Abstract
Yersinia pestis, the bacterial agent of plague, forms a biofilm in the foregut of its flea vector to produce a transmissible infection. The closely related Yersinia pseudotuberculosis, from which Y. pestis recently evolved, can colonize the flea midgut but does not form a biofilm in the foregut. Y. pestis biofilm in the flea and in vitro is dependent on an extracellular matrix synthesized by products of the hms genes; identical genes are present in Y. pseudotuberculosis. The Yersinia Hms proteins contain functional domains present in Escherichia coli and Staphylococcus proteins known to synthesize a poly-β-1,6-N-acetyl-d-glucosamine biofilm matrix. In this study, we show that the extracellular matrices (ECM) of Y. pestis and staphylococcal biofilms are antigenically related, indicating a similar biochemical structure. We also characterized a glycosyl hydrolase (NghA) of Y. pseudotuberculosis that cleaved β-linked N-acetylglucosamine residues and reduced biofilm formation by staphylococci and Y. pestis in vitro. The Y. pestis nghA ortholog is a pseudogene, and overexpression of functional nghA reduced ECM surface accumulation and inhibited the ability of Y. pestis to produce biofilm in the flea foregut. Mutational loss of this glycosidase activity in Y. pestis may have contributed to the recent evolution of flea-borne transmission.
The plague bacillus Yersinia pestis is maintained in flea-rodent transmission cycles in many parts of the world. Plague transmission is facilitated by the ability of Y. pestis to form a bacterial biofilm on the spines that line the interior surface of the flea's proventriculus, a valve guarding the entrance to the midgut that opens and closes rhythmically during blood feeding (23, 29). Growth and consolidation of the biofilm among the proventricular spines interfere with the patency of the proventricular valve and the passage of blood into the midgut, resulting in regurgitative transmission of bacteria into the bite site when an infected flea attempts to feed (2, 3). Eventually, the proventriculus can become completely blocked with Y. pestis biofilm. Blocked fleas make frequent, persistent attempts to take a blood meal before they die from starvation, and this altered feeding behavior increases the chances of transmission.
A biofilm is a dense aggregate of microorganisms embedded in an extracellular matrix (ECM) and usually attached to a surface (10). Biofilm formation in the flea and in vitro is mediated by the Y. pestis hms gene products, which are responsible for an extracellular material produced at growth temperatures of ≤26°C (13, 23, 29, 40). Homologous genes in other bacteria, including the ica genes of Staphylococcus aureus and Staphylococcus epidermidis and the pga genes of Escherichia coli, act to synthesize an ECM of poly-β-1,6-linked N-acetyl-d-glucosamine (β-1,6-GlcNAc) that is essential for biofilm formation (11, 21, 50).
Flea-borne transmission, as well as the increased virulence of Y. pestis, coevolved within the past 20,000 years, the time frame in which Y. pestis diverged from Yersinia pseudotuberculosis, a relatively benign food- and waterborne intestinal pathogen (1, 35). The close phylogenetic relationship between these two species is reflected in their high degree of genomic identity. For example, Y. pestis contains only 32 chromosomal genes that do not have identical or highly similar orthologs in Y. pseudotuberculosis, and the nucleotide sequences of the hms genes are identical in the two species (8, 39). Despite this, Y. pseudotuberculosis produces less-cohesive biofilms than Y. pestis under some in vitro conditions and never colonizes the flea proventriculus even though most strains produce a chronic infection in the midgut (16).
Y. pestis contains many pseudogenes and genes disrupted by insertion sequence elements that are intact genes in Y. pseudotuberculosis. This suggests that Y. pestis is in the early stages of genome decay, eliminating genes no longer needed to survive outside of its insect or mammalian hosts (8, 39, 51). One of the Y. pseudotuberculosis genes (YPTB1123) that appears to be a pseudogene in Y. pestis is predicted to encode a family 20 glycosyl hydrolase enzyme and was annotated as chitobiase (chb) (8, 22, 39). Notably, another family 20 glycosyl hydrolase, dispersin B of Actinobacillus, cleaves poly-β-1,6-GlcNAc and disrupts biofilm formation in Y. pestis and other bacteria containing genes similar to hms (28, 31). Given the importance of biofilm production to Y. pestis transmission, we hypothesized that mutational loss of chb was selectively favored during the evolution of Y. pestis from Y. pseudotuberculosis because it resulted in a more stable biofilm in the flea vector. In this study, we demonstrated that the structures of the Hms-dependent ECM and the poly-β-1,6-GlcNAc ECM of S. epidermidis are similar and characterized the YPTB1123 gene product as a glycosyl hydrolase whose activity interferes with biofilm formation.
MATERIALS AND METHODS
Bacterial strains.
Y. pestis KIM6+ and KIM6 (19), Y. pseudotuberculosis IP32953 (8), and S. epidermidis 1457 and its isogenic Ica− derivative (36) were used. Y. pestis KIM6 is an Hms− derivative of KIM6+ that lacks the Pgm locus that contains the hmsHSFR operon. Both strains lack the pYV virulence plasmid, which is not required to produce a transmissible infection in fleas (23).
Immunofluorescence assays.
Y. pestis KIM6+ and KIM6 were grown on heart infusion agar (Difco) containing 0.2% galactose at 21 and 37°C. Wild-type and Ica− S. epidermidis bacteria were grown in tryptic soy broth at 37°C. Bacterial suspensions were prepared after 24 h, spotted on glass slides, allowed to air dry, and incubated with a 1:100 dilution of polyclonal antiserum generated against purified Ica-dependent ECM polysaccharide (PIA) of S. epidermidis, from which nonspecific antibodies had been removed by absorption with whole bacteria and cell extracts of Ica− S. epidermidis (49). After 1 h of incubation at 37°C, the slides were washed and reincubated with a 1:50 dilution of fluorescein isothiocyanate-labeled goat antirabbit secondary antibody (Pierce Biotechnology). One hour later, the slides were washed and redried before examination by fluorescence and phase-contrast microscopy.
Cloning and deletion of YPTB1123 (nghA).
The predicted nghA open reading frame including upstream and downstream regions was PCR amplified from Y. pseudotuberculosis IP32953 genomic DNA using the primers 5′-GCA CTA CAC CCT GTA TGA CAA CC-3′ and 5′-GCG TTA CAC ATC AGG CTG TTG-3′ with Pfu polymerase (Stratagene). The PCR product was cloned into the high-copy-number pCR4Blunt cloning vector (Invitrogen; ∼200 copies per cell) to generate the plasmid pCRBnghA and into the low-copy-number (6 to 8 copies per cell) pLG338 cloning vector (44) to generate the plasmid pLGnghA. Each plasmid was used to transform Y. pestis KIM6+ via electroporation. The entire open reading frames plus upstream and downstream regions of other Y. pseudotuberculosis-specific glycosyl hydrolases (Table 1) were cloned into the pCR4Blunt plasmid.
TABLE 1.
Comparison of putative glycosidases for the annotated genomes of Y. pseudotuberculosis IP32953 and Y. pestis CO92a
| Family | Predicted enzyme(s) | Y. pstb ORF | Y. pestis ORF | Gene name |
|---|---|---|---|---|
| 1 | β-Glucosidase, β-galactosidase, β-mannosidase | YPTB1290 | YPO1254 | bglA |
| 2 | β-Galactosidase | YPTB2415 | YPO1654 | lacZ |
| 3 | β-N-Acetylhexosaminidase, β-glucosidase | YPTB1055 | YPO2803 | bglB |
| β-N-Acetylhexosaminidase, β-glucosidase | YPTB2449 | YPO1615 | ycfO/nagZ | |
| β-N-Acetylhexosaminidase, β-glucosidase | YPTB3439 | YPO0616 | bglX | |
| 4 | α-Glucosidase, α-galactosidase, 6P-β-glucosidase | YPTB3735 | YPO0166 | chbF |
| 5 | Chitosanase, cellulase, endo-1,6-β-glucosidase, endo-1,6-β-galactanase | YPTB3269 | YPO0757 IS insertion | |
| 8 | Chitosanase, cellulase, endo-1,4-β-xylanase | YPTB3837 | YPO3998 frameshift | bcsZ |
| 13 | α-Amylase | YPTB3786 | YPO3941 | glgX |
| 1,4-α-Glucan branching | YPTB3787 | YPO3942 | glgB | |
| Trehalose-6-phosphate hydrolase | YPTB3537 | YPO3696 | treC | |
| α-Amylase | YPTB3909 | YPO4080 | malS | |
| Maltodextrin glucosidase | YPTB0922 | YPO3200 | malZ | |
| 18 | Chitinase, endo-β-N-acetylglucosaminidase | YPTB3365 | Absent | chiC |
| 20 | β-Hexosamidase | YPTB1123 | YPO2632 frameshift | chb (nghA) |
| 23 | Lysozyme, type G | YPTB0595 | YPO0452 | slt |
| Lysozyme, type G | YPTB2346 | YPO2438 | mltC | |
| Lysozyme, type G | YPTB2681 | YPO2957 | mltB | |
| Lysozyme, type G | YPTB2880 | YPO2922 | slt | |
| Lysozyme, type G | YPTB2968 | YPO1078 | mltD | |
| Lysozyme, type G | YPTB3226 | YPO0954 | mltC | |
| 24 | Lysozyme | YPTB1755 | YPO2098 | |
| Lysozyme | YPTB1805 | Absent | ||
| 31 | α-Glucosidase | YPTB3093 | YPO0848 | |
| 36 | α-Galactosidase | YPTB1608 | YPO1581 | rafA |
| 42 | β-Galactosidase | YPTB3097 | YPO0852 | bgaB |
| 53 | Endo-1,4-β-galactanase | YPTB3098 | YPO0853 | |
| 73 | Endo-β-N-acetylglucosaminidase, N-acetylmuramoylhydrolase | YPTB1680 | YPO1807 | flgJ |
| 77 | 4-α-Glucanotransferase | YPTB3774 | YPO0126 | malQ |
| 94 | Cellobiose phosphorylase | YPTB3445 | YPO0610 |
Genome data were compiled using the carbohydrate-active-enzyme server (http://www.cazy.org). Genes predicted to be absent or nonfunctional in Y. pestis are highlighted in bold. Y. pstb, Y. pseudotuberculosis; ORF, open reading frame.
A 2.2-kb deletion from pCRBnghA that eliminated a 2.2-kb internal fragment of the nghA gene was generated by inverse PCR using the primers 5′-CCG CTT GCC AGT ACC GGG GGC CAG AGT GGT GGC-3′ and 5′-GCC CAG TCC TGG CCT TTG ATC TCA TCC CCG GTG-3′ and religation. A fragment containing the deleted allele was subcloned into the suicide vector pCVD442 (15), which was then introduced into E. coli S17-1. The suicide vector construct was transferred to Y. pseudotuberculosis IP32953 by conjugation. Y. pseudotuberculosis transconjugants in which allelic exchange had occurred were PCR tested to verify deletion of nghA.
Colloidal gold labeling.
Silicon chips were applied briefly to bacterial lawns after 24 h of growth at room temperature. Adherent bacteria on the chips were washed with phosphate-buffered saline (PBS) and blocked with 1% bovine serum albumin-PBS for 2 min in a Pelco 3451 laboratory microwave oven (Ted Pella Inc., Redding CA) at 150 W, 37°C under vacuum (all additional steps were carried out with the same microwave settings). Samples were incubated in the microwave with anti-PIA antiserum diluted 1:100 in blocking buffer two times (2 min each), washed with blocking solution three times (1 min each), and incubated with goat antirabbit 10-nm colloidal gold (BBInternational, Cardiff, United Kingdom) diluted 1:100 two times (2 min each) before rinsing three times (1 min each) with PBS and once for 1 min with distilled H2O.
Scanning electron microscopy.
Samples were prepared as described previously (18) with the following modifications. Immunogold-labeled samples were fixed twice (2 min each) at 150 W at 24°C under vacuum with 75 mM lysine acetate, 0.075% (wt/vol) alcian blue, 2% paraformaldehyde, and 2.5% glutaraldehyde, buffered with 0.1 M sodium cacodylate. Samples were washed two times (3 min each) with 0.1 M sodium cacodylate, and postfixed with 3 rounds of 1% osmium tetroxide-0.8% potassium ferricyanide in 0.1 M sodium cacodylate, alternating with two rounds of 1% tannic acid in distilled H2O for bulk conductivity (two times, 2 min for each round) at 80 W and 24°C under vacuum in the microwave oven. Samples were washed once for 5 min with 0.1 M sodium cacodylate and twice for 5 min with distilled H2O prior to dehydration with a graded ethanol series of 50%, 75%, and 95% and three with 100% for 40 s each at 80 W and 24°C. Samples were critical-point-dried under CO2 with a Bal-Tec cpd 030 drier (Balzers, Liechtenstein) and mounted on aluminum studs. Samples for backscatter imaging were left uncoated, and samples for secondary imaging were sputter coated with 35 Å of iridium in an IBS/TM200S ion beam sputterer (VCR Group, South San Francisco, CA) prior to viewing at 5 kV on a Hitachi S-5200 field emission in-lens scanning electron microscope in either backscatter composite or secondary imaging modes (Hitachi, Tokyo, Japan).
Expression and purification of NghA.
To express NghA, the open reading frame was PCR amplified using the primers 5′-ATG AGG TTA GTA ATG AAC AAA T-3′and 5′-GAT GTT AAC TGG CTC GGC ACG-3′ and the product cloned into the pBAD-Thio-HIS cloning vector (Invitrogen), which adds a C-terminal six-His tag. E. coli TOP10 cells (Invitrogen) containing the plasmid were grown in 500 ml of Luria broth, induced with 0.2% arabinose at mid-log phase, and grown overnight at 28°C. Recombinant His-tagged protein was purified from the cell pellet under native conditions using nickel-nitrilotriacetic acid columns (Qiagen) according to the instructions of the manufacturer. The purity of the eluted protein was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining. Protein concentrations were determined by using the BCA protein assay kit (Pierce).
Glycosyl hydrolase assays.
The synthetic substrates 4-nitrophenyl-N-acetyl-β-d-glucosaminide, 4-nitrophenyl-N-acetyl-β-d-galactosaminide, 4-nitrophenyl-N-acetyl-α-d-glucosaminide, and 4-nitrophenyl-N-acetyl-α-d-galactosaminide (Sigma) were dissolved in 50 mM sodium phosphate buffer (pH 5.9) at a 5 mM final concentration (31). Nine hundred microliters of substrate solution was added to 100 μl buffer containing 4 μg purified NghA protein. After incubation at 37°C for 0 to 80 min, reactions were stopped by adding 5 μl 10 N NaOH. To determine inhibitory effects of excess glucose, N-acetylglucosamine, chitobiose, or staphylococcal PIA, 100 μl of 4-nitrophenyl-N-acetyl-β-d-glucosaminide substrate was added to 100 μl enzyme solution containing 4 μg purified NghA with or without 0.4% glucose or N-acetylglucosamine; 10 mM N,N′-diacetylchitobiose (Sigma); or 10 mg PIA. PIA was prepared from S. epidermidis 1457 as described previously (48) by heating cell pellets at 100°C for 10 min in 0.5 M EDTA, pH 8.0, centrifugation, dialysis of the supernatant, and lyophilization. Y. pestis and Y. pseudotuberculosis cell extracts were prepared by centrifuging 1 ml bacterial culture containing ∼1.0 × 108 bacteria/ml, resuspending the cell pellets in 500 μl sodium phosphate reaction buffer, adding 20 μl of 0.2% sodium dodecyl sulfate and 40 μl chloroform, and vortexing for 10 s. For enzyme assays, 100 μl of the cell extract mixture was added to 100 μl of 4-nitrophenyl-N-acetyl-β-d-glucosaminide substrate solution. Reactions with excess glucose, N-acetylglucosamine, chitobiose, or PIA and with cell extracts were stopped by adding 800 μl 1 M NaCO3. Enzymatic activity (release of the 4-nitrophenyl indicator group) was measured spectrophotometrically at 405 nm. The absorbance at 550 nm was also measured, as was the absorbance at 600 nm of the original culture from which the extract was derived. These values were used to calculate β-hexosaminidase units in the cell extracts, where 1 unit equals 10,000 × {[A405 − (1.75 × A550)]/(time × A600)} (reaction time in minutes).
In vitro biofilm assays.
To determine the ability of NghA to inhibit biofilm formation, purified recombinant NghA was concentrated in TMH liquid medium (46) without gluconate using a Millipore 30,000-molecular-weight-cutoff filter. Biofilm formation or dispersal of S. epidermidis and Y. pestis KIM6+ in 96-well polystyrene microtiter plates was measured (28) in the presence or absence of 50 μg/ml NghA. Y. pestis biofilms were grown in TMH with 0.2% galactose at 21°C. The adherent biofilm was washed three times with water, stained with safranin, and measured spectrophotometrically at 490 nm (16). S. epidermidis biofilms were grown in tryptic soy broth at 37°C, stained with crystal violet, washed three times with water, and measured at 605 nm. Biofilms were measured in three wells at each time point. To determine the effect of NghA expression, biofilms of Y. pestis KIM6+ carrying the plasmid pLG338, pCR4Blunt, pLGnghA, or pCRBnghA were grown in microtiter plates in TMH medium containing 10 μg/ml hemin and measured as described above. Quantitative differences between strains were assessed by using a paired, two-tailed t test.
Flea infections.
Fleas were infected by allowing them to feed on heparinized mouse blood containing 1 × 108 to 5 × 108 Y. pestis/ml in a membrane feeder device (16, 23). Fleas (50 females and 50 males) were maintained for 28 days and checked twice weekly for proventricular blockage (presence of fresh blood in the esophagus but not in the midgut) immediately after they had been allowed to feed on an uninfected mouse. Additional samples of 20 female fleas from each experiment were collected at 0, 7, and 28 days after the infectious blood meal to determine the percentage of infected fleas (16, 23). Three or four independent infection experiments were done with each strain.
RESULTS
Antigenic cross-reactivity of the ECM of Yersinia and staphylococcal biofilms.
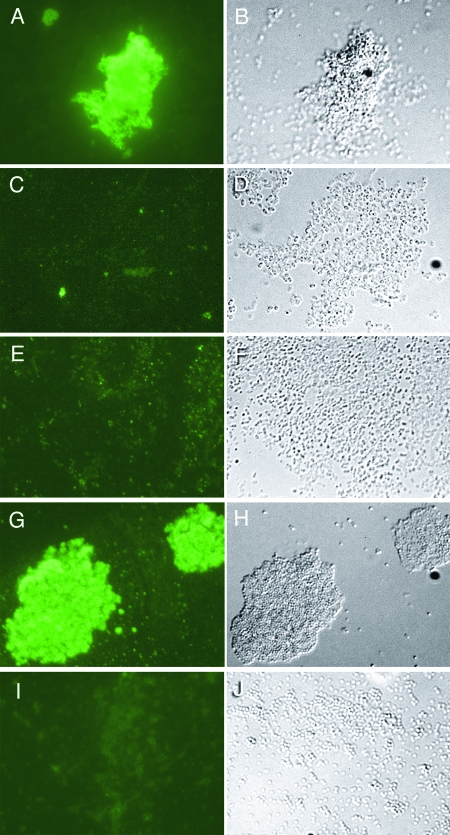
The chemical structure of the Y. pestis Hms-dependent ECM has not been defined, but the similarity between the hms, ica, and pga genes suggests that it too is a β-1,6-GlcNAc polymer. To assess the biochemical relatedness of the Yersinia and S. epidermidis ECMs, we tested whether antibody raised against PIA, the Ica-dependent β-1,6-GlcNAc ECM of S. epidermidis biofilm, would cross-react with the Hms-dependent ECM of Y. pestis. The anti-PIA antiserum reacted strongly with the Y. pestis strain containing the hms genes grown at 21°C but not at 37°C (Fig. 1A to D). The antibody did not bind to the Hms− Y. pestis strain, which is unable to form biofilm or colonize the proventriculus of fleas, at either temperature (Fig. 1E and F). This result is highly suggestive that the biofilm ECM of the two species have similar poly-β-1,6-GlcNAc-based structures.
FIG. 1.
Antibody to the poly-β-1,6-N-acetylglucosamine ECM of S. epidermidis biofilm cross-reacts with Y. pestis ECM. Immunofluorescence assays of Hms+ (A to D) or Hms− (E and F) Y. pestis or Ica+ (G and H) or Ica− (I and J) S. epidermidis using polyclonal antibody generated against purified Ica-dependent ECM polysaccharide of S. epidermidis are shown. Y. pestis strains were cultured at 21°C (A, B, E, and F) or 37°C (C and D); the Hms-dependent ECM is expressed only at 26°C or below (40). The matching phase-contrast image is shown to the right of each immunofluorescence image. Magnification, ×600.
The Y. pseudotuberculosis YPTB1123 gene encodes a β-N-acetylglucosaminidase.
Y. pseudotuberculosis, like Y. pestis, forms robust Hms-dependent biofilms in many in vitro environments and on the outer mouthparts of Caenorhabditis elegans (13, 30). However, Y. pseudotuberculosis never forms biofilm on the flea proventriculus to cause blockage of the digestive tract, even though it can colonize the flea midgut (16). We reasoned that the inability of Y. pseudotuberculosis to colonize the proventriculus could in part be due to alteration or instability of the β-1,6-GlcNAc ECM polymer. The predicted amino acid sequence of the Y. pseudotuberculosis YPTB1123 gene product contains the signature carbohydrate-binding and catalytic domains of family 20 glycosyl hydrolases, including the conserved glutamate and aspartate residues that are essential for activity (41). The protein also contains an N-terminal signal peptide sequence but no predicted transmembrane domains, indicating that it is most likely localized to the periplasm (6).
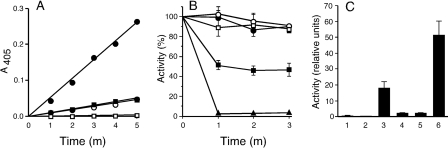
To determine whether YPTB1123 encodes a glycosyl hydrolase, we cloned this gene from Y. pseudotuberculosis, isolated recombinant protein, and tested it for its ability to hydrolyze various glycosidic linkages of 4-nitrophenyl-labeled synthetic hexosamine substrates. The protein had greatest hydrolytic activity against N-acetyl-β-d-glucosaminide, less activity against N-acetyl-β-d-galactosaminide and N-acetyl-α-d-glucosaminide, and no activity against N-acetyl-α-d-galactosaminide (Fig. 2A). β-d-Glucosaminidase activity was strongly inhibited by the addition of chitobiose to the reaction and to a lesser extent by GlcNAc but not by glucose (Fig. 2B). Extracts enriched for staphylococcal PIA did not inhibit enzymatic activity (Fig. 2B), suggesting that the active site of the enzyme has less affinity for the mature β-1,6-N-acetyl-d-glucosamine PIA polymer, which is substantially deacetylated (49). These results are consistent with the activity of a β-N-acetylhexosaminidase with affinity for chitobiose; we therefore refer to YPTB1123 as nghA (β-N-acetyl-d-glucosamine hydrolase A).
FIG. 2.
Y. pseudotuberculosis NghA is a family 20 glycosyl hydrolase with highest activity against β-N-acetylglucosamine linkages and an affinity for chitobiose. (A) Release of 4-nitrophenyl (NP) adduct from 4-NP-N-acetyl-β-d-glucosaminide (filled circles), 4-NP-N-acetyl-α-d-glucosaminide (open circles), 4-NP-N-acetyl-β-d-galactosaminide (filled squares), or 4-NP-N-acetyl-α-d-galactosaminide (open squares) was measured during incubation at 37°C with purified recombinant NghA at the indicated times. (B) Release of 4-NP by NghA from 4-NP-N-acetyl-β-d-glucosaminide in the presence of 0.4% glucose (open squares), 10 mM N-acetylglucosamine (closed squares), 10 mM chitobiose (triangles), or 20 mg/ml PIA extract from wild-type S. epidermidis (circles). (C) Hydrolysis of 4-NP-N-acetyl-β-d-glucosaminide by cell extracts of Y. pestis grown at 21°C (1) or 37°C (2), Y. pseudotuberculosis grown at 21°C (3) or 37°C (4), or Y. pestis containing the plasmids pLGnghA (5) or pCRBnghA (6) grown at 21°C. Relative β-hexosaminidase units were calculated as described in Materials and Methods. Results in panels A and C are the means ± standard deviations of a representative experiment performed in triplicate; results in panel B are the means ± standard errors of the means for three independent experiments performed in triplicate.
Y. pseudotuberculosis NghA inhibits Yersinia and staphylococcal biofilm formation.
The enzymatic activity of NghA suggested it might interfere with production or stability of the ECM. We first tested whether Y. pseudotuberculosis produces detectable β-hexosaminidase activity. Lysates of Y. pseudotuberculosis grown at 21°C had higher β-hexosaminidase activities than those from cultures grown at 37°C (Fig. 2C). Our goal was to determine whether restoration of a functional nghA gene to Y. pestis results in altered ECM production and decreased biofilm formation in vitro or in vivo. For these experiments, Y. pestis was transformed with pCRBnghA or pLGnghA, recombinant high- and low-copy-number plasmids, respectively, that contain the Y. pseudotuberculosis nghA gene and promoter region. The high-copy-number plasmid conferred approximately 2.5-fold-greater β-hexosaminidase activity to Y. pestis than Y. pseudotuberculosis, whereas the low-copy-number plasmid resulted in a level approximately fourfold lower than that of Y. pseudotuberculosis and the wild-type (NghA−) Y. pestis parent strain had none (Fig. 2C).
We then tested the effect of nghA restoration on Y. pestis ECM by immunoelectron microscopy using gold-labeled anti-PIA antibody. The majority of wild-type cells were extensively labeled with gold particles (Fig. 3A). Secondary imaging gave a higher resolution of the surface morphology independently of the gold particles; these images showed that the antibody-reactive Hms-dependent material was associated primarily with extensive bumps on the surface of the bacteria (Fig. 3B). In contrast, Hms− cells were uniformly unlabeled and smooth (Fig. 3C and D). Restoration of nghA resulted in reduced, more variable immunogold labeling, with some indication of possible sloughing of the Hms-dependent matrix (Fig. 3E and G). This was accompanied by reduced cell surface ECM (Fig. 3F and H), a consistent finding that correlated with the nghA copy number—the surface of the bacteria carrying the high-copy vector appeared smooth, much like the Hms− strain (Fig. 3D and H).
FIG. 3.
NghA expression prevents accumulation of Hms-dependent ECM. Y. pestis KIM6+ (Hms+) (A and B), KIM6 (Hms−) (C and D), or Y. pestis KIM6+ containing plasmid pLGnghA (E and F) or pCRBnghA (G and H) were assayed with gold-labeled anti-PIA antibody and analyzed by scanning electron microscopy in backscatter composite imaging mode (left panels) or secondary imaging mode (right panels). Scale bar = 0.5 μm (left panels) or 0.1 μm (right panels).
In vitro biofilm production of the different strains was evaluated in polystyrene microtiter plates. At 24 h, there were no differences in the biofilms formed by NghA+ strains or the wild-type (NghA−) strains containing empty vectors. However, biofilms of Y. pestis overexpressing NghA (high copy number) measured at 48 and 72 h were significantly reduced (P < 0.0001) compared to those of Y. pestis expressing less NghA (low copy number) or no NghA (empty vectors) (Fig. 4A).
FIG. 4.
NghA interferes with Y. pestis and S. epidermidis biofilm formation. (A) Quantitation of adherent biofilm produced by Y. pestis KIM6+ containing plasmid pCR4Blunt (filled squares), pLG338 (filled circles), pCRBnghA (open squares), or pLGnghA (open circles). (B and C) Quantitation of adherent biofilm produced by Y. pestis (B) or S. epidermidis (C) in triplicate wells of polystyrene microtiter plates in the presence (filled circles) or absence (open circles) of 50 μg/ml purified recombinant NghA. Results are the means ± standard deviations from two independent experiments (A) or a single representative experiment (B and C) performed in triplicate.
To determine whether extracellular NghA impedes biofilm formation, we added it at t = 0 to the Y. pestis and S. epidermidis inocula in the microtiter plate quantitative biofilm assay. Similar to Actinobacillus dispersin B (28), exogenous NghA prevented in vitro biofilm formation for both species (Fig. 4B and C). However, adding NghA to preformed biofilms (after 24 or 48 h for S. epidermidis or Y. pestis, respectively) did not result in their disruption or dispersal (data not shown), consistent with the observation that the mature, presumably highly deacetylated form of the poly-β-1,6-N-acetyl-d-glucosamine ECM is not a competitive substrate for NghA (Fig. 2B).
Overexpression of Y. pseudotuberculosis NghA in Y. pestis results in decreased proventricular blockage of fleas.
Biofilm development and maturation constitute a complex physiological process that is sensitive to environmental conditions and substrate characteristics (5). Therefore, we next measured the effect of NghA activity in the relevant biological context, the ability of Y. pestis to infect and produce a proventricular biofilm in the rat flea Xenopsylla cheopis, an important vector of plague worldwide (Fig. 5). The Y. pestis strain overexpressing the nghA gene (high copy number) blocked significantly fewer fleas than the wild-type strain (Fig. 5A). The mean blockage rate of fleas infected with the Y. pestis strain transformed with nghA on the low-copy-number plasmid was also reduced, but the difference was not statistically significant (Fig. 5A). Expression of functional nghA in Y. pestis did not affect the incidence of chronic digestive tract infection and the average bacterial load in fleas (Fig. 5B).
FIG. 5.
Transformation of Y. pestis with functional nghA inhibits proventricular biofilm formation but not midgut colonization in fleas. (A) Percentage of fleas that developed proventricular blockage during a 28-day period after infection with Y. pestis (filled squares) or Y. pestis transformed with a high-copy-number (open squares) or low-copy-number (circles) plasmid containing nghA. Horizontal lines indicate means. (B) Flea midgut colonization rates (means ± standard errors of means) from experiments shown in panel A.
Although expression of functional nghA in Y. pestis appeared to destabilize biofilm formation in the flea, deletion of nghA was not sufficient by itself to enable Y. pseudotuberculosis to produce a stable biofilm in fleas. Fleas infected with a Y. pseudotuberculosis ΔnghA strain never developed proventricular blockage, and this strain showed no defect in biofilm formation in polystyrene microtiter plates (data not shown). The Y. pestis and Y. pseudotuberculosis genomes contain additional genes that are predicted to encode glycosyl hydrolases of other families (Table 1). Four of these appear to be functional in Y. pseudotuberculosis but are pseudogenes or absent in Y. pestis. One encodes a phage-related lysozyme that is not likely to be active against the Hms-dependent ECM. The other three encode predicted family 5 and family 8 chitosanases or cellulases (YPTB3269 and YPTB3837) and a family 18 chitinase (YPTB3365). However, transformation of Y. pestis with individual plasmids containing these three genes did not affect biofilm formation in vitro or the ability to block fleas (data not shown).
DISCUSSION
Our results provide further evidence that Y. pestis produces a transmissible infection in its flea vector by the same mechanism by which staphylococci infect catheters and other indwelling medical devices (21). In both cases, a poly-β-1,6-GlcNAc ECM may enhance aggregation on a hydrophobic surface. The proventricular spines are covered with cuticle, the same acellular, hydrophobic material that makes up the insect exoskeleton. As predicted by the similarity between the ECM biosynthetic genes of staphylococci and yersiniae, anti-PIA antibody cross-reacted strongly with Y. pestis ECM (Fig. 1 and 3). Bobrov et al. (7) have reported similar evidence that the S. epidermidis and Y. pestis ECMs are structurally related β-1,6-GlcNAc polysaccharides. Both Y. pestis and Y. pseudotuberculosis are capable of forming in vitro biofilms, mediated by the ECM synthesized by their identical hms genes (13, 16, 30). However, only Y. pestis produces biofilm in the digestive tract of fleas. During the transition to its vector-borne lifestyle, therefore, Y. pestis adapted to grow as an adherent biofilm on proventricular cuticle. This adaptation could have involved alterations in environmental sensing, signal transduction, and biofilm gene regulation pathways; cell surface characteristics; or specific adhesins; all of which can have environment-specific effects on biofilm formation (5, 25, 45). In this study, we characterized an N-acetyl-β-glucosaminidase enzyme from Y. pseudotuberculosis, showed that it interferes with ECM production, and examined whether its loss in Y. pestis contributed to the evolution of flea-borne transmission.
The Y. pseudotuberculosis YPTB1123 open reading frame was annotated as a putative chitobiase (chb) gene because its predicted amino acid sequence is highly similar to that of Chb of Serratia marcescens (39). In Serratia and other bacteria, Chb participates in the catabolism of chitin, in which extracellular chitinases release chitobiose and higher β-1,4-linked chito-oligosacccharides that are then hydrolyzed by Chb to produce GlcNAc (32, 37). E. coli and other species are able to use chitobiose as a sole carbon source; the genes involved in chitobiose phosphotransferase uptake and utilization are encoded in the chbBCARFG operon (33). Both Y. pseudotuberculosis and Y. pestis contain an orthologous chbBCARG chitobiose phosphotransferase uptake locus (Y. pestis YPO2678 to YPO2682), but the putative phosphochitobiase gene chbF (YPO0166) is not linked to this operon and is less similar to E. coli chbF. Interestingly, Y. pseudotuberculosis also contains an ortholog of one of the S. marcescens chitinases, chiC, that is absent in Y. pestis (Table 1). This suggests that the primary function of these genes in Y. pseudotuberculosis relates to degradation of environmental chitin and that their loss in Y. pestis was neutral because they were no longer required for the flea-mammal life cycle. However, our data indicate that YPTB1123 can prevent accumulation of the poly-β-1,6-GlcNAc ECM of Yersinia biofilms, our rationale for referring to it by the more general Ngh descriptive. Thus, loss of nghA may have had the positive consequence of enhancing biofilm formation or stability in the flea and represent a selected rather than a neutral mutation. The orthologous gene in all five of the Y. pestis strains whose genome sequences have been reported contains a frameshift mutation after codon 182 and is predicted to encode a truncated, inactive protein (9, 14, 39, 43). Since the five strains are representative of the three major branches of the Y. pestis evolutionary tree (1), the gene loss appears to have occurred early in the divergence of Y. pestis from Y. pseudotuberculosis.
Although few studies have specifically examined synthesis and assembly of the Yersinia Hms ECM (7), synthesis of poly-β-1,6 N-acetyl-d-glucosamine by staphylococci and E. coli and the functions of the Ica and Pga proteins are better understood. IcaA/PgaC (similar to HmsR) assemble short polymers from UDP-N-acetylglucosamine in the cytoplasm. In staphylococci, IcaD is also required for polymerization, and IcaC could link these polymers into longer chains and/or increase the processivity of the IcaAD enzyme (20). In E. coli, PgaD (similar to HmsS) is also localized to the inner membrane and likely assists PgaC in polymerization (27). The polymer is deacetylated by IcaB/PgaB (similar to HmsF), either at the cell surface after export in the case of staphylococci (47) or prior to export and transport through the outer membrane porin PgaA (similar to HmsH) in the case of E. coli (27). In Y. pestis, NghA could hydrolyze growing polymers at any point during their initial assembly in the cytoplasm, transport through the periplasm, or extracellular anchoring to the cell envelope, thereby reducing ECM levels and biofilm formation (Fig. 2 and 3). Purified NghA apparently has low affinity for fully formed (presumably deacetylated) PIA (Fig. 2) and does not destabilize already formed, mature Y. pestis or S. epidermidis biofilms yet is still able to prevent biofilm formation during growth (Fig. 4). One possible explanation for these results is that deacetylation or some other late modification of the polymer reduces its substrate affinity for NghA. Taken together, our results favor a model in which intracellular or secreted NghA interferes with subunit polymerization and accumulation, rather than degradation, of the ECM.
An effect of nghA function on Y. pestis biofilm was detected in the flea, but a significant reduction in flea blockage resulted only when nghA was present in high copy number (Fig. 5). This result is somewhat unexpected, since in Y. pseudotuberculosis, this gene is chromosomal and therefore occurs in a single copy. However, the levels of NghA activity conferred by a low-copy-number plasmid in Y. pestis were lower than those of wild-type Y. pseudotuberculosis under the conditions we tested (Fig. 2C), suggesting that there may be pre- or posttranscriptional regulatory differences that reduce the amount of NghA made by Y. pestis. Additionally, previous experiments performed with C. elegans indicate a difference in the matrices made by Y. pestis and Y. pseudotuberculosis (12). If, because of this difference, NghA is more active on Y. pseudotuberculosis than on Y. pestis ECM, higher levels of NghA would then be needed to observe an effect on Y. pestis biofilm accumulation. Thus, a fitness advantage of nghA mutation may have been greater in the progenitor strain. Nonetheless, fixation of alleles within populations can be driven by even slight selective advantages, especially within small effective populations of bacteria adapting to novel environments (4, 26, 34). Furthermore, the transmission efficiency and vector competence of fleas for Y. pestis is low, even for X. cheopis, considered to be the most proficient plague vector because it becomes blocked more frequently than other flea species (1, 35). Thus, at the ecological level, even a small augmentative effect on flea-borne transmission due to a loss of NghA activity may have been sufficient to confer a selective advantage to the Y. pestis progenitor strain. Previously identified steps in the evolutionary transition of Y. pestis from a food- and waterborne pathogen to an arthropod-borne pathogen include acquisition of two Y. pestis-unique plasmids that encode a phospholipase D required for bacterial survival and replication in the flea midgut and a plasminogen activator that enhanced invasion from an intradermal flea bite site (24, 42), as well as a reduction in toxicity toward the flea vector (17). By increasing the stability of the Y. pestis biofilm ECM in the flea, the loss of glycosyl hydrolase activity may have been an incremental step in the evolution of vector-borne transmission. Thus, like the recently described gene rcsA (46a), nghA may represent an antitransmission factor, analogous to antivirulence genes that enhance bacterial fitness in mammalian hosts (38), and its inactivation in Y. pestis may be an example of selective gene loss that contributed to the emergence of flea-borne transmission.
Acknowledgments
We thank Cuong Vuong and Michael Otto for providing the anti-PIA antiserum and the S. epidermidis strains used in this study. We also thank Viveka Vadyvaloo and Frank Gherardini for critical reading of the manuscript.
This work was supported by the Division of Intramural Research, NIAID, NIH, and the Ellison Medical Foundation.
Footnotes
Published ahead of print on 17 October 2008.
REFERENCES
- 1.Achtman, M., G. Morelli, P. Zhu, T. Wirth, I. Diehl, B. Kusecek, A. J. Vogler, D. M. Wagner, C. J. Allender, W. R. Easterday, V. Chenal-Francisque, P. Worsham, N. R. Thomson, J. Parkhill, L. E. Lindler, E. Carniel, and P. Keim. 2004. Microevolution and history of the plague bacillus, Yersinia pestis. Proc. Natl. Acad. Sci. USA 10117837-17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacot, A. W. 1915. Further notes on the mechanism of the transmission of plague by fleas. J. Hyg. Plague 14(Suppl. 4)774-776. [PMC free article] [PubMed] [Google Scholar]
- 3.Bacot, A. W., and C. J. Martin. 1914. Observations on the mechanism of the transmission of plague by fleas. J. Hyg. Plague 13(Suppl. 3)423-439. [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett, R. D., R. C. MacLean, and G. Bell. 2006. Mutations of intermediate effect are responsible for adaptation in evolving Pseudomonas fluorescens populations. Biol. Lett. 2236-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beloin, C., S. Da Re, and J. M. Ghigo. August 2005, posting date. Chapter 8.3.1.3. Colonization of abiotic surfaces. In A. Böck, R. Curtis III, J. B. Kaper, F. C. Neidhardt, K. Nyström, E. Rudd, and C. L. Squires (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org/.
- 6.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340783-795. [DOI] [PubMed] [Google Scholar]
- 7.Bobrov, A. G., O. Kirillina, S. Forman, D. Mack, and R. D. Perry. 2008. Insights into Yersinia pestis biofilm development: topology and co-interaction of Hms inner membrane proteins involved in exopolysaccharide production. Environ. Microbiol. 101419-1432. [DOI] [PubMed] [Google Scholar]
- 8.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 10113826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chain, P. S., P. Hu, S. A. Malfatti, L. Radnedge, F. Larimer, L. M. Vergez, P. Worsham, M. C. Chu, and G. L. Andersen. 2006. Complete genome sequence of Yersinia pestis strains Antiqua and Nepal516: evidence of gene reduction in an emerging pathogen. J. Bacteriol. 1884453-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49711-745. [DOI] [PubMed] [Google Scholar]
- 11.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 675427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darby, C., S. L. Ananth, L. Tan, and B. J. Hinnebusch. 2005. Identification of gmhA, a Yersinia pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect. Immun. 737236-7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darby, C., J. W. Hsu, N. Ghori, and S. Falkow. 2002. Caenorhabditis elegans: plague bacteria biofilm blocks food intake. Nature 417243-244. [DOI] [PubMed] [Google Scholar]
- 14.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 1844601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 594310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson, D. L., C. O. Jarrett, B. W. Wren, and B. J. Hinnebusch. 2006. Serotype differences and lack of biofilm formation characterize Yersinia pseudotuberculosis infection of the Xenopsylla cheopis flea vector of Yersinia pestis. J. Bacteriol. 1881113-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson, D. L., N. R. Waterfield, V. Vadyvaloo, D. Long, E. R. Fischer, R. Ffrench-Constant, and B. J. Hinnebusch. 2007. Acute oral toxicity of Yersinia pseudotuberculosis to fleas: implications for the evolution of vector-borne transmission of plague. Cell Microbiol. 92658-2666. [DOI] [PubMed] [Google Scholar]
- 18.Fassel, T. A., and C. E. Edmiston, Jr. 1999. Bacterial biofilms: strategies for preparing glycocalyx for electron microscopy. Methods Enzymol. 310194-203. [DOI] [PubMed] [Google Scholar]
- 19.Fetherston, J. D., P. Schuetze, and R. D. Perry. 1992. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol. Microbiol. 62693-2704. [DOI] [PubMed] [Google Scholar]
- 20.Gerke, C., A. Kraft, R. Sussmuth, O. Schweitzer, and F. Gotz. 1998. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J. Biol. Chem. 27318586-18593. [DOI] [PubMed] [Google Scholar]
- 21.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Gotz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 201083-1091. [DOI] [PubMed] [Google Scholar]
- 22.Henrissat, B., and A. Bairoch. 1993. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 293781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinnebusch, B. J., R. D. Perry, and T. G. Schwan. 1996. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273367-370. [DOI] [PubMed] [Google Scholar]
- 24.Hinnebusch, B. J., A. E. Rudolph, P. Cherepanov, J. E. Dixon, T. G. Schwan, and A. Forsberg. 2002. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 296733-735. [DOI] [PubMed] [Google Scholar]
- 25.Horswill, A. R., P. Stoodley, P. S. Stewart, and M. R. Parsek. 2007. The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Anal. Bioanal Chem. 387371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imhof, M., and C. Schlotterer. 2001. Fitness effects of advantageous mutations in evolving Escherichia coli populations. Proc. Natl. Acad. Sci. USA 981113-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh, Y., J. D. Rice, C. Goller, A. Pannuri, J. Taylor, J. Meisner, T. J. Beveridge, J. F. Preston III, and T. Romeo. 2008. Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-beta-1,6-N-acetyl-d-glucosamine. J. Bacteriol. 1903670-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh, Y., X. Wang, B. J. Hinnebusch, J. F. Preston III, and T. Romeo. 2005. Depolymerization of beta-1,6-N-acetyl-d-glucosamine disrupts the integrity of diverse bacterial biofilms. J. Bacteriol. 187382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarrett, C. O., E. Deak, K. E. Isherwood, P. C. Oyston, E. R. Fischer, A. R. Whitney, S. D. Kobayashi, F. R. DeLeo, and B. J. Hinnebusch. 2004. Transmission of Yersinia pestis from an infectious biofilm in the flea vector. J. Infect. Dis. 190783-792. [DOI] [PubMed] [Google Scholar]
- 30.Joshua, G. W., A. V. Karlyshev, M. P. Smith, K. E. Isherwood, R. W. Titball, and B. W. Wren. 2003. A Caenorhabditis elegans model of Yersinia infection: biofilm formation on a biotic surface. Microbiology 1493221-3229. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan, J. B., C. Ragunath, N. Ramasubbu, and D. H. Fine. 2003. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity. J. Bacteriol. 1854693-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keyhani, N. O., and S. Roseman. 1999. Physiological aspects of chitin catabolism in marine bacteria. Biochim. Biophys. Acta 1473108-122. [DOI] [PubMed] [Google Scholar]
- 33.Keyhani, N. O., and S. Roseman. 1997. Wild-type Escherichia coli grows on the chitin disaccharide, N,N′-diacetylchitobiose, by expressing the cel operon. Proc. Natl. Acad. Sci. USA 9414367-14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin, B. R., and C. T. Bergstrom. 2000. Bacteria are different: observations, interpretations, speculations, and opinions about the mechanisms of adaptive evolution in prokaryotes. Proc. Natl. Acad. Sci. USA 976981-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorange, E. A., B. L. Race, F. Sebbane, and B. J. Hinnebusch. 2005. Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. J. Infect. Dis. 1911907-1912. [DOI] [PubMed] [Google Scholar]
- 36.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 623244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuo, Y., M. Kurita, J. K. Park, K. Tanaka, T. Nakagawa, M. Kawamukai, and H. Matsuda. 1999. Purification, characterization and gene analysis of N-acetylglucosaminidase from Enterobacter sp. G-1. Biosci. Biotechnol. Biochem. 631261-1268. [DOI] [PubMed] [Google Scholar]
- 38.Maurelli, A. T. 2007. Black holes, antivirulence genes, and gene inactivation in the evolution of bacterial pathogens. FEMS Microbiol. Lett. 2671-8. [DOI] [PubMed] [Google Scholar]
- 39.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413523-527. [DOI] [PubMed] [Google Scholar]
- 40.Perry, R. D., M. L. Pendrak, and P. Schuetze. 1990. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J. Bacteriol. 1725929-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prag, G., Y. Papanikolau, G. Tavlas, C. E. Vorgias, K. Petratos, and A. B. Oppenheim. 2000. Structures of chitobiase mutants complexed with the substrate di-N-acetyl-d-glucosamine: the catalytic role of the conserved acidic pair, aspartate 539 and glutamate 540. J. Mol. Biol. 300611-617. [DOI] [PubMed] [Google Scholar]
- 42.Sebbane, F., C. O. Jarrett, D. Gardner, D. Long, and B. J. Hinnebusch. 2006. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc. Natl. Acad. Sci. USA 1035526-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song, Y., Z. Tong, J. Wang, L. Wang, Z. Guo, Y. Han, J. Zhang, D. Pei, D. Zhou, H. Qin, X. Pang, Y. Han, J. Zhai, M. Li, B. Cui, Z. Qi, L. Jin, R. Dai, F. Chen, S. Li, C. Ye, Z. Du, W. Lin, J. Wang, J. Yu, H. Yang, J. Wang, P. Huang, and R. Yang. 2004. Complete genome sequence of Yersinia pestis strain 91001, an isolate avirulent to humans. DNA Res. 11179-197. [DOI] [PubMed] [Google Scholar]
- 44.Stoker, N. G., N. F. Fairweather, and B. G. Spratt. 1982. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene 18335-341. [DOI] [PubMed] [Google Scholar]
- 45.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56187-209. [DOI] [PubMed] [Google Scholar]
- 46.Straley, S. C., and W. S. Bowmer. 1986. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect. Immun. 51445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Sun, Y. C., B. J. Hinnebusch, and C. Darby. 2008. Experimental evidence for negative selection in the evolution of a Yersinia pestis pseudogene. Proc. Natl. Acad. Sci. USA 1058097-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vuong, C., S. Kocianova, J. M. Voyich, Y. Yao, E. R. Fischer, F. R. DeLeo, and M. Otto. 2004. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 27954881-54886. [DOI] [PubMed] [Google Scholar]
- 48.Vuong, C., and M. Otto. 2008. The biofilm exopolysaccharide polysaccharide intercellular adhesin—a molecular and biochemical approach. Methods Mol. Biol. 43197-106. [DOI] [PubMed] [Google Scholar]
- 49.Vuong, C., J. M. Voyich, E. R. Fischer, K. R. Braughton, A. R. Whitney, F. R. DeLeo, and M. Otto. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 6269-275. [DOI] [PubMed] [Google Scholar]
- 50.Wang, X., J. F. Preston III, and T. Romeo. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 1862724-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wren, B. W. 2003. The yersiniae—a model genus to study the rapid evolution of bacterial pathogens. Nat. Rev. Microbiol. 155-64. [DOI] [PubMed] [Google Scholar]