Abstract
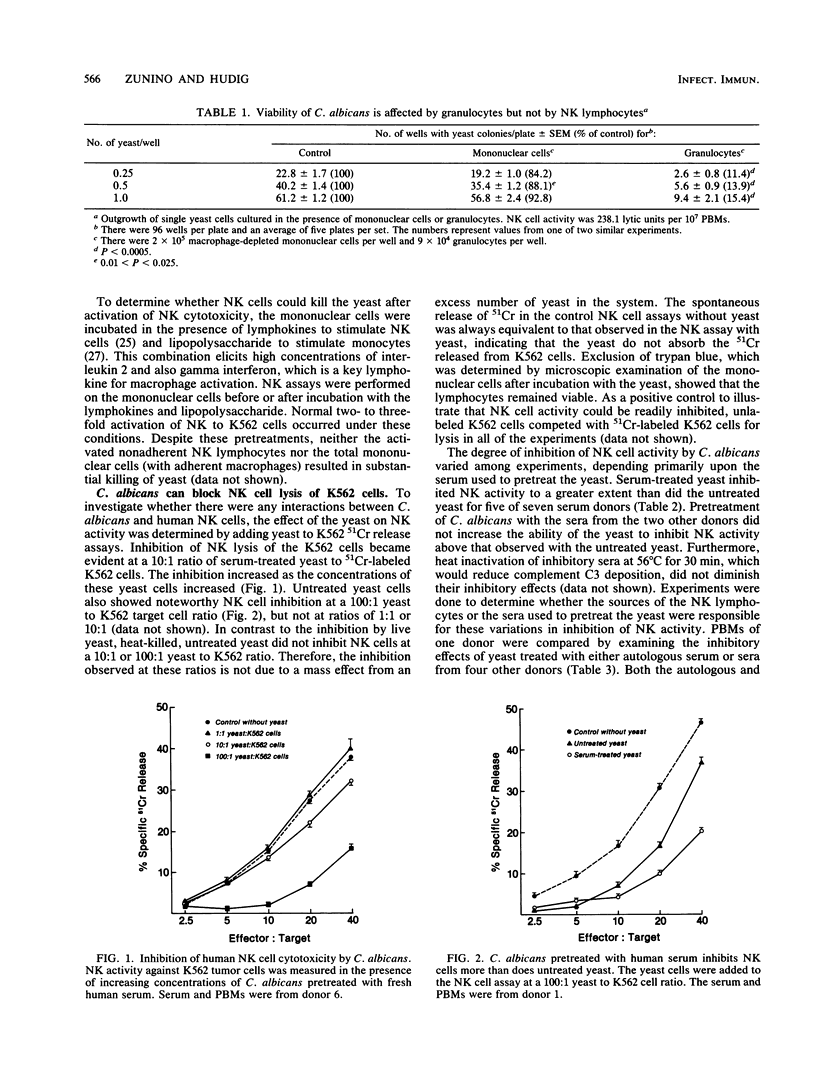
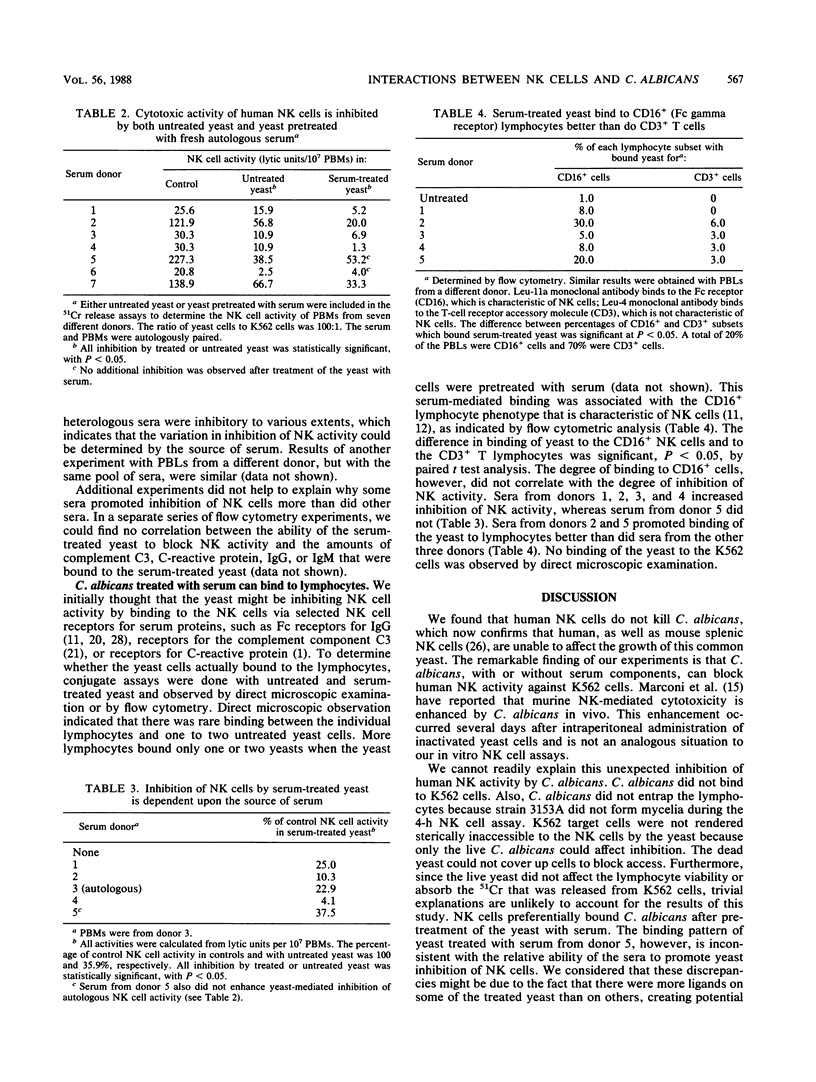
Rodent natural killer (NK) lymphocytes are cytotoxic to certain fungi. We investigated whether human NK cells are cytotoxic to the yeast Candida albicans. We found that human peripheral blood lymphocytes possessing NK cell activity had little or no effect on the viability of the yeast. Unopsonized C. albicans, however, were able to block NK cell-mediated cytotoxicity at a ratio of 100 yeast to one K562 erythroleukemia cell. C. albicans was not toxic to the lymphocytes nor did it take up isotope released by the K562 cells. Furthermore, C. albicans that was pretreated with human serum blocked NK cell activity more than did untreated C. albicans. Binding of the yeasts to NK cells could account for the blocking effect of serum-treated yeasts, but not for that of the untreated yeasts. Flow cytometry indicated that there was preferential binding of C. albicans to NK lymphocytes but not to T cells when the yeasts were pretreated with human serum. In this report we affirm the results of the study by Vecchiarelli et al. (A. Vecchiarelli, F. Bistoni, E. Cenci, S. Perito, and A. Cassone, Sabouraudia 23:377-387, 1985), that the first report of rodent NK cell activity against the yeast Cryptococcus neoformans (J. W. Murphy and D. O. McDaniel, J. Immunol. 128:1577-1583, 1982) cannot be extrapolated to a general phenomenon of unprimed lymphocyte-mediated destruction of all species of yeast. Our data extend the observations to humans and also suggest that in vivo interactions between NK lymphocytes and opportunistic fungal pathogens may affect NK cell function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum L. L., James K. K., Glaviano R. R., Gewurz H. Possible role for C-reactive protein in the human natural killer cell response. J Exp Med. 1983 Jan 1;157(1):301–311. doi: 10.1084/jem.157.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carine K., Hudig D. Assessment of a role for phospholipase A2 and arachidonic acid metabolism in human lymphocyte natural cytotoxicity. Cell Immunol. 1984 Aug;87(1):270–283. doi: 10.1016/0008-8749(84)90151-5. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne A., Odds F. C. Interactions of Candida albicans yeast cells, germ tubes and hyphae with human polymorphonuclear leucocytes in vitro. J Gen Microbiol. 1984 Mar;130(3):465–471. doi: 10.1099/00221287-130-3-465. [DOI] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Jimenez B. E., Murphy J. W. In vitro effects of natural killer cells against Paracoccidioides brasiliensis yeast phase. Infect Immun. 1984 Nov;46(2):552–558. doi: 10.1128/iai.46.2.552-558.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975 Apr 15;15(4):596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Marconi P., Scaringi L., Tissi L., Boccanera M., Bistoni F., Bonmassar E., Cassone A. Induction of natural killer cell activity by inactivated Candida albicans in mice. Infect Immun. 1985 Oct;50(1):297–303. doi: 10.1128/iai.50.1.297-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect Immun. 1985 Oct;50(1):50–57. doi: 10.1128/iai.50.1.50-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. A., Hosking C. S. The role of complement and antibody in opsonization and intracellular killing of Candida albicans. Clin Exp Immunol. 1984 Aug;57(2):307–314. [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Trinchieri G. Antibody 3G8, specific for the human neutrophil Fc receptor, reacts with natural killer cells. J Immunol. 1984 Mar;132(3):1410–1415. [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Jondal M. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. II. Is the complement receptor necessarily present on the killer cells? Int J Cancer. 1977 Sep 15;20(3):353–358. doi: 10.1002/ijc.2910200306. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A., Bistoni F., Cenci E., Perito S., Cassone A. In-vitro killing of Candida species by murine immunoeffectors and its relationship to the experimental pathogenicity. Sabouraudia. 1985 Oct;23(5):377–387. doi: 10.1080/00362178585380541. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Chapman H. A., Jr, Hibbs J. B., Jr Characterization of the effects of endotoxin on macrophage tumor cell killing. J Immunol. 1978 Jul;121(1):72–80. [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]
- Wright S. C., Weitzen M. L., Kahle R., Granger G. A., Bonavida B. Studies on the mechanism of natural killer cytotoxicity. II. coculture of human PBL with NK-sensitive or resistant cell lines stimulates release of natural killer cytotoxic factors (NKCF) selectively cytotoxic to NK-sensitive target cells. J Immunol. 1983 May;130(5):2479–2483. [PubMed] [Google Scholar]


