Abstract
O125 is an enteropathogenic Escherichia coli (EPEC) serogroup, which includes the O125ac:H6 serotype, defined as atypical EPEC. Strains of this serotype displayed the aggregative adherence (AA) pattern with HEp-2, Caco-2, T84, and HT-29 cells, possessed all the LEE region genes, and expressed intimin, Tir, and EspABD, although the attaching-effacing lesion was not detected in vitro. These results confirm that E. coli O125ac:H6 is atypical EPEC that displays the AA pattern and indicate the necessity of testing for EPEC genes combined with the determination of the adherence pattern for atypical EPEC identification.
Infectious diarrheal diseases are still a major public health concern, affecting mainly children in developing countries (19). Escherichia coli as the etiological agent of such infections is referred to as diarrheagenic E. coli and classified into six pathotypes: enteropathogenic E. coli (EPEC), enterotoxigenic E. coli, enteroinvasive E. coli, enteroaggregative E. coli (EAEC), Shiga toxin-producing E. coli, and diffusely adherent E. coli (17).
Among these pathotypes, EPEC has been responsible for a vast number of cases of acute infantile diarrhea in Brazil and several other countries (1, 11, 13, 21, 27). The diagnosis of EPEC is commonly based on the serological determination of the lipopolysaccharide (O) antigen. However, the 12 EPEC O serogroups are composed of serotypes that may include different pathogens (32).
The EPEC serogroup O125 has been isolated from cases of diarrhea throughout the world (9, 11, 13, 30, 31). This serogroup mainly encompasses serotypes associated with the EAEC pathotype, i.e., E. coli strains displaying the aggregative adherence (AA) pattern with HeLa cells and that are reactive with the EAEC diagnostic probe (5). However, among the diversity of serotypes, O125ac:H6 shows an exclusive profile, including strains described as displaying a nondefined adherence pattern with HeLa cells and harboring the EPEC adhesin intimin-encoding gene (eae) (5). Further characterization of this serotype has demonstrated that these strains display the AA pattern with HEp-2 cells in the 6-h assay and lack EAEC virulence markers, including the EAEC probe (8). Since these strains carry the eae gene but not the EPEC adherence factor plasmid, they are classified as atypical EPEC (5, 8, 32). Therefore, the main objective of this study was to investigate the adherence patterns with different epithelial cell lines and the atypical EPEC attributes of strains belonging to this serotype.
For this purpose, six O125ac:H6 strains isolated in Brazil (strains EC292/84 and 1794/80), Germany (strains CB1924 and CB5304), and Australia (strains CB3114 and CB3338) were selected. The strains isolated from cases of diarrhea in Brazil were described previously (5). Strains CB1924 and CB5304 were isolated from cases of infantile diarrhea in Germany and belong to Lothar Beutin's laboratory collection. Strains CB3114 and CB3338 were isolated from a case of infantile diarrhea and from a healthy baby in Australia, respectively, and were kindly donated by Karl Bettelheim (University of Melbourne, Melbourne, Australia).
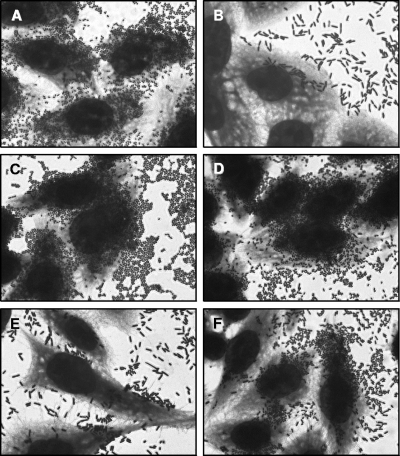
Initially, the pattern of adherence to HEp-2 cells was determined in 3- and 6-h assays, following the protocol described by Cravioto et al. (4). Figure 1 shows that all six strains displayed the AA pattern, since they adhered to the cells and to the coverslip surface in a stacked-brick pattern (24) after 6 h of incubation. However, bacteria were predominantly found adhered to the coverslip surface, which has been considered a variation of the AA pattern (14). It is interesting to note that the adherence to HeLa cells was much less intense than that displayed on HEp-2 cells (data not shown), which can explain the early description of this serotype as demonstrating a noncharacteristic adherence pattern with HeLa cells (5).
FIG. 1.
AA pattern with HEp-2 cells (6-h assay) of six atypical EPEC strains belonging to the O125ac:H6 serotype. Strains EC292/84 (A), 1794/80 (B), CB1924 (C), CB3114 (D), CB3338 (E), and CB5304 (F) are shown. Cells were subjected to Giemsa and May-Grünwald staining. Original magnification, ×1,000.
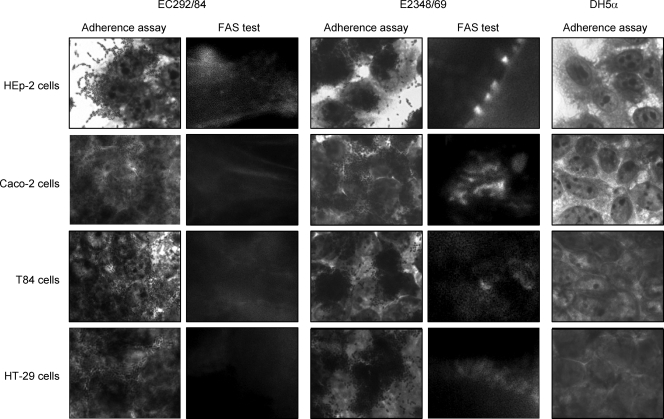
Expression of AA in the adherence assay is the main characteristic that defines the EAEC pathotype (17). However, strains belonging to the O125ac:H6 serotype have been described as harboring the eae gene (5, 8). For this reason, the capacity to induce the histopathological lesion on epithelial cells, known as the attaching-effacing (AE) lesion (23), was investigated by means of the fluorescent-actin staining (FAS) assay, which detects actin accumulation under the adherent bacteria (18). All six strains were unable to cause the AE lesion in HEp-2 cells after 3 or 6 h of bacterium-cell interaction. Figure 2 shows the FAS-negative reaction of a representative strain (EC292/84) after a 6-h period.
FIG. 2.
Adherence and FAS assays (6-h assays). HEp-2, Caco-2, T84, and HT-29 polarized cells were infected with the representative O125ac:H6 atypical EPEC strain EC292/84. EPEC E2348/69 and E. coli DH5α were used as positive and negative control strains, respectively. Cells were subjected to Giemsa and May-Grünwald staining (adherence assays) or labeled with fluorescein isothiocyanate-phalloidin (FAS assays). Original magnification, ×1,000.
Due to the fact that a difference in AA pattern expression between HEp-2 and HeLa cells was observed with our strains, we also investigated the adherence pattern and the capacity of these strains to cause AE lesions with some intestinal cell lines cultivated in vitro. For this purpose, strains EC292/84 and 1794/80 were selected for the 3- and 6-h adherence assays employing Caco-2, T84, and HT29 polarized cell lines cultivated in vitro (25, 26). As shown in Fig. 2, the representative strain EC292/84 demonstrated a similar AA pattern, as displayed by both strains on HEp-2 cells, with these three other cell lines, as well as the inability to cause the AE lesion, indicated by the negative FAS test.
Recently Bai et al. (2) demonstrated that strains belonging to the O125:H6 serotype lack the ability to utilize either the Nck or TccP/TccP2 pathway to activate the N-WASP protein and are therefore unable to activate actin polymerization in vitro, the basis of the AE lesion. However, such a phenotype could be observed by employing human intestinal biopsies, demonstrating that strains of this serotype colonize the intestinal mucosa via Nck- and TccP-independent mechanisms. Our data acquired by employing three different polarized cell lines of intestinal origin support the idea that in vitro assays of the capacity to cause AE lesions has limitations (2). In fact, strains EC292/84 and 1794/80 were negative for the tccP and tccP2 genes, as determined by PCR (see the supplemental material), and tyrosine phosphorylation of the intimin translocated receptor (16) was not observed in these strains, which is in agreement with previous reports (2, 28). However, these previous works have not distinguished the O subgroups (ab and ac) of the O125:H6 strains.
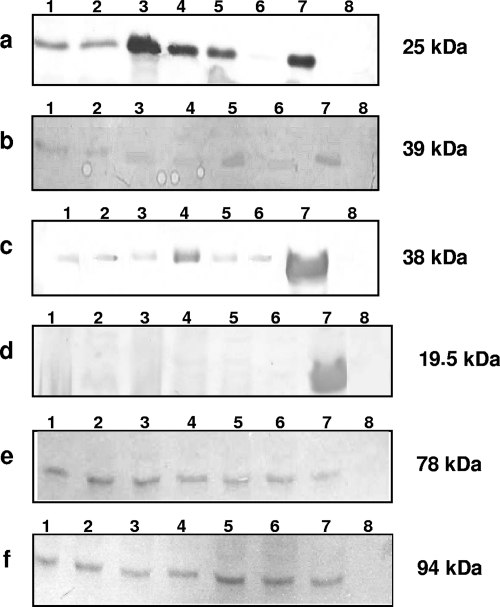
All genes necessary to mediate the expression of the AE lesion are located in a pathogenicity island known as the locus of enterocyte effacement (LEE) (22). The presence of 31 genes of LEE was investigated by PCR for strains EC292/84 and 1794/80 (see the supplemental material). All genes assayed were detected in both strains, demonstrating the presence of the LEE genes. The expression of the main proteins involved in the establishment of the AE lesion was also examined, employing immunoblots of secreted or whole-cell proteins and specific polyclonal antisera (10, 20, 29, 33). As presented in Fig. 3, the expression of intimin, Tir, and EspABD was observed in all six strains, as was the lack of bundle-forming pilus expression, supporting the classification of O125ac:H6 as atypical EPEC. These data indicate that the main LEE-encoded proteins are expressed in vitro but there are differences in signal transduction between cultured epithelial cells and intestinal mucosa, since the AE lesion is expressed only in human biopsies (2).
FIG. 3.
Western immunoblot analyses of EspA (a), EspD (b), EspB (c), bundle-forming pilus (d), Tir (e), and intimin (f) in O125ac:H6 atypical EPEC strains. Secreted (a to c) or whole-cell (d to f) proteins were separated on 10% sodium dodecyl sulfate-polyacrylamide gels, and the transferred nitrocellulose membranes were immunodetected with the corresponding polyclonal antiserum. Lanes: 1, EC292/84; 2, 1794/80; 3, CB1924; 4, CB5304; 5, CB3114; 6, CB3338; 7, positive controls (E2348/69 [a to d and f] or EDL933 [e]); 8, negative controls (UMD872 [a], UMD870 [b], UMD864 [c], JPN15 [d], or E. coli DH5α [e and f]). The apparent molecular masses are indicated on the right.
Finally, the presence of three additional EAEC virulence factors was investigated for all the strains, since the lack of the aatA, aggA, aafA, aggR, aap, shf, pet, pic, irp2, and astA genes has been previously reported (8). The AAF/III usher (agg3C) and pilin (agg3A) subunits (3), the EAEC major subunit of type IV pili (pilS) (6), and the aaiA gene of a pathogenicity island inserted at pheU site of the chromosome of EAEC 042 (7) were sought by PCR (see the supplemental material), and none of them was detected. Therefore, our strains are devoid of the main plasmid and chromosomal virulence markers of EAEC described so far (15).
We conclude that the strains of serotype O125ac:H6 are atypical EPEC strains which express the AA pattern in different cultured epithelial cells. The fact that they are devoid of all EAEC virulence markers contributes to this classification. Atypical EPEC strains displaying aggregative, diffuse, or localized adherence have been described, indicating the heterogeneity of this subgroup of EPEC (12, 33). The characterization of the adhesin mediating the AA pattern of this serotype is currently under investigation in our laboratories.
In summary, our data clearly demonstrate the importance of complementing the serological diagnosis of EPEC by employing adherence assays and genetic detection of virulence markers. Moreover, O-antigen subgrouping is also indicated.
Supplementary Material
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant 04/12136-5 to W.P.E. and a fellowship to S.F.B.).
Footnotes
Published ahead of print on 15 October 2008.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Araujo, J. M., G. F. Tabarelli, K. R. S. Aranda, S. H. Fabbricotti, U. Fagundes-Neto, C. M. F. Mendes, and I. C. A. Scaletsky. 2007. Typical enteroaggregative and atypical enteropathogenic types of Escherichia coli are the most prevalent diarrhea-associated pathotypes among Brazilian children. J. Clin. Microbiol. 453396-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, L., S. Schüller, A. Whale, A. Mousnier, O. Marches, L. Wang, T. Ooka, R. Heuschkel, F. Torrente, J. B. Kaper, T. A. T. Gomes, J. Xu, A. D. Phillips, and G. Frankel. 2008. Enteropathogenic Escherichia coli O125:H6 triggers attaching and effacing lesions on human intestinal biopsy specimens independently of Nck and TccP/TccP2. Infect. Immun. 76361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernier, C., P. Gounon, and C. Le Bouguénec. 2002. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect. Immun. 704302-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cravioto, A., R. J. Gross, S. M. Scotland, and B. Rowe. 1979. An adhesive factor found in strains of Escherichia coli belonging to the traditional enteropathogenic serotypes. Curr. Microbiol. 395-99. [Google Scholar]
- 5.do Valle, G. R. F., T. A. T. Gomes, K. Irino, and L. R. Trabulsi. 1997. The traditional enteropathogenic Escherichia coli (EPEC) serogroup O125 comprises serotypes which are mainly associated with the category of enteroaggregative E. coli. FEMS Microbiol. Lett. 15295-100. [DOI] [PubMed] [Google Scholar]
- 6.Dudley, E. G., C. Abe, J. M. Ghigo, P. Latour-Lambert, J. C. Hormazabal, and J. P. Nataro. 2006. An IncI1 plasmid contributes to the adherence of atypical enteroaggregative Escherichia coli strain C1096 to cultured cells and abiotic surfaces. Infect. Immun. 742102-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley, E. G., N. R. Thomson, J. Parkhill, N. P. Morin, and J. P. Nataro. 2006. Proteomic and microarray characterization of the AggR regulon identifies a pheU pathogenicity island in enteroaggregative Escherichia coli. Mol. Microbiol. 611267-1282. [DOI] [PubMed] [Google Scholar]
- 8.Elias, W. P., S. F. Barros, C. G. Moreira, L. R. Trabulsi, and T. A. T. Gomes. 2002. Enteroaggregative Escherichia coli strains among classical enteropathogenic Escherichia coli O serogroups. J. Clin. Microbiol. 403540-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giammanco, A., M. Maggio, G. Giammanco, R. Morelli, F. Minelli, F. Scheutz, and A. Caprioli. 1996. Characteristics of Escherichia coli strains belonging to enteropathogenic E. coli serogroups isolated in Italy from children with diarrhea. J. Clin. Microbiol. 34689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gismero-Ordoñez, J., M. Dall'Agnol, L. R. Trabulsi, and J. A. Girón. 2002. Expression of the bundle-forming pilus by enteropathogenic Escherichia coli strains of heterologous serotypes. J. Clin. Microbiol. 402291-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes, T. A. T., P. A. Blake, and L. R. Trabulsi. 1989. Prevalence of Escherichia coli strains with localized, diffuse, and aggregative adherence to HeLa cells in infants with diarrhea and matched controls. J. Clin. Microbiol. 27266-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes, T. A. T., K. Irino, D. M. Girão, V. B. C. Girão, B. E. C. Guth, T. M. I. Vaz, F. C. Moreira, S. H. Chinarelli, and M. A. M. Vieira. 2004. Emerging enteropathogenic Escherichia coli strains? Emerg. Infect. Dis. 101851-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes, T. A. T., V. Rassi, K. L. MacDonald, S. R. T. S. Ramos, L. R. Trabulsi, M. A. M. Vieira, B. E. C. Guth, J. A. Candeias, C. Ivey, and M. R. F. Toledo. 1991. Enteropathogens associated with acute diarrheal disease in urban infants in São Paulo, Brazil. J. Infect. Dis. 164331-337. [DOI] [PubMed] [Google Scholar]
- 14.Gomes, T. A. T., M. A. M. Vieira, C. M. Abe, D. Rodrigues, P. M. Griffin, and S. R. T. S. Ramos. 1998. Adherence patterns and adherence-related DNA sequences in Escherichia coli isolates from children with and without diarrhea in São Paulo city, Brazil. J. Clin. Microbiol. 363609-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington, S. M., E. G. Dudley, and J. P. Nataro. 2006. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol. Lett. 25412-18. [DOI] [PubMed] [Google Scholar]
- 16.Hernandes, R. T., R. M. Silva, S. M. Carneiro, F. A. Salvador, M. C. D. C. Fernandes, A. C. B. Padovan, D. Yamamoto, R. A. Mortara, W. P. Elias, M. R. S. Briones, and T. A. T. Gomes. 2008. The localized adherence pattern of an atypical enteropathogenic Escherichia coli is mediated by intimin omicron and unexpectedly promotes HeLa cell invasion. Cell. Microbiol. 10415-425. [DOI] [PubMed] [Google Scholar]
- 17.Kaper, J. B., J. P. Nataro, and H. L. T. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2123-140. [DOI] [PubMed] [Google Scholar]
- 18.Knutton, S. K., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 571290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosek, M., C. Bern, and R. L. Guerrant. 2003. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. W. H. O. 81197-204. [PMC free article] [PubMed] [Google Scholar]
- 20.Mairena, E. C., B. C. Neves, L. R. Trabulsi, and W. P. Elias. 2004. Detection of LEE 4 region-encoded genes from different enteropathogenic and enterohemorrhagic Escherichia coli serotypes. Curr. Microbiol. 48412-418. [DOI] [PubMed] [Google Scholar]
- 21.Mathewson, J. J., R. A. Oberhelman, H. L. Dupont, F. Javier de la Cabada, and E. V. Garibay. 1987. Enteroadherent Escherichia coli as a cause of diarrhea among children in Mexico. J. Clin. Microbiol. 251917-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 921664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 411340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nataro, J. P., J. B. Kaper, R. Robins-Browne, V. Prado, P. Vial, and M. M. Levine. 1987. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr. Infect. Dis. J. 6829-831. [DOI] [PubMed] [Google Scholar]
- 25.Nataro, J. P., S. Hicks, A. D. Phillips, P. A. Vial, and C. L. Sears. 1996. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect. Immun. 644761-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro-Garcia, F., C. Sears, C. Eslava, A. Cravioto, and J. P. Nataro. 1999. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect. Immun. 672184-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okeke, I. N., A. Lamikanra, H. Steinruck, and J. B. Kaper. 2000. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J. Clin. Microbiol. 387-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooka, T., M. A. M. Vieira, Y. Ogura, L. Beutin, R. La Ragione, P. M. van Diemen, M. P. Stevens, I. Aktan, S. Cawthraw, A. Best, R. T. Hernandes, G. Krause, T. A. T. Gomes, T. Hayashi, and G. Frankel. 2007. Characterization of tccP2 carried by atypical enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 271126-135. [DOI] [PubMed] [Google Scholar]
- 29.Sanches, M. I., R. Keller, E. L. Hartland, D. M. M. Figueiredo, M. Batchelor, M. B. Martinez, G. Dougan, M. M. S. Carneiro-Sampaio, G. Frankel, and L. R. Trabulsi. 2000. Human colostrum and serum contain antibodies reactive to the intimin-binding region of the enteropathogenic Escherichia coli translocated intimin receptor. J. Pediatr. Gastroenterol. Nutr. 3073-77. [DOI] [PubMed] [Google Scholar]
- 30.Scotland, S. M., G. A. Willshaw, H. R. Smith, R. J. Gross, and B. Rowe. 1989. Adhesion to cells in culture and plasmid profiles of enteropathogenic Escherichia coli isolated from outbreaks and sporadic cases of infant diarrhoea. J. Infect. 19237-249. [DOI] [PubMed] [Google Scholar]
- 31.Smith, H. R., S. M. Scotland, N. Stokes, and B. Rowe. 1990. Examination of strains belonging to enteropathogenic Escherichia coli serogroups for genes encoding EPEC adherence factor and Vero cytotoxins. J. Med. Microbiol. 31235-240. [DOI] [PubMed] [Google Scholar]
- 32.Trabulsi, L. R., R. Keller, and T. A. T. Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieira, M. A. M., J. R. C. Andrade, L. R. Trabulsi, A. C. P. Rosa, A. M. G. Dias, S. R. T. S. Ramos, G. Frankel, and T. A. T. Gomes. 2001. Phenotypic and genotypic characteristics of Escherichia coli strains of non-enteropathogenic E. coli (EPEC) serogroups that carry EAE and lack the EPEC adherence factor and Shiga toxin DNA probe sequences. J. Infect. Dis. 183762-772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.