Abstract
Insertional transposon mutations in the sll0804 and slr1306 genes were found to lead to a loss of optimal photoautotrophy in the cyanobacterium Synechocystis sp. strain PCC 6803 grown under ambient CO2 concentrations (350 ppm). Mutants containing these insertions (4BA2 and 3ZA12, respectively) could grow photoheterotrophically on glucose or photoautotrophically at elevated CO2 concentrations (50,000 ppm). Both of these mutants exhibited an impaired affinity for inorganic carbon. Consequently, the Sll0804 and Slr1306 proteins appear to be putative components of the carbon-concentrating mechanism in Synechocystis sp. strain PCC 6803.
The growth rate of cyanobacteria depends on the amount of available inorganic carbon (Ci), i.e., CO2 and HCO3−. An effective photosynthetic carbon-concentrating mechanism (CCM) has evolved to elevate the CO2 concentration in the vicinity of the CO2-fixing enzyme Rubisco. This enables efficient CO2 fixation despite Rubisco's inherent low affinity for CO2 (12). Genetic and physiological studies of cyanobacterial mutants that require high CO2 concentrations for growth have identified a number of genes directly related to the CCM (11, 12, 15).
Synechocystis sp. strain PCC 6803 is a unicellular, naturally transformable cyanobacterium that can grow photoheterotrophically in the presence of glucose (4, 14, 17). This cyanobacterium has proved to be an ideal model organism for study of the mechanism and regulation of oxygenic photosynthesis (5). Analysis of the Synechocystis sp. strain PCC 6803 genome indicates that nearly half of the 3,260 genes present are of unknown function (6). In our laboratory we are interested in identifying novel genes required for optimal oxygenic photosynthesis (3, 18). In this study we report the apparent involvement of two genes, sll0804 and slr1306, in the CCM of Synechocystis sp. strain PCC 6803.
Two photoautotrophy mutants, 4BA2 and 3ZA12, were previously described (18) as containing single transposon insertions in the sll0804 and slr1306 genes, respectively. Both mutants were kanamycin resistant, and neither one exhibited polar effects due to the transposon insertion (18). In our current studies, the kanamycin-resistant wild-type strain K3 was used as a control (13). This strain contains a kanamycin resistance cassette located 5′ to the psbB gene. Growth conditions were as previously described (18). To test the ability of cells to grow at different CO2 concentrations, cells of the K3, 4BA2, and 3ZA12 strains were spotted in a 10-fold dilution series onto petri dishes containing BG-11 medium formulated for either photoautotrophic or photoheterotrophic growth (in 5 mM glucose plus 10 μM DCMU [3-(3,4-dichlorophenyl)-1,1-dimethylurea]) (18). The plates were incubated for 2 weeks under different CO2 concentrations at room temperature and a light intensity of ca. 25 μmol m−2 s−1.
For O2 evolution measurements in the presence of variable amounts of Ci, the 4BA2, 3ZA12, and K3 cells were grown photoheterotrophically on glucose-containing liquid BG-11 and then transferred to standard BG-11 medium for 2 days and bubbled with either 350 or 50,000 ppm CO2. Oxygen evolution rates were measured by polarography after addition of various concentrations of sodium bicarbonate. These experiments were performed with a Hansatech oxygen electrode at a chlorophyll concentration of 10 μg/ml at 24°C and with a light intensity of 800 μmol photons m−2 s−1. Cells were assayed in BG-11 from which Na2CO3 was deleted but with the addition of 20 mM NaCl (2). The assay buffer was bubbled with N2 gas for more than 2 hours before use. Chlorophyll concentrations were determined using the method of Lichtenthaler (8), while cell numbers were estimated using the equations of Williams (17).
Semiquantitative reverse transcription-PCR (RT-PCR) was performed with the two-step RT-PCR kit (USB Corporation, Cleveland, OH) using primers specific for the sll0804, slr1306, ccmN, cmpA, sbtA, psbA, rbcL and rnpB genes. The RNase P subunit B gene (rnpB) was used as a control for transcript abundance. Total RNA from K3 and the 4BA2 and 3ZA12 mutants grown photoautotrophically at a concentration of 350 or 50,000 ppm CO2 was isolated using the RiboPure-Bacteria kit (Ambion Inc., Austin, TX).
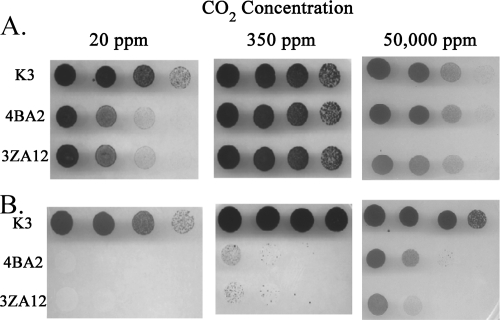
Figure 1 illustrates the growth of the mutant and wild-type strains under both photoheterotrophic and photoautotrophic conditions at various atmospheric CO2 concentrations. A 10-fold dilution series is shown (3 × 106 to 3 × 103 cells per spot). Both mutants grew photoheterotrophically at nearly the same apparent rate as the K3 control strain at all tested CO2 concentrations (Fig. 1A). In contrast, the mutants were unable to grow photoautotrophically at a CO2 concentration of 20 ppm and grew very poorly at 350 ppm, but they grew significantly better at a CO2 concentration of 50,000 ppm (Fig. 1B), although not as well as the wild type, which grew well at all tested CO2 concentrations. These results indicate that both the 4BA2 and 3ZA12 mutants require high CO2 concentrations to grow photoautotrophically. Complementation of both mutants by their respective wild-type genes restored their abilities to grow photoautotrophically at near-wild-type rates with limiting CO2 concentrations (data not shown).
FIG. 1.
Growth of the control strain K3 and the 4BA2 and 3ZA12 mutants under photoheterotrophic and photoautotrophic conditions. A. Growth under photoheterotrphic conditions (5 mM glucose plus 10 μM DCMU) and various CO2 concentrations. B. Growth under photoautotrophic conditions and various CO2 concentrations. Dilution series of cells (3 × 106 to 3 × 103) from the different strains were spotted onto agar plates and grown for 2 weeks. CO2 concentrations are shown above and the individual strains are shown to the left.
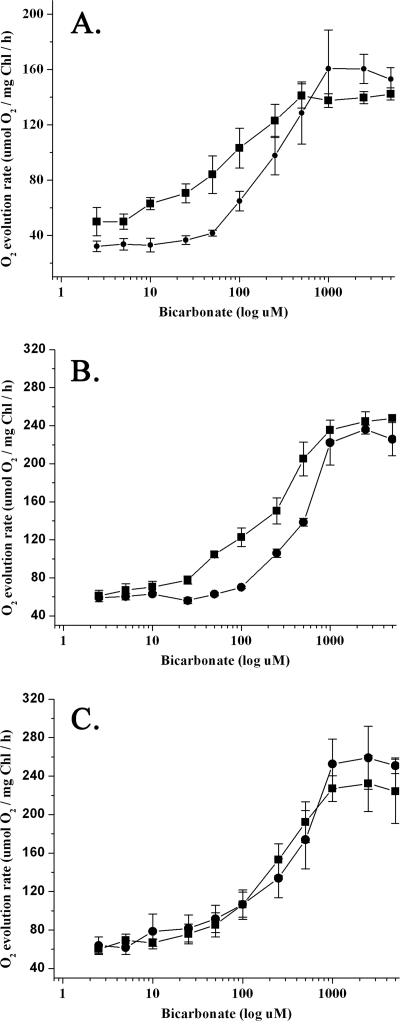
Mutant and control cells grown in air or high CO2 were assayed for changes in their steady-state photosynthetic O2 evolution rates upon the addition of various amounts of NaHCO3. These results are shown in Fig. 2 and summarized in Table 1. The Vmax values of O2 evolution appear to be similar for the high-CO2 and air-grown cells for all strains when results were normalized to the chlorophyll content of the different strains. Interestingly, both mutants contained about 40% less chlorophyll on a per cell basis than did the wild type. The K0.5 values for bicarbonate, however, were significantly different between the high-CO2 and air-grown cells in all three strains. As expected, the K0.5 value of the K3 strain was ∼10 times higher in the high-CO2 cells than in the air-grown cells. This indicated a strong induction of the CCM at low CO2 concentrations. The K0.5 values of 4BA2 and 3ZA12, however, were only two to three times higher in the high-CO2 cells than in the air-grown cells. This indicated that the CCM in the mutant strains was induced poorly in both air-grown mutant cell lines. Additionally, while the K0.5 values of the high-CO2 cells were very similar between the 4BA2, 3ZA12, and K3 strains, the K0.5 values of the air-grown mutant strains were ∼4 times higher than the control strain. These differences in K0.5 indicate that both the 4BA2 and 3ZA12 mutants exhibited significantly lower affinities for Ci than did the wild type.
FIG. 2.
Photosynthetic O2 evolution rates of the wild-type K3 strain and the 4BA2 and 3ZA12 mutants at various concentrations of externally provided bicarbonate. Wild-type strain K3 (A) and the mutant strains 4BA2 (B) and 3ZA12 (C) are shown. The strains were grown photoautotrophically with either a high CO2 concentration (50,000 ppm; •) or ambient CO2 concentration (350 ppm; ▪). n = 3 to 5, and error bars show ±1.0 standard deviation.
TABLE 1.
Kinetic parameters for Ci utilization by the control strain K3 and the mutant strains 4BA2 and 3ZA12 grown under ambient air (350 ppm CO2) and high-CO2 (50,000 ppm) conditionsa
| Strain | Vmax (μmol/mg chl/h) | K0.5 (μM HCO3−) | Chl content (μg chl/109 cells) |
|---|---|---|---|
| K3air | 142 ± 4 | 25 ± 2 | 0.104 |
| K3CO2 | 154 ± 14 | 251 ± 97 | ND |
| 4BA2air | 248 ± 3 | 98 ± 2 | 0.063 |
| 4BA2CO2 | 226 ± 17 | 272 ± 12 | ND |
| 3ZA12air | 232 ± 24 | 108 ± 8 | 0.066 |
| 3ZA12CO2 | 252 ± 6 | 250 ± 27 | ND |
The apparent maximal velocity (Vmax) and the bicarbonate concentration which yielded 50% of the apparent maximal oxygen evolution activity (K0.5) were determined by examination of the direct plots shown in Fig. 2. The values in the table are means ± 1.0 standard deviation (n = 3 to 5). Chlorophyll content and cell numbers were determined as described in the text. ND, not determined.
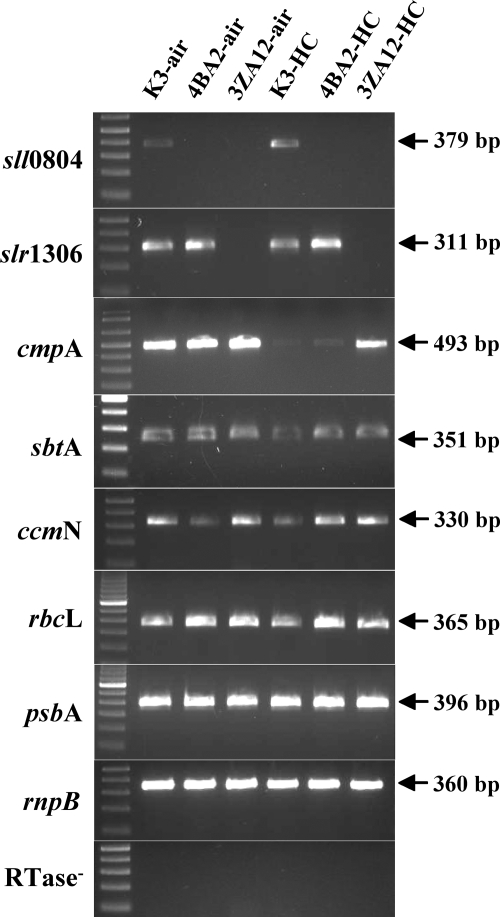
RT-PCR was used to evaluate the transcript levels of sll0804 and slr1306 at ambient air (350 ppm) and high (50,000 ppm) CO2 concentrations. As expected, we were unable to detect the sll0804 and slr1306 transcripts in their respective 4BA2 and 3ZA12 mutants (Fig. 3). All three transcripts were detected in the K3 strain at both high and low CO2 concentrations. The level of the sll0804 transcript in the wild-type strain was 3.6 times higher in the high-CO2 cells than in the air-grown cells, while there was little difference observed for the slr1306 transcript with either CO2 concentration. Interestingly, we could not detect the sll0804 transcript in the 3ZA12 mutant cells but did detect the slr1306 transcript in the 4BA2 mutant cells (Fig. 3). The absence of the sll0804 transcript in the 3ZA12 mutant and the presence of the slr1306 transcript in the 4BA2 mutant indicates that the Slr1306 protein may act upstream of the Sll0804 component. One hypothesis consistent with this observation is that the Slr1306 protein acts as a positive regulator, either directly or indirectly, of sll0804 expression.
FIG. 3.
Semiquantitative RT-PCR analysis of the total RNA extracted from the wild-type K3 strain and the 4BA2 and 3ZA12 mutants. The strains were grown photoautotrophically under high CO2 (50,000 ppm; HC) and ambient CO2 (350 ppm; air) concentrations. Strains and CO2 concentrations are indicated at the top; the sizes and locations of the PCR fragments are indicated to the right, and the genes analyzed are shown on the left. In the absence of reverse transcriptase (RTase−), no bands were observed.
RT-PCR was also used to examine the expression of a number of other CCM components and control proteins. Interestingly, several other CCM component genes exhibited differential expression in the 4BA2 and 3ZA12 mutants. CmpA is a component of the BCT1 high-affinity HCO3− transporter (11), while SbtA is a high-affinity, Na+-dependent HCO3− transporter (15). Both of these are induced under low-CO2 conditions. We observed this induction pattern in our control strain, K3, with the cmpA transcript being strongly upregulated and the sbtA transcript being modestly upregulated under low-CO2 conditions. A different pattern was observed in the 4BA2 and 3ZA12 mutants. While cmpA was poorly expressed under high-CO2 conditions in the 4BA2 mutant, it was expressed at a high level in the 3ZA12 mutant. Under high-CO2 conditions the sbtA transcript also appeared to be expressed at higher levels in both the 4BA2 and 3ZA12 mutants. A similar pattern was observed for the ccmN transcript. CcmN is a carboxysome component of unknown function (for a review, see reference 12). In our hands this transcript is moderately upregulated under low-CO2 conditions in the control strain K3. In the 4BA2 and 3ZA12 mutants the ccmN transcript is expressed at moderately high levels even under high-CO2 conditions. It should be noted that the control transcripts psbA (a membrane protein) and rnpB (a cytoplasmic component) do not exhibit differential regulation and are present in equivalent amounts in both mutants and the control cell line under both high- and low-CO2 conditions. These observations further strengthen the hypothesis that the Sll0804 and Slr1306 proteins are putative components of the cyanobacterial CCM.
The sll0804 gene is predicted to encode a protein of 453 amino acid residues. Using the SMART program (7), three transmembrane domains (amino acids 307 to 329, 349 to 371, and 386 to 408) and a coiled-coil domain (amino acids 268 to 296) were predicted within the protein. In addition, an ATP/GTP-binding domain containing a G1 box (GLVSRGKS), G2 box (T), G3 box (DTPG), and G4 box (KIDL) was located between amino acids 59 and 211 of the Sll0804 protein. A homology search using the BLAST program (1) revealed that homologues of the Sll0804 protein are present in a wide variety of both α- and β-cyanobacterial species, including Cyanothece sp., Microcystis aeruginosa, Thermosynechococcus strain BP1, Synechococcus strain CC9902, Prochlorococcus marinus MIT9313, etc. Within Synechocystis sp. strain PCC 6803 several putative paralogues of the Sll0804 protein were identified, including hypothetical proteins encoded by sll0503 (55% similarity and 38% identity) and slr1462 (47% similarity and 27% identity).
The slr1306 gene is predicted to encode a protein containing 485 amino acid residues. The Slr1306 protein was also examined with the SMART program and found to contain a putative N-terminal signal peptide of 37 amino acids and three domains with low compositional complexity (seg domains). An ATP/GTP-binding domain (P loop) was also identified between amino acid residues 128 and 139. BLAST analysis indicated that homologues of the Slr1306 protein were also found in a variety of other cyanobacterial species, including Cyanothece sp., Microcystis aeruginosa, Synechococcus sp. strain PCC 7002, Anabaena sp. strain PCC 7120, and Nostoc punctiforme ATCC 29133. Within Synechocystis sp. strain PCC 6803 several putative paralogues of the Slr1306 protein were identified, including hypothetical proteins encoded by slr1462 (47% similarity and 26% identity) and sll0503 (45% similarity and 23% identity).
Growth of many aquatic photosynthetic microorganisms, such as the cyanobacterium Synechocystis sp. strain PCC 6803 examined in this study, depends on the activity of a CCM to provide adequate inorganic carbon for photosynthetic carbon fixation (12). Mutations in one or more of the genes involved in the CCM of cyanobacteria often lead to changes of cellular physiology and growth phenotype, such as low growth rates at limiting CO2 concentrations (9-11, 15, 16). The 4BA2 and 3ZA12 mutants reported in this study lost their abilities to grow photoautotrophically under the low-CO2 condition, but they regained the ability to grow photoautotrophically under the high-CO2 conditions. Additionally, the mutants exhibited incomplete induction of the CCM at low CO2 concentrations, with a consequent decrease in their apparent affinity for Ci. These phenotypes are the result of single transposon insertions in the sll0804 or slr1306 genes and are complemented by transformation with the respective wild-type genes. These results demonstrate that the sll0804 or slr1306 gene is required for optimal growth of Synechocystis sp. strain PCC 6803 at ambient CO2 concentrations. We hypothesize that these proteins are components of the cyanobacterial CCM.
Footnotes
Published ahead of print on 17 October 2008.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benschop, J. J., M. R. Badger, and G. D. Price. 2003. Characterization of CO2 and HCO3− uptake in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 77117-126. [DOI] [PubMed] [Google Scholar]
- 3.Bricker, T. M., S. Zhang, S. M. Laborde, P. R. Mayer III, L. K. Frankel, and J. V. Moroney. 2004. The malic enzyme is required for optional photoautotrophic growth of Synechocystis sp. strain PCC 6803 under continuous light but not under a diurnal light regimen. J. Bacteriol. 18611483-11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grigorieva, G., and S. Shestokov. 1982. Transformation in the cyanobacterium Synechocystis sp. 6803. FEMS Microbiol. Lett. 13367-370. [Google Scholar]
- 5.Ikeuchi, M., and S. Tabata. 2001. Synechocystis sp. PCC 6803—a useful tool in the study of the genetics of cyanobacteria. Photosynth. Res. 7073-83. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakmura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakzaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3109-136. [DOI] [PubMed] [Google Scholar]
- 7.Letunic, I., R. R. Copley, S. Schmidt, F. D. Ciccarelli, T. Doerks, J. Schultz, C. P. Ponting, and P. Bork. 2004. Smart 4.0: towards genomic data integration. Nucleic Acid Res. 32142-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenthaler, H. K. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148350-382. [Google Scholar]
- 9.Ogawa, T. 1990. Mutants of Synechocystis PCC 6803 defective in inorganic carbon transport. Plant Physiol. 94760-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohkawa, H., G. D. Price, M. R. Badger, and T. Ogawa. 2000. Mutation of ndh genes leads to inhibition of CO2 uptake rather than HCO3− uptake in Synechocystis sp. strain PCC 6803. J. Bacteriol. 1822591-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omata, T., G. D. Price, M. R. Badger, M. Okamura, S. Gohta, and T. Ogawa. 1999. Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc. Natl. Acad. Sci. USA 9613571-13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price, G. D., M. R. Badger, F. J. Woodger, and B. M. Long. 2008. Advances in understanding the cyanobacterial CO2-concentrating mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 591441-1461. [DOI] [PubMed] [Google Scholar]
- 13.Putnam-Evans, C., and T. M. Bricker. 1992. Site-directed mutagenesis of the CPa-1 protein of photosystem II: alteration of the basic residue pair 384,385R to 384,385G leads to a defect associated with the oxygen-evolving complex. Biochemistry 3111482-11488. [DOI] [PubMed] [Google Scholar]
- 14.Rippka, R., J. Deruelles, J. B. Waterbury, M. Herdman, and R. Y. Sranier. 1979. Genetic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1111-61. [Google Scholar]
- 15.Shibata, M., H. Katoh, M. Sonoda, H. Ohkawa, M. Shimoyama, H. Fukuzawa, A. Kaplan, and T. Ogawa. 2002. Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: function and phylogenetic analysis. J. Biol. Chem. 27718658-18664. [DOI] [PubMed] [Google Scholar]
- 16.Shibata, M., H. Ohkawa, T. Kaneko, H. Fukuzawa, S. Tabata, A. Kaplan, and T. Ogawa. 2001. Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc. Natl. Acad. Sci. USA 9811789-11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams, J. G. K. 1988. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 167766-778. [Google Scholar]
- 18.Zhang, S., S. M. Laborde, L. K. Frankel, and T. M. Bricker. 2004. Identification of four novel genes required for efficient photoautotrophic growth of the cyanobacterium Synechocystis sp. PCC 6803 by in vitro transposon mutagenesis. J. Bacteriol. 186875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]