Abstract
The Helicobacter pylori protein HP0958 is essential for flagellum biogenesis. It has been shown that HP0958 stabilizes the σ54 factor RpoN. The aim of this study was to further investigate the role of HP0958 in flagellum production in H. pylori. Global transcript analysis identified a number of flagellar genes that were differentially expressed in an HP0958 mutant strain. Among these, the transcription of the major flagellin gene flaA was upregulated twofold, suggesting that HP0958 was a negative regulator of the flaA gene. However, the production of the FlaA protein was significantly reduced in the HP0958 mutant, and this was not due to the decreased stability of the FlaA protein. RNA stability analysis and binding assays indicated that HP0958 binds and destabilizes flaA mRNA. The HP0958 mutant was successfully complemented, confirming that the mutant phenotype described was due to the lack of HP0958. We conclude that HP0958 is a posttranscriptional regulator that modulates the amount of the flaA message available for translation in H. pylori.
Helicobacter pylori is a gram-negative microaerophilic epsilon-proteobacterium that colonizes the human gastric mucosa. H. pylori infection elicits gastric inflammation and may cause gastritis, peptic ulcers, duodenal ulcers, and gastric adenocarcinoma (5, 10, 20, 59). The motility of H. pylori is an essential requirement for colonization of the gastric niche (13, 14). The pathogenic bacterium is motile by means of multiple polar sheathed flagella (5, 17, 18). In addition to motility, flagella in some bacteria mediate adhesion to, and interaction with, host cells (26). The interaction between H. pylori and gastric cells induces a cytokine response in gastric epithelial cells via Toll-like receptors (TLR) (25, 53). However, the flagellar filament in H. pylori is not recognized by TLR5 (16).
In the Enterobacteriaceae, the flagellar apparatus is the result of the sequential assembly of more than 40 flagellar proteins (3, 30, 56). Flagellar biogenesis in H. pylori shares common features with those of model organisms like Salmonella and Escherichia spp. (40, 41), and most of the relevant genes for structural and regulatory proteins required for H. pylori flagellum biogenesis have been annotated (37, 56). The transcription of flagellar genes is under the control of three main RNA polymerase sigma factors, σ28, σ54, and σ80, and is also modulated by an anti-σ28 factor, FlgM (8, 9, 27, 37, 42, 51). The master regulator FlhC4D2, present in most Enterobacteriaceae, has not been annotated in H. pylori, highlighting the distinct regulatory network in this organism (37). Three flagellar gene classes have been defined (37). Early flagellar proteins required in flagellar biogenesis (class I) are under the transcriptional regulation of σ80 (37). RpoN (σ54) controls the transcription of the middle flagellar structural genes (class II), including the gene for the hook-length protein FliK (37). RpoN activity is regulated by its activator FlgR and its cognate histidine kinase FlgS (HP0244) (8, 54), similarly to the FleR/FleS system in Pseudomonas aeruginosa (46). The transcription of the late flagellar genes (class III), including the gene encoding the major flagellin FlaA, is under the control of FliA (σ28) (37). FliA activity is tightly repressed by the anti-σ28 factor FlgM (9). This repression is relieved when FlgM is secreted from the cell upon completion of the hook structure (29), though FlgM secretion has not actually been demonstrated for H. pylori. The hook length is under the control of FliK in Salmonella (15), which we recently identified as the product of the gene HP0906 in H. pylori (49). Completion of the hook structure modulates the activity of the RpoN regulon in H. pylori.
Based on the data from a high-throughput screen of a yeast two-hybrid system (Hybrigenics PimRider database), HP0958 also interacts with the sigma factor RpoN (44). HP0958 was defined as a novel flagellar gene in H. pylori (42, 48). It contains a zinc-finger motif, suggesting possible interactions with nucleic acids or proteins (48). The deletion of HP0958 impairs the transcription of genes encoding flagellar components, such as the hook protein FlgE, the major flagellin FlaA, and the minor flagellin FlaB (48). Pereira and Hoover showed that HP0958 controls RpoN activity at the protein level by stabilizing RpoN (42). The transcription of the HP0958 gene was not affected in a number of flagellar mutants analyzed in a previous investigation (37).
In the present study, we performed a global transcript analysis of mutants lacking the HP0958 gene. This led us to further investigate the role of the HP0958 protein in flagellin production and to uncover a new regulatory role for this protein in H. pylori flagellum biogenesis.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains used in the present study are listed in Table 1. H. pylori strain P79 (33), a streptomycin mutant of the P1 wild-type strain, was generously provided by R. Haas. H. pylori strains were cultured as previously described (49). A CCUG17874-derivative H. pylori mutant lacking the HP0958 gene was previously described (48, 49). The P79 strain-derivative H. pylori mutant lacking the HP0958 gene was generated as previously described by Ryan et al. for strain CCUG17874 (48). Transformants from the present study were selected on CBA (Columbia agar base) plates supplemented with antibiotics: 10 μg/ml chloramphenicol (Sigma) and 50 μg/ml kanamycin (Sigma). One Shot TOP10 chemically competent Escherichia coli cells (Invitrogen, CA) were propagated on Luria-Bertani (LB) agar plates or LB broth at 37°C supplemented with the following antibiotics: 50 μg/ml kanamycin (Sigma), 100 μg/ml ampicillin (Merck, Germany), and 10 μg/ml chloramphenicol (Sigma).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| H. pylori | ||
| CCUG17874 | Wild-type strain | Culture collection, University of Gothenborg, Sweden |
| NCTC 26695 | Wild-type strain | NCTC, United Kingdom |
| hp0958 KOa | CCUG17874 Δhp0958::Cmr | 48 |
| P1 | Wild-type strain | 38 |
| P79 | P1 Strr | 21 |
| P79-0958KO | P79 Δhp0958::Cmr | This study |
| P79-0958/pIR203K04 | P79 Δhp0958::Cmr with pIR203K04 (Kanr) | This study |
| P79-0958/pIR1041 | P79 Δhp0958::Cmr with pIR1041 (Kanr) | This study |
| P79-0958/pIR1563 | P79 Δhp0958::Cmr with pIR1563 (Kanr) | This study |
| P79-0958/pIR1186 | P79 Δhp0958::Cmr with pIR1186 (Kanr) | This study |
| E. coli | ||
| One Shot TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araΔ139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Rosetta 2 DE3 pLysS | F−ompT hsdSB(rB− mB−) gal dcm (DE3) pLysSRARE2 (Cmr) | Novagen, Germany |
| Plasmids | ||
| pIR203K04 | Kanamycin resistance cassette (Kanr) | 32 |
| pIR1041 | pIR203K04 with the hp0958 gene under the control of the hp1041 promoter | This study |
| pIR1563 | pIR203K04 with the hp0958 gene under the control of the hp1563 promoter | This study |
| pIR1186 | pIR203K04 with the hp0958 gene under the control of the hp1186 promoter | This study |
| pGEX-6P-3 | N-terminally GST-tagged expression vector (Ampr) | GE Healthcare, United Kingdom |
| pDC006 | pGEX-6P-3 hp0958 (Ampr) | This study |
KO, knockout.
Extraction of genomic DNA from H. pylori.
Genomic DNA from 2-day-old H. pylori plate cultures was extracted by following the DNeasy tissue kit instructions (Qiagen). The genomic DNA was then concentrated by vacuum-drying and quantified using the Nanodrop ND-1000 (Nanodrop).
Preparation of whole cell fractions.
H. pylori cells were harvested from 20-h liquid cultures and pelleted for 15 s at 13,000 rpm. The cell pellets were then washed in 1 ml sterile phosphate-buffered saline (PBS). After a further 15-s centrifugation at 13,000 rpm, the supernatant was discarded. The washed cell pellets were resuspended in 250 μl PBS. The optical density at 600 nm of the samples was measured and then standardized as required. Cell preparations were boiled for 5 min and stored at −80°C.
Protein electrophoresis and immunoblotting.
A standard protocol was used to perform sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (50) and immunoblotting. Proteins from 12.5% acrylamide gels were transferred onto nitrocellulose membranes by electroblotting (57). Polyclonal antibody directed against H. pylori flagellin A and hook protein was used as primary antibody (39). Anti-rabbit antibody conjugated to horseradish peroxidase (Sigma) was used as the secondary antibody. Hydrogen peroxide and 4-chloro-1-naphthol (Sigma) were employed for detection.
RNA isolation from H. pylori.
Total RNA was extracted from 20-h H. pylori liquid cultures using the RNeasy mini kit (Qiagen). Briefly, H. pylori cells were harvested and centrifuged for 15 s at 13,000 rpm. Cell pellets were then resuspended in 750 μl RNAprotect bacteria reagent (Qiagen). The remainder of the protocol was performed per the manufacturer's instructions. RNA quality was assessed using a Bioanalyzer 2100 instrument (Agilent Technologies) and quantified by NanoDrop ND-1000 (Nanodrop). Total RNA samples were DNase treated using a DNA-free kit (Ambion).
H. pylori microarray.
The design and construction of the H. pylori microarray (BμG@S HPv1.0.0; Bacterial Microarray Group at St. George's, University of London) was completed using the approaches described by Hinds et al. (22, 23). In brief, the PCR products were designed to include amplicons representing all 1,576 open reading frames in H. pylori NCTC 26695 (56) and all 1,495 open reading frames in H. pylori J99 (3) using the approach described by Hinds et al. (23). The microarrays were constructed by robotic spotting of the PCR products in duplicate on UltraGaps amino-silane coated glass slides (Corning) using a MicroGrid II (BioRobotics) (23) and were post-print processed according to the slide manufacturer's instructions.
Type II microarray analysis.
To compare the transcriptional profiles of the wild-type and HP0958 mutant strains, the H. pylori whole-genome microarray was used in a common reference or type II experimental design, whereby Cy5-labeled cDNA from each strain was cohybridized to an array with a Cy3-labeled genomic DNA reference. Nucleic acid labeling and microarray hybridizations were undertaken according to BμG@S standard protocols (22). In brief, for the common reference, 5 μg of wild-type CCUG17874 genomic DNA was labeled with dCTP Cy3 using random primers (Promega) and a DNA polymerase I large Klenow fragment (Invitrogen). Cy5-labeled cDNA was generated from 4 μg of total RNA during first-strand synthesis using random primers (Promega) and Superscript II reverse transcriptase (Invitrogen). The Cy3- and Cy5-labeled nucleic acid mixtures were then copurified using the MinElute PCR purification kit (Qiagen), mixed in a hybridization solution of 4× saline-sodium citrate buffer (SSC) and 0.3% SDS and hybridized under a LifterSlip (Erie Scientific Company) for 18 h at 65°C. Microarray slides were washed once in 1× SSC, 0.06% SDS at 65°C for 2 min and twice in 0.06× SSC for 2 min, dried by centrifugation, and scanned using a dual laser Affymetrix 428 scanner (Affymetrix, United Kingdom). The images and data were analyzed using the GeneDirector software package, which includes ImaGene and GeneSight v2.0 (Biodiscovery, El Segundo, CA). Genes designated as missing or uncertain in CCUG17874 were filtered out. Array hybridizations for the wild type and for the mutant were performed in triplicate. Ratios for the wild-type DNA versus the wild-type RNA and for the wild-type DNA versus the mutant RNA were exported to Excel (Microsoft). Following the within-slide normalization in GeneSight, the genes containing empty values were discarded. Quantile normalization was then performed to make the entire distribution of the values identical between each array slide (6). The triplicates for the wild type and for the mutant were averaged, and the final log2 ratios were calculated. One-way analysis of variance was used to calculate statistical confidences. Genes with a P value less than 0.05 and a change greater than 2.00-fold were highlighted as differentially expressed in the mutant.
Quantitative analysis of transcription by RT-PCR.
Quantitative real-time PCR (qRT-PCR) was performed as a confirmatory test on selected genes following global transcript analysis by microarray. RT-PCR primers were designed using the Primer3 software package (47) and are listed in Table S1 in the supplemental material. A total of 500 ng of RNA was reverse transcribed using Improm-II reverse transcriptase (Promega) and 500 ng random hexamers as described in the manufacturer's manual. qRT-PCR was performed on flagellar genes using the primers listed in Table S1 in the supplemental material. The reaction mixture was prepared as described in the manufacturer's protocol. Briefly, amplification by qRT-PCR was performed in a final volume of 12.5 μl, including 1 μl cDNA, 50 nM of each primer, 6.25 μl 2× master mix (Biogen, United Kingdom), and 1/60,000 Sybr green I (Biogene, United Kingdom). qRT-PCRs were run and monitored using an ABI 7000 thermocycler and ABI Prism 7000 SDS software (both from Applied Biosystems). The reactions were performed in triplicate (technical replicates) from at least two independent RNA preparations (biological replicates). Relative changes in expression were calculated as described by Pfaffl (43). The era gene was used as a housekeeping gene (52). Each transcript abundance was therefore calculated relative to the era gene transcript abundance.
Bacterial cytoplasmic protein extract preparation.
H. pylori cytoplasmic protein extracts were prepared using a modified protocol described by Donahue et al. (11). Cells were grown for 20 h in broth, and cell pellets were resuspended in a buffer containing 20 mM Tris-acetate (pH 7.9), 50 mM potassium acetate, 5 mM Na2EDTA, 1 mM dithiothreitol (DTT), and protease inhibitor cocktail (Complete Mini; Roche, Germany). H. pylori cell suspensions were bead beaten for 1 min and then centrifuged at 13,000 rpm for 5 min. The supernatants were harvested, and the protein concentration was quantified using the BCA protein assay kit (Pierce) and stored at −80°C.
Protein overexpression and purification.
The HP0958 gene of H. pylori CCUG17874 was overexpressed in Escherichia coli as a glutathione S-transferase (GST) fusion protein using the pGEX-6P-3 vector (GE Healthcare Ltd., United Kingdom). The primers are listed in Table S1 of the supplemental material. E. coli Rosetta 2 DE3 pLysS cells (Novagen, Germany) were grown at 37°C to an optical density at 600 nm of 0.4 to 0.6 and induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Melford Laboratories Ltd., United Kingdom). After induction, the cells were grown at 18°C for an additional 10 to 15 h. The cells were harvested by centrifugation and resuspended in PBS (Gibco). The cells were then lysed by a freeze/thaw treatment according to the Novagen pET system manual, and the supernatant was harvested following centrifugation at 13,000 × g for 20 min. The soluble fraction was incubated with glutathione Sepharose 4B at 4°C overnight. The GST tag was then removed by adding PreScission Protease (GE Healthcare Ltd.). Once the GST tag had been removed, HP0958 was purified on an ÄKTA purifier for fast protein liquid chromatography (GE Healthcare Ltd.), using a HiTrap Q FF column (GE Healthcare Ltd.) in a 20 mM Tris-HCl buffer at pH 9.50. HP0958 was further purified by gel filtration chromatography in 10 mM potassium phosphate buffer at pH 7.0 using a Superdex 75 10/300 GL column (GE Healthcare Ltd.). HP0958 purity was assessed after each purification step by SDS-polyacrylamide gel electrophoresis. The protein concentrations were measured using the BCA protein assay kit (Pierce).
Native RNA agarose gel electrophoresis.
RNA samples were resolved using horizontal agarose gel electrophoresis. A 1% (wt/vol) agarose gel was prepared with diethyl pyrocarbonate-treated 1× MOPS buffer, containing 20 mM 3-(N-morpholino) propane-sulfonic acid, 5 mM sodium acetate, and 1 mM EDTA at pH 7.0. The native RNA gels were run at 90 V for about 45 min in 1× MOPS running buffer. Following staining with ethidium bromide (Sigma), the samples were visualized under UV irradiation.
RNA-binding assay by EMSA.
The binding of the HP0958 protein and RNA was investigated using an electrophoretic gel migration shift assay (EMSA). flaA mRNA transcripts and variants were synthesized using a modified protocol of the Riboprobe in vitro transcription system (Promega). DNA templates for in vitro transcription were amplified by PCR, and the T7 promoter was fused to the 5′ end of the DNA fragments in another PCR. The primers are listed in Table S1 in the supplemental material. Next, the linear T7 promoter-attached DNA fragments were used for in vitro transcription per the manufacturer's instructions. The size and quality of the in vitro-transcribed messages were assessed using RNA gel electrophoresis. Different amounts of HP0958 protein were incubated in a total volume of 15 μl binding buffer containing 30 ng flaA Riboprobe transcript, 20 mM Tris-acetate (pH 7.9), 50 mM potassium acetate, 10 mM magnesium acetate, 1 mM DTT, 5 mM Na2EDTA, 200 μM S-(5-adenosyl)-l-methionine chloride (Sigma), and 40 U RNasin (Promega). The range of the HP0958 protein concentration used was based on previous EMSAs (12). The mixture was incubated at room temperature for 20 min. The RNA was then resolved by native agarose gel electrophoresis. To detect RNA species, Northern hybridization was performed with a labeled PCR probe corresponding to the riboprobe (see below). In a further EMSA, additional EDTA (final concentration, 50 mM) or DTT (final concentration, 10 mM) was added to the incubation mixture.
Northern blotting and hybridization.
Separated RNAs were transferred from agarose gels to a positively charged nylon membrane (Hybond; GE Healthcare) by overnight capillary transfer (50). RNAs were covalently bound to the nylon membrane by UV cross-linking using the Stratalinker UV linker (Stratagene). RNA detection was performed using the ECL system (GE Healthcare). All steps were performed per the manufacturer's protocol. DNA probes were amplified by PCR. A 10-μl solution containing 100 ng DNA probe was prepared and boiled for 5 min and incubated for 5 min on ice. Ten microliters of labeling reagent and 10 μl of glutaraldehyde solution were added. The mixture was incubated at 37°C for 20 min. The nylon membrane was prehybridized at 42°C for at least 45 min in ECL hybridization buffer (GE Healthcare) in a hybridization oven. The 30-μl labeled probe was then added to the hybridization buffer. Following overnight hybridization, the membrane was washed once in 5× SSC buffer and three times in wash buffer (6 M urea, 0.4% SDS, 0.5× SSC [pH 7.0]) at 42°C and twice in 5× SSC buffer for 10 min at room temperature. The membrane was drained to remove excess secondary wash buffer. Equal volumes of reagent 1 (GE Healthcare) and reagent 2 (GE Healthcare) were mixed and added directly to the blotted side of the membrane. The blot, completely covered with the developing solution, was incubated for 1 min at room temperature. The excess developing solution was then removed, and the blot was placed in a detection cassette.
Protein stability assay.
CCUG17874 and the HP0958 mutant were grown in liquid culture for 20 h in a microaerobic environment. Tetracycline (60 μg/ml) was then added to stop protein synthesis. Aliquots were collected at different times following tetracycline treatment. Whole cell fractions were then prepared as previously described and analyzed by immunoblotting.
RNA stability assay.
CCUG17874 and the HP0958 mutant were cultured in liquid culture for 20 h in a microaerobic environment. Rifampin (80 μg/ml) was added to stop transcription. Aliquots were collected at different times following rifampin treatment. Total RNA was then extracted as described above and subsequently analyzed by Northern blotting (50).
Molecular cloning.
Standard procedures were used to perform cloning experiments with E. coli. The strains, plasmids, and primers used are listed in Table 1 and in Table S1 in the supplemental material. The vector pIR203K04, a generous gift from D. J. McGee, contains a kanamycin resistance marker and was designed to introduce DNA fragments into an intergenic region of the H. pylori chromosome (32). The promoter regions of the genes HP1186 (carbonic anhydrase), flhA, and ahpC (alkyl hydroperoxide reductase) were spliced to the HP0958 gene by single overlapping extension PCR (24). The amplicons were digested with BamHI and ClaI and ligated to the linearized pIR203K04 plasmid. The ligated products were first introduced into E. coli as a cloning host and then into the H. pylori P79 mutant lacking HP0958. Transformants were selected on kanamycin (50 μg/ml) and analyzed by colony PCR, motility assay, and immunoblotting.
Motility assay.
H. pylori strains and mutants were grown for 2 days on CBA plates and then stabbed on brucella soft agar plates containing 0.3% (wt/vol) agar and 5% (vol/vol) heat-inactivated fetal bovine serum (Sigma). Motility plates were incubated at 37°C in an atmosphere containing 5% CO2 and periodically observed.
Bioinformatic analysis and RNA analysis using secondary-structure modeling.
The predicted secondary structures were obtained using RNAdraw (35). This prediction program is based on the lowest free energy of formation and models the secondary structures of RNA molecules, such as hairpins. Alignments of the protein sequences with Clustal W (55) were performed using the Institute for Genome Research (TIGR) public database.
Microarray data accession numbers.
The BμG@S HPv1.0.0 array design is available in BμG@Sbase (accession no. A-BUGS-18; http://bugs.sgul.ac.uk/A-BUGS-18) and also ArrayExpress (accession no. A-BUGS-18). Fully annotated microarray data have been deposited in BμG@Sbase (accession no. E-BUGS- 73; http://bugs.sgul.ac.uk/E-BUGS-73) and also ArrayExpress (accession no. E-BUGS-73).
RESULTS
Transcriptional analysis of the HP0958 mutant.
The flagellar apparatus involves more than 40 flagellar proteins in H. pylori (37, 56). The disruption of the HP0958 gene was previously shown to impair motility in H. pylori and to interfere at different levels in flagellar regulatory circuitry (42, 48). HP0958 was shown to regulate RpoN at a posttranscriptional level by apparently protecting RpoN from proteolysis (42). However, previous studies of HP0958-dependent gene expression have been restricted to candidate approaches and targeted methods (qRT-PCR and gene fusions) and thus were limited to only three selected RpoN-dependent genes, flgE, flaB, and HP1120, encoding a hypothetical protein (42, 48). To provide a genome-wide understanding of HP0958 involvement in flagellar regulation, we performed type 2 microarray experiments. A total of 44 genes were differentially expressed in the HP0958 mutant compared to those in the wild type (see Table S2 in the supplemental material). Twenty-four genes were downregulated, and 20 genes were upregulated. Seventeen out of the 44 genes had been annotated as hypothetical. In Table 2, we present the relative transcriptional levels in the mutant of all known H. pylori flagellar genes by hierarchical class (37). As indicated by these data, HP0958 does not play a major role in regulating class I genes. Pereira and Hoover showed that the mutation of HP0958 caused the downregulation of the class 2 genes flaB and HP1120 (42). Our global transcript analysis reveals that most of the RpoN-dependent (class 2) genes were significantly downregulated (Table 2), though rpoN expression itself was unaffected in the HP0958 mutant, in agreement with our previous findings (48). Thus, the role of HP0958 in flagellar regulation appeared to be mainly mediated by the protein stabilization of RpoN. Strikingly, however, the class 2 genes HP0114, HP0295 (flaB homolog), flgK (encoding hook-associated protein HAP1), and HP1120 (encoding a hypothetical protein) were transcribed at the wild-type level, which is in contradiction with a previous study (37). For flgK (HP1119) and HP1120, this may be due to read-through from the upregulated flgM gene (HP1122). HP1121 was also transcribed at the wild-type level. Another noteworthy finding was that flgJ/HP1233, part of the RpoN regulon, was 1.6-fold upregulated in the HP0958 mutant. HP1233 encodes for a putative flagellar muraminidase, present only in the Helicobacter genus.
TABLE 2.
Array-based analysis of flagellar gene expression in an HP0958 mutanta
| Proposed flagellar class | TIGR ORF | Putative gene product (gene) | Change (fold) in the Δhp0958 strain | P |
|---|---|---|---|---|
| Class 1 | Hp26695-0019 | Chemotaxis protein (cheV) | 1.071 | 0.34 |
| Hp26695-0082 | Methyl-accepting chemotaxis transducer (tlpC) | 0.911 | 0.24 | |
| Hp26695-0099 | Methyl-accepting chemotaxis protein (tlpA) | 0.831 | 0.19 | |
| Hp26695-0103 | Methyl-accepting chemotaxis protein (tlpB) | 1.098 | 0.48 | |
| Hp26695-0173 | Flagellar biosynthetic protein (fliR) | 1.059 | 0.61 | |
| Hp26695-0244 | Signal-transducing protein, histidine kinase (atoS) | 0.692 | 0.08 | |
| Hp26695-0246 | Flagellar basal-body P-ring protein (flgI) | — | — | |
| Hp26695-0325 | Flagellar basal-body L-ring protein (flgH) | 1.236 | 0.17 | |
| Hp26695-0326 | CMP-N-acetylneuraminic acid synthetase (neuA) | 0.881 | 0.21 | |
| Hp26695-0327 | Flagellar protein G (flaG) | 0.805 | 0.28 | |
| Hp26695-0351 | Basal-body M-ring protein (fliF) | 1.178 | 0.03 | |
| Hp26695-0352 | Flagellar motor switch protein (fliG) | 1.329 | 0.01 | |
| Hp26695-0391 | Purine-binding chemotaxis protein (cheW) | 1.494 | 0.02 | |
| Hp26695-0392 | Histidine kinase (cheA) | 1.518 | 0.07 | |
| Hp26695-0393 | Chemotaxis protein (cheV) | 1.457 | 0.03 | |
| Hp26695-0584 | Flagellar motor switch protein (fliN) | 1.098 | 0.52 | |
| Hp26695-0599 | Hemolysin secretion protein precursor (hylB) | 0.950 | 0.83 | |
| Hp26695-0616 | Chemotaxis protein (cheV) | 1.209 | 0.23 | |
| Hp26695-0684 | Flagellar biosynthesis protein (fliP) | 0.252 | 0.04 | |
| Hp26695-0685 | Flagellar biosynthetic protein (fliP) | 0.989** | 0.49 | |
| Hp26695-0703 | Response regulator | 0.997 | 0.99 | |
| Hp26695-0714 | RNA polymerase sigma-54 factor (rpoN) | 1.032* | 0.92 | |
| Hp26695-0770 | Flagellar biosynthetic protein (flhB) | 0.754 | 0.36 | |
| Hp26695-0815 | Flagellar motor rotation protein (motA) | 1.431 | 0.03 | |
| Hp26695-0816 | Flagellar motor rotation protein (motB) | 1.281** | 0.24 | |
| Hp26695-0840 | flaA1 protein | 0.962 | 0.80 | |
| Hp26695-1041 | Flagellar biosynthesis protein (flhA) | 0.969 | 0.59 | |
| Hp26695-1067 | Chemotaxis protein (cheY) | 1.552 | 0.24 | |
| Hp26695-1092 | Flagellar basal-body rod protein (flgG) | 1.407 | 0.12 | |
| Hp26695-1286 | Conserved hypothetical secreted protein (fliZ) | 1.832 | 0.33 | |
| Hp26695-1419 | Flagellar biosynthetic protein (fliQ) | 0.684 | 0.05 | |
| Hp26695-1420 | Flagellar export protein ATP synthase (flil) | 0.934 | 0.65 | |
| Hp26695-1462 | Secreted protein involved in flagellar motility | 1.291 | 0.01 | |
| Hp26695-1575 | Homolog of FlhB protein (flhB2) | 0.964 | 0.59 | |
| Hp26695-1585 | Flagellar basal-body rod protein (flgG) | 1.014 | 0.93 | |
| Class 2 | Hp26695-0114 | Hypothetical protein | 0.808 | 0.38 |
| Hp26695-0115 | Flagellin B (flaB) | 0.503* | 0.00 | |
| Hp26695-0295 | Flagellin B homolog (fla) | 0.889 | 0.25 | |
| Hp26695-0869 | Hydrogenase expression/formation protein (hypA) | 0.540 | 0.00 | |
| Hp26695-0870 | Flagellar hook (flgE) | 0.450 | 0.01 | |
| Hp26695-0906 | Hook length control regulator (fliK) | 0.514 | 0.01 | |
| Hp26695-1076 | Hypothetical protein | 0.683 | 0.05 | |
| Hp26695-1119 | Flagellar hook-associated protein 1 (HAP1) (flgK) | 0.981 | 0.55 | |
| Hp26695-1120 | Hypothetical protein | 1.014 | 0.92 | |
| Hp26695-1154 | Hypothetical protein (operon with murG) | 0.724 | 0.05 | |
| Hp26695-1155 | Transferase, peptidoglycan synthesis (murG) | 0.711 | 0.04 | |
| Hp26695-1233 | Putative flagellar muraminidase (flgJ) | 1.617 | 0.00 | |
| Class 3 | Hp26695-0472 | Outer membrane protein (omp11) | 1.698* | 0.13 |
| Hp26695-0601 | Flagellin A (flaA) | 2.12* | 0.01 | |
| Hp26695-1051 | Hypothetical protein | 1.175 | 0.25 | |
| Hp26695-1052 | UDP-3-0-acyl-N-acetylglucosamine deacetylase (envA) | 1.134* | 0.58 | |
| Intermediate class | Hp26695-0165 | Hypothetical protein | 1.155 | 0.75 |
| Hp26695-0166 | Response regulator (ompR) | 1.254 | 0.41 | |
| Hp26695-0366 | Spore coat polysaccharide biosynthesis protein C | 0.838 | 0.37 | |
| Hp26695-0367 | Hypothetical protein | 0.896 | 0.20 | |
| Hp26695-0488 | Hypothetical protein | 0.789 | 0.04 | |
| Hp26695-0907 | Hook assembly protein, flagella (flgD) | 0.715 | 0.01 | |
| Hp26695-0908 | Flagellar hook (flgE) | 0.794 | 0.06 | |
| Hp26695-1028 | Hypothetical protein | 1.196 | 0.43 | |
| Hp26695-1029 | Hypothetical protein | 1.037 | 0.76 | |
| Hp26695-1030 | FliY protein (fliY) | 1.157 | 0.36 | |
| Hp26695-1031 | Flagellar motor switch protein (fliM) | 1.083 | 0.22 | |
| Hp26695-1032 | Alternative transcription initiation factor, sigma-28 (fliA) | 1.112* | 0.32 | |
| Hp26695-1033 | Hypothetical protein | 0.929 | 0.45 | |
| Hp26695-1034 | ATP-binding protein (ylxH) | 0.954 | 0.76 | |
| Hp26695-1035 | Flagellar biosynthesis protein (flhF) | 1.135 | 0.35 | |
| Hp26695-1122 | Anti-sigma-28 factor (flgM) | 1.496* | 0.02 | |
| Hp26695-1440 | Hypothetical protein | 0.398* | 0.00 | |
| Hp26695-1557 | Flagellar basal-body protein (fliE) | 0.584* | 0.01 | |
| Hp26695-1558 | Flagellar basal-body rod protein (flgC) (proximal rod protein) | 0.527* | 0.00 | |
| Hp26695-1559 | Flagellar basal-body rod protein (flgB) (proximal rod protein) | 0.644* | 0.00 | |
| Hp26695-0751 | (flaG2) | 1.621 | 0.01 | |
| Hp26695-0752 | Flagellar cap protein (fliD) | 1.492 | 0.24 | |
| Hp26695-0753 | Flagellar chaperone (fliS) | 1.760 | 0.00 | |
| Hp26695-0754 | Flagellar chaperone (fliT) | — | — | |
| Unassigned | Hp26695-0410 | Flagellar sheath-associated protein (hpaA2) | 1.170 | 0.48 |
| Hp26695-0492 | Flagellar sheath-associated protein (hpaA3) | 0.556 | 0.01 | |
| Hp26695-0797 | Flagellar sheath-associated protein (hpaA) | 1.278 | 0.13 |
Relative expression levels for all known flagellar genes were tabulated. Changes and P values were calculated from three independent biological replicates as described in Materials and Methods. Open reading frames (ORFs) and gene annotations are based on the TIGR database (56). Genes were assigned to flagellar classes as previously proposed (37). Changes considered significant (P value less than or equal to 0.05) are in bold. Dashes indicate values excluded during array data analysis due to variation or technical problems with array features. *, Confirmatory analysis by qRT-PCR was performed; **, values were missing in the microarray analysis and were investigated by qRT-PCR.
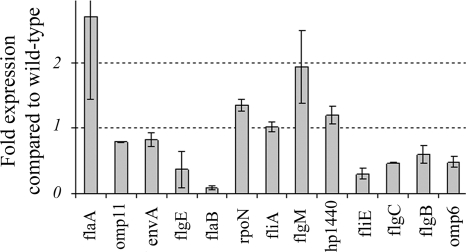
In previous studies, the transcription of the intermediate class genes was shown to be under the control of σ54 and σ28. As shown in Table 2, four genes in the intermediate class, HP1440, fliE, flgC, and flgB, were downregulated in the HP0958 mutant but below the twofold-change cutoff. The fliE, flgC, and flgB genes code for flagellar basal-body proteins. The downregulation of fliE, flgC, and flgB was confirmed by qRT-PCR (Fig. 1). Although the microarray data indicated that HP1440 was downregulated, RT-PCR indicated upregulation in the mutant, which we cannot currently explain.
FIG. 1.
Confirmation of transcriptional changes in selected flagellar genes in the HP0958 mutant using qRT-PCR. Changes and standard deviations were calculated using the era transcript abundance as a reference. qRT-PCRs were performed on at least two biological replicates.
The array experiments indicated that the transcription of flaA increased twofold in the HP0958 mutant, and this was confirmed by qRT-PCR (Fig. 1). This upregulation of flaA expression was independent of levels of σ28, which was transcribed at the wild-type level. Pereira and Hoover also reported increased flaA transcription in an HP0958 mutant, which they suggested could indicate an additional regulatory effect of HP0958 in the broader FliA regulon (42). However, our array data clearly show that the class 3 genes omp11, HP1051 (hypothetical), and envA were not significantly differentially transcribed in the HP0958 mutant. Despite the increase in flaA transcription described herein, previous analyses have reported reduced FlaA protein production in an HP0958 mutant (48). Among other surface-protein-related genes were omp6 (Fig. 1) and omp23 that express two outer membrane proteins that we noted were downregulated. omp6 expresses a porin of the Hop protein family, involved in the colonization and persistence of H. pylori in the gastric mucosa (36, 58).
FlaA stability is unaffected in the HP0958 mutant.
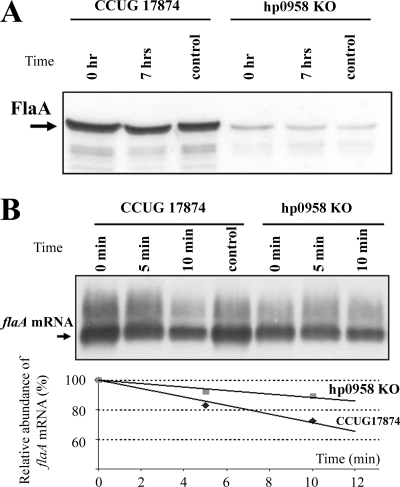
A striking contradiction in our findings was the apparent doubling of flaA transcription in the HP0958 mutant, despite our previously reported significant reduction in FlaA protein production in this mutant (48). To investigate if the modulation of flagellin production was at the protein level, a protein stability assay was performed. Protein synthesis in H. pylori was stopped by adding tetracycline, and aliquots were taken at various time points for analysis by immunoblotting (Fig. 2). After 7 h of treatment with tetracycline, there was no significant difference in stability of the flagellin protein between the wild type and the HP0958 mutant. This indicated that the low abundance of FlaA protein in the HP0958 mutant was not the result of reduced protein stability and that the regulatory effect on flaA occurred at a posttranscriptional or translational level.
FIG. 2.
Stability assays. (A) Mutation of HP0958 does not reduce FlaA protein stability. FlaA protein levels in the HP0958 mutant and the wild type were analyzed by immunoblotting at various time points after the addition of tetracycline. Two independent stability assays were performed. The control samples were treated for 7 h with ethanol, the solvent for tetracycline. (B) Lack of HP0958 increases flaA mRNA stability in the HP0958 mutant compared to the wild-type strain. flaA mRNA levels in the HP0958 mutant and CCUG17874 were analyzed by Northern blotting at various time points after the addition of rifampin. Time is in min. The control samples were treated with methanol (rifampin solvent) for 10 min. Band intensities were analyzed by densitometry. KO, knockout.
RNA stability.
Since flagellin stability was apparently unaffected by HP0958 mutation, we next investigated the stability of the flaA message (Fig. 2). After 10 min of treatment with rifampin, it appeared that the flaA message was less stable in the wild type than in the HP0958 mutant. Analysis of the Northern blot by densitometry indicated that the flaA message was degraded at a rate approximately 2.5-fold higher in the wild type than in the HP0958 mutant. In the wild type, the half-life of the flaA message was calculated at 17.3 min, compared to 42.2 min in the HP0958 mutant. We also confirmed the increased flaA mRNA stability in the HP0958 mutant by a qRT-PCR approach (data not shown), whereby the half-life was 19.2 min in the mutant and 28.2 min in the wild type.
The HP0958 protein binds to the flaA message.
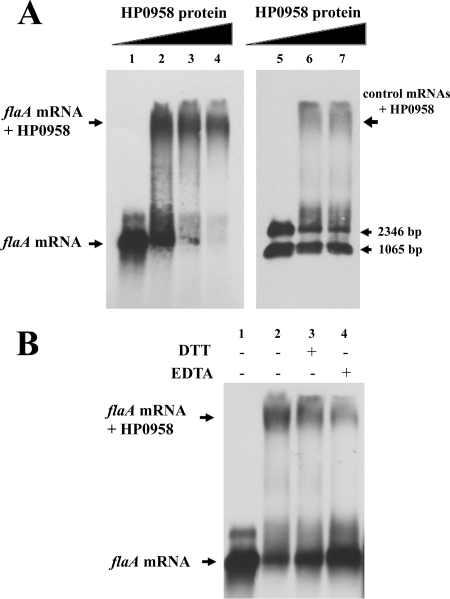
Our previous bioinformatics analyses (48) indicated that HP0958 belongs to the zinc ribbon superfamily of proteins (28), incorporating proteins from COG1579. Proteins in this family typically use the zinc-finger motif to interact with other proteins or nucleic acid (28). This highlighted the potential involvement of HP0958 in flagellar regulatory processes. Multiple alignments of the HP0958 protein sequence and its orthologs highlighted the conservation of two main domains. One of them showed the following pattern: C-X2-C-X(17 to 20)-C-X2-C, suggesting a zinc-motif type that might interact with nucleic acid. We thus hypothesized that HP0958 may have a regulatory effect on flaA mRNA by interacting directly with the flaA message. To test for an interaction between the flaA transcript and HP0958, EMSAs were performed using in vitro-transcribed mRNA and HP0958. The HP0958 protein was purified to >99% purity, adjudged by silver staining (D. L. Caly, S. A. Moore, and P. W. O'Toole, unpublished data). The synthesis of the in vitro-transcribed flaA transcript introduced several nucleotides at the 5′ end, but predictive models suggested that these bases are not involved in secondary-structure formation and would not alter the native conformation of the flaA mRNA. Because we used a less-sensitive (nonradiolabeled) detection system than some published studies, we scaled up the components of the EMSA reactions proportionately, e.g., compared to the amounts used for studying FljA-fliC mRNA interaction in Salmonella (2). Thus, we increased the RNA amount 40-fold from 1.25 fmol to 57 fmol and the protein concentration 50-fold from 264 nM to 13 μM.
Upon the incubation of flaA with purified HP0958, a mobility shift of the flaA transcript was observed (Fig. 3). The major shift band suggested the direct association of HP0958 with the flaA message. A similar experiment using two control RNA species was performed (Fig. 3). Small amounts of a diffuse shift were produced, approximately eightfold less intense than the HP0958-flaA shift. The higher affinity of HP0958 for flaA RNA suggested that a particular RNA sequence or secondary structure was involved. In Caulobacter crescentus, the RNA chaperone FlbT binds to the 5′ untranscribed region of the fljK flagellin mRNA to regulate flagellin synthesis posttranscriptionally (4). Following a similar method used to analyze the C. crescentus transcript, we identified a potential stem-loop structure located 12 nucleotides upstream of the ribosome binding site of HP0958 (not shown). When the stem-loop was deleted from the in vitro-transcribed flaA message, a gel shift pattern identical to the full-length transcript was observed (data not shown). The addition of EDTA to the reaction reduced the HP0958-flaA riboprobe association, suggesting that HP0958 requires cations to interact with the flaA message. Densitometry analysis indicated that the shifted band was approximately 3.5-fold weaker when incubated with additional EDTA. That supports the hypothesis that HP0958 may be an RNA-binding protein with a zinc-finger motif. DTT can form complexes with zinc ions, and the addition of DTT has been shown to reduce the free zinc ion concentration in reaction mixtures (1). The addition of DTT reduced the amount of the shifted species by 1.3-fold compared to that of the control EMSA, supporting the involvement of a zinc-finger motif in the RNA-protein association.
FIG. 3.
Gel shift assays of purified HP0958 binding to the flaA transcript. (A) EMSA shows that HP0958 binds to the flaA transcript. In vitro transcripts added to incubations for EMSA were 30 ng flaA mRNA (lanes 1 to 4) and 30 ng control mRNAs (lanes 5 to 7). HP0958 protein concentrations added to EMSAs were 1,200 ng (lane 2), 3,600 ng (lanes 3 and 6), and 6,000 ng (lanes 4 and 7). (B) The flaA mRNA and HP0958 association is affected by EDTA and DTT. Lane 1, 30 ng flaA transcript; lanes 2 to 4, 30 ng flaA transcript and 6,000 ng HP0958 protein. Lanes where additional EDTA (50 mM) or DTT (10 mM) were added are labeled with a plus sign.
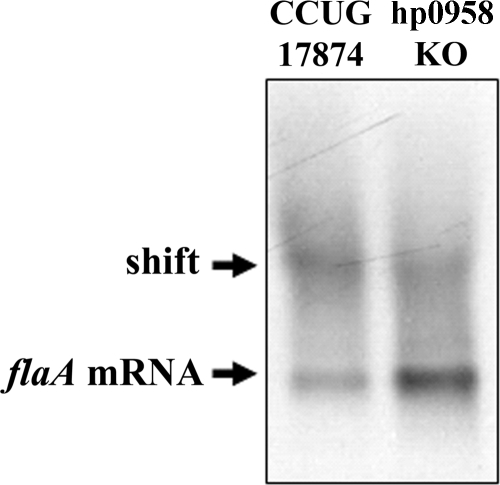
To examine the HP0958-flaA mRNA association under more physiological conditions, cytoplasmic fractions from the wild type and the HP0958 mutant were prepared and incubated with the flaA riboprobe (Fig. 4). Shifted bands were observed in both samples. However, the shifted band obtained with the wild-type cell extract was stronger than that in the HP0958 mutant.
FIG. 4.
Loss of HP0958 reduces the flaA transcript shift by H. pylori cytoplasmic extract. A total of 30 ng flaA in vitro transcript and 1 μg total protein were used for both EMSAs. KO, knockout.
Complementation of the HP0958 mutant.
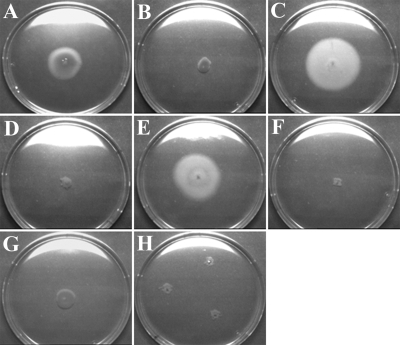
Previous attempts to complement an HP0958 mutant by expressing the gene under the control of the ureA promoter were unsuccessful, suggesting that the overexpression of HP0958 interferes with its normal function (42). To eliminate the possible involvement of second-site mutations in our HP0958 mutant, we attempted to complement the mutation by expressing the HP0958 gene under the control of three well-characterized promoters, controlling the expression of flhA (a component of the flagellar apparatus), HP1186 (alpha-carbonic anhydrase) (60), and ahpC (alkyl hydroperoxide reductase) (34). The complementation plasmids were each introduced into a derivative of the P79 strain in which the HP0958 gene had been insertionally inactivated. We used strain P79 because it supports the replication of the vector used for complementation (21), whereas strain 17874, in which transcription analyses were performed, does not. The phenotypes of the three complemented strain P79 mutants were analyzed by motility assay (Fig. 5) and immunoblotting (Fig. 6). The complemented mutants in which the HP0958 gene was under the control of the promoters of flhA and the carbonic anhydrase gene were not motile and flagellin synthesis was not restored. The mutant that was transformed with the HP0958 gene under the control of the ahpC promoter was motile, and FlaA protein synthesis was restored to the wild-type level or above. This confirms that the mutant phenotype was due only to the loss of the HP0958 gene and that the wild-type phenotype may be restored in an HP0958 mutant in a manner dependent on HP0958 expression levels.
FIG. 5.
Restoration of motility by the complementation of an HP0958 mutant, when hp0958 was put under the control of the ahpC gene promoter. (A) The CCUG17874 wild-type strain; (B) the CCUG17874-hp0958 KO; (C) the P79 wild-type strain; (D) the P79-hp0958 KO; (E) P79-0958/pIR1563; (F) P79-0958/pIR203K04; (G) P79-0958/pIR1186; (H) P79-0958/pIR1041.
FIG. 6.
Restoration of FlaA production by the complementation of an HP0958 mutant is promoter dependent. Lane 1, Protein marker; lane 2, the CCUG17874 wild-type strain; lane 3, the CCUG17874-hp0958 KO; lane 4, the P79 wild-type strain; lane 5, the P79-0958 KO; lane 6, P79-0958/pIR203K04; lane 7, P79-0958/pIR1041; lane 8, P79-0958/pIR1186; lane 9, P79-0958/pIR1563.
DISCUSSION
HP0958 has been previously identified as an essential gene for flagellum production in H. pylori (42, 48), and we aimed to further investigate its role in this process. Pereira and Hoover showed that the stable accumulation of RpoN required HP0958 (42). Consistent with this role, our global transcript analysis indicated that most of the RpoN-dependent genes were downregulated in the HP0958 mutant. The downregulation of the intermediate class genes fliE, flgC, and flgB indicated that they may be under the additional control of RpoN. The position of the HP0958 gene itself, however, in flagellar gene hierarchy, is still unclear. The transcription of class I regulators was unaffected in the HP0958 mutant, showing that HP0958 is below this level or outside the normal hierarchy altogether. The HP0958 gene is in the middle of an operon containing several housekeeping genes (48), which would likely be dependent on the major sigma factor RpoD.
It was previously hypothesized that HP0958 may control the complete FliA flagellar regulon, based on targeted studies with flaA (42). The utilization of an array approach allowed us to demonstrate that HP0958 does not regulate the FliA regulon but only the flaA gene. The flaA gene is transcribed at normal levels in a mutant lacking RpoN (37), ruling out the indirect RpoN-related modulation of flaA transcription in the HP0958 mutant. However, in a mutant lacking the RpoN activator FlgR, the transcription of flaA was upregulated (54), while the deletion of FlgS also caused an increase in flaA transcription but no changes in the expression of other class III genes (37). Thus, it is clear that flaA transcription is under some additional controls involving the RpoN regulators but independent of the sigma factor itself.
FlaA protein stability was not affected in the HP0958 mutant, indicating that the modulation of FlaA production was not mediated at the protein level—a possibility that needed to be excluded, given the fact that HP0958 protects RpoN from proteolysis (42). A slight caveat for this interpretation is that the HP0958 mutant lacks flagella (48). Thus, FlaA protein localizes differently in the wild type and in the mutant, likely being cytoplasmic in the latter. Nonetheless, if HP0958 was a FlaA-protective chaperone, one would still expect to see an enhanced degradation of cytoplasmic FlaA protein in the mutant. The present study supports the hypothesis that FlaA protein synthesis is controlled at a posttranscriptional stage. Our data indicate that the HP0958 protein binds to flaA mRNA, resulting in a decreased stability of the transcript but higher levels of translation and flagellin production. We know of two relevant precedents for protein-modulated RNA stability and translation efficiency involved in flagellin synthesis. In Caulobacter crescentus, the flagellin transcript is posttranscriptionally regulated by an interaction of the protein FlbT with the 5′ untranscribed region of flagellin (FljK) mRNA (4). The binding of FlbT to this transcript was dependent on a predicted loop structure, and mutations in this loop region that prevented FlbT binding resulted in the increased stability of fljK mRNA. This system differs from our demonstrated interaction of HP0958 with H. pylori flaA mRNA, where the deletion of a predicted stem-loop did not abolish HP0958-flaA mRNA interaction. However, the association of the protein-mRNA interaction with decreased mRNA stability is common to our findings for H. pylori and the Caulobacter posttranscription regulation example. In the latter, it was suggested (4) that FlbT competes with another factor for binding to the fljK transcript, and the outcome of this competition determines if the transcript is degraded or translated. Our data from EMSA experiments with H. pylori cytoplasmic extracts suggest that a protein(s) other than HP0958 also binds to the flaA transcript, since there is a weak shift with extracts from the HP0958 mutant (Fig. 4). A more complex role for the regulatory function of HP0958 in H. pylori flagellin synthesis regulation is therefore possible.
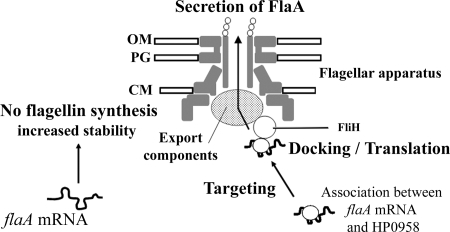
Posttranscriptional control of flagellin synthesis is also a feature of flagellar phase variation in Salmonella enterica (2, 7, 61), whereby the FljA protein binds to the mRNA for the alternative flagellin protein FliC and blocks its translation (2). However, by analysis of bypass mutations that affected fliC mRNA structure, it was discovered that some such mutations block FliC incorporation in the flagellum but still permit its translation. The authors concluded that mRNAs for the alternative flagellins might compete for occupancy at an assembly site and that the outcome of this competition was influenced by the FljA protein (2). Although the Salmonella system is much better characterized, permitting such a model to be developed, we think it significant that the yeast two-hybrid interaction network data for H. pylori (44) show an interaction of HP0958 with FliH. The FliH protein is a presumptive inhibitor of the flagellar export ATPase FliI (19), and we have recently determined the molecular basis for the H. pylori FliH-FliI association (31). The interaction between HP0958 and FliH suggests a possible targeting of the flaA message to the flagellar export apparatus, where the translation and secretion of FlaA proteins would jointly occur (Fig. 7). Substrate recognition by the Yersinia type III secretion system machinery is known to involve signals within the cognate mRNAs (reviewed in reference 45) but which require translation, and some Yersinia type III secretion system effector molecules have controversially been suggested to be cotranslationally secreted (45). Following the coupled translation and secretion of FlaA protein, flaA mRNA would then be degraded. The disruption of HP0958-mediated addressing of the flaA message would leave it biologically inert in the cytoplasm. Compared to mRNA actively being turned over at the FlaA secretion apparatus, this cytoplasmic mRNA might be less susceptible to RNase, potentially explaining its higher stability in the HP0958 mutant than in the wild type. If HP0958 bound with greater affinity to the flaA transcript than to RpoN, then commencement of the production of the flaA transcript (by relief of FlgM inhibition of FliA after completion of the hook-basal body) would act as a switch to turn off the RpoN regulon. This model thus presents a number of independent lines of approach for experimental testing of its validity.
FIG. 7.
Proposed mechanistic model for the role of HP0958 in H. pylori flagellum biogenesis. The model proposes increased turnover of the flaA transcript when in a translation-competent state promoted by HP0958 (right side of the figure) and greater stability of the transcript when HP0958 is absent (left side of the figure). OM, outer membrane; PG, peptidoglycan; CM, cytoplasmic membrane.
Pereira and Hoover reported that the overproduction of HP0958 protein interfered with its activity (42). In the present study, the HP0958 mutant was successfully complemented only when put under the control of the ahpC promoter (of those tested), confirming that a controlled level of HP0958 protein is important for cellular function. Biochemical analysis in our lab indicates that HP0958 protein forms dimers and hexamers in vitro and that the monomer conformation may be more active than the dimer and hexamer conformations in the flaA transcript gel shift assay (data not shown). Further investigation of the physicochemical properties of HP0958 is warranted. The RNA-binding ability of HP0958 was also shown to be sensitive to EDTA, supporting the involvement of the HP0958 zinc-finger motif in the binding, and we have recently confirmed the presence of zinc in the HP0958 protein (Caly, Moore, and O'Toole, unpublished data). An investigation of the HP0958 protein structure will help us to understand its function and to identify the regions of the HP0958 molecule involved in the association with flaA mRNA, the σ54 sigma factor RpoN, and FliH.
Supplementary Material
Acknowledgments
Work in P.W.O.'s lab is supported by a Science Foundation Ireland grant from the Research Frontiers Programme.
We acknowledge R. Haas and D. J. McGee for providing strains and plasmids and The Wellcome Trust for supporting BμG@S (Bacterial Microarray Group at St. George's, University of London). We thank M. W. Mangan for valuable discussions.
Footnotes
Published ahead of print on 17 October 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aballay, A., N. G. Arenas, A. F. Quest, and L. S. Mayorga. 1997. A factor with a zinc- and phorbol ester-binding domain is necessary for endosome fusion. Exp. Cell Res. 23528-34. [DOI] [PubMed] [Google Scholar]
- 2.Aldridge, P. D., C. Wu, J. Gnerer, J. E. Karlinsey, K. T. Hughes, and M. S. Sachs. 2006. Regulatory protein that inhibits both synthesis and use of the target protein controls flagellar phase variation in Salmonella enterica. Proc. Natl. Acad. Sci. USA 10311340-11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alm, R. A., L.-S. L. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397176-180. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, P. E., and J. W. Gober. 2000. FlbT, the post-transcriptional regulator of flagellin synthesis in Caulobacter crescentus, interacts with the 5′ untranslated region of flagellin mRNA. Mol. Microbiol. 3841-52. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, M. J. 1997. Ecology of Helicobacter pylori in the human stomach. J. Clin. Investig. 100759-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolstad, B. M., R. A. Irizarry, M. Astrand, and T. P. Speed. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19185-193. [DOI] [PubMed] [Google Scholar]
- 7.Bonifield, H. R., and K. T. Hughes. 2003. Flagellar phase variation in Salmonella enterica is mediated by a posttranscriptional control mechanism. J. Bacteriol. 1853567-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmachary, P., M. G. Dashti, J. W. Olson, and T. R. Hoover. 2004. Helicobacter pylori FlgR is an enhancer-independent activator of σ54-RNA polymerase holoenzyme. J. Bacteriol. 1864535-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colland, F., J.-C. Rain, P. Gounon, A. Labigne, P. Legrain, and H. De Reuse. 2001. Identification of the Helicobacter pylori anti-sigma 28 factor. Mol. Microbiol. 41477-487. [DOI] [PubMed] [Google Scholar]
- 10.Cover, T. L., and M. J. Blaser. 1992. Helicobacter pylori and gastroduodenal disease. Annu. Rev. Med. 43135-145. [DOI] [PubMed] [Google Scholar]
- 11.Donahue, J. P., D. A. Israel, R. M. Peek, M. J. Blaser, and G. G. Miller. 2000. Overcoming the restriction barrier to plasmid transformation of Helicobacter pylori. Mol. Microbiol. 371066-1074. [DOI] [PubMed] [Google Scholar]
- 12.Doyle, M., and C. J. Dorman. 2006. Reciprocal transcriptional and posttranscriptional growth-phase-dependent expression of sfh, a gene that encodes a paralogue of the nucleoid-associated protein H-NS. J. Bacteriol. 1887581-7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton, K. A., D. R. Morgan, and S. Krakowka. 1992. Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J. Med. Microbiol. 37123-127. [DOI] [PubMed] [Google Scholar]
- 14.Eaton, K. A., S. Suerbaum, C. Josenhans, and S. Krakowka. 1996. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect. Immun. 642445-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferris, H. U., and T. Minamino. 2006. Flipping the switch: bringing order to flagellar assembly. Trends Microbiol. 14519-526. [DOI] [PubMed] [Google Scholar]
- 16.Galkin, V. E., X. Yu, J. Bielnicki, J. Heuser, C. P. Ewing, P. Guerry, and E. H. Egelman. 2008. Divergence of quaternary structures among bacterial flagellar filaments. Science 320382-385. [DOI] [PubMed] [Google Scholar]
- 17.Geis, G., H. Leying, S. Suerbaum, U. Mai, and W. Opferkuch. 1989. Ultrastructure and chemical analysis of Campylobacter pylori flagella. J. Clin. Microbiol. 27436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geis, G., S. Suerbaum, B. Forsthoff, H. Leying, and W. Opferkuch. 1993. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J. Med. Microbiol. 38371-377. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Pedrajo, B., G. M. Fraser, T. Minamino, and R. M. Macnab. 2002. Molecular dissection of Salmonella FliH, a regulator of the ATPase FliI and the type III flagellar protein export pathway. Mol. Microbiol. 45967-982. [DOI] [PubMed] [Google Scholar]
- 20.Graham, D. Y., G. M. Lew, D. G. Evans, D. J. Evans, Jr., and P. D. Klein. 1991. Effect of triple therapy (antibiotics plus bismuth) on duodenal ulcer healing. A randomized controlled trial. Ann. Intern. Med. 115266-269. [DOI] [PubMed] [Google Scholar]
- 21.Heuermann, D., and R. Haas. 1998. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257519-528. [DOI] [PubMed] [Google Scholar]
- 22.Hinds, J., K. G. Laing, J. A. Mangan, and P. D. Butcher. 2002. Glass slide microarrays for bacterial genomes, p. 83-99. In B. W. Wren and N. Dorrell (ed.), Methods in microbiology: functional microbial genomics. Academic Press, London, United Kingdom.
- 23.Hinds, J., K. G. Laing, J. A. Mangan, and P. D. Butcher. 2002. Microarrays for microbes: the BμG@S approach. Comp. Funct. Genomics 3333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 7751-59. [DOI] [PubMed] [Google Scholar]
- 25.Huang, J., P. W. O'Toole, P. Doig, and T. J. Trust. 1995. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect. Immun. 631732-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inglis, T. J., T. Robertson, D. E. Woods, N. Dutton, and B. J. Chang. 2003. Flagellum-mediated adhesion by Burkholderia pseudomallei precedes invasion of Acanthamoeba astronyxis. Infect. Immun. 712280-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josenhans, C., E. Niehus, S. Amersbach, A. Horster, C. Betz, B. Drescher, K. T. Hughes, and S. Suerbaum. 2002. Functional characterization of the antagonistic flagellar late regulators FliA and FlgM of Helicobacter pylori and their effects on the H. pylori transcriptome. Mol. Microbiol. 43307-322. [DOI] [PubMed] [Google Scholar]
- 28.Krishna, S. S., I. Majumdar, and N. V. Grishin. 2003. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res. 31532-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutsukake, K. 1994. Excretion of the anti-sigma factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol. Gen. Genet. 243605-612. [DOI] [PubMed] [Google Scholar]
- 30.Kutsukake, K., Y. Ohya, and T. Iino. 1990. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J. Bacteriol. 172741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane, M. C., P. W. O'Toole, and S. A. Moore. 2006. Molecular basis of the interaction between the flagellar export proteins FliI and FliH from Helicobacter pylori. J. Biol. Chem. 281508-517. [DOI] [PubMed] [Google Scholar]
- 32.Langford, M. L., J. Zabaleta, A. C. Ochoa, T. L. Testerman, and D. J. McGee. 2006. In vitro and in vivo complementation of the Helicobacter pylori arginase mutant using an intergenic chromosomal site. Helicobacter 11477-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, W. K., Y. S. An, K. H. Kim, S. H. Kim, J. Y. Song, B. D. Ryu, Y. J. Choi, Y. H. Yoon, S. C. Baik, K. H. Rhee, and M. J. Cho. 1997. Construction of a Helicobacter pylori-Escherichia coli shuttle vector for gene transfer in Helicobacter pylori. Appl. Environ. Microbiol. 634866-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundstrom, A. M., and I. Bolin. 2000. A 26 kDa protein of Helicobacter pylori shows alkyl hydroperoxide reductase (AhpC) activity and the mono-cistronic transcription of the gene is affected by pH. Microb. Pathog. 29257-266. [DOI] [PubMed] [Google Scholar]
- 35.Matzura, O., and A. Wennborg. 1996. RNAdraw: an integrated program for RNA secondary structure calculation and analysis under 32-bit Microsoft Windows. Comput. Appl. Biosci. 12247-249. [DOI] [PubMed] [Google Scholar]
- 36.McGuinness, B. T., P. R. Lambden, and J. E. Heckels. 1993. Class 1 outer membrane protein of Neisseria meningitidis: epitope analysis of the antigenic diversity between strains, implications for subtype definition and molecular epidemiology. Mol. Microbiol. 7505-514. [DOI] [PubMed] [Google Scholar]
- 37.Niehus, E., H. Gressmann, F. Ye, R. Schlapbach, M. Dehio, C. Dehio, A. Stack, T. F. Meyer, S. Suerbaum, and C. Josenhans. 2004. Genome-wide analysis of transcriptional hierarchy and feedback regulation in the flagellar system of Helicobacter pylori. Mol. Microbiol. 52947-961. [DOI] [PubMed] [Google Scholar]
- 38.Odenbreit, S., M. Till, and R. Haas. 1996. Optimized BlaM-transposon shuttle mutagenesis of Helicobacter pylori allows the identification of novel genetic loci involved in bacterial virulence. Mol. Microbiol. 20361-373. [DOI] [PubMed] [Google Scholar]
- 39.O'Toole, P. W., M. Kostrzynska, and T. J. Trust. 1994. Non-motile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook production. Mol. Microbiol. 14691-703. [DOI] [PubMed] [Google Scholar]
- 40.O'Toole, P. W., M. C. Lane, and S. Porwollik. 2000. Helicobacter pylori motility. Microbes Infect. 21207-1214. [DOI] [PubMed] [Google Scholar]
- 41.Pallen, M. J., C. W. Penn, and R. R. Chaudhuri. 2005. Bacterial flagellar diversity in the post-genomic era. Trends Microbiol. 13143-149. [DOI] [PubMed] [Google Scholar]
- 42.Pereira, L., and T. R. Hoover. 2005. Stable accumulation of σ54 in Helicobacter pylori requires the novel protein HP0958. J. Bacteriol. 1874463-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rain, J. C., L. Selig, H. De Reuse, V. Battaglia, C. Reverdy, S. Simon, G. Lenzen, F. Petel, J. Wojcik, V. Schachter, Y. Chemama, A. Labigne, and P. Legrain. 2001. The protein-protein interaction map of Helicobacter pylori. Nature 409211-215. [DOI] [PubMed] [Google Scholar]
- 45.Ramamurthi, K. S., and O. Schneewind. 2003. Substrate recognition by the Yersinia type III protein secretion machinery. Mol. Microbiol. 501095-1102. [DOI] [PubMed] [Google Scholar]
- 46.Ritchings, B. W., E. C. Almira, S. Lory, and R. Ramphal. 1995. Cloning and phenotypic characterization of fleS and fleR, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect. Immun. 634868-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132365-386. [DOI] [PubMed] [Google Scholar]
- 48.Ryan, K. A., N. Karim, M. Worku, S. A. Moore, C. W. Penn, and P. W. O'Toole. 2005. HP0958 is an essential motility gene in Helicobacter pylori. FEMS Microbiol. Lett. 24847-55. [DOI] [PubMed] [Google Scholar]
- 49.Ryan, K. A., N. Karim, M. Worku, C. W. Penn, and P. W. O'Toole. 2005. Helicobacter pylori flagellar hook-filament transition is controlled by a FliK functional homolog encoded by the gene HP0906. J. Bacteriol. 1875742-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 51.Scarlato, V., I. Delany, G. Spohn, and D. Beier. 2001. Regulation of transcription in Helicobacter pylori: simple systems or complex circuits? Int. J. Med. Microbiol. 291107-117. [DOI] [PubMed] [Google Scholar]
- 52.Sebert, M. E., L. M. Palmer, M. Rosenberg, and J. N. Weiser. 2002. Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH two-component system and contributes to nasopharyngeal colonization. Infect. Immun. 704059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith, M. F., Jr., A. Mitchell, G. Li, S. Ding, A. M. Fitzmaurice, K. Ryan, S. Crowe, and J. B. Goldberg. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa-B activation and chemokine expression by epithelial cells. J. Biol. Chem. 27832552-32560. [DOI] [PubMed] [Google Scholar]
- 54.Spohn, G., and V. Scarlato. 1999. Motility of Helicobacter pylori is coordinately regulated by the transcriptional activator FlgR, an NtrC homolog. J. Bacteriol. 181593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388539-547. [DOI] [PubMed] [Google Scholar]
- 57.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tufano, M. A., F. Rossano, P. Catalanotti, G. Liguori, C. Capasso, M. T. Ceccarelli, and P. Marinelli. 1994. Immunobiological activities of Helicobacter pylori porins. Infect. Immun. 621392-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veldhuyzen van Zanten, S. J., and P. M. Sherman. 1994. Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and nonulcer dyspepsia: a systematic overview. CMAJ 150177-185. [PMC free article] [PubMed] [Google Scholar]
- 60.Wen, Y., J. Feng, D. R. Scott, E. A. Marcus, and G. Sachs. 2007. The HP0165-HP0166 two-component system (ArsRS) regulates acid-induced expression of HP1186 α-carbonic anhydrase in Helicobacter pylori by activating the pH-dependent promoter. J. Bacteriol. 1892426-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto, S., and K. Kutsukake. 2006. FljA-mediated posttranscriptional control of phase 1 flagellin expression in flagellar phase variation of Salmonella enterica serovar Typhimurium. J. Bacteriol. 188958-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.