Abstract
During a 3-year surveillance, six household members (five humans and the family dog) yielded 14 Escherichia coli clones. Virulence genes, group B2, and having caused cystitis (in the mother or dog) corresponded to colonization endpoints (number of samples, colonies, hosts, and dates). The dog's cystitis clone was the most extensively recovered clone.
Extraintestinal pathogenic Escherichia coli (ExPEC) causes most episodes of urinary tract infection (UTI) in humans, dogs, and cats (9, 25, 26, 32). In E. coli UTI, the immediate source of the causative strain is usually the host's own fecal flora (4, 9, 23, 30). Household members, including pets, commonly share E. coli clones, suggesting within-household transmission (2, 3, 5, 6, 13, 24). Although pets sometimes carry ExPEC clones that cause UTI in human household members (6, 13, 24), the reverse scenario has not been reported.
We investigated this phenomenon in relation to an acute UTI episode involving the pet dog of a household that 2 years previously had been studied around the mother's acute cystitis episode (6). We analyzed fecal E. coli collected from household members at the time of the dog's UTI episode and 1 and 11 months later and then compared newly and previously recovered clones.
Subjects and methods.
The subjects studied included six cohabiting individuals: a mother (age 53 years), a father (age 52 years), two daughters (both age 15 years), a son (age 13 years), and a dog (6). Fecal samples were submitted concurrent with the dog's UTI episode (dog, mother, and father) and again 1 and 11 months later (six subjects). The dog's acute urine sample was collected by the family's veterinary clinic in a sterile container during spontaneous voiding. Procedures for recovering five E. coli colonies (as available) per sample were as described previously (6), except that MacConkey agar was used instead of eosin-methylene blue agar. Unique clones were resolved by random amplified polymorphic DNA analysis and XbaI pulsed-field gel electrophoresis (6). Sequence types (STs) were newly defined by seven-gene multilocus sequence typing (http://web.mpiib-berlin.mpg.de/mlst/dbs/Ecoli/). The E. coli phylogenetic group, 12 UTI-associated O types, and 55 ExPEC virulence factor genes were determined by PCR (1, 7, 16, 18). The virulence score was the number of virulence genes detected.
Shared clones were considered those recovered concurrently or sequentially from multiple hosts. Colonization was considered the presence of a clone in feces. Long-term persistence was considered the presence of a clone in samples separated by ≥1 year. Colonization behaviors included a clone's cumulative number of hosts, samples, sampling time points, and colonies. Comparisons were tested by using Fisher's exact test or the Mann-Whitney U test, as appropriate. Correlations were assessed by using Spearman's correlation coefficient.
Case history.
In March 2007, the family dog abruptly began straining to void and exhibited urinary frequency, small-volume urination, and gross hematuria. Microscopic urinalysis showed pyuria and bacteriuria. Clinical manifestations resolved promptly with amoxicillin-clavulanate therapy. Urine culture yielded >105 CFU/ml of susceptible E. coli.
Clone distribution.
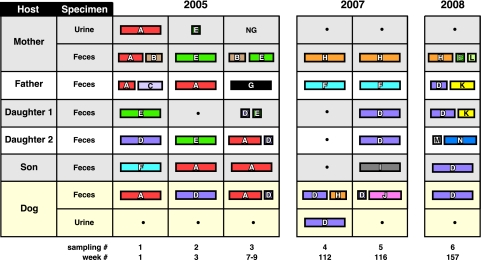
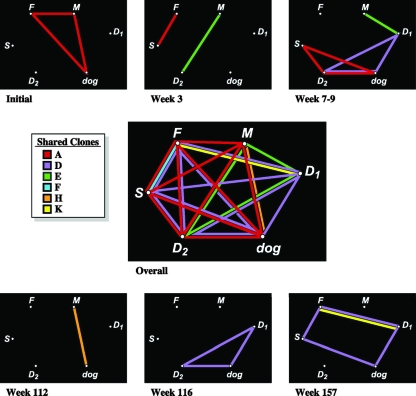
Over the 3-year study interval, 14 unique E. coli clones were identified within the household by analysis of five E. coli colonies (as available) from each acute and follow-up fecal sample and the dog's and mother's urine samples (Fig. 1). Individual hosts yielded three to six clones each, three or four of which they shared with other hosts (Table 1). Strain sharing was more extensive when considered sequentially rather than by sampling point; over the 3-year study period, each host shared, cumulatively, one to three clones with every other household member (Fig. 2). Six clones were involved in strain sharing (among up to five hosts each for the two UTI clones), whereas six clones appeared at multiple (two to six) sampling points (Tables 1 and 2). Four clones (including the dog's UTI clone) were long-term persisters, i.e., appeared repeatedly in the same or different hosts over a period of 1 year (clone H), 2 years (clone F), or 3 years (clones D and E).
FIG. 1.
Distribution of E. coli clones by host over time among five human household members and their pet dog. Each color represents a different unique clone (a total of 14 clones, arbitrarily designated A to L). The width of each rectangle is proportional to the number of colonies accounted for by the particular clone among the total number of colonies analyzed from the particular sample (usually, five colonies per sample). Sequential sampling number (#) and week number, counting from the mother's initial UTI episode, are shown below the chart. Rectangles with heavy black borders indicate symptomatic UTI episodes (mother, sampling 1; dog, sampling 4). Results for samplings 1 to 3 were reported previously (6). The daughters were redesignated here according to age (1 = older, 2 = younger, the reverse of the previously used designations [6]). Gray and white background = humans; buff background = dog.
TABLE 1.
Distribution of 14 unique E. coli clones among six household members over six sampling points (3 years)
| Host | Sampling point(s)a at which clone was encountered in indicated host
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab | B | C | Db | Eb | Fb | G | Hb | I | J | Kb | L | M | N | |
| Mother | 1 | 1, 3 | 2, 3, 6 | 4, 5, 6 | 6 | |||||||||
| Father | 1, 2 | 1 | 6 | 4, 5 | 3 | 6 | ||||||||
| Daughter 1c | 3, 5, 6 | 1, 3 | 6 | |||||||||||
| Daughter 2c | 3 | 1, 3, 5 | 2 | 6 | 6 | |||||||||
| Son | 2, 3 | 6 | 1 | 5 | ||||||||||
| Dog | 1, 3 | 2, 3, 4, 5, 6 | 4 | 5 | ||||||||||
Sampling points are numbered 1 (initial, week 1), 2 (week 3), 3 (week 7 or 9), 4 (week 112), 5 (week 115), and 6 (week 157). Results for sampling points 1, 2, and 3 were reported previously (6). Daughter 1 was not sampled at point 2, whereas at point 3 she was sampled at week 9 (versus week 7 for all other subjects). None of the three children was sampled at point 4.
Clones encountered in multiple hosts (shared clones) are underlined. Clones A and D caused acute UTI in the mother (sampling point 1) and dog (sampling point 4), respectively.
The daughters were redesignated here according to age (1 = older, 2 = younger, the reverse of the previously used designations [6]).
FIG. 2.
Clone-sharing relationships among six household members at each of six sampling points and overall. A colored line indicates detection of the corresponding clone (see the color code key) in both individuals connected by the line, either concurrently at the same sampling point (top and bottom small boxes for sampling points 1 to 6) or at any time during the study (center large box). F, father; M, mother; S, son; D1, daughter 1; D2, daughter 2. Results for samplings 1 to 3 were reported previously (6). Daughters were redesignated here according to age (1 = older, 2 = younger, the reverse of the previously used designations [6]).
TABLE 2.
Colonization behavior and molecular characteristics of 14 E. coli clones recovered from five human members and a canine member of a householda
| Clone | UTI | Clone present, no.
|
Molecular characteristicsb
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Colonies | Hosts | Time points | Phy. | ST | O | pap | F type(s) | sfa | foc | iha | fim | hlyD | hlyF | sat | astA | iroN | fyuA | ireA | iutA | kpsM II | K1 | K5 | cvaC | usp | traT | ompT | iss | malX | H7 fliC | pic | tsh | vat | hra | clbB | clbN | ||
| C | − | 1 | 3 | 1 | 1 | A | New | O12 | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| E | − | 7 | 21 | 3 | 4 | A | 399 | O7 | − | − | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| K | − | 2 | 5 | 2 | 1 | A | 10 | + | 16 | − | − | + | − | + | − | + | − | − | + | − | + | + | − | − | − | − | − | + | − | + | − | − | − | − | − | − | − | |
| N | − | 1 | 4 | 1 | 1 | A | 746 | − | − | − | + | + | − | − | − | − | − | + | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | ||
| H | − | 4 | 15 | 2 | 3 | B1 | 33 | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| L | − | 1 | 1 | 1 | 1 | B1 | 99 | − | − | − | + | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| A | + | 9 | 38 | 5 | 3 | B2 | 95 | O1 | + | 11 | − | − | − | + | − | − | − | − | − | + | + | − | + | + | − | − | + | + | + | − | + | + | + | − | − | − | + | − |
| D | + | 14 | 47 | 5 | 6 | B2 | 73 | O6 | − | + | + | + | + | − | − | + | − | + | + | − | + | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | + | |
| F | − | 3 | 15 | 2 | 3 | B2 | 95 | O1 | + | 11 | − | − | − | + | − | − | − | − | − | + | + | − | + | + | − | − | + | + | + | − | + | + | − | − | + | − | + | + |
| I | − | 1 | 5 | 1 | 1 | B2 | 95 | O1 | + | 11 | − | − | − | + | − | + | − | − | + | + | + | + | + | + | − | + | + | + | + | + | + | − | − | − | + | − | − | − |
| B | − | 2 | 4 | 1 | 2 | D | New | − | − | − | − | + | − | + | − | − | + | − | − | + | − | − | − | − | − | + | + | + | − | − | − | + | + | − | − | − | ||
| G | − | 1 | 5 | 1 | 1 | D | 403 | O2 | + | 10, 48 | − | − | + | + | + | − | + | − | − | + | − | + | + | − | + | − | − | + | − | − | + | − | − | − | − | + | − | − |
| J | − | 1 | 4 | 1 | 1 | D | 565 | − | − | − | − | + | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| M | − | 1 | 1 | 1 | 1 | D | 69 | + | 16 | − | − | + | + | − | − | + | − | − | + | − | + | + | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | |
Clones are sorted alphabetically within each phylogenetic group. UTI, UTI clone. Samples (number of samples), colonies (total number of colonies, up to five per sample), hosts (number of hosts), and time points (number of sampling points) indicate the extent of recovery of the clone. A plus sign indicates the presence of the trait, and a minus sign indicates the absence of the trait. Typing results for clones A to G were reported in part previously (6).
All tested representatives of a given clone yielded identical results. One representative of each clone per host per sample type was tested. Phy., phylogenetic group; O, O type (among 12 tested types); pap, P fimbriae (all positive isolates contained papA [structural subunit], papC [assembly], papEG [tip pilins], and papG allele II [adhesin variant]); F type, specific papA allele; sfa, S and F1C fimbriae; foc, F1C fimbriae; iha, adhesin-siderophore; fim, type 1 fimbriae; hlyD, alpha hemolysin; hlyF, variant hemolysin; sat, secreted autotransporter toxin (serine protease); astA, enteroaggregative E. coli toxin; iroN, salmochelin receptor; fyuA, yersiniabactin receptor; iutA, aerobactin receptor; kpsM II, group 2 capsule; K1 and K5, capsule variants; cvaC, microcin V; usp, uropathogenicity-specific protein; traT, serum resistance associated; ompT, outer membrane protease T; iss, increased serum survival; malX, pathogenicity island marker; H7; fliC, flagellar variant; pic, protein involved in intestinal colonization (serine protease); tsh, temperature-sensitive hemagglutinin (serine protease); vat, vacuolating autotransporter (serine protease); hra, heat-resistant agglutinin; clbB and clbN, colibactin synthesis island. Traits sought but not detected included papG alleles I and III, F types (papA alleles) other than those listed, sfaS (S fimbriae), afa/dra (Dr adhesins), afaE8 (variant afimbrial adhesin), bmaE (M fimbriae), gafD (G fimbriae), F17 fimbriae, clpG (CS31A adhesin), cnf (cytotoxic necrotizing factor), cdtB (cytolethal distending toxin), kpsM III (group 3 capsule), K15 (capsule variant), rfc (O4 lipopolysaccharide synthesis), and ibeA (invasion of brain endothelium).
Virulence typing.
The 14 clones represented all four phylogenetic groups and exhibited diverse O types and virulence profiles (Table 3). Group B2 clones had higher virulence scores than other clones (median, 14 [range, 12 to 16] versus 4 [range, 1 to 11]; P = 0.002). By multilocus sequence typing, clone D (the dog's UTI clone) represented ST73 (O6:K2:H1), a prominent pathogen in humans and pets (8). Three other clones (A [the mother's UTI clone], F, and I) represented ST95 (O1/O2/O18:K1:H7), a prominent pathogen in humans and poultry (14, 19, 20). Clone M represented ST69 or “clonal group A,” a multidrug-resistant pathogen that emerged recently (10, 11, 22).
TABLE 3.
Colonization behaviors in relation to UTI status and other bacterial traits among 14 E. coli clones recovered from five human members and a canine member of a household over 3 yearsa
| Traitb (no. of clones with trait) | Median no. of samples/clone (range)
|
P value | Median no. of colonies/clone (range)
|
P value | Median no. of hosts/ clone (range)
|
P value | Median no. of time points/clone (range)
|
P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait present | Trait absent | Trait present | Trait absent | Trait present | Trait absent | Trait present | Trait absent | |||||
| UTI clone (2) | 11.5 (9-14) | 1 (1-7) | 0.01 | 42.5 (38-47) | 4.5 (1-21) | 0.02 | 5 (5-5) | 1 (1-3) | 0.01 | 4.5 (3-6) | 1 (1-4) | 0.04 |
| Group B2 (4) | 6 (1-14) | 1 (1-7) | 0.09 | 26.5 (5-47) | 4 (1-21) | 0.03 | 3.5 (1-5) | 1 (1-3) | 3 (1-6) | 1 (1-4) | ||
| pic (2) | 11.5 (9-14) | 1 (1-7) | 0.01 | 42.5 (38-47) | 4.5 (1-21) | 0.02 | 5 (5-5) | 1 (1-3) | 0.01 | 4.5 (3-6) | 1 (1-4) | 0.04 |
| usp (4) | 6 (1-14) | 1 (1-7) | 0.09 | 26.5 (5-47) | 4 (1-21) | 0.03 | 3.5 (1-5) | 1 (1-3) | 3 (1-6) | 1 (1-4) | ||
| clbB (3) | 9 (3-14) | 1 (1-7) | 0.01 | 38 (15-47) | 4 (1-21) | 0.01 | 5 (2-5) | 1 (1-3) | 0.01 | 3 (3-6) | 1 (1-4) | 0.02 |
| clbN (2) | 8.5 (3-14) | 1 (1-9) | 0.066 | 31 (15-47) | 4.5 (1-38) | 0.088 | 3.5 (2-5) | 1 (1-5) | 0.099 | 4.5 (3-6) | 1 (1-4) | 0.04 |
| malX (6) | 2.5 (1-14) | 1 (1-7) | 10 (5-47) | 4 (1-21) | 0.03 | 2 (1-5) | 1 (1-3) | 2 (1-6) | 1 (1-4) | |||
Samples, total of number of samples that yielded the clone at any time during the study; colonies, total number of colonies accounted for by the clone during the study; hosts, total number of hosts that yielded the clone at any time during the study; time points, number of sampling points at which the clone was encountered in one or more hosts.
The traits shown are those that yielded P < 0.05 by the Mann-Whitney U test (exact, two tailed) for an association with one or more of the colonization behavior endpoints. P values of <0.10 are shown. UTI clone, urine isolate from mother's or dog's acute UTI episode. Group B2, phylogenetic group B2; pic, protein involved in colonization (serine protease); usp, uropathogenicity-specific protein; clbB and clbN, colibactin synthesis; malX, pathogenicity island marker. Additional traits that yielded only borderline associations (0.10 > P > 0.05) with one or more of the colonization behaviors included sfa/foc (S and F1C fimbriae; P = 0.07 for dates, samples, and colonies) and the H7 fliC allele (flagellin; P = 0.099 for hosts, P = 0.088 for samples). No assessed trait yielded a borderline or significant negative association with any of the colonization behavior endpoints.
Compared with non-UTI clones, the two UTI clones exhibited borderline (P < 0.10) or significant (P < 0.05) associations with group B2 (100% versus 17% [P = 0.066]), certain virulence genes (pic, 100% versus 0% [P = 0.01]; usp, 100% versus 17% [P = 0.066]; clbB, 100% versus 8% [P = 0.03]), the aggregate virulence score (median, 14 versus 7 [P = 0.099]), and the number of samples, colonies, hosts, and/or time points (Table 3). Likewise, group B2 and certain virulence genes (pic, usp, clbB, clbN, and malX) exhibited borderline or significant associations with the number of samples, colonies, hosts, and/or time points (Table 3). Virulence scores were correlated with the numbers of colonies (P = 0.01) and samples (P = 0.09). In contrast, no borderline or significant negative associations were observed among the study variables.
Comment.
This study, which complements a previous survey of this household (6) by capturing a second UTI episode (uniquely in the dog), doubling the sample size, and greatly extending the follow-up duration, yielded several novel and important findings. First, the dog's UTI clone colonized four of the five human household members and was the most widely shared clone. Second, clone sharing was quite extensive overall, involving 6 of a total of 14 clones and all six household members, with most host pairs sharing multiple clones. Third, the two UTI clones were encountered much more frequently and extensively as asymptomatic intestinal colonizers than as uropathogens, calling into question what triggered the UTI episodes. Fourth, four clones exhibited prolonged persistence (1 to 3 years) within the household, being isolated either continuously or intermittently from the same or different hosts over the interval. Finally, group B2, virulence gene content, and UTI clone status exhibited borderline or significant associations with multiple colonization endpoints, suggesting that colonization and transmission fitness may contribute to UTI pathogenesis, whereas virulence-associated traits may promote colonization and transmission.
Documentation of UTI in a companion animal caused by an E. coli strain concurrently present in a human household contact is, to our knowledge, novel. This represents an interesting counterpoint to previously documented UTI episodes caused in humans by E. coli strains that colonized the family's dog or cat (6, 13, 24). These findings suggest that UTI may sometimes be a zoonosis in either direction (human to pet or pet to human). In this regard, certain virulent E. coli serotypes or STs are commonly encountered among both humans and companion animals (8, 12, 14, 15, 17, 21, 31, 32). The two putatively zoonotic clones identified here represent two such STs, i.e., ST73 (clone D) and ST95 (clone A).
The extremely prolonged (1- to 3-year) within-household persistence demonstrated for clones D, E, F, and H suggests that identification of external sources for colonizing ExPEC clones may prove challenging, given the possibly great separation in time between acquisition and detection. Notably, the apparent persistence of clone D in certain hosts (the dog and both daughters) over 2 to 3 years is among the longest reported colonizations of any host(s) with the same E. coli clone (27-29).
Study limitations include the small numbers, five-colony typing approach (which detects mainly high-prevalence clones), long intervals between certain sampling points, absence of information regarding host exposures and interactions, and lack of environmental sampling. Strengths include the participation of all household members, the protracted study period, the capture of two acute UTI episodes, extensive molecular typing, and a sample size sufficient for statistical analysis.
In summary, we observed long-term intestinal colonization of multiple human household members and the family dog with an E. coli clone that caused acute UTI in the dog. We also documented other dog-human strain sharing, long-term persistence and possible (nonsexual) host-to-host transmission of additional clones, and statistical associations among virulence traits, UTI occurrence, and colonization endpoints. These findings provide added evidence that the household functions as an integrated microbiological unit, demonstrate long-term persistence potential for certain ExPEC clones within individuals and households, support a possible link between virulence and colonization or transmission, and imply that, in some instances, UTI may represent a zoonosis in either direction.
Acknowledgments
This material is based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs.
Dave Prentiss (Minneapolis Veterans Affairs Medical Center) helped prepare the figures. We thank the study subjects for their participation.
Possible conflicts of interest are that J. R. Johnson has received grants, consultancies, and/or honoraria from Merck, Bayer, Ortho-McNeil, Wyeth-Ayerst, Rochester Medical, and Procter & Gamble. The rest of us have no conflicts of interest.
Footnotes
Published ahead of print on 22 October 2008.
REFERENCES
- 1.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 664555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foxman, B., S. D. Manning, P. Tallman, R. Bauer, L. Zhang, J. S. Koopman, B. Gillespie, J. D. Sobel, and C. F. Marrs. 2002. Uropathogenic Escherichia coli are more likely than commensal E. coli to be shared between heterosexual sex partners. Am. J. Epidemiol. 1561133-1140. [DOI] [PubMed] [Google Scholar]
- 3.Foxman, B., L. Zhang, P. Tallman, B. C. Andree, A. M. Geiger, J. S. Koopman, B. W. Gillespie, K. A. Palin, J. D. Sobel, C. K. Rode, C. A. Bloch, and C. F. Marrs. 1997. Transmission of uropathogens between sex partners. J. Infect. Dis. 175989-992. [DOI] [PubMed] [Google Scholar]
- 4.Grüneberg, R. N. 1969. Relationship of infecting urinary organism to the faecal flora in patients with symptomatic urinary infection. Lancet 2766-768. [DOI] [PubMed] [Google Scholar]
- 5.Johnson, J. R., J. J. Brown, U. B. Carlino, and T. A. Russo. 1998. Colonization with and acquisition of uropathogenic Escherichia coli strains as revealed by polymerase chain reaction-based detection. J. Infect. Dis. 1771120-1124. [DOI] [PubMed] [Google Scholar]
- 6.Johnson, J. R., and C. Clabots. 2006. Sharing of virulent Escherichia coli clones among household members of a woman with acute cystitis. Clin. Infect. Dis. 43e101-e108. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, J. R., A. Gajewski, A. J. Lesse, and T. A. Russo. 2003. Extraintestinal pathogenic Escherichia coli as a cause of invasive nonurinary infections. J. Clin. Microbiol. 415798-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, J. R., B. Johnston, C. R. Clabots, M. A. Kuskowski, E. Roberts, and C. DebRoy. 2008. Virulence genotypes and phylogenetic background of Escherichia coli serogroup O6 isolates from humans, dogs, and cats. J. Clin. Microbiol. 46417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson, J. R., N. Kaster, M. A. Kuskowski, and G. V. Ling. 2003. Identification of urovirulence traits in Escherichia coli by comparison of urinary and rectal E. coli isolates from dogs with urinary tract infection. J. Clin. Microbiol. 41337-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, J. R., A. R. Manges, T. T. O'Bryan, and L. R. Riley. 2002. A disseminated multi-drug resistant clonal group of extraintestinal pathogenic Escherichia coli as a cause of pyelonephritis. Lancet 3592249-2251. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, J. R., A. C. Murray, M. A. Kuskowski, S. Schubert, M.-F. Prere, B. Picard, R. Colodner, R. Raz, and Trans-Global Initiative for Antimicrobial Resistance Analysis (TIARA) Investigators. 2005. Distribution and characteristics of Escherichia coli clonal group A. Emerg. Infect. Dis. 11141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, J. R., T. T. O'Bryan, D. A. Low, G. Ling, P. Delavari, C. Fasching, T. A. Russo, U. Carlino, and A. L. Stell. 2000. Evidence of commonality between canine and human extraintestinal pathogenic Escherichia coli that express papG allele III. Infect. Immun. 683327-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, J. R., K. Owens, A. Gajewsi, and C. Clabots. 2008. Escherichia coli colonization patterns among human household members and pets, with attention to acute urinary tract infection. J. Infect. Dis. 197218-224. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, J. R., K. L. Owens, C. R. Clabots, S. J. Weissman, and S. B. Cannon. 2006. Phylogenetic relationships among clonal groups of extraintestinal pathogenic Escherichia coli as assessed by multi-locus sequence analysis. Microbes Infect. 81702-1713. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, J. R., A. Stell, and P. Delavari. 2001. Canine feces as a reservoir of extraintestinal pathogenic Escherichia coli. Infect. Immun. 691306-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181261-272. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, J. R., A. L. Stell, P. Delavari, A. C. Murray, M. Kuskowski, and W. Gaastra. 2001. Phylogenetic and pathotypic similarities between Escherichia coli isolates from urinary tract infections in dogs and extraintestinal infections in humans. J. Infect. Dis. 183897-906. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, J. R., A. L. Stell, F. Scheutz, T. T. O'Bryan, T. A. Russo, U. B. Carlino, C. C. Fasching, J. Kavle, L. van Dijk, and W. Gaastra. 2000. Analysis of F antigen-specific papA alleles of extraintestinal pathogenic Escherichia coli using a novel multiplex PCR-based assay. Infect. Immun. 681587-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, J. R., S. J. Weissman, A. L. Stell, E. Tritchina, D. E. Dykhuizen, and E. V. Sokurenko. 2001. Clonal and pathotypic analysis of archetypal Escherichia coli cystitis isolate NU14. J. Infect. Dis. 1841556-1565. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, T. J., S. Kariyawasam, Y. Wannemuehler, P. Mangiamele, S. J. Johnson, C. Doetkott, J. A. Skyberg, A. M. Lynne, J. R. Johnson, and L. K. Nolan. 2007. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J. Bacteriol. 1893228-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low, D. A., B. A. Braaten, G. V. Ling, D. L. Johnson, and A. L. Ruby. 1988. Isolation and comparison of Escherichia coli strains from canine and human patients with urinary tract infections. Infect. Immun. 562601-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manges, A. R., J. R. Johnson, B. Foxman, T. T. O'Bryan, K. E. Fullerton, and L. W. Riley. 2001. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N. Engl. J. Med. 3451007-1013. [DOI] [PubMed] [Google Scholar]
- 23.Moreno, E., A. Andreu, T. Perez, M. Sabate, J. R. Johnson, and G. Prats. 2006. Relationship between Escherichia coli strains causing urinary tract infection in women and the dominant faecal flora of the same hosts. Epidemiol. Infect. 1341015-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, A. C., M. A. Kuskowski, and J. R. Johnson. 2004. Virulence factors predict Escherichia coli colonization patterns among human and animal household members. Ann. Intern. Med. 140848-849. [DOI] [PubMed] [Google Scholar]
- 25.Russo, T. A., and J. R. Johnson. 2000. A proposal for an inclusive designation for extraintestinal pathogenic Escherichia coli: ExPEC. J. Infect. Dis. 1811753-1754. [DOI] [PubMed] [Google Scholar]
- 26.Russo, T. A., and J. R. Johnson. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: an overlooked epidemic. Microbes Infect. 5449-456. [DOI] [PubMed] [Google Scholar]
- 27.Sears, H. J., and I. Brownlee. 1952. Further observations of the persistence of individual strains of Escherichia coli in the intestinal tract of man. J. Bacteriol. 6347-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sears, H. J., I. Brownlee, and J. K. Uchiyama. 1950. Persistence of individual strains of Escherichia coli in the intestinal tract of man. J. Bacteriol. 59293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sears, H. J., H. Janes, R. Saloum, I. Brownlee, and L. F. Lamoreaux. 1956. Persistence of individual strains of Escherichia coli in man and dog under varying conditions. J. Bacteriol. 71370-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto, S., T. Tsukamoto, A. Terai, H. Kurazono, Y. Takeda, and O. Yoshida. 1997. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J. Urol. 1571127-1129. [PubMed] [Google Scholar]
- 31.Yuri, K., K. Nakata, H. Katae, T. Tsukamoto, and A. Hasegawa. 1999. Serotypes and virulence factors of Escherichia coli strains isolated from dogs and cats. J. Vet. Med. Sci. 6137-40. [DOI] [PubMed] [Google Scholar]
- 32.Yuri, K., K. Nakata, H. Katae, S. Yamamoto, and A. Hasegawa. 1998. Distribution of uropathogenic virulence factors among Escherichia coli strains isolated from dogs and cats. J. Vet. Med. Sci. 60287-290. [DOI] [PubMed] [Google Scholar]