Abstract
The transcriptional regulator SugR from Corynebacterium glutamicum represses genes of the phosphoenolpyruvate-dependent phosphotransferase system (PTS). Growth experiments revealed that the overexpression of sugR not only perturbed the growth of C. glutamicum on the PTS sugars glucose, fructose, and sucrose but also led to a significant growth inhibition on ribose, which is not taken up via the PTS. Chromatin immunoprecipitation combined with DNA microarray analysis and gel retardation experiments were performed to identify further target genes of SugR. Gel retardation analysis confirmed that SugR bound to the promoter regions of genes of the glycolytic enzymes 6-phosphofructokinase (pfkA), fructose-1,6-bisphosphate aldolase (fba), enolase (eno), pyruvate kinase (pyk), and NAD-dependent l-lactate dehydrogenase (ldhA). The deletion of sugR resulted in increased mRNA levels of eno, pyk, and ldhA in acetate medium. Enzyme activity measurements revealed that SugR-mediated repression affects the activities of PfkA, Fba, and LdhA in vivo. As the deletion of sugR led to increased LdhA activity under aerobic and under oxygen deprivation conditions, l-lactate production by C. glutamicum was determined. The overexpression of sugR reduced l-lactate production by about 25%, and sugR deletion increased l-lactate formation under oxygen deprivation conditions by threefold. Thus, SugR functions as a global repressor of genes of the PTS, glycolysis, and fermentative l-lactate dehydrogenase in C. glutamicum.
Corynebacterium glutamicum, which was isolated as an l-glutamate-excreting soil bacterium (1, 39), is a predominantly aerobic, biotin-auxotrophic, gram-positive bacterium widely used for the industrial production of more than 2 million tons of amino acids per year, mainly l-glutamate and l-lysine (31, 43). A general view of this nonpathogenic bacterium, which has become a model organism for the Corynebacterineae, a suborder of Actinomycetales that also comprises the genus Mycobacterium (54), can be found in two recent monographs (9, 17).
C. glutamicum is able to grow on a variety of sugars, sugar alcohols, and organic acids as sole carbon and energy sources (64). As in many other gram-positive and gram-negative bacteria, the phosphoenolpyruvate-dependent phosphotransferase system (PTS) is the major sugar uptake system (15, 37, 45, 47). The PTS-mediated glucose, fructose, and sucrose uptake in C. glutamicum operates by phosphoryl group transfer from phosphoenolpyruvate via EI (encoded by ptsI) and HPr (ptsH) to the sugar-specific permeases EIIGlc, EIIFru, and EIISuc, respectively (ptsG, ptsF, and ptsS, respectively). Unlike many other bacteria, C. glutamicum usually coutilizes the carbon sources present in mixtures without showing diauxic growth (64). Glucose as the preferred carbon source has been shown to be cometabolized with acetate (63), l-lactate (55), or fructose (14). When glucose is coutilized with another carbon source, e.g., acetate or fructose (16, 63), its uptake is reduced due to the repression of ptsG by the recently identified transcriptional repressor SugR (19). It was shown that SugR not only acts as a repressor of ptsG expression in C. glutamicum but also controls genes of the fructose- and sucrose-specific PTS permeases (fruR-fruK-ptsF and ptsS, respectively) (19) as well as genes of the general components of the PTS (ptsH and ptsI) (21, 59). The binding of SugR to the ptsG promoter was found to be negatively affected by millimolar concentrations of fructose-6-phosphate (19), while micromolar concentrations of fructose-1-phosphate and millimolar concentrations of glucose-6-phosphate and fructose-1,6-bisphosphate were shown to negatively affect the binding of SugR to the ptsI-fruR intergenic region (21). An 8-bp motif upstream of ptsG was suggested to be part of the SugR binding site (19). This motif upstream of ptsG is part of a 23-bp AC-rich motif which was shown to be required for the binding of SugR to the ptsI-fruR intergenic region and which also is present upstream of the SugR targets ptsS and ptsH (21). In Escherichia coli and Bacillus subtilis, regulators like Crp (cyclic AMP [cAMP] receptor protein) and CcpA (catabolite control protein A) not only regulate pts genes but also are global regulators of carbon metabolism in these bacteria. CcpA is the master regulator of carbon catabolite regulation in B. subtilis (30, 53) and regulates more than 300 genes by either activation (e.g., the α-acetolactate synthase gene alsS and the acetate kinase gene ackA) or repression (e.g., the gluconate operon repressor gene gntR) (29, 44, 53). Activation and repression mediated by CcpA may utilize different conformational changes of the protein (53). Because ccpA mutants are unable to activate glycolysis or carbon overflow metabolism, CcpA appears to control a superregulon of glucose catabolism in this organism (61). In E. coli, Crp, one of the global regulators known to regulate >50% of this bacterium's transcription units (26), is activated by cAMP, which is synthesized from ATP by adenylate cyclase (cyaA) (40). Chromatin immunoprecipitation combined with DNA microarray analysis (ChIP-to-chip analysis) and DNA microarrays showed that Crp binds to dozens of regions in the E. coli chromosome, e.g., rbsD (d-ribose high-affinity transport system), gnt (gluconate transporter), aceA and aceB (isocitrate lyase, malate synthase), gnd (6-phosphogluconate dehydrogenase), and pckA (phosphoenolpyruvate carboxykinase) (25, 26).
In this study, it was determined that in addition to regulating pts genes the DeoR-type transcriptional regulator SugR also regulates genes of the central carbon metabolism in C. glutamicum. Thus, SugR was shown to be a pleiotropic regulator with its regulon comprising genes of the PTS, glycolysis, and fermentative l-lactate dehydrogenase in C. glutamicum.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The strains and plasmids used are listed in Table 1 and the oligonucleotides used are listed in Table 2. The C. glutamicum type strain ATCC 13032 (36) was used as the wild type (WT). Growth experiments were performed using CgXII minimal medium as described previously (19). As carbon and energy sources, 100 mM glucose, 100 mM fructose, 50 mM sucrose, and 120 mM ribose were used. For all cloning purposes, E. coli DH5α was used as the host, and for the overproduction of SugR, E. coli BL21(DE3) (56) was used. The E. coli strains were cultivated aerobically in Luria-Bertani (LB) medium (49) at 37°C.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source and/or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | F−thi-1 endA1 hsdR17(r− m−) supE44 ΔlacU169 (φ80lacZΔM15) recA1 gyrA96 relA1 | Bethesda Research Laboratories; 28 |
| BL21(DE3) | ompT hsdSB(rB− mB−) gal dcm (DE3) | Novagen |
| C. glutamicum strains | ||
| WT | ATCC 13032; wild-type strain, auxotrophic for biotin | ATCC |
| ΔsugR WT | In-frame deletion of the sugR gene of WT | 19 |
| WT sugRStrep | pk19mobsacB- sugRStrep integration in WT | This work |
| Plasmids | ||
| pGEM-T | Ampr; PCR cloning vector (PT7, PSP6) | Promega, WI, USA |
| pGEM-T-sugRStrep | Ampr; pGEM-T with 424-bp fragment of the 3′ end of sugR with a C-terminal StrepTag II sequence | 19 |
| pk19mobsacB | Kanr; mobilizable E. coli vector for the construction of insertion and deletion mutants in C. glutamicum (oriVE.c., sacB, lacZα) | 50 |
| pk19mobsacB- sugRStrep | Kanr; pk19mobsacB with the 424-bp integration construct comprising the 3′ end of sugR with added codons for a C-terminal StrepTag II | This work |
| pVWEx1 | Kanr; Ptac, lacIq | 48 |
| pVWEx1-sugR | Kanr; pVWEx1 with an 807-bp fragment of the sugR gene and an artificial RBS | 19 |
| pET16b | Ampr; overproduction of proteins with an N-terminal decahistidine tag in E. coli (pBR322 oriVE.c., PT7, lacI) | Novagen |
| pET16b-SugRHis | Ampr; pET16b with a 786-bp fragment of the gene sugR with a N-terminal decahistidine tag | 19 |
oriVE.c., oriV from E. coli; RBS, ribosome binding site.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence (5′ → 3′) | Sequence feature
|
Use | ||
|---|---|---|---|---|---|
| Nucleotide in NC003450 (underlined) | Restriction site (bold) | Tag (italics) | |||
| sugR | CGGAATTCTAGCTCCAAGCAGTGGTCGATCG | 2038164 | EcoRI | ChIP-to-chip | |
| sugR-Strep | CGGGATCCTTATTTTTCGAACTGCGGGTGGCTCCAAGCGCTTTCTGCAATCACAACTTCTACATCG | 2038591 | BamHI | StrepTag II | ChIP-to-chip |
| M13-rev | CACACAGGAAACAGCTATGACCATG | Integration control | |||
| P0441-for | GCTAAAAAAGTAGAAATTATCGACC | 387199 | Gel shift | ||
| P0441-rev | GCCGGCTCCGAGTACTAC | 387730 | Gel shift | ||
| P1110-for | GTGATGCCTGCGGTTGCTG | 1033597 | Gel shift | ||
| P1110-rev | CAAATGAGATCACCATGATGACGGC | 1034029 | Gel shift | ||
| P1111-for | CTAAAACTTAACAAGCGCAACCCCC | 1034739 | Gel shift | ||
| P1111-rev | CCGCGGGAGTCGAGAATTTC | 1034998 | Gel shift | ||
| P1142_1143-for | GCGGTGATTCGAGCCGCTAAATCAAG | 1060191 | Gel shift | ||
| P1142_1143-rev | GTGCTGGCTGAGACTAGCTGGC | 1059882 | Gel shift | ||
| P1408_1409-for | GCGACGACGGTGGCAGTGCTGACC | 1314735 | Gel shift | ||
| P1408_1409-rev | CCGGGGCAGTCGCCGCCTGAC | 1315095 | Gel shift | ||
| F4(ptsG)-for | GCATAATCTGACAGTGTGTCCGTTTTC | 1422834 | Gel shift | ||
| F4(ptsG)-rev | GGCTCCCCCGCAATAGATTTGTGG | 1423019 | Gel shift | ||
| P1773_1774-for | GATCGTGTCCAAGGGTTCTCCTCC | 1663548 | Gel shift | ||
| P1773_1774-rev | GCGTACAGTGAGCGCCTGAAGTTC | 1664450 | Gel shift | ||
| P2115-for | GTCGCTTTTCAGGTTCCCGC | 2037511 | Gel shift | ||
| P2115-rev | CTCAACTGCCGTTAATGAGGCAATC | 2037865 | Gel shift | ||
| P2121-for | TCGGACCTTGACCCGATGTCTGGTC | 2045575 | Gel shift | ||
| P2121-rev | CGTGCGTGCAGGCCAACGGAG | 2045811 | Gel shift | ||
| P2157-for | GGCTTTAGGAGCCTAGTGGC | 2073290 | Gel shift | ||
| P2157-rev | CCAGCAATCACTGCGATAGTGATTAG | 2072856 | Gel shift | ||
| P2291-for | GTTTCAACCATAGGCCTGACCTGGC | 2207298 | Gel shift | ||
| P2291-rev | CTAGCCACCGCTGGGCCTAG | 2207043 | Gel shift | ||
| P3068-for | CCCCGATAGTGTATGTGCTGAC | 2955520 | Gel shift | ||
| P3068-rev | CTTAGCACGATCGAGCATCTCGTTA | 2955223 | Gel shift | ||
| P3169_3170-for | GGTAAGACCGCAGCGTAGCTTTTGG | 3054803 | Gel shift | ||
| P3169_3170-rev | CCTTATTCTTGGTCGGCGCCTCG | 3053838 | Gel shift | ||
| P3219-for | GTTGCCAGGCGAGTGGTGAGC | 3113669 | Gel shift | ||
| P3219-rev | CATCTCCTGCGCCAATGAGGACAATC | 3113343 | Gel shift | ||
| P3366_3367-for | GCTGTGAACGACCCAAAACTCAAACTTAG | 3242913 | Gel shift | ||
| P3366_3367-rev | CGGTCGAGTTCTATGCGTTCTGC | 3242640 | Gel shift | ||
| cg2228_for | GTTCGCTACGTCCGAGTGATCACC | 2146341 | Negative control for gel shift | ||
| cg2228_rev_short | CTCAGGCATGATGATGTCAGGC | 2146264 | Negative control for gel shift | ||
| cg2228_rev | GTCCCCCACGATTGTTTAAAAGTC | 2145944 | Negative control for gel shift | ||
| P1145_for | CGTTTCGCAGTACGGAGCG | 1063987 | Gel shift | ||
| P1145_rev | CCTTCACTTCACCCATGGTGTC | 1063566 | Gel shift | ||
| P2837_for | CGCAGCAATGTGATAACAGAGG | 2727080 | Gel shift | ||
| P2837_rev | GGCACACCATGGGTTTCAAAG | 2726526 | Gel shift | ||
| P3048-for | GATTCCTGCTGGACCTGCAGCG | 2938454 | Gel shift | ||
| P3048-rev | CGAAGCTGCGGTTGACCGTGG | 2937840 | Gel shift | ||
| P2831_2833-for | CGGTGATGTTGTTGTACACATTG | 2721953 | Gel shift | ||
| P2831_2833-rev | CAGCGCCGAACGAATAGC | 2721178 | Gel shift | ||
| P1283_1284-for | CGCCTTTAACAAACCCTGTTGAG | 1182041 | Gel shift | ||
| P1283_1284-rev | GCAGCGAGAGAGATGCAC | 1181626 | Gel shift | ||
| P2795_2796-for | GAGTTTGGCAGCTTCCACG | 2684967 | Gel shift | ||
| P2795_2796-rev | GAACAACACTATTGATTGGACCTTC | 2684599 | Gel shift | ||
| P2262-for | TCCCTGATTTCATCGGAGGG | 2176106 | Gel shift | ||
| P2262-rev | GCGAGAACCACGAGGAC | 2175455 | Gel shift | ||
| P0680_0681-for | GGTCCGCGCTTGGACAGT | 600827 | Gel shift | ||
| P0680_0681-rev | GTGTTATGGGCGATGGCATC | 601020 | Gel shift | ||
| P0645-for | ACCCGCCCTCCATCTGCAT | 568268 | Gel shift | ||
| P0645-rev | GAATGGGCATCCACCGGT | 568032 | Gel shift | ||
Recombinant DNA work.
The enzymes for recombinant DNA work were obtained from Roche Diagnostics (Mannheim, Germany). The oligonucleotides were obtained from Operon (Cologne, Germany). Standard methods like PCR, restriction, and ligation were carried out according to reference 49. Plasmids from E. coli were isolated with the QIAprep spin miniprep kit (Qiagen, Hilden, Germany). E. coli was transformed by the RbCl method (27), and C. glutamicum was transformed by electroporation (19, 62). DNA sequencing was performed by Agowa GmbH (Berlin, Germany).
Construction of sugR-StrepTag strain.
To generate a strain derived from C. glutamicum ATCC 13032, which synthesizes C-terminally StrepTag-tagged SugR from the genomic sugR locus, the plasmid pK19mobsacB-sugRStrep was constructed. Base pairs 349 to 777 of sugR were amplified using primers sugR and sugR-Strep (Table 2), introducing the StrepTag II sequence at the C terminus of the protein (N-SAWSHPQFEK-C) (52). The PCR product was subcloned into the pGEM-T vector (Promega, Wisconsin) and was cloned as an EcoRI/BamHI fragment into the pK19mobsacB vector (50). C. glutamicum was transformed with the resulting plasmid, pK19mobsacB-sugRStrep, by electroporation, and the site-specific integration of the plasmid into the sugR genomic locus was verified by PCR using the primers sugR and M13 (Table 2). As expected, only C. glutamicum WT-sugRStrep yielded a PCR product of the expected size, while no signal was obtained with ATCC 13032 WT.
Overproduction and purification of SugR.
The C. glutamicum SugR protein containing an N-terminal decahistidine tag was overproduced in E. coli BL21(DE3) by use of the expression plasmid pET16b-SugRHis and purified by Ni2+-chelate affinity chromatography as described previously by Engels and Wendisch (19).
Gel shift assays.
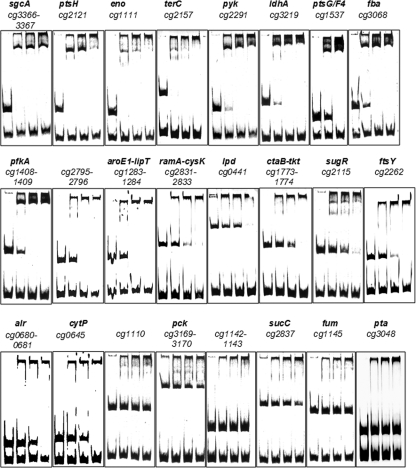
Gel shift assays with SugRHis were performed as described previously (19). Briefly, purified SugRHis (in concentrations ranging from 0 to 3.3 μM) was mixed with various promoter fragments (186 to 967 bp; final concentrations, 7 to 37 nM [see Fig. 2 below]) in a total volume of 20 μl and contained 50 mM Tris-HCl, 10% (vol/vol) glycerol, 50 mM KCl, 10 mM MgCl2, 0.5 mM EDTA, pH 7.5. A 78- or a 398-bp cg2228 promoter fragment (90 or 20 nM, respectively) served as the negative control. The primers used for amplification of the promoter fragments are listed in Table 2. All PCR products used in the gel shift assays were purified with the PCR purification kit (Qiagen, Hilden, Germany) and eluted in 10 mM Tris-HCl, pH 8.5. After incubation for 30 min at room temperature, the samples were separated on a 10% native polyacrylamide gel at room temperature and a constant 170 V by use of 1× 89 mM Tris base, 89 mM boric acid, 2 mM EDTA, pH 8.3 as the electrophoresis buffer. The gels were subsequently stained with SYBR green I according to the instructions of the supplier (Sigma, Rödermark, Germany) and photographed.
FIG. 2.
Binding of SugRHis to candidate target genes. DNA fragments (186 to 967 bp; final concentration, 7 to 37 nM) covering the promoter regions of further putative target genes of SugR were incubated for 30 min at room temperature without SugRHis protein (first lanes) or with a 30-fold (second lanes), 60-fold (third lanes), or 90-fold (fourth lanes) molar excess of purified SugRHis protein before separation by native polyacrylamide gel electrophoresis (10%) and staining with SYBR green I. In the case of the ptsG/F4 fragment, the first lane is without SugRHis protein and the second and third lanes are with 30- and 60-fold molar excesses of purified SugRHis protein, respectively. A 78- or 398-bp cg2228 promoter fragment (90 or 20 nM, respectively) served as the negative control. Oligonucleotides used for the amplification of these fragments via PCR are listed in Table 2.
ChIP-to-chip and transcriptome analysis.
From a fresh LB agar plate with C. glutamicum WT as the control strain and the C. glutamicum WT-sugRStrep strain, the first preculture, 5 ml of LB medium, was inoculated. After the cells were washed in culture medium without any carbon source, the second preculture and the main culture were inoculated to an optical density at 600 nm (OD600) of 0.5 either in 500 ml CgXII minimal medium in eight separate flasks, which contained 0.03 g/liter protocatechuic acid and 0.2 mg/liter biotin, or in LB medium. As carbon and energy sources, 100 mM glucose, 100 mM fructose, or 50 mM sucrose was used. The main cultures were cultivated to mid-exponential phase (OD600 of 4 to 6). Fifty-milliliter portions of these main cultures were harvested by centrifugation (10 min, 11,325 × g, 4°C), and the cells were washed with 50 ml buffer A (100 mM Tris, 1 mM EDTA, pH 8.0). Subsequently, the cells were resuspended in 10 ml of buffer A that was supplemented with 1% (vol/vol) formaldehyde. After incubation for 20 min at room temperature, glycine was added to a final concentration of 125 mM, and the cultures were incubated for another 5 min. Then, the cells were harvested (10 min, 3,500 × g, 4°C) and washed twice in buffer A. The cell pellet was stored at −20°C until use. After thawing, cells were resuspended in 10 ml of buffer A with one pill of complete Mini protease inhibitor (Roche, Mannheim, Germany) and 100 μg RNase A and disrupted by six passages at 172 MPa through a French pressure cell (SLM Aminco Spectronic Instruments, Rochester, NY). The chromosomal DNA of the lysate was sheared by sonication (two times for 30 s with Branson sonifier W-250 [Danbury, CT] with a pulse length of 40% and an intensity of 1) to give an average fragment size of 200 to 750 bp. Cell debris was removed by centrifugation (20 min, 5,300 × g, 4°C) and ultracentrifugation (1 h, 150,000 × g, 4°C). The cytosolic fraction was then used for immunoprecipitation and therefore was spiked with 5 μg/ml avidin and incubated for 5 min on ice. The SugRStrep-DNA complexes in the supernatant were precipitated by loading the supernatant onto an equilibrated (two times washed with 10 ml buffer W [100 mM Tris, 1 mM EDTA, 100 mM NaCl, pH 8.0]) column with 4 ml 50% (wt/vol) StrepTactin-Sepharose (IBA GmbH, Göttingen, Germany). After the column was washed three times with 10 ml buffer W, the bound SugRStrep-DNA complexes were eluted in eight 1-ml fractions of buffer E (100 mM Tris, 1 mM EDTA, 100 mM NaCl, 2.5 mM desthiobiotin, pH 8.0). After 1% sodium dodecyl sulfate was added to the elution fractions, they were incubated overnight at 65°C and then treated for 3 h at 55°C with proteinase K (400 μg/ml). The DNA was purified by phenol-chloroform extraction, precipitated with ethanol, washed with 70% (vol/vol) ethanol, dried, and resuspended in 100 μl EB buffer (10 mM Tris-HCl, pH 8.5; Qiagen, Hilden, Germany). Fluorescent labeling of genomic DNA was performed as described previously (20, 51). Preparation and labeling of RNA for transcriptome analysis, hybridization to the C. glutamicum whole-genome microarray, and array scanning were performed as described previously (19). The enrichment factors were the ratios of the hybridization signal from the labeled DNA of the SugRStrep-DNA complexes isolated from C. glutamicum WT-sugRStrep divided by the hybridization signal from the mock experiment with C. glutamicum WT.
Determination of glucose, fructose, and ribose concentrations.
d-Glucose and d-fructose were quantified enzymatically with the d-glucose/d-fructose kit (R-Biopharm, Darmstadt, Germany) according to the manufacturers' instructions as described previously in reference 19. d-Ribose was separated by high-performance liquid chromatography with an organic acid resin column (300 by 8 mm, 10-μm inner diameter, 25 Å; CS Chromatographie Service; Langerwehe, Germany) at 60°C by use of injection volumes of 5 μl, 5 mM H2SO4 as the mobile phase, a flow rate of 1.0 ml/min, and an overall run time of 15 min. Substances were detected via a refractive index detector 1200 series (Agilent Technologies, Santa Clara, CA). Concentrations were determined by comparing the sample probes with external standards.
Measurement of enzyme activities.
For measurements of enzyme activities, the C. glutamicum WT, WTΔsugR, WT(pVWEx1), and WT(pVWEx1-sugR) strains were cultivated in LB medium to OD600s of 2 to 3.5. The cells were harvested by centrifugation (10 min, 4°C, 3,220 × g), washed twice in 100 mM Tris-HCl, pH 7.3, plus 10% (vol/vol) glycerol, and stored at −70°C until use. For the preparation of cell extracts, the cell pellet was resuspended in 500 μl of the washing buffer and the cells were mechanically disrupted by bead beating three times for 20 s with 0.5 g of zirconia-silica beads (diameter, 0.1 mm; Roth, Karlsruhe, Germany) by use of a Silamat S5 (Vivadent, Ellwangen, Germany). After centrifugation (45 min, 4°C, 12,100 × g), the supernatant was used immediately for the enzyme assay. Protein concentrations were determined with the Bradford assay kit (Bio-Rad Laboratories, Hercules, Canada) with bovine serum albumin used as the standard.
Determination of the specific activity of the 6-phosphofructokinase PfkA (EC 2.7.1.11) in crude extracts was performed as described previously (57). The two different enzyme tests described in reference 57 were named test A (coupling to pyruvate kinase/lactate dehydrogenase) and test B (coupling to aldolase, triosephosphate isomerase, and glycerol-3-phosphate dehydrogenase) in this study. Both assay mixtures (500-μl total volumes) contained 100 mM Tris-HCl, pH 7.5, 0.2 mM NADH, 10 mM MgCl2, 1 mM ATP, and 20 to 75 μl of crude extract. In addition, the mixture for test A contained 2.75 U/ml NAD-dependent l-lactate dehydrogenase and 2.2 U/ml pyruvate kinase, and the mixture for test B contained 0.4 U/ml aldolase, 3.06 U/ml α-glycerol-3-phosphate dehydrogenase, and 0.033 U/ml triosephosphate isomerase. The reaction was started by the addition of 4 mM fructose-6-phosphate, and the increase in absorption at 340 nm [ɛ340 nm(NADH) = 6.3 mM−1 cm−1] was monitored at 30°C for 5 to 30 min using a Shimadzu UV-1202 spectrophotometer (Shimadzu, Duisburg, Germany).
Determination of the specific activity of the fructose-1,6-bisphosphate aldolase Fba (EC 4.1.2.13) in crude extracts was performed as described in reference 6. The assay mixture (500-μl total volume) contained 100 mM Tris-HCl, pH 7.4, 0.13 mM NADH, 1.67 U/ml α-glycerol-3-phosphate dehydrogenase, 0.018 U/ml triosephosphate isomerase, and 20 to 50 μl of crude extract. The reaction was started by the addition of 2 mM fructose-1,6-bisphosphate, and the increase in absorption at 340 nm was monitored at 30°C for 5 to 10 min using a Shimadzu UV-1202 spectrophotometer (Shimadzu, Duisburg, Germany).
Determination of the specific activity of the NAD-dependent l-lactate dehydrogenase LdhA (EC 1.1.1.27) in crude extracts was performed as described previously (8). The assay mixture (500-μl total volume) contained 20 mM MOPS (morpholinepropanesulfonic acid), pH 7.0, 0.2 mM NADH, and 1 to 20 μl of crude extract. The reaction was started by the addition of 30 mM pyruvate, and the increase in absorption at 340 nm was monitored at 30°C for 5 min using a Shimadzu UV-1202 spectrophotometer (Shimadzu, Duisburg, Germany).
Determination of the specific activity of the pyruvate kinase Pyk (EC 2.7.1.40) in crude extracts was performed as described previously (35). The assay mixture (500-μl total volume) contained 200 mM Tris-HCl, pH 7.0, 5 mM NADH, 200 mM MgCl2, 20 mM ATP, 110 U/ml NAD-dependent l-lactate dehydrogenase, and 1 to 20 μl of crude extract. The reaction was started by the addition of 240 mM phosphoenolpyruvate, and the increase in absorption at 340 nm was monitored at 30°C for 5 to 30 min using a Shimadzu UV-1202 spectrophotometer (Shimadzu, Duisburg, Germany).
Determination of the specific activity of the fumarase Fum (EC 4.2.1.2) in crude extracts was performed as described previously (22). The assay mixture (500-μl total volume) contained 100 mM sodium phosphate buffer, pH 7.3, and 0.5 to 2 μl of crude extract. The reaction was started by the addition of 50 mM l-malate, and the increase in absorption at 240 nm [ɛ240 nm(fumarate) = 2.44 mM−1 cm−1] was monitored at 26°C for 5 min using a Shimadzu UV-1202 spectrophotometer (Shimadzu, Duisburg, Germany).
Transketolase was assayed as described previously (58). The reaction mixture contained 50 mM Tris-HCl buffer, pH 7.5, 0.24 mM NADH, 0.01 mM thiamine pyrophosphate, 1.0 mM MgCl2, 0.5 mM xylulose-5-phosphate, 0.5 mM ribulose-5-phosphate, and 20 μg of a mixture of triosephosphate isomerase and glycerol-3-phosphate dehydrogenase in a total volume of 0.5 ml. The reaction was started by the addition of the enzyme, and the decrease in NADH was monitored at 340 nm.
Transaldolase was assayed as described previously (58). The reaction mixture contained 50 mM Tris-HCl buffer, pH 7.5, 5 mM EDTA, 0.5 mM erythrose-4-phosphate, 4.0 mM fructose-6-phosphate, 0.2 mM NADH, and 20 μg of a mixture of triosephosphate isomerase and glycerol-3-phosphate dehydrogenase in a total volume of 0.5 ml. The reaction was started by the addition of the enzyme, and the decrease in NADH was monitored at 340 nm.
l-Lactic acid production.
For l-lactic acid production, the C. glutamicum WT and WT ΔsugR strains along with strains WT(pVWEx1) and WT(pVWEx1-sugR) were cultivated aerobically at 30°C for about 16 h in 100 ml LB complex medium with 4% (wt/vol) glucose as the carbon source. When appropriate, media were supplemented with 50 μg/ml kanamycin. The precultures were harvested by centrifugation (5 min, 11,325 × g, 4°C), cells were washed in CgXII medium (pH 7.2) without any carbon source, and the washed cells were resuspended in 1 ml of the same medium and used to inoculate the oxygen deprivation culture with 80 ml CgXII minimal medium, pH 7.2, with 200 mM glucose, 0.03 g/liter protocatechuic acid, and 0.2 mg/liter biotin; with 30 mM potassium nitrate as the electron acceptor; with 1 μg/ml resazurin as the oxygen indicator; and when appropriate with 50 μg/ml kanamycin and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The medium was flushed for 24 h with nitrogen prior to inoculation. The cell suspension was subsequently incubated at 30°C in a lidded 100-ml medium bottle with gentle shaking for 3 hours.
RESULTS
Effect of sugR overexpression on growth on fructose, sucrose, or ribose.
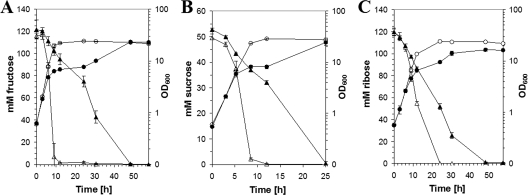
SugR binds to promoters of PTS genes and was previously shown to affect the utilization of the PTS substrate glucose in vivo (19). To test whether SugR also affects the utilization of the PTS substrates sucrose and fructose in vivo, the growth of a C. glutamicum strain overexpressing sugR was compared to that of the control strain on minimal medium with 100 mM fructose or 50 mM sucrose (Fig. 1A and B). The strain overexpressing sugR grew significantly slower and formed less biomass than the control strain on 100 mM fructose. Moreover, the strain overexpressing sugR utilized the added fructose with an uptake rate that was decreased approximately twofold in comparison to what was seen for the control strain (Fig. 1A). The overexpression of sugR also perturbed the growth of C. glutamicum on sucrose minimal medium, and the sucrose uptake rate was reduced about 13% compared to that of the control strain (Fig. 1B). The more pronounced effect of sugR overexpression on fructose utilization might be due to the fact that SugR represses the fruR-fruK-ptsF operon, encoding both the fructose-specific PTS component and 1-phosphofructokinase, while SugR represses only ptsS and not the sucrose-6-phosphate hydrolase gene scrB (18). Taken together, the overexpression of sugR had a negative influence on the utilization of all known PTS substrates (glucose, fructose, and sucrose) by C. glutamicum.
FIG. 1.
Role of SugR for growth of C. glutamicum on minimal medium with fructose, sucrose, or ribose. Growth of C. glutamicum WT(pVWEx1) (open symbols) and WT(pVWEx1-sugR) (filled symbols) on CgXII minimal medium containing 100 mM fructose (A), 50 mM sucrose (B), or 120 mM ribose (C). The cultures were induced 3 hours after inoculation by the addition of 1 mM IPTG. The optical densities (circles) and the fructose, sucrose, and ribose concentrations (triangles) are indicated.
To test whether the control of substrate utilization by SugR is limited to PTS substrates, the empty vector control strain and the strain overexpressing sugR were grown on minimal medium containing 120 mM ribose, which is taken up into the cell via an ABC transport system, as the sole carbon and energy source (Fig. 1C). C. glutamicum WT(pVWEx1-sugR) grew slower on ribose minimal medium than the control strain WT(pVWEx1) and formed less biomass (Fig. 1C). Whereas the negative effect of sugR overexpression on the PTS substrates glucose, fructose, and sucrose can be explained by SugR control of its known targets, the PTS genes, the negative effect on the non-PTS substrate ribose suggested that SugR controls the expression of additional target genes, e.g., genes coding for ribose uptake proteins or enzymes of the central carbon metabolism.
Identification of possible SugR targets using ChIP-to-chip analysis.
In order to identify further direct target genes of SugR on the genome-wide scale, a modified method of ChIP-to-chip analysis (34) was applied. The C. glutamicum WT-sugRStrep strain, which produces an affinity-tagged SugR protein instead of native SugR, was constructed in order to facilitate the purification of SugRStrep-DNA complexes formed in vivo. A vector containing the 3′ end of the sugR gene extended by codons for the addition of a C-terminal StrepTag was inserted into the sugR locus, thus allowing sugRStrep expression from the native sugR promoter. Protein-DNA complexes formed in vivo in the C. glutamicum WT-sugRStrep strain and in the control C. glutamicum WT strain were cross-linked by treating intact cells with formaldehyde. After cell disruption and DNA sharing by sonification, SugRStrep-DNA complexes were enriched by StrepTactin-Sepharose chromatography. After the reversal of the cross-links, the coprecipitated DNA was purified, fluorescently labeled, and hybridized to C. glutamicum DNA microarrays.
Table 3 lists all genes showing average enrichment factors of two or more in three independent ChIP-to-chip analyses during growth on LB (P values of ≤0.05) and those genes or genomic regions which were enriched in three additional ChIP-to-chip experiments during growth on CgXII minimal medium containing either 100 mM glucose, 100 mM fructose, or 50 mM sucrose. Enrichment of the known SugR target genes ptsG and ptsH as well as sugR itself and the gene for the fourth EII permease in C. glutamicum, possibly transporting an as-yet-unknown substrate (cg3366, sgcA), could be shown by the ChIP-to-chip experiments. Possibly due to the absence of suitable probes on the DNA microarrays, the enrichment factors for ptsI and the operon fruR-fruK-ptsF were only 1.6-fold (data not shown), and enrichment of ptsS was not identified. The ChIP-to-chip analysis identified a number of genes encoding enzymes of the central carbon metabolism as candidate SugR target genes, i.e., the genes for trehalose phosphatase (otsB, cg2909), 6-phosphofructokinase (pfkA [lies divergent to cg1408]), fructose-1,6-bisphosphate aldolase (cg3068, fba), enolase (cg1111, eno), pyruvate kinase (cg2291, pyk), fermentative NAD-dependent l-lactate dehydrogenase (cg3219, ldhA), which is crucial for anaerobic l-lactate production (32), dihydrolipoamide dehydrogenase (cg0441, lpd), and the genomic region between the genes for polyprenyltransferase and transketolase (cg1774, tkt).
TABLE 3.
Genes enriched in SugRStrep-DNA complexes identified by ChIP-to-chip analysisa
| Gene IDb | Gene name, gene product, and/or deduced function | ChIP-to-chip enrichment factor with:
|
|||
|---|---|---|---|---|---|
| Glucose | Fructose | Sucrose | LB | ||
| cg0641 | fabG2, probable short-chain dehydrogenase, secreted | 4.27 | 3.41 | 3.25 | 13.62 |
| cg0682 | Predicted ATPase or kinase | 2.77 | 2.50 | 2.65 | 8.91 |
| cg1110 | Conserved hypothetical protein | 5.07 | 6.51 | 7.26 | 8.58 |
| cg1111 | eno, enolase | 4.27 | 4.72 | 4.60 | 10.64 |
| cg1408 | Hypothetical membrane protein | 2.01 | 2.14 | 1.06 | 6.35 |
| cg2157P | terC, membrane protein TerC | 2.76 | 2.60 | 2.52 | 7.75 |
| cg3068P | fba, fructose-1,6-bisphosphate aldolase | 4.12 | 3.76 | 3.46 | 28.24 |
| cg3219 | ldhA, l-lactate dehydrogenase | 2.81 | 3.93 | 2.35 | 4.28 |
| cg0441P | lpd, diaminolipoamide dehydrogenase | 1.38 | 1.23 | 0.95 | 3.97 |
| cg0448 | Hypothetical protein | 1.12 | 1.10 | 1.07 | 2.07 |
| cg1174P | tkt, transketolase | 1.93 | 1.36 | 1.18 | 7.28 |
| cg1283 | aroE2, putative Shikimate/Quinate 5-dehydrogenase | 1.17 | 1.23 | 0.98 | 2.39 |
| cg1493 | Conserved hypothetical protein | 1.13 | 0.99 | 1.05 | 3.58 |
| cg1537P | ptsG, glucose-specific IIABC PTS component | 1.24 | 1.13 | 0.98 | 3.79 |
| cg1696 | Permease of the major facilitator superfamily | 1.34 | 1.29 | 1.07 | 4.80 |
| cg2115P | sugR, transcriptional regulator DeoR family | 1.30 | 1.45 | 1.45 | 4.39 |
| cg2121 | ptsH, phosphocarrier protein HPr | 1.91 | 1.87 | 1.47 | 9.64 |
| cg2262P | ftsY, signal recognition particle GTPase | 1.29 | 1.10 | 1.07 | 2.55 |
| cg2291 | pyk, pyruvate kinase | 1.32 | 1.26 | 1.12 | 2.41 |
| cg2794 | Conserved hypothetical protein | 1.33 | 1.26 | 1.45 | 3.49 |
| cg2831P-cg2833P | ramA, transcriptional regulator LuxR-family/cysK, cysteine synthase | 1.22 | 1.28 | 1.51 | 2.47 |
| cg2908 | Hypothetical trehalose-binding protein | 1.46 | 1.29 | 0.98 | 2.85 |
| cg2909 | otsB, trehalose-phosphatase | 1.56 | 1.48 | 1.39 | 4.48 |
| cg3366 | sgcA, putative phosphotransferase enzyme II, A component | 1.12 | 1.19 | 1.74 | 2.33 |
| cg0974 | Conserved hypothetical protein | 1.43 | 3.15 | 0.80 | |
| cg1074 | Conserved hypothetical protein | 2.30 | 1.83 | 0.25 | 2.71 |
| cg1142 | Na+/proline, Na+/panthothenate symporter | 2.03 | 1.06 | ||
Genes or genomic regions listed showed enrichment factors of two or more in three independent ChIP-to-chip analyses during growth on LB (P ≤ 0.05). DNA enriched in SugRStrep-DNA complexes by a factor of two or more was considered as a candidate target bound by SugR in vivo. In addition, those genes which were enriched in three additional ChIP-to-chip experiments during growth on CgXII minimal medium containing 100 mM glucose, 100 mM fructose, or 50 mM sucrose are listed.
P, enrichment factors based on intergenic regions, of which few were present on the DNA microarray.
Of the candidate SugR target genes identified by ChIP-to-chip analysis (Table 3), only PTS genes (ptsG, ptsI/fruR-fruK-ptsF, and ptsS) were found to be differentially expressed in a comparison between the WT and WT ΔsugR strains during growth in LB medium (19). As the absence of SugR showed the greatest effect on ptsG expression during growth in acetate medium (19), the gene expressions of the WT and WT ΔsugR strains were compared during growth in acetate minimal medium (Table 4). Statistically significant gene expression changes of a factor of three or more were observed for four genes (cg1673, cg2425, cg3368, and cg2071) showing low mRNA levels for the WT ΔsugR strain compared to the WT. High mRNA levels in the WT ΔsugR strain compared to the WT were determined for 24 genes: ptsG, ptsH, ptsI, fruR-fruK-ptsF, fruK2, ptsS, ldhA, pyk, eno, pyc, lldD, dtsR, sufD, metE, fas-IB, rplO, rpsQ, pepB, ssuB, cg1112, cg1977, and cg2430. Thus, evidence from ChIP-to-chip and transcriptome analysis suggests that, besides repressing the PTS genes, SugR also represses pyk, eno, and ldhA.
TABLE 4.
Genes whose average mRNA ratios were altered by a factor of three or morea in acetate minimal medium for C. glutamicum WT compared with the ΔsugR mutant
| Gene IDb | Gene name, gene product, and/or deduced function | mRNA ratio (ΔsugR mutant/WT)c |
|---|---|---|
| cg2925 | ptsS, sucrose-specific enzyme II of the PTS | 50.7 |
| cg2119 | pfkB, 1-phosphofructokinase protein | 47.8 |
| cg2120 | ptsF, fructose-specific enzyme II of the PTS | 32.3 |
| cg2118 | fruK, DeoR-type transcriptional regulator | 26.9 |
| cg2117 | ptsI, enzyme I of the PTS | 11.1 |
| cg2121 | ptsH, phosphocarrier protein HPr | 9.0 |
| cg3219 | ldhA, NAD-dependent l-lactate dehydrogenase | 6.7 |
| cg0812 | dtsR1, acetyl/propionyl-CoA carboxylase beta chain | 6.6 |
| cg0957 | fas-IB, fatty acid synthase | 5.9 |
| cg2116 | Putative phosphofructokinase | 5.4 |
| cg1290 | metE, 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase | 4.8 |
| cg3218 | pyk, pyruvate kinase | 4.3 |
| cg1763 | sufD, components of an uncharacterized iron-regulated ABC-type transporter | 3.6 |
| cg1112 | Septum formation initiator, secreted protein | 3.6 |
| cg0791 | pyc, pyruvate carboxylase | 3.5 |
| cg0634 | rplO, 50S ribosomal protein L15 | 3.5 |
| cg2430 | Hypothetical protein | 3.5 |
| cg1111 | eno, enolase | 3.3 |
| cg3227 | lldD, quinone-dependent l-lactate dehydrogenase | 3.1 |
| cg2419 | pepB, leucyl aminopeptidase | 3.1 |
| cg1379 | ssuB, aliphatic sulfonates ATP-binding ABC transporter protein | 3.1 |
| cg0604 | rpsQ, 30S ribosomal protein S17 | 3.0 |
| cg1977 | Putative secreted protein | 3.0 |
| cg1537 | ptsG, glucose-specific enzyme II of the PTS | 3.0 |
| cg1673 | ppmN, polyprenol-phosphate-mannose synthase | 0.3 |
| cg2425 | Predicted permease | 0.3 |
| cg3368 | ABC transporter permease protein | 0.2 |
| cg2071 | int2′, putative phage integrase (N-terminal fragment) | 0.2 |
| cg2115 | sugR, transcriptional regulator of sugar metabolism | 0.1 |
P < 0.05.
Gene numbering, gene designations, and descriptions of gene products are for NC006958.
The mRNA ratios represent average values obtained from three DNA microarray experiments performed with RNA isolated from three independent cultures in acetate minimal medium.
Gel retardation analysis of candidate SugR target genes.
In order to test for a direct interaction of SugR with the promoter regions of the possible new target genes in vitro, gel retardation analysis was performed with purified SugRHis protein and DNA fragments covering the corresponding promoter regions. Of the candidate SugR target genes deduced from ChIP-to-chip analysis, primarily genes encoding enzymes of central carbon metabolism were chosen for gel retardation analysis. In addition, the succinyl-coenzyme A (CoA) synthetase gene sucC and the phosphotransacetylase gene pta were included, as our previous transcriptome analysis suggested control by SugR (19). Complete gel shifts were observed at a 30-fold molar excess of SugR for sgcA, ptsH, eno, and terC and at a 60-fold excess for pyk, ldhA, ptsG, fba, pfkA, and the intergenic regions between cg2795-cg2796 and aroE1-lipT (Fig. 2). At a 90-fold excess of SugR, almost complete gel shifts were observed for ramA-cysK, lpd, ctaB-tkt, sugR, ftsY, alr, and cytP (Fig. 2). SugR binding to the promoter fragments of the phosphoenolpyruvate carboxykinase gene (pck), the succinyl-CoA synthetase (sucC) and the fumarase (fum) genes, and the phosphotransacetylase gene (pta) was very weak or even absent.
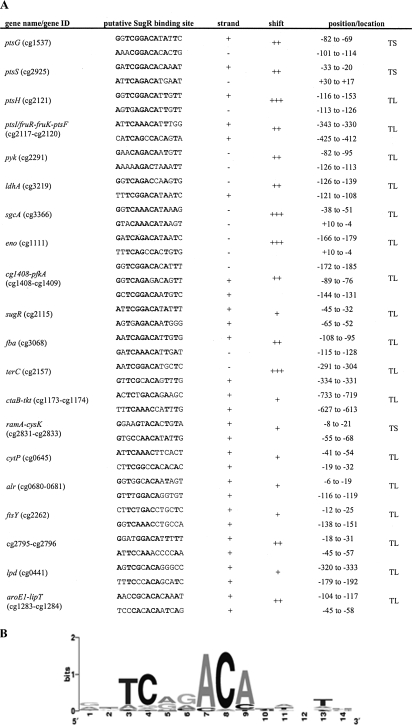
To identify a consensus SugR binding site, the promoter sequences of those genes which were bound by SugR as evidenced by ChIP-to-chip and gel retardation analysis were compared for sequence similarities using the free MEME software (http://meme.sdsc.edu/) (4). Two putative SugR binding motives were identified in each promoter fragment with the consensus sequence shown in Fig. 3B. The binding sites have in common that they are located at or near the transcriptional start sites, suggesting that SugR acts as a repressor of those genes.
FIG. 3.
SugR binding sites in the DNA fragments verified by band shift analysis. (A) The SugR binding sites shown in this figure were identified by a motif search using the MEME software (http://meme.sdsc.edu/) (4) and the promoter fragments used in the gel shift analysis. The column labeled “shift” indicates whether a complete gel shift occurred at a 30-fold (+++), 60-fold (++), or 90-fold (+) excess of SugR to the corresponding DNA fragments. The positions of the binding sites relative to the transcriptional start site (TS) or the translational start site (TL) are given by the numbers in the “position/location” column. (B) A frequency plot of the deduced consensus sequence conservation at each position of the 14-bp motif, where the height of each symbol within the stack reflects the relative frequency of the corresponding nucleotide at that position.
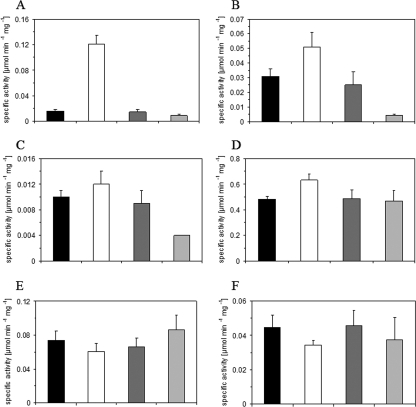
Specific activities of NAD-dependent l-lactate dehydrogenase, 6-phosphofructokinase, fructose-1,6-bisphosphate aldolase, transketolase, transaldolase, pyruvate kinase, and fumarase in C. glutamicum WT and WT ΔsugR strains and WT(pVWEx1) and WT(pVWEx1-sugR).
The specific activities of NAD-dependent l-lactate dehydrogenase, 6-phosphofructokinase, pyruvate kinase, and fructose-1,6-bisphosphate aldolase were determined for strains lacking sugR or overexpressing sugR because SugR was found to bind to the promoter regions of their genes. Transketolase and transaldolase activities were determined, as SugR bound upstream of the putative tkt-tal-zwf-opcA-devB operon. Fumarase activity was also determined, although SugR binding was very weak (or absent). As shown in Fig. 4, the activities of the tested enzymes were comparable in C. glutamicum WT and WT(pVWEx1). In the C. glutamicum WT ΔsugR strain, the LdhA, Fba, PfkA, and Pyk activities were increased 8.1-fold, 1.6-fold, 1.2-fold, and 1.3-fold, respectively, compared to what was seen for the WT (Fig. 4A to D). In C. glutamicum WT(pVWEx1-sugR), the specific activities of LdhA, Fba, and PfkA were 65%, 16%, and 44%, respectively, of the specific activities measured for the empty vector control (Fig. 4A to C). The specific activity of Pyk was not significantly changed due to the overexpression of sugR (Fig. 4D), indicating that the role of SugR for pyk expression is not absolutely clear. The specific activities of transketolase, transaldolase, and fumarase were comparable for all strains tested (Fig. 4 and data not shown). Taken together, the results indicate that SugR acts as a repressor of the genes for NAD-dependent l-lactate dehydrogenase, 6-phosphofructokinase, and fructose-1,6-bisphosphate aldolase and affects their activities in vivo.
FIG. 4.
Specific activities of NAD-dependent l-lactate dehydrogenase (LdhA) (A), fructose-1,6-bisphosphate aldolase (Fba) (B), 6-phosphofructokinase (PfkA) (C), pyruvate kinase (Pyk) (D), transketolase (Tkt) (E), and transaldolase (Tal) (F) in the C. glutamicum WT (black bars), WTΔsugR (white bars), WT(pVWEx1) (dark gray bars), and WT(pVWEx1-sugR) (light gray bars) strains during aerobic growth on LB. All data are arithmetic means with absolute errors of at least four determinations from one or two independent cultivations.
Influence of deletion and overexpression of sugR on l-lactate formation by C. glutamicum.
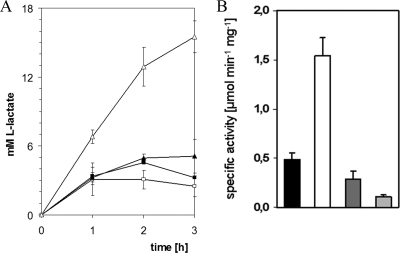
The formation of l-lactate, which is secreted into the medium during anaerobiosis or as a by-product during glutamate production (37, 55), requires ldhA (32), which was shown to be repressed by SugR (Fig. 2 and 4A). To test the physiological relevance of ldhA control by SugR, levels of l-lactate formation by the C. glutamicum WT and WT ΔsugR strains and WT(pVWEx1) and WT(pVWEx1-sugR) were compared during aerobic growth on glucose and under oxygen deprivation conditions. During the aerobic growth on glucose of the C. glutamicum WT, WT ΔsugR, and WT(pVWEx1) strains, l-lactate transiently accumulated to maximal concentrations of 19 ± 2 mM, 24 ± 3 mM, and 23 ± 2 mM, respectively, after 12 h, while during the cultivation of WT(pVWEx1-sugR), l-lactate could not be detected (<0.5 mM [data not shown]). Under oxygen deprivation conditions, the WT ΔsugR deletion mutant showed a threefold-increased l-lactate formation compared to the WT, whereas the strain overexpressing sugR formed approximately 30% less l-lactate than the control (Fig. 5A). The specific activity of fermentative NAD-dependent l-lactate dehydrogenase was about twofold lower for WT(pVWEx1) than for WT(pVWEx1-sugR) and about threefold higher for the WT ΔsugR strain than for the WT (Fig. 5B). Thus, SugR repression of ldhA is important for aerobic and anaerobic l-lactate formation by C. glutamicum.
FIG. 5.
Production of l-lactate (A) and specific activities of NAD-dependent l-lactate dehydrogenase LdhA (B) during growth on glucose under oxygen deprivation conditions. (A) Concentrations of l-lactate produced by C. glutamicum WT (closed triangles), the WT ΔsugR mutant (open triangles), WT(pVWEx1) (closed squares), and WT(pVWEx1-sugR) (open squares) are indicated. The data represent averages of two independent cultivations. (B) NAD-dependent l-lactate dehydrogenase (LdhA) in the C. glutamicum WT (black bars), WTΔsugR (white bars), WT(pVWEx1) (dark gray bars), and WT(pVWEx1-sugR) (light gray bars) strains during growth on glucose under oxygen deprivation conditions. All data are arithmetic means with absolute errors of at least four determinations from three independent cultivations.
DISCUSSION
SugR has previously been shown to repress the PTS genes for the glucose-, fructose-, and sucrose-specific enzymes II (19) and for the general components enzyme I and HPr (21, 59). The ChIP-to-chip and gel retardation analysis shown here revealed that SugR represses not only PTS genes but also a number of further genes, which mainly encode enzymes of the central carbon metabolism. It was shown here that the genes encoding 6-phosphofructokinase (pfkA), fructose-1,6-bisphosphate aldolase (fba), and NAD-dependent l-lactate dehydrogenase (ldhA) belong to the SugR regulon (Table 3; Fig. 2). Thus, SugR coordinately controls the expression of genes for the uptake of carbohydrates via the PTS and for their further metabolism in the central pathways of glycolysis (pfkA, fba, and ldhA). The genes pfkA, fba, and ldhA are not known targets of the carbon regulators AcnR (represses aconitase gene acn [41]), GntR1 and GntR2 (repress gluconate utilization genes gntP, gntK, and gnd and activate ptsG and ptsS [10]), GlxR (represses gntP, gntK, and isocitrate lyase and malate synthase genes aceA and aceB [38, 42]), and LldR (represses the l-lactate utilization operon cg3226-lldD [23]). RamB was shown to repress its own gene, aceA, aceB, the acetate activation operon pta-ack, and the alcohol dehydrogenase gene adhA and to activate aceE, which encodes subunit E1 of pyruvate dehydrogenase (2, 7, 24). The occurrence of RamB binding motifs suggests that ptsG, the tricarboxylic acid (TCA) cycle genes gltA and acn, and the phosphoenolpyruvate carboxykinase and malic enzyme genes pck and malE are regulated by RamB (3). RamA activates adhA, aceA, aceB, pta-ack, and ramB (2, 3, 11, 12) and, in addition, likely regulates the TCA cycle genes sdhCAB for succinate dehydrogenase and acn, as well as pck and malE, as RamA binding site motifs are found in the promoter regions of these genes (3).
The regulons of the carbon catabolite regulators cAMP receptor protein-cAMP complex from E. coli and CcpA from B. subtilis comprise genes of glycolysis and the TCA cycle and are larger than the SugR regulon from C. glutamicum. In E. coli, at least 200 genes which encode enzymes of many different pathways including glycolysis and the TCA cycle are controlled by the cAMP receptor protein-cAMP complex (25, 26). The carbon catabolite control protein CcpA of B. subtilis positively regulates genes for glycolytic enzymes and carbon overflow pathways and represses genes of the TCA cycle and for utilization of carbon sources other than glucose (13). In both E. coli and B. subtilis, carbon catabolite control is a dominant regulatory mechanism responsible for diauxic growth phenomena. In contrast, in C. glutamicum, which generally coutilizes carbon sources present in substrate mixtures, the carbon regulators SugR, RamA, and RamB primarily fine-tune central metabolic pathways for simultaneous substrate utilization.
A conserved sequence motif (Fig. 3B) is found two times in the promoter regions of all identified putative SugR targets and coincides with sequences upstream of ptsG (19) and in the intergenic region between ptsI and fruR (21), shown by mutational analysis to be essential for SugR binding. The locations and the relative orientations between the two sequence motifs within a given promoter region vary (Fig. 3A). In vitro evidence suggests that a TG(T)2-5G sequence might additionally be involved in SugR binding in C. glutamicum R, a related strain providing high lactate yields (60). Typical representatives of the DeoR-type family proteins, to which SugR from C. glutamicum belongs, are DeoR from E. coli, which binds to a 16-bp palindromic sequence, 5′-TGTTAGAA·TTCTAACA-3′, in two of the three operator sites, namely, O1, O2, and OE, forming a single or double DNA loop (46), and FruR from Lactococcus lactis, which potentially binds to four repeating nonpalindromic sequences upstream of the fructose-PTS gene cluster (5). Currently, it is not known how the different orientations and locations of the binding sequence motifs affect the action of the SugR from C. glutamicum in vivo and if SugR occurs in different multimeric forms, as described for DeoR of E. coli.
SugR control of PTS genes is physiologically relevant, as the utilization of glucose (19), fructose, or sucrose (Fig. 1) is negatively affected by the overexpression of sugR, while the deletion of sugR resulted in increased glucose uptake during growth on glucose-acetate mixtures (19). In addition, the physiological significance of the SugR control of non-PTS genes became obvious by the facts that sugR overexpression perturbed the utilization of ribose (Fig. 1) and that the deletion of sugR resulted in increased l-lactate formation under oxygen deprivation conditions (Fig. 5). l-Lactate is both a metabolic product and a carbon substrate for growth. The growth of C. glutamicum on l-lactate requires quinone-dependent l-lactate dehydrogenase LldD (cg3227; EC 1.1.2.3) (55). The cg3226-lldD operon, which contains a gene for a putative lactate transport system besides lldD, is repressed by the FadR-type transcriptional regulator LldR in the absence of its effector l-lactate (23). C. glutamicum is able to secrete l-lactate into the medium, e.g., as a by-product during glutamate and lysine production (37, 55) or under oxygen deprivation conditions (32). l-Lactate formation requires the NAD-dependent l-lactate dehydrogenase LdhA (32) and ldhA mRNA levels increased about ninefold under oxygen deprivation conditions (33), as expected for fermentative enzymes. However, the regulatory mechanism for the anaerobic induction or aerobic repression of ldhA is currently unknown. Here, it was shown that ldhA is a target of SugR and that SugR represses ldhA (Table 3; Fig. 2 and 4A). In the absence of SugR, LdhA activities were about eightfold higher than in C. glutamicum WT (Fig. 4A), which was associated with a threefold-increased l-lactate formation on glucose medium under oxygen deprivation conditions (Fig. 4). As the glycolytic intermediates glucose-6-phosphate, fructose-6-phosphate, fructose-1,6-bisphosphate, and fructose-1-phosphate interfere with SugR binding to its target promoters (19, 21), SugR control of ldhA ensures that ldhA expression is maximal under oxygen deprivation conditions only if the supply of carbohydrate growth substrates entering glycolysis is sufficient.
Acknowledgments
We thank Michael Bott and Hermann Sahm for their continuous support and Maria Bucsenez, Melanie Brocker, and Tino Polen for technical assistance. Part of the described work belongs to the dissertation of Verena Engels at the faculty of Mathematics and Natural Sciences of the Heinrich-Heine-Universität Düsseldorf.
Footnotes
Published ahead of print on 10 October 2008.
REFERENCES
- 1.Abe, S., K.-I. Takayama, and S. Kinoshita. 1967. Taxonomical studies on glutamic acid-producing bacteria. J. Gen. Appl. Microbiol. 13279-301. [Google Scholar]
- 2.Arndt, A., and B. J. Eikmanns. 2007. The alcohol dehydrogenase gene adhA in Corynebacterium glutamicum is subject to carbon catabolite repression. J. Bacteriol. 1897408-7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arndt, A., and B. J. Eikmanns. 2008. Regulation of carbon metabolism in Corynebacterium glutamicum, p. 155-182. In A. Burkovski (ed.), Corynebacteria: genomics and molecular biology, in press. Caister Academic Press, Wymondham, United Kingdom.
- 4.Bailey, T. L., and C. Elkan. 1995. The value of prior knowledge in discovering motifs with MEME. Proc. Int. Conf. Intell. Syst. Mol. Biol. 321-29. [PubMed] [Google Scholar]
- 5.Barrière, C., M. Veiga-da-Cunha, N. Pons, E. Guedon, S. A. van Hijum, J. Kok, O. P. Kuipers, D. S. Ehrlich, and P. Renault. 2005. Fructose utilization in Lactococcus lactis as a model for low-GC gram-positive bacteria: its regulator, signal, and DNA-binding site. J. Bacteriol. 1873752-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmeyer, H. U. (ed.). 1974. Methods of enzymatic analysis, vol. I, p. 430. Verlag Chemie GmbH, Weinheim, Germany. [Google Scholar]
- 7.Blombach, B., A. Cramer, B. J. Eikmanns, and M. Schreiner. RamB is an activator of the pyruvate dehydrogenase complex subunit E1p gene in Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol., in press. [DOI] [PubMed]
- 8.Bunch, P. K., F. Mat-Jan, N. Lee, and D. P. Clark. 1997. The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 143187-195. [DOI] [PubMed] [Google Scholar]
- 9.Burkovski, A. Corynebacteria: genomics and molecular biology, in press. Caister Academic Press, Wymondham, United Kingdom.
- 10.Cramer, A., M. Auchter, J. Frunzke, M. Bott, and B. J. Eikmanns. 2007. RamB, the transcriptional regulator of acetate metabolism in Corynebacterium glutamicum, is subject to regulation by RamA and RamB. J. Bacteriol. 1891145-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer, A., and B. J. Eikmanns. 2007. RamA, the transcriptional regulator of acetate metabolism in Corynebacterium glutamicum, is subject to negative autoregulation. J. Mol. Microbiol. Biotechnol. 1251-59. [DOI] [PubMed] [Google Scholar]
- 12.Cramer, A., R. Gerstmeir, S. Schaffer, M. Bott, and B. J. Eikmanns. 2006. Identification of RamA, a novel LuxR-type transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol. 1882554-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutscher, J., A. Galinier, and I. Martin-Verstraete. 2002. Carbohydrate uptake and metabolism, p. 129-150. In A. L. Sonenshein, J. A. Hoch, and R. Losik (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 14.Dominguez, H., M. Cocaign-Bousquet, and N. D. Lindley. 1997. Simultaneous consumption of glucose and fructose from sugar mixtures during batch growth of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 47600-603. [Google Scholar]
- 15.Dominguez, H., and N. D. Lindley. 1996. Complete sucrose metabolism requires fructose phosphotransferase activity in Corynebacterium glutamicum to ensure phosphorylation of liberated fructose. Appl. Environ. Microbiol. 623878-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez, H., C. Rollin, A. Guyonvarch, J. L. Guerquin-Kern, M. Cocaign-Bousquet, and N. D. Lindley. 1998. Carbon-flux distribution in the central metabolic pathways of Corynebacterium glutamicum during growth on fructose. Eur. J. Biochem. 25496-102. [DOI] [PubMed] [Google Scholar]
- 17.Eggeling, L., and M. Bott. 2005. Handbook on Corynebacterium glutamicum. CRC Press, Boca Raton, FL.
- 18.Engels, V., T. Georgi, and V. F. Wendisch. 2008. ScrB (Cg2927) is a sucrose-6-phosphate hydrolase essential for sucrose utilization by Corynebacterium glutamicum. FEMS Microbiol. Lett. 28980-89. [DOI] [PubMed] [Google Scholar]
- 19.Engels, V., and V. F. Wendisch. 2007. The DeoR-type regulator SugR represses expression of ptsG in Corynebacterium glutamicum. J. Bacteriol. 1892955-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinberg, A. P., and B. Vogelstein. 1983. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 1326-13. [DOI] [PubMed] [Google Scholar]
- 21.Gaigalat, L., J. P. Schlüter, M. Hartmann, S. Mormann, A. Tauch, A. Pühler, and J. Kalinowski. 2007. The DeoR-type transcriptional regulator SugR acts as a repressor for genes encoding the phosphoenolpyruvate:sugar phosphotransferase system (PTS) in Corynebacterium glutamicum. BMC Mol. Biol. 8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genda, T., S. Watabe, and H. Ozaki. 2006. Purification and characterization of fumarase from Corynebacterium glutamicum. Biosci. Biotechnol. Biochem. 701102-1109. [DOI] [PubMed] [Google Scholar]
- 23.Georgi, T., V. Engels, and V. F. Wendisch. 2008. Regulation of l-lactate utilization by the FadR-type regulator LldR of Corynebacterium glutamicum. J. Bacteriol. 190963-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstmeir, R., A. Cramer, P. Dangel, S. Schaffer, and B. J. Eikmanns. 2004. RamB, a novel transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol. 1862798-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosset, G., Z. Zhang, S. Nayyar, W. A. Cuevas, and M. H. Saier, Jr. 2004. Transcriptome analysis of Crp-dependent catabolite control of gene expression in Escherichia coli. J. Bacteriol. 1863516-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grainger, D. C., D. Hurd, M. Harrison, J. Holdstock, and S. J. Busby. 2005. Studies of the distribution of Escherichia coli cAMP-receptor protein and RNA polymerase along the E. coli chromosome. Proc. Natl. Acad. Sci. USA 10217693-17698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanahan, D. 1985. Techniques for transformation of E. coli, p. 109-135. In D. M. Glover (ed.), DNA cloning, vol. 1. IRL Press, Oxford, United Kingdom. [Google Scholar]
- 28.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 29.Henkin, T. M. 1996. The role of CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol. Lett. 1359-15. [DOI] [PubMed] [Google Scholar]
- 30.Henkin, T. M., F. J. Grundy, W. L. Nicholson, and G. H. Chambliss. 1991. Catabolite repression of alpha-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacl and galR repressors. Mol. Microbiol. 5575-584. [DOI] [PubMed] [Google Scholar]
- 31.Hermann, T. 2003. Industrial production of amino acids by coryneform bacteria. J. Biotechnol. 104155-172. [DOI] [PubMed] [Google Scholar]
- 32.Inui, M., S. Murakami, S. Okino, H. Kawaguchi, A. A. Vertes, and H. Yukawa. 2004. Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J. Mol. Microbiol. Biotechnol. 7182-196. [DOI] [PubMed] [Google Scholar]
- 33.Inui, M., M. Suda, S. Okino, H. Nonaka, L. G. Puskas, A. A. Vertes, and H. Yukawa. 2007. Transcriptional profiling of Corynebacterium glutamicum metabolism during organic acid production under oxygen deprivation conditions. Microbiology 1532491-2504. [DOI] [PubMed] [Google Scholar]
- 34.Iyer, V. R., C. E. Horak, C. S. Scafe, D. Botstein, M. Snyder, and P. O. Brown. 2001. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature 409533-538. [DOI] [PubMed] [Google Scholar]
- 35.Jetten, M. S., M. E. Gubler, S. H. Lee, and A. J. Sinskey. 1994. Structural and functional analysis of pyruvate kinase from Corynebacterium glutamicum. Appl. Environ. Microbiol. 602501-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalinowski, J., B. Bathe, D. Bartels, N. Bischoff, M. Bott, A. Burkovski, N. Dusch, L. Eggeling, B. J. Eikmanns, L. Gaigalat, A. Goesmann, M. Hartmann, K. Huthmacher, R. Krämer, B. Linke, A. C. McHardy, F. Meyer, B. Möckel, W. Pfefferle, A. Pühler, D. A. Rey, C. Rückert, O. Rupp, H. Sahm, V. F. Wendisch, I. Wiegräbe, and A. Tauch. 2003. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of l-aspartate-derived amino acids and vitamins. J. Biotechnol. 1045-25. [DOI] [PubMed] [Google Scholar]
- 37.Kiefer, P., E. Heinzle, and C. Wittmann. 2002. Influence of glucose, fructose and sucrose as carbon sources on kinetics and stoichiometry of lysine production by Corynebacterium glutamicum. J. Ind. Microbiol. Biotechnol. 28338-343. [DOI] [PubMed] [Google Scholar]
- 38.Kim, H. J., T. H. Kim, Y. Kim, and H. S. Lee. 2004. Identification and characterization of glxR, a gene involved in regulation of glyoxylate bypass in Corynebacterium glutamicum. J. Bacteriol. 1863453-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinoshita, S., S. Udaka, and M. Shimono. 1957. Studies on the amino acid fermentation. I. Production of l-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol. 3193-205. [PubMed] [Google Scholar]
- 40.Kolb, A., S. Busby, H. Buc, S. Garges, and S. Adhya. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62749-795. [DOI] [PubMed] [Google Scholar]
- 41.Krug, A., V. F. Wendisch, and M. Bott. 2005. Identification of AcnR, a TetR-type repressor of the aconitase gene acn in Corynebacterium glutamicum. J. Biol. Chem. 280585-595. [DOI] [PubMed] [Google Scholar]
- 42.Letek, M., N. Valbuena, A. Ramos, E. Ordónez, J. A. Gil, and L. M. Mateos. 2006. Characterization and use of catabolite-repressed promoters from gluconate genes in Corynebacterium glutamicum. J. Bacteriol. 188409-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leuchtenberger, W., K. Huthmacher, and K. Drauz. 2005. Biotechnological production of amino acids and derivatives: current status and prospects. Appl. Microbiol. Biotechnol. 691-8. [DOI] [PubMed] [Google Scholar]
- 44.Miwa, Y., K. Nagura, S. Eguchi, H. Fukuda, J. Deutscher, and Y. Fujita. 1997. Catabolite repression of the Bacillus subtilis gnt operon exerted by two catabolite-responsive elements. Mol. Microbiol. 231203-1213. [DOI] [PubMed] [Google Scholar]
- 45.Moon, M. W., H. J. Kim, T. K. Oh, C. S. Shin, J. S. Lee, S. J. Kim, and J. K. Lee. 2005. Analyses of enzyme II gene mutants for sugar transport and heterologous expression of fructokinase gene in Corynebacterium glutamicum ATCC 13032. FEMS Microbiol. Lett. 244259-266. [DOI] [PubMed] [Google Scholar]
- 46.Mortensen, L., G. Dandanell, and K. Hammer. 1989. Purification and characterization of the deoR repressor of Escherichia coli. EMBO J. 8325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parche, S., A. Burkovski, G. A. Sprenger, B. Weil, R. Krämer, and F. Titgemeyer. 2001. Corynebacterium glutamicum: a dissection of the PTS. J. Mol. Microbiol. Biotechnol. 3423-428. [PubMed] [Google Scholar]
- 48.Peters-Wendisch, P. G., B. Schiel, V. F. Wendisch, E. Katsoulidis, B. Möckel, H. Sahm, and B. J. Eikmanns. 2001. Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol. 3295-300. [PubMed] [Google Scholar]
- 49.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 50.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multipurpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 14569-73. [DOI] [PubMed] [Google Scholar]
- 51.Sindelar, G. 2003. Globale Expressionsanalysen zur Charakterisierung der Lysin-Produktion in Corynebacterium glutamicum. Dissertation. Heinrich-Heine-Universität Düsseldorf, Jülich, Germany.
- 52.Skerra, A., and T. G. Schmidt. 2000. Use of the StrepTag and streptavidin for detection and purification of recombinant proteins. Methods Enzymol. 326271-304. [DOI] [PubMed] [Google Scholar]
- 53.Sprehe, M., G. Seidel, M. Diel, and W. Hillen. 2007. CcpA mutants with differential activities in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1296-105. [DOI] [PubMed] [Google Scholar]
- 54.Stackebrandt, E., F. A. Rainey, and N. L. Ward-Rainey. 1997. Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int. J. Syst. Bacteriol. 47479-491. [Google Scholar]
- 55.Stansen, C., D. Uy, S. Delaunay, L. Eggeling, J. L. Goergen, and V. F. Wendisch. 2005. Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl. Environ. Microbiol. 715920-5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189113-130. [DOI] [PubMed] [Google Scholar]
- 57.Sugimoto, S., and I. Shiio. 1989. Fructose metabolism and regulation of 1-phosphofructokinase and 6-phosphofructokinase in Brevibacterium flavum. Agric. Biol. Chem. 531261-1268. [Google Scholar]
- 58.Sugimoto, S., and I. Shiio. 1989. Regulation of enzymes for erythrose 4-phosphate synthesis in Brevibacterium flavum. Agric. Biol. Chem. 532081-2087. [Google Scholar]
- 59.Tanaka, Y., N. Okai, H. Teramoto, M. Inui, and H. Yukawa. 2008. Regulation of the expression of phosphoenolpyruvate: carbohydrate phosphotransferase system (PTS) genes in Corynebacterium glutamicum R. Microbiology 154264-274. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka, Y., H. Teramoto, M. Inui, and H. Yukawa. 2008. Regulation of expression of general components of the phosphoenolpyruvate: carbohydrate phosphotransferase system (PTS) by the global regulator SugR in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 78309-318. [DOI] [PubMed] [Google Scholar]
- 61.Tobisch, S., D. Zuhlke, J. Bernhardt, J. Stulke, and M. Hecker. 1999. Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis. J. Bacteriol. 1816996-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Rest, M. E., C. Lange, and D. Molenaar. 1999. A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl. Microbiol. Biotechnol. 52541-545. [DOI] [PubMed] [Google Scholar]
- 63.Wendisch, V. F., A. A. de Graaf, H. Sahm, and B. J. Eikmanns. 2000. Quantitative determination of metabolic fluxes during coutilization of two carbon sources: comparative analyses with Corynebacterium glutamicum during growth on acetate and/or glucose. J. Bacteriol. 1823088-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wendisch, V. F. 2006. Genetic regulation of Corynebacterium glutamicum metabolism. J. Microbiol. Biotechnol. 16999-1009. [Google Scholar]