Abstract
Rapid human immunodeficiency virus (HIV) antibody tests support the effort to expand access to HIV testing and counseling services in remote, rural, and poor parts of the world. We validated the Capillus HIV-1/HIV-2 (Trinity Biotech PLC, Bray, County Wicklow, Ireland) and Determine HIV-1/2 (Abbott Laboratories, Abbott Park, IL) rapid tests in a reference laboratory using patient samples from Tanzania and evaluated the performance of the tests under field conditions in northern Tanzania. We used the resulting data to study sequential and parallel testing algorithms. In the validation study, sensitivity, specificity, the predictive value of a positive test (PV+), and the predictive value of a negative test (PV−) were all 100% for Capillus and Determine. In the field evaluation among 12,737 clients, sensitivity, specificity, PV+, and PV− were 99.7%, 99.8%, 98.7%, and 99.9%, respectively, for Capillus and 99.6%, 99.9%, 99.5%, and 99.9%, respectively, for Determine. A sequential testing algorithm that did not confirm a negative initial Capillus result with a Determine result cost $7.77 per HIV diagnosis but missed 0.3% of HIV infections. A sequential testing algorithm that did not confirm a negative initial Determine result with a Capillus result cost $7.64 per HIV diagnosis but missed 0.4% of HIV infections. A parallel testing algorithm cost $13.46 per HIV diagnosis but detected more HIV-infected clients.
Human immunodeficiency virus (HIV) voluntary counseling and testing (VCT) is an important tool for both HIV prevention and care in sub-Saharan Africa. VCT use has been associated with a reduction in high-risk sexual behavior and of risk for HIV transmission (6, 30). VCT also provides the means for persons to learn their HIV status in order to access treatment and care services (5, 9). However, these benefits hinge on the accurate diagnosis of HIV infection. False-negative results lead to delayed entry into care or failure to enter care, while false-positive results place economic, social, and emotional burdens on patients. As efforts to expand HIV testing services to reach the entire populations of countries with generalized HIV epidemics gain momentum (5), so the number of tests conducted increases. Increasing the amount of HIV testing will mean that even small shortcomings in the sensitivity and specificity of assays or testing algorithms may lead to false-positive or false-negative results for large numbers of people.
The development and deployment of rapid HIV antibody tests have allowed the expansion of access to HIV testing and counseling services in remote, rural, and poor areas where more complex and expensive means of diagnosing HIV infection are difficult to implement (7). The use of rapid HIV antibody tests has been endorsed by the World Health Organization, and they have been adopted into national guidelines for HIV VCT in many countries in sub-Saharan Africa (1, 18). Capillus HIV-1/HIV-2 (Trinity Biotech PLC, Bray, County Wicklow, Ireland), Determine HIV-1/2 (Abbott Laboratories, Abbott Park, IL), and other rapid HIV antibody tests have been evaluated in several studies and have been found to have sensitivities and specificities exceeding 99.5% (8, 12, 14, 16, 19, 20, 22, 27, 28, 31, 32). These rapid HIV antibody tests may be implemented in several types of diagnostic algorithms employing one or more rapid tests used in sequence or in parallel (1). Understanding the performance of rapid HIV antibody tests in different testing algorithms is critical to inform local and national HIV testing policies. In Tanzania, as in many other resource-poor settings, national VCT guidelines recommend a sequential testing approach in which a single rapid test, if negative, is not confirmed with a second test but is reported as a negative result. If the first test is positive, it is confirmed with a second, different rapid test, with discordant results resolved with an enzyme-linked immunosorbent assay (ELISA) (1, 18). Since negative results for the first rapid test are not confirmed, the sequential approach has the potential to miss some cases of HIV infection.
We validated the Capillus and Determine rapid HIV antibody tests and evaluated their performance under field conditions in northern Tanzania in a large cohort over a 5-year time period. We assessed the performance of a sequential testing approach and compared the diagnostic accuracy of the sequential algorithm to an alternative parallel testing algorithm in which all samples were tested with two rapid tests. We further considered the incremental cost of implementing the parallel algorithm and the cost per case of HIV infection identified for each algorithm.
MATERIALS AND METHODS
Validation study.
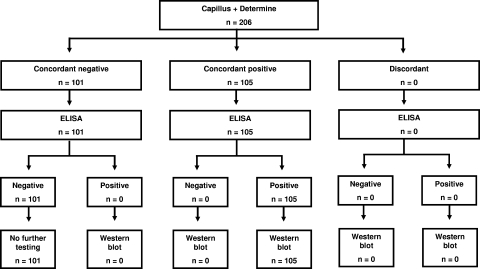
Blood samples for the validation study were collected from medical inpatients at Kilimanjaro Christian Medical Centre (KCMC) and clients of Kikundi cha Wanawake Kilimanjaro Kupambana na UKIMWI (KIWAKKUKI; Women Against AIDS in Kilimanjaro). The medical inpatients were unselected and had symptoms expected among patients admitted to the hospital. Whole blood was tested using both the Capillus HIV-1/HIV-2 (Trinity Biotech PLC, Bray, County Wicklow, Ireland) and Determine HIV-1/2 (Abbott Laboratories, Abbott Park, IL) rapid HIV antibody tests. If both rapid tests were negative, the sample was tested with an ELISA (Vironostika Uni-Form II plus O Ab; bioMérieux, Durham, NC). If the ELISA was negative, no further testing was done. If the ELISA was positive, a Western blot (Genetic Systems HIV-1 Western blot kit; Bio-Rad, Hercules, CA) was planned to be done (Fig. 1). If both rapid tests were positive or were discordant, the sample was tested with an ELISA and a Western blot. Sample collection was planned to continue until a minimum of 100 HIV-infected and 100 HIV-uninfected subjects was reached. Laboratory technologists were blinded to the results of other HIV antibody tests performed on the same sample.
FIG. 1.
Validation study algorithm and results.
Field evaluation.
KIWAKKUKI began offering HIV VCT services on 13 March 2003 at its AIDS Information Centre in downtown Moshi. The characteristics of KIWAKKUKI VCT clients have been described in detail elsewhere (3, 24). All counseling procedures were performed in accordance with guidelines provided by the Tanzanian Ministry of Health's National AIDS Control Programme (18). A 2-ml blood sample was drawn from each patient and tested using both the Capillus and Determine rapid HIV antibody tests. Concordant results were reported, whereas for discordant results, sequential testing on the sample was performed at the KCMC clinical laboratory using an ELISA (Vironostika HIV Uni-Form II plus O Ab; bioMérieux, Durham, NC). For quality-control purposes, an ELISA was also performed on every 10th sample at the KCMC laboratory regardless of the Capillus and Determine results.
Sequential and parallel testing algorithms.
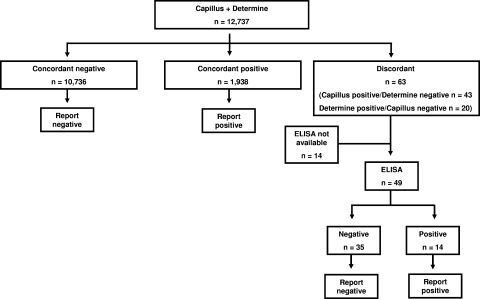
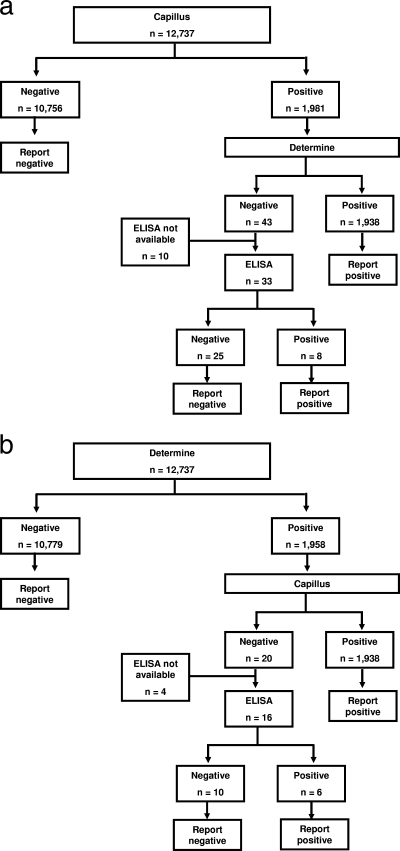
In the field evaluation, we implemented a strategy of parallel rapid testing, in which all samples were tested in parallel using both the Capillus and Determine assays; concordant rapid test results were accepted, while discordant results were resolved with ELISA (see Fig. 3). We compared the parallel testing strategy with the performance that would have been observed with four alternative testing strategies for determining HIV infection status: strategy 1, testing with Capillus alone with no second test; strategy 2, testing with Determine alone with no second test; strategy 3, initial testing with Capillus with positive results confirmed by Determine and no additional testing for negative results (Fig. 2a); and strategy 4, initial testing with Determine with positive results confirmed by Capillus and no additional testing for negative results (Fig. 2b). Strategy 3 reflected the Tanzanian national testing guideline at the time. We calculated the sensitivity, specificity, positive predictive value (PV+), and negative predictive value (PV−), along with the exact 95% confidence interval, of each alternative testing strategy relative to the parallel testing strategy in determining the HIV infection status. To examine the clinical relevance of discordant rapid HIV antibody test results, we reviewed the results of subsequent rapid HIV antibody tests among the group of patients with initial discordant results who returned subsequently for repeat testing.
FIG. 3.
Algorithm and results using Capillus and Determine rapid HIV antibody tests in parallel.
FIG. 2.
(a) Algorithm and results using a Capillus screening rapid HIV antibody test followed by Determine sequential testing of positive samples. (b) Algorithm and results using a Determine screening rapid HIV antibody test followed by Capillus sequential testing of positive samples.
Cost-effectiveness analyses.
We calculated the cost-effectiveness of the parallel testing strategy and each of the four alternative strategies. Cost-effectiveness was defined as the cost per case of HIV infection correctly identified. For inputs, we assumed a cost of $1.00 per Capillus assay, $1.00 per Determine assay, and $17.00 per ELISA, including labor (approximate prices in Tanzania at the time of writing).
Statistical analyses.
Study data were entered into an Excel database (Microsoft Corporation, Seattle, WA) and analyzed using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Sensitivity and specificity were calculated in the validation study using an ELISA (for concordant results) and Western blotting (for discordant results) as the gold standard. Sensitivity and specificity were calculated in the field evaluation using two rapid tests (for concordant results) and an ELISA (for discordant results) as the gold standard. Confidence intervals were calculated using exact methods.
Research ethics.
The validation study was part of a protocol approved by the KCMC Research Ethics Committee, the Tanzania National Institutes for Medical Research National Research Ethics Coordinating Committee, and an institutional review board (IRB) of Duke University Medical Center. The field evaluation used on-shelf data obtained from KIWAKKUKI records. All links to personal identifiers were removed and held by a privacy officer not involved in the study. A Duke University Health System IRB exempted this part of the study from IRB review.
RESULTS
Validation study.
In order to meet the enrollment targets for HIV-infected and HIV-uninfected subjects, samples were collected from 206 subjects of whom 105 (51%) were ultimately shown to be HIV infected and 101 (49%) were ultimately shown to be HIV uninfected. For all patients, there was 100% concordance between the Capillus, Determine, ELISA, and Western blot results (Fig. 1). The sensitivity, specificity, PV+, and PV− were 100% for Capillus and Determine, both when used individually and in combination.
Field evaluation.
Between 13 March 2003 and 14 December 2007, a total of 12,737 clients received VCT at KIWAKKUKI and had the results of both the Capillus and Determine rapid tests available. Of these, 1,938 (15.2%) had concordant positive HIV rapid tests and 10,736 (84.3%) had concordant negative HIV rapid tests. Of the 63 (0.5%) clients with discordant rapid HIV test results, 43 (68.3%) clients had a positive Capillus result and a negative Determine result and 20 (31.7%) clients had a negative Capillus result and a positive Determine result. The results of the ELISA testing of samples with discordant rapid HIV antibody tests are shown in Fig. 2a and b. Based on these findings, for Capillus, sensitivity in the field evaluation was 99.7% (exact 95% confidence interval, 99.3 to 99.9%), specificity 99.8% (99.7 to 99.8%), PV+ 98.7% (98.1 to 99.2%), and PV− 99.9% (99.9 to 100.0%). For Determine, sensitivity in the field evaluation was 99.6% (99.2 to 99.8%), specificity 99.9% (99.8 to 100.0%), PV+ 99.5% (99.1 to 99.8%), and PV− 99.9% (99.1 to 100.0%) (Table 1). Of the 63 clients with discordant rapid HIV antibody test results, 5 (7.9%) returned subsequently for repeat testing and had results available. Of these 5, 4 (80.0%) tested concordant negative on the repeat test, and one had persistently discordant results. ELISA testing of every 10th sample identified 10 instances of concordant rapid test results with a discordant ELISA. These results were resolved with repeat testing in accordance with national guidelines.
TABLE 1.
Sensitivity and specificity of Capillus and Determine rapid HIV tests
| Test(s) | Result | HIV infected
|
HIV uninfected
|
Total | ||||
|---|---|---|---|---|---|---|---|---|
| Second rapid test | ELISA | Second rapid test and/or ELISAa | Second rapid test | ELISA | Second rapid test and/or ELISAa | |||
| Capillus | Positive | 1,938 | 114 | 1,946 | 25 | 25 | 25 | 1,971 |
| Negative | 6 | 6 | 6 | 10,736 | 461 | 10,746 | 10,752 | |
| Total | 1,952 | 10,771 | 12,723 | |||||
| Determine | Positive | 1,938 | 112 | 1,944 | 10 | 10 | 10 | 1,954 |
| Negative | 8 | 8 | 8 | 10,736 | 476 | 10,761 | 10,769 | |
| Total | 1,952 | 10,771 | 12,723 | |||||
Fourteen ELISA results were not available.
Sequential versus parallel testing algorithms.
The parallel testing algorithm with ELISA resolution of discordant results (Fig. 3) identified 1,952 cases of HIV infection among the 12,737 clients in the cohort. The same data set and assay results were used to study each algorithm. In a sequential testing algorithm with an initial Capillus test where negative Capillus results were not confirmed and only positive Capillus results were confirmed by Determine (Fig. 2a), 1,946 cases would have been identified. This algorithm would have failed to identify six (0.3%) HIV-infected clients (Table 2). An alternative sequential algorithm that relied on an initial Determine test that was not confirmed if negative and was confirmed by Capillus (Fig. 2b) if positive would have identified 1,944 cases and would have missed 8 (0.4%) HIV-infected clients (Table 2).
TABLE 2.
Performance of alternative rapid HIV antibody test algorithms in field investigation
| Testing algorithm | HIV infecteda
|
HIV uninfected
|
Sensitivity (%) | Specificity (%) | PV+ (%)b | PV− (%)b | ||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||||
| Capillus alone | 1,946 | 6 | 25 | 10,746 | 99.7 | 99.8 | 98.7 | 99.9 |
| Determine alone | 1,944 | 8 | 10 | 10,761 | 99.6 | 99.9 | 99.5 | 99.9 |
| Sequential (initial Capillus)c | 1,946 | 6 | 0 | 10,771 | 99.7 | 100.0 | 100.0 | 99.9 |
| Sequential (initial Determine)d | 1,944 | 8 | 0 | 10,771 | 99.6 | 100.0 | 100.0 | 99.9 |
Based on two rapid HIV antibody tests (if concordant) or ELISA (if two rapid HIV antibody tests are discordant).
Predictive values are calculated for this study population with 15.1% HIV seroprevalence and would vary in populations with different prevalence.
As shown in Fig. 2a.
As shown in Fig. 2b.
Cost-effectiveness of sequential versus parallel testing algorithms.
The cost-effectiveness of sequential versus parallel testing algorithms is shown in Table 3. The cost per case identified using the Capillus or Determine tests alone are each $6.54. The cost per case identified in a sequential testing algorithm using an initial Capillus test or an initial Determine test are $7.77 and $7.64, respectively. A parallel testing algorithm costs $13.46 per case identified.
TABLE 3.
Comparison of the cost-effectiveness of alternative algorithms for rapid HIV testing
| Testing algorithm | No. of cases identified (%) | Total cost ($) | Cost per case ($) |
|---|---|---|---|
| Capillus alone | 1,946 (99.7) | 12,723.00 | 6.54 |
| Determine alone | 1,944 (99.6) | 12,723.00 | 6.54 |
| Sequential (initial Capillus) | 1,946 (99.7) | 15,119.00 | 7.77 |
| Sequential (initial Determine) | 1,944 (99.6) | 14,847.00 | 7.64 |
| Parallel (double rapid tests) | 1,952 (100.0) | 26,279.00 | 13.46 |
DISCUSSION
We demonstrate that the Capillus and Determine rapid HIV antibody tests are accurate diagnostic tests both when validated in a central laboratory against ELISA and Western blotting and when implemented under field conditions in a large cohort, although sensitivity, specificity, PV+, and PV− were less than 100% under field conditions. Furthermore, we show that a commonly used sequential testing algorithm, for which a negative initial rapid test result is not confirmed with a second rapid test, identifies 0.3 to 0.4% fewer HIV infections than a testing algorithm that uses the two rapid tests in parallel on all samples.
Although the sensitivity, specificity, PV+, and PV− for Capillus and Determine were slightly lower under field conditions than in the validation study, the performance of the two tests did not differ substantially and was consistent with that reported by other investigators (8, 12, 14, 16, 19, 20, 22, 27, 28, 31, 32). There are several possible explanations for sensitivity and specificity levels below 100% for Capillus and Determine. First, it is known that standard antibody-based HIV diagnostic tests are not 100% sensitive with patients with early or acute HIV infection. When the performance of Capillus and Determine has been studied in acute HIV infection panels, the Determine assay appears to be more sensitive than the Capillus assay (8, 15, 23). The HIV seroincidence in the KIWAKKUKI VCT cohort has been estimated to be approximately 2 per 100 person-years (26). Despite the likelihood that some VCT clients included in this study were experiencing acute HIV infection at the time of HIV testing, among clients with discordant HIV rapid test results the combination of positive Determine and negative Capillus results was not more common than positive Capillus and negative Determine results. Furthermore, in the small group of clients who had initially discordant rapid HIV antibody test results and returned for repeat testing, none moved from having discordant to concordant positive results. Thus, it is unlikely that acute HIV infection played a major role in the performance of the rapid HIV antibody tests in our study. HIV-1 subtype diversity in the testing population has been shown to affect the performance of antibody-based HIV diagnostic tests and has led to recommendations that rapid HIV antibody tests be evaluated in the local population prior to widespread implementation (19, 25). In Tanzania, where HIV-1 subtypes A, C, and D and their recombinants predominate (2, 10, 11, 21, 27, 29), it is possible that subtype diversity plays a role in the performance of rapid HIV antibody tests. Although we did not evaluate HIV subtypes among HIV-infected clients, the accuracy of both the Capillus and Determine tests in our study suggests that the role of subtype diversity is likely to be minimal. Finally, sample collection, labeling, transport, and testing conditions may play a role in the performance of any diagnostic test under field conditions. Each of these steps may be challenged in resource-constrained settings (13, 17).
Sequential HIV testing algorithms that do not confirm the result of an initial negative rapid test with a second rapid test are widely employed (1, 18). Because our field evaluation was done in a setting where a parallel testing strategy with two rapid tests was used, the availability of both rapid test results and ELISA results for discordant samples allowed us to examine how such a sequential strategy would have performed in the same population. We showed that a sequential testing algorithm that did not confirm a negative Capillus result with a Determine test would fail to detect 0.3% of HIV-infected clients and that a sequential testing algorithm that did not confirm a negative Determine result with a Capillus test would fail to detect 0.4% of HIV-infected clients. Although such sequential algorithms have been recommended and widely adopted (1, 18, 31), our study suggests that this strategy will fail to detect some HIV-infected clients. While the proportion of missed infections is small, when applied to the large testing populations that the serostatus approach to HIV prevention and care demands, the numbers of missed HIV diagnoses can become substantial (4). Indeed, in a hypothetical country with a population of 30 million and an HIV seroprevalence of 7% with universal HIV testing, our data suggest that a testing strategy that does not confirm the negative result of the initial rapid HIV antibody test would fail to detect 6,300 to 8,400 HIV-infected persons. Both assays yielded false-positive results, but because both sequential and parallel algorithms call for the use of a second rapid HIV antibody test, neither strategy offered an advantage over the other in identifying false-positive results.
We determined that a parallel testing strategy would cost $13.46 per HIV-infected client identified, compared with $7.77 for a sequential testing strategy using an initial Capillus test and $7.64 for a sequential testing strategy using an initial Determine test. Policy makers must decide if the additional cost of $5.69 to $5.82 per HIV diagnosis for a parallel testing strategy is offset by the prevention and treatment benefits to those HIV-infected persons who would be missed by using a sequential testing algorithm that did not confirm negative results of an initial rapid HIV antibody test.
We demonstrate that both the Capillus and Determine rapid HIV antibody tests perform well in both the reference laboratory and under field conditions in Tanzania in a cohort of >12,000 VCT clients. We suggest that testing algorithms that do not confirm the negative result of an initial test with a second rapid test miss a small proportion of HIV-infected clients. While the proportion of missed HIV infections is small, the absolute number of undetected HIV infections is substantial when applied to a large or national VCT program. Policy makers must decide whether the additional cost of adopting a parallel rapid HIV antibody testing strategy is offset by the prevention and treatment benefits to those additional HIV-infected persons detected by this testing strategy.
Acknowledgments
VCT services at KIWAKKUKI were funded by Roche Laboratories, and initial analyses were supported by a global health research training grant from the North Carolina GlaxoSmithKline Foundation. Investigator support was obtained from the Fogarty International Center (D43 PA-03-018 to N.M.T. and J.A.C.), the Duke Clinical Trials Unit and Clinical Research Sites (U01 AI069484-01 to N.M.T. and J.A.C.), the International Studies on AIDS-Associated Co-Infections award (ISAAC) (U01 AI-03-036 to I.A.A., C.O.O., E.N., N.M.T., A.B.M., J.F.S., and J.A.C.), and a Duke University Stead scholarship (to M.K.M.).
Footnotes
Published ahead of print on 15 October 2008.
REFERENCES
- 1.Anonymous. 1997. Joint United Nations Programme on HIV/AIDS (UNAIDS)-WHO revised recommendations for the selection and use of HIV antibody tests. Wkly. Epidemiol. Rec. 7281-87. [PubMed] [Google Scholar]
- 2.Arroyo, M. A., M. Hoelscher, E. Sanders-Buell, K.-H. Herbinger, E. Samky, L. Maboko, O. Hoffmann, M. R. Robb, D. L. Birx, and F. E. McCutchan. 2004. HIV type 1 subtypes among blood donors in the Mbeya region of southwest Tanzania. AIDS Res. Hum. Retrovir. 20895-901. [DOI] [PubMed] [Google Scholar]
- 3.Chu, H. Y., J. A. Crump, J. Ostermann, R. B. Oenga, D. K. Itemba, A. Mgonga, S. Mtweve, J. A. Bartlett, J. F. Shao, and N. M. Thielman. 2005. Sociodemographic and clinical characteristics of clients presenting for HIV voluntary counseling and testing in Moshi, Tanzania. Int. J. STD AIDS 16691-696. [DOI] [PubMed] [Google Scholar]
- 4.De Cock, K. M., E. Marum, and D. Mbori-Ngacha. 2003. A serostatus approach to HIV/AIDS prevention and care in Africa. Lancet 3621847-1849. [DOI] [PubMed] [Google Scholar]
- 5.De Cock, K. M., D. Mbori-Ngacha, and E. Marum. 2002. Shadow on the continent: public health and HIV/AIDS in Africa in the 21st century. Lancet 36067-72. [DOI] [PubMed] [Google Scholar]
- 6.Denison, J. A., K. R. O'Reilly, G. P. Schmid, C. E. Kennedy, and M. D. Sweat. 2007. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990-2005. AIDS Behav. 12363-373. [DOI] [PubMed] [Google Scholar]
- 7.Ekwueme, D. U., S. D. Pinkerton, D. R. Holtgrave, and B. M. Branson. 2003. Cost comparison of three HIV counseling and testing technologies. Am. J. Prev. Med. 25112-121. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira, O. C., C. Ferreira, M. Riedel, M. R. V. Widolin, A. Barbosa-Junior, and HIV Rapid Test Study Group. 2005. Evaluation of rapid tests for anti-HIV detection in Brazil. AIDS 19S70-S75. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey-Faussett, P., D. Maher, Y. D. Mukadi, P. Nunn, J. Perriens, and M. Raviglione. 2002. How human immunodeficiency virus voluntary testing can contribute to tuberculosis control. Bull. W. H. O. 80939-945. [PMC free article] [PubMed] [Google Scholar]
- 10.Herbinger, K.-H., M. Gerhardt, S. Piyasirisilp, D. Mloka, M. A. Arroyo, H. Hoffmann, L. Maboko, D. L. Birx, D. Mmbando, F. E. McCutchan, and M. Hoelscher. 2006. Frequency of HIV type 1 dual infection and HIV diversity: analysis of low- and high-risk populations in Mbeya region, Tanzania. AIDS Res. Hum. Retrovir. 22599-606. [DOI] [PubMed] [Google Scholar]
- 11.Kiwelu, I. E., B. Renjifo, B. Chaplin, N. Sam, W. M. M. M. Nkya, J. Shao, S. Kapiga, and M. Essex. 2003. HIV type 1 subtypes among bar and hotel workers in Moshi, Tanzania. AIDS Res. Hum. Retrovir. 1957-64. [DOI] [PubMed] [Google Scholar]
- 12.Koblavi-Dème, S., C. Maurice, D. Yavo, T. S. Sibailly, K. N′Guessan, Y. Kamelan-Tano, S. Z. Wiktor, T. H. Roels, T. Chorba, and J. N. Nkengasong. 2001. Sensitivity and specificity of human immunodeficiency virus rapid serologic assays and testing algorithms in an antenatal clinic in Abidjan, Ivory Coast. J. Clin. Microbiol. 391808-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landman, K. Z., G. D. Kinabo, W. Schimana, W. M. Dolmans, M. E. Swai, J. F. Shao, and J. A. Crump. 2006. Capacity of health-care facilities to deliver HIV treatment and care services, northern Tanzania, 2004. Int. J. STD AIDS 17459-462. [DOI] [PubMed] [Google Scholar]
- 14.Lien, T. X., N. T. K. Tien, G. F. Chanpong, C. T. Cuc, V. T. Yen, R. Soderquist, and A. Corwin. 2000. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 62301-309. [DOI] [PubMed] [Google Scholar]
- 15.Louie, B., E. Wong, J. D. Klausner, S. Liska, F. Hecht, T. Dowling, M. Obeso, S. S. Phillips, and M. W. Pandori. 2008. Assessment of rapid tests for detection of human immunodeficiency virus-specific antibodies in recently infected individuals. J. Clin. Microbiol. 461494-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menard, D., A. Mairo, M.-J. Mandeng, P. Doyemet, T. Koyazegbe, C. Rochigneux, and A. Talarmin. 2005. Evaluation of rapid HIV testing strategies in under equipped laboratories in the Central African Republic. J. Virol. Methods 12675-80. [DOI] [PubMed] [Google Scholar]
- 17.Mfinanga, G. S., B. Mutayoba, G. Mbogo, A. Kahwa, G. Kimaro, P. P. Mhame, C. Mwangi, and M. N. Malecela. 2007. Quality of HIV laboratory testing in Tanzania: a situation analysis. Tanzan. Health Res. Bull. 944-47. [DOI] [PubMed] [Google Scholar]
- 18.National AIDS Control Programme. 2005. National guidelines for voluntary counselling and testing, 2005. The United Republic of Tanzania Ministry of Health, Dar es Salaam, Tanzania.
- 19.Phillips, S., T. C. Granade, C.-P. Pau, D. Candal, D. J. Hu, and B. S. Parekh. 2000. Diagnosis of human immunodeficiency virus type 1 infection with different subtypes using rapid tests. Clin. Diagn. Lab. Immunol. 7698-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plate, D. K., and Rapid HIV Test Evaluation Working Group. 2007. Evaluation and implementation of rapid HIV tests: the experience in 11 African countries. AIDS Res. Hum. Retrovir. 231491-1498. [DOI] [PubMed] [Google Scholar]
- 21.Ramadhani, H. O., N. M. Thielman, K. Z. Landman, E. M. Ndosi, F. Gao, J. L. Kirchherr, R. Shah, H. J. Shao, S. C. Morpeth, J. D. McNeill, J. F. Shao, J. A. Bartlett, and J. A. Crump. 2007. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin. Infect. Dis. 451492-1498. [DOI] [PubMed] [Google Scholar]
- 22.Ramalingam, S., R. Kannangai, A. A. Raj, M. V. Jesudason, and G. Sridharan. 2002. Rapid particle agglutination test for human immunodeficiency virus: hospital-based evaluation. J. Clin. Microbiol. 401553-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samdal, H. H., B.-G. Gutigard, D. Labay, S. I. Wiik, K. Skaug, and A. G. Skar. 1996. Comparison of the sensitivity of four rapid assays for the detection of antibodies to HIV-1/HIV-2 during seroconversion. Clin. Diagn. Virol. 755-61. [DOI] [PubMed] [Google Scholar]
- 24.Thielman, N. M., H. Y. Chu, J. Ostermann, D. K. Itemba, A. Mgonga, S. Mtweve, J. A. Bartlett, J. F. Shao, and J. A. Crump. 2005. Cost-effectiveness of free HIV-1 voluntary counseling and testing through a community-based AIDS service organization in northern Tanzania. Am. J. Public Health 96114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorstensson, R., S. Andersson, S. Lindback, F. Dias, F. Mhalu, H. Gaines, and G. Biberfeld. 1998. Evaluation of 14 commercial HIV-1/HIV-2 antibody assays using serum panels of different geographical origin and clinical stage including a unique seroconversion panel. J. Virol. Methods 70139-151. [DOI] [PubMed] [Google Scholar]
- 26.Tribble, A. C., J. A. Crump, A. Mgonja, D. K. Itemba, K. Z. Landman, E. M. Ndosi, J. F. Shao, J. A. Bartlett, and N. M. Thielman. 2006. Characteristics, behavior change, and HIV seroincidence among clients presenting for repeat voluntary counseling and testing in Moshi, Tanzania, abstr. WePe0382. Abstr. XVI Int. AIDS Conf. International AIDS Society, Toronto, Canada.
- 27.Urassa, W., S. Nozohoor, S. Jaffer, K. Karama, F. Mhalu, and G. Biberfeld. 2002. Evaluation of an alternative confirmatory strategy for the diagnosis of HIV infection in Dar es Salaam, Tanzania, based on simple rapid assays. J. Virol. Methods 100115-120. [DOI] [PubMed] [Google Scholar]
- 28.van den Berk, G. E. L., P. H. J. Frissen, R. M. Regez, and P. J. G. M. Rietra. 2003. Evaluation of the rapid immunoassay Determine HIV 1/2 for detection of antibodies to human immunodeficiency virus types 1 and 2. J. Clin. Microbiol. 413868-3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasan, A., B. Renjifo, E. Hertzmark, B. Chaplin, G. Msamanga, M. Essex, and W. Fawzi. 2006. Different rates of disease progression of HIV type 1 infection in Tanzania based on infecting subtype. Clin. Infect. Dis. 42843-852. [DOI] [PubMed] [Google Scholar]
- 30.The Voluntary HIV-1 Counselling and Testing Efficacy Study Group. 2000. Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet 2000103-112. [PubMed] [Google Scholar]
- 31.Wilkinson, D., N. Wilkinson, C. Lombard, D. Martin, A. Smith, K. Floyd, and R. Ballard. 1997. On-site HIV testing in resource-poor settings: is one rapid test enough? AIDS 11377-381. [DOI] [PubMed] [Google Scholar]
- 32.Windsor, I. M., M. L. G. dos Santos, L. I. De La Hunt, A. A. Wadee, S. Khumalo, F. Radebe, Y. Dangor, and R. C. Ballard. 1997. An evaluation of the capillus HIV-1/HIV-2 latex agglutination test using serum and whole blood. Int. J. STD AIDS 8192-195. [DOI] [PubMed] [Google Scholar]