (performed as described in Materials and Methods on the ΔfolE::Kanr strain transformed with plasmids expressing M. maripaludis folE2 or S. solfataricus folE or with the pBAD24 control). The expected size of the PCR product from detecting ΔfolE::Kanr is about 1.5 kb, while the same primers amplify a 1.2-kb product in the WT strain.
Archaeal and bacterial folE genes are functionally equivalent.
If GCYH-I is the first enzyme of pre-Q0 biosynthesis, the corresponding gene should also be found in all Archaea. Analysis of the distribution of folE, folE2, and queCDE genes in Archaea is presented in Fig. 3. A few archaea (such as Ferroplasma acidarmanus and the Sulfolobus) have a folE1 gene, and in F. acidarmanus it clusters with queDEF homologs (Fig. 2A). All other archaea contain folE2 homologs. To test if the folE genes found in archaeal genomes could functionally replace their bacterial counterparts in vivo, the folE gene from Sulfolobus solfataricus P2 and the folE2 gene from M. maripaludis were cloned into pBAD24 and transformed into the E. coli ΔfolE::Kanr strain. The transformants were plated on LB supplemented with dT, Amp, and Kan and screened for the capacity to grow on LB without dT in the presence of various concentrations of arabinose. The presence of the ΔfolE::KanR allele and of the pBAD24 derivatives in the transformants was confirmed by PCR as described in Materials and Methods (Fig. 4C). The dT auxotrophy phenotype of the ΔfolE strain was complemented by both families of archaeal folE genes (Fig. 4C). A large amount of arabinose (0.2%) was needed to observe the complementation, which is not surprising as Sulfolobus solfataricus is thermophilic and M. maripaludis has a high GC content; hence, the activity of the S. solfataricus enzyme and expression of the M. maripaludis gene could be low in the AT-rich mesophilic E. coli. tRNA was then extracted from the two complemented strains (VDC5200 and VDC5198) and analyzed by LC-MS. As shown in Fig. 4B, complementation of the Q− phenotype was observed in the expression of the M. maripaludis ortholog (VDC5198), even though the complementation level was not as high as with the bacterial folE genes (∼20% of WT levels). Only trace amounts were observed in the folE S. solfataricus complementation experiment (<3% of WT levels) (data not shown), and further analysis with improved expression levels of the enzyme is needed to confirm that this gene can complement the Q− phenotype of the folE strain even though it clearly complements the dT auxotrophy phenotype (Fig. 4C).
GCYH-I is involved in G+ biosynthesis.
The complementation results for E. coli showed that the folE genes of archaeal and bacterial origin are functionally equivalent for Q biosynthesis. To test if folE is required for G+ biosynthesis, we compared the modification profile in tRNA extracted from H. volcanii strain H26 or from a derivative that contains a deletion of the entire folE2 gene (HVO_2348 in the UCSC database) that had been previously constructed (B. El Yacoubi, G. Phillips, C. Haas, I. K. Blaby, Y. Cruz, J. Greenberg, and V. de Crécy-Lagard, submitted for publication). As shown in Fig. 5A, the peak observed for the protonated molecular weight of G+ (325 m/z), corresponding to the 25.7-min peak detected on the UV trace present in the WT H. volcanii H26 strain, was reduced nearly 58-fold in the ΔfolE2 derivative (VDC3235). A slight growth defect was repeatedly observed in the ΔfolE2 strain grown at 45°C in Hv-rich medium supplemented with dT (Fig. 5B). Further studies are needed to establish the statistical relevance of this result and distinguish between an effect of the reduced levels of G+ or of the absence of folates.
FIG. 5.
(A) Results of LC-MS-MS analysis of tRNA extracted from H. volcanii strains showing the presence or absence of the G+ peak on the UV trace and the extraction ion chromatogram (small window) for the WT H. volcanii and the ΔfolE2 (VDC3235) strain. AU/uV, absorbance units per μV. (B) Growth of both strains in Hv-YPC (yeast-peptone-Casamino Acids) supplemented with dT at 45°C was followed by measuring optical density (A600 nm). Growth of three independent clones for each strain was followed. The average of the three growth curves is presented; the error bars correspond to the plus-or-minus standard deviation. O.D., optical density.
DISCUSSION
The combination of the results of comparative genomic analysis (clustering and phylogenetic distribution) with the absence of Q in bulk tRNA extracted from the E. coli folE mutants demonstrates that GCYH-I is required for Q biosynthesis. The analysis of bulk tRNA extracted from a folE2 H. volcanii mutant showed a 60-fold reduction of G+, suggesting that GCYH-I is also critical for G+ biosynthesis. We have shown that the H. volcanii folE2 deletion is auxotrophic for folate-dependent metabolites (El Yacoubi et al., submitted), making it unlikely that a nonspecific enzyme or a yet-to-be-discovered folE gene is the cause of the residual G+ content. Another possibility currently under investigation is that the small amount of G+ could be due to a salvage pathway, as the growth medium used contained bacterial and meat extracts that could contain pre-Q0 (51).
Grochowski and coworkers (17) have shown that the GCYH-IB enzyme of Methanocaldococcus jannaschii (MptA) has GCYH-I activity in vitro. However, the product of the MtpA-driven reaction was found to be 7,8-dihydroneopterin-cyclic phosphate, not 7,8-dihydroneopterin triphosphate as observed for the bacterial GCYH-IA and IB enzymes (10, 12). Given that the M. maripaludis folE2 (65% identical to M. jannaschii MptA) complements the dT auxotrophy phenotype of the E. coli ΔfolE strain, 7,8-dihydroneopterin, the next biosynthetic intermediate, must be produced in the cell. Several possibilities can be proposed to explain this result: (i) in vivo, the M. maripaludis MptA produces enough 7,8-dihydroneopterin triphosphate to enter the folate pathway, or (ii) an endogenous phosphatase can generate 7,8-dihydroneopterin from 7,8-dihydroneopterin-cyclic phosphate directly, bypassing the folate pathway phosphorylases. To determine if 7,8-dihydroneopterin triphosphate is an intermediate of the pre-Q0 pathway both in Archaea and Bacteria or if some Archaea go through the cyclic intermediate will require more experiments to identify the intermediates in vivo.
All sequenced prototrophic Archaea have a folE1 or a folE2 gene. Folate is known to be present at high concentrations in extremely halophilic Archaea (53), whereas other Archaea use related compounds, such as methanopterin, as a C1 donor (54-56). It has been proposed that GCYH-IB in methanogens is involved in methanopterin biosynthesis (17), and our data are consistent with a role for GCYH-IB in G+ biosynthesis as well. The situation is somewhat complicated by the presence of GCYH-III, which is found in some Archaea and is proposed to be involved in riboflavin and F420 biosynthesis (15). Comparative genomics analysis of the GCYH-III family strengthens this prediction; clustering of the GCYH-III genes with F420 and riboflavin biosynthetic genes is observed, but not clustering with methanopterin or G+ biosynthetic genes (data not shown). There is also an inverse distribution of GCYH-III and GCYH-II (classically involved in riboflavin synthesis) genes in Archaea (Fig. 3). Hence Archaea have used two nonorthologous solutions for both GCYH-I and GCYH-II activities.
Several tRNA modifications share biosynthetic pathways with primary metabolites, allowing the potential fine-tuning of translation with the metabolic state of the cell. The best-documented example is the biosynthesis of i6A [N6-(A2-isopentenyl)adenosine] derivatives. Competition for dimethylallyl pyrophosphate, an intermediate of both the isoprenoid and the i6A pathways, can increase frameshifting (6) when the relevant tRNAs lack i6A. Another example is the biosynthesis of ho5U and its derivatives, which are linked to aromatic amino acid synthesis through the common intermediate chorismate. Finally, several tRNA modification enzymes contain iron-sulfur clusters (28); one can predict that these biosynthetic pathways will be affected by iron availability and other factors, such as oxidation, that affect the synthesis of iron-sulfur clusters in the cell (28). The fact that THF and Q share a common intermediate links translational accuracy to the level of the GTP pool and to the accumulation of 7,8-dihydroneopterin triphosphate. Little is known about the regulation of GCYH-I enzymes in bacteria. In mammals, GCYH-I is under negative-feedback inhibition through interactions with the GCYH feedback regulatory protein (24), but this is not observed in bacteria as they lack this protein, and the overexpression of folate or BH4 biosynthetic genes results in overproduction of the final products (52, 57). In E. coli, the expression of the folE gene has been shown to be under the control of the negative regulator MetJ (30). The folE1 gene (mtrA) of B. subtilis is in an operon with the TRAP-encoding gene (mtrB). TRAP regulates the Tryp/folate operon in B. subtilis (4), but it is not known how the mtrAB operon itself is regulated. In any case, the levels of Q could certainly be affected by the competition for an intermediate of the folate pathway, and further studies are needed to explore this possibility. As for known mechanisms of regulation of Q levels in the cell, two different types of pre-Q1 binding riboswitches have recently been discovered upstream of Q biosynthesis and potential salvage genes (32, 41) These riboswitches are found only in some gram-positive bacteria, suggesting that other types of regulatory mechanisms are yet to be discovered.
Acknowledgments
This work was supported by the National Science foundation (grant number MCB-05169448) and by the National Institutes of Health (grant number R01 GM70641-01).
We thank Iris Porat and William Whitman for M. maripaludis genomic DNA, Ken Stedman for S. solfataricus genomic DNA, Julie Maupin for advice regarding H. volcanii manipulations, Andrew Hanson for insightful discussions, and Jonathan Eisen for making the sequence of Haloferax volcanii publicly available before publication.
Footnotes
Published ahead of print on 17 October 2008.
REFERENCES
- 1.Allers, T., H.-P. Ngo, M. Mevarech, and R. G. Lloyd. 2004. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl. Environ. Microbiol. 70943-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 22006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babitzke, P., P. Gollnick, and C. Yanofsky. 1992. The mtrAB operon of Bacillus subtilis encodes GTP cyclohydrolase I (MtrA), an enzyme involved in folic acid biosynthesis, and MtrB, a regulator of tryptophan biosynthesis. J. Bacteriol. 1742059-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai, Y., D. T. Fox, J. A. Lacy, S. G. Van Lanen, and D. Iwata-Reuyl. 2000. Hypermodification of tRNA in Thermophilic archaea. Cloning, overexpression, and characterization of tRNA-guanine transglycosylase from Methanococcus jannaschii. J. Biol. Chem. 27528731-28738. [DOI] [PubMed] [Google Scholar]
- 6.Benko, A. L., G. Vaduva, N. C. Martin, and A. K. Hopper. 2000. Competition between a sterol biosynthetic enzyme and tRNA modification in addition to changes in the protein synthesis machinery causes altered nonsense suppression. Proc. Natl. Acad. Sci. USA 9761-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 313497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cossins, E. A., and L. Chen. 1997. Folates and one-carbon metabolism in plants and fungi. Phytochemistry 45437-452. [DOI] [PubMed] [Google Scholar]
- 9.Crain, P. F. 1990. Preparation and enzymatic hydrolysis of DNA and RNA for mass spectrometry. Methods Enzymol. 193782-790. [DOI] [PubMed] [Google Scholar]
- 10.Elstner, E. F., and R. J. Suhadolnik. 1971. The biosynthesis of the nucleoside antibiotics. IX. Purification and properties of guanosine triphosphate 8-formylhydrolase that catalyzes production of formic acid from the ureido carbon of guanosine triphosphate. J. Biol. Chem. 2466973-6981. [PubMed] [Google Scholar]
- 11.Elstner, E. F., and R. J. Suhadolnik. 1975. Guanosine triphosphate-8-formylhydrolase. Methods Enzymol. 43515-520. [DOI] [PubMed] [Google Scholar]
- 12.El Yacoubi, B., S. Bonnett, J. N. Anderson, M. A. Swairjo, D. Iwata-Reuyl, and V. de Crécy-Lagard. 2006. Discovery of a new prokaryotic type I GTP cyclohydrolase family. J. Biol. Chem. 28137586-37593. [DOI] [PubMed] [Google Scholar]
- 13.Frey, B., J. A. McCloskey, W. Kersten, and H. Kersten. 1988. New function of vitamin B12: cobamide-dependent reduction of epoxyqueuosine to queuosine in tRNAs of Escherichia coli and Salmonella typhimurium. J. Bacteriol. 1702078-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaur, R., and U. Varshney. 2005. Genetic analysis identifies a function for the queC (ybaX) gene product at an initial step in the queuosine biosynthetic pathway in Escherichia coli. J. Bacteriol. 1876893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, D. E., H. Xu, and R. H. White. 2002. A member of a new class of GTP cyclohydrolases produces formylaminopyrimidine nucleotide monophosphates. Biochemistry 4115074-15084. [DOI] [PubMed] [Google Scholar]
- 16.Green, J. C., B. P. Nichols, and R. G. Matthews. 1996. Folate biosynthesis, reduction, and polyglutamylation, p. 665-673. In F. C. Neidhart (ed.), Escherichia coli and Salmonella, cellular and molecular biology. American Society for Microbiology, Washington, DC.
- 17.Grochowski, L. L., H. Xu, K. Leung, and R. H. White. 2007. Characterization of an Fe2+-dependent archaeal-specific GTP cyclohydrolase, MptA, from Methanocaldococcus jannaschii. Biochemistry 466658-6667. [DOI] [PubMed] [Google Scholar]
- 18.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1774121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzenmeier, G., C. Schmid, and A. Bacher. 1990. Cloning and expression of the putative gene coding for GTP cyclohydrolase I from Escherichia coli. FEMS Microbiol. Lett. 54231-234. [DOI] [PubMed] [Google Scholar]
- 20.Kauri, T., R. Wallace, and D. J. Kushner. 1990. Nutrition of the halophilic archaebacterium, Haloferax volcanii. Syst. Appl. Microbiol. 1314-18. [Google Scholar]
- 21.Kersten, H., and W. Kersten. 1990. Biosynthesis and function of queuine and queuosine tRNAs, p. B69-B108. In K. C. T. Kuo (ed.), Chromatography and modification of nucleosides, part B. Elsevier, Amsterdam, The Netherlands.
- 22.Kinzie, S. D., B. Thern, and D. Iwata-Reuyl. 2000. Mechanistic studies of the tRNA-modifying enzyme QueA: a chemical imperative for the use of AdoMet as a “ribosyl” donor. Org. Lett. 21307-1310. [DOI] [PubMed] [Google Scholar]
- 23.Klaus, S. M., E. R. Kunji, G. G. Bozzo, A. Noiriel, R. D. de la Garza, G. J. Basset, S. Ravanel, F. Rebeille, J. F. Gregory III, and A. D. Hanson. 2005. Higher plant plastids and cyanobacteria have folate carriers related to those of trypanosomatids. J. Biol. Chem. 28038457-38463. [DOI] [PubMed] [Google Scholar]
- 24.Kolinsky, M. A., and S. S. Gross. 2004. The mechanism of potent GTP cyclohydrolase I inhibition by 2,4-diamino-6-hydroxypyrimidine: requirement of the GTP cyclohydrolase I feedback regulatory protein. J. Biol. Chem. 27940677-40682. [DOI] [PubMed] [Google Scholar]
- 25.Krumdieck, C. L., E. Shaw, and C. M. Baugh. 1966. The biosynthesis of 2-amino-4-hydroxy-6-substituted pteridines. J. Biol. Chem. 241383-387. [PubMed] [Google Scholar]
- 26.Kuchino, Y., H. Kasai, K. Nihei, and S. Nishimura. 1976. Biosynthesis of the modified nucleoside Q in transfer RNA. Nucleic Acids Res. 3393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lederberg, J., L. L. Cavalli, and E. M. Lederberg. 1952. Sex compatibility in Escherichia coli. Genetics 37720-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lill, R., and U. Muhlenhoff. 2008. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu. Rev. Biochem. 77669-700. [DOI] [PubMed] [Google Scholar]
- 29.Lucock, M. 2000. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol. Genet. Metab. 71121-138. [DOI] [PubMed] [Google Scholar]
- 30.Marincs, F., I. W. Manfield, J. A. Stead, K. J. McDowall, and P. G. Stockley. 2006. Transcript analysis reveals an extended regulon and the importance of protein-protein co-operativity for the Escherichia coli methionine repressor. Biochem. J. 396227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarty, R. M., and V. Bandarian. 2008. Deciphering deazapurine biosynthesis: pathway for pyrrolopyrimidine nucleosides toyocamycin and sangivamycin. Chem. Biol. 15790-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer, M. M., A. Roth, S. M. Chervin, G. A. Garcia, and R. R. Breaker. 2008. Confirmation of a second natural preQ1 aptamer class in Streptococcaceae bacteria. RNA 14685-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Nar, H., R. Huber, G. Auerbach, M. Fischer, C. Hosl, H. Ritz, A. Bracher, W. Meining, S. Eberhardt, and A. Bacher. 1995. Active site topology and reaction mechanism of GTP cyclohydrolase I. Proc. Natl. Acad. Sci. USA 9212120-12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noguchi, S., Y. Nishimura, Y. Hirota, and S. Nishimura. 1982. Isolation and characterization of an Escherichia coli mutant lacking tRNA-guanine transglycosylase. Function and biosynthesis of queuosine in tRNA. J. Biol. Chem. 2576544-6550. [PubMed] [Google Scholar]
- 36.Okada, N., S. Noguchi, H. Kasai, N. Shindo-Okada, T. Ohgi, T. Goto, and S. Nishimura. 1979. Novel mechanism of post-transcriptional modification of tRNA. J. Biol. Chem. 2543067-3073. [PubMed] [Google Scholar]
- 37.Okada, N., S. Noguchi, S. Nishimura, T. Ohgi, T. Goto, P. F. Crain, and J. A. McCloskey. 1978. Structure determination of a nucleoside Q precursor isolated from E. coli tRNA: 7-(aminomethyl)-7-deazaguanosine. Nucleic Acids Res. 52289-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overbeek, R., T. Begley, R. M. Butler, J. V. Choudhuri, H. Y. Chuang, M. Cohoon, V. de Crécy-Lagard, N. Diaz, T. Disz, R. Edwards, M. Fonstein, E. D. Frank, S. Gerdes, E. M. Glass, A. Goesmann, A. Hanson, D. Iwata-Reuyl, R. Jensen, N. Jamshidi, L. Krause, M. Kubal, N. Larsen, B. Linke, A. C. McHardy, F. Meyer, H. Neuweger, G. Olsen, R. Olson, A. Osterman, V. Portnoy, G. D. Pusch, D. A. Rodionov, C. Ruckert, J. Steiner, R. Stevens, I. Thiele, O. Vassieva, Y. Ye, O. Zagnitko, and V. Vonstein. 2005. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 335691-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomerantz, S. C., and J. A. McCloskey. 1990. Analysis of RNA hydrolyzates by liquid chromatography-mass spectrometry. Methods Enzymol. 193796-824. [DOI] [PubMed] [Google Scholar]
- 40.Reader, J. S., D. Metzgar, P. Schimmel, and V. de Crécy-Lagard. 2004. Identification of four genes necessary for biosynthesis of the modified nucleoside queuosine. J. Biol. Chem. 2796280-6285. [DOI] [PubMed] [Google Scholar]
- 41.Roth, A., W. C. Winkler, E. E. Regulski, B. W. K. Lee, J. Lim, I. Jona, J. E. Barrick, A. Ritwik, J. N. Kim, R. Welz, D. Iwata-Reuyl, and R. R. Breaker. 2007. A riboswitch selective for the queuosine precursor preQ1 contains an unusually small aptamer domain. Nat. Struct. Mol. Biol. 14308. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 43.Schneider, K. L., K. S. Pollard, R. Baertsch, A. Pohl, and T. M. Lowe. 2006. The UCSC archaeal genome browser. Nucleic Acids Res. 34D407-D410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shindo-Okada, N., N. Okada, T. Ohgi, T. Goto, and S. Nishimura. 1980. Transfer ribonucleic acid guanine transglycosylase isolated from rat liver. Biochemistry 19395-400. [DOI] [PubMed] [Google Scholar]
- 45.Slany, R. K., M. Bosl, P. F. Crain, and H. Kersten. 1993. A new function of S-adenosylmethionine: the ribosyl moiety of AdoMet is the precursor of the cyclopentenediol moiety of the tRNA wobble base queuine. Biochemistry 327811-7817. [DOI] [PubMed] [Google Scholar]
- 46.Slany, R. K., M. Bosl, and H. Kersten. 1994. Transfer and isomerization of the ribose moiety of AdoMet during the biosynthesis of queuosine tRNAs, a new unique reaction catalyzed by the QueA protein from Escherichia coli. Biochimie 76389-393. [DOI] [PubMed] [Google Scholar]
- 47.Smulson, M. E., and R. J. Suhadolnik. 1967. The biosynthesis of the 7-deazaadenine ribonucleoside, tubercidin, by Streptomyces tubercidicus. J. Biol. Chem. 2422872-2876. [PubMed] [Google Scholar]
- 48.Suhadolnik, R. J., and T. Uematsu. 1970. Biosynthesis of the pyrrolopyrimidine nucleoside antibiotic, toyocamycin. J. Biol. Chem. 2454365-4371. [PubMed] [Google Scholar]
- 49.Thony, B., G. Auerbach, and N. Blau. 2000. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 3471-16. [PMC free article] [PubMed] [Google Scholar]
- 50.Van Lanen, S. G., J. S. Reader, M. A. Swairjo, V. de Crécy-Lagard, B. Lee, and D. Iwata-Reuyl. 2005. From cyclohydrolase to oxidoreductase: discovery of nitrile reductase activity in a common fold. Proc. Natl. Acad. Sci. USA 1024264-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe, M., M. Matsuo, S. Tanaka, H. Akimoto, S. Asahi, S. Nishimura, J. R. Katz, T. Hashizume, P. F. Crain, J. A. McCloskey, and N. Okada. 1997. Biosynthesis of archaeosine, a novel derivative of 7-deazaguanosine specific to archaeal tRNA, proceeds via a pathway involving base replacement of the tRNA polynucleotide chain. J. Biol. Chem. 27220146-20151. [DOI] [PubMed] [Google Scholar]
- 52.Wegkamp, A., M. Starrenburg, W. M. de Vos, J. Hugenholtz, and W. Sybesma. 2004. Transformation of folate-consuming Lactobacillus gasseri into a folate producer. Appl. Environ. Microbiol. 703146-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White, R. H. 1988. Analysis and characterization of the folates in the nonmethanogenic archaebacteria. J. Bacteriol. 1704608-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White, R. H. 1991. Distribution of folates and modified folates in extremely thermophilic bacteria. J. Bacteriol. 1731987-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White, R. H. 1997. Purine biosynthesis in the domain Archaea without folates or modified folates. J. Bacteriol. 1793374-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Worrell, V. E., and D. P. Nagle, Jr. 1988. Folic acid and pteroylpolyglutamate contents of archaebacteria. J. Bacteriol. 1704420-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto, K., E. Kataoka, N. Miyamoto, K. Furukawa, K. Ohsuye, and M. Yabuta. 2003. Genetic engineering of Escherichia coli for production of tetrahydrobiopterin. Metab. Eng. 5246-254. [DOI] [PubMed] [Google Scholar]
- 58.Yim, J. J., and G. M. Brown. 1976. Characteristics of guanosine triphosphate cyclohydrolase I purified from Escherichia coli. J. Biol. Chem. 2515087-5094. [PubMed] [Google Scholar]