Abstract
We have developed a real-time genotyping and quantitative PCR (RT-GQ-PCR) assay to genotype cytomegalovirus (CMV) and quantify viral loads simultaneously in solid organ transplant (SOT) recipients. Special minor-groove DNA-binding probes were designed based on sequence polymorphism in the gB gene to increase genotyping specificity for gB1 to gB4. For validation, 28 samples with known genotypes determined by restriction fragment analysis (RFA) and 121 with unknown genotypes were tested. All samples were from SOT patients with CMV viremia. A 100% concordance for genotyping was achieved by using the RT-GQ-PCR with known genotypes determined by RFA. The RT-GQ-PCR identified more cases of CMV infections with mixed genotypes than RFA did. No cross-reaction between genotypes was observed. All four gB genotypes were detected in the 121 samples of unknown genotype. gB1 was the predominant single genotype (n = 61, 50.4%), followed by gB2 (n = 26, 21.0%), gB3, (n = 11, 9.1%), and gB4 (n = 3, 2.5%). Mixed-genotype infections were detected in 17% (20/121) of the samples. Patients with mixed-genotype infections had significantly higher CMV viral loads than those with single-genotype infections (P = 0.019). The RT-GQ-PCR assay was found to be highly sensitive and specific, with a wide dynamic range (2.7 to 10.7 log10 copies/ml) and very good precision (coefficient of variation, ∼1.78%). With the prominent feature of concurrent CMV gB genotyping and quantitation in a single reaction, the new assay provides a rapid and cost-effective method for monitoring CMV infection in SOT recipients.
Despite an improved understanding of risk factors for cytomegalovirus (CMV) disease and significant advances resulting from the use of preemptive and prophylactic antiviral therapy for the prevention of CMV after solid-organ transplantation (SOT), CMV infection continues to cause morbidity and mortality in this setting (8, 13, 23, 27, 30, 32, 33, 37). The pathogenesis of CMV disease in SOT recipients is likely based on the interplay between viral factors and host responses. A potential viral factor that may play a role in virulence and pathogenesis is sequence polymorphism in the viral genome. In addition, evaluation of viral genomic variants may be a useful epidemiologic tool if sufficient loci are evaluated. In some instances, the clustered nature of these polymorphisms allows for discrimination of distinct strains or genotypes. A common method of CMV genotyping is based on variations in the UL55 gene that encodes CMV glycoprotein B (gB). Previous studies of immunocompromised patients with CMV infections have shown that infections with different genotypes occur and that reinfection with other CMV strains can occur in individuals who have been previously infected with different CMV strains (2, 6, 14, 15, 20, 21, 25, 29). Therefore, in SOT recipients with CMV disease, not one but a variety of CMV strains may cause infection, including the recipient's own pretransplant latent virus, as well as donor-acquired strains of virus. Studies in which CMV infection caused by such multiple CMV strains analyzed systematically during the posttransplantation period are lacking. Understanding the relationship between CMV infection and different CMV strains at different stages of a recipient's life may be very important for understanding the pathogenesis and improving the clinical management of CMV disease. The protective immune response to CMV could be highly strain specific (10, 26), and therefore reinfection with different CMV strains might influence the clinical course of the infection differently compared with reactivation of the recipient's own latent CMV strains (7).
CMV gB is a major envelope glycoprotein of CMV encoded by the UL55 gene. CMV gB has been implicated in host cell entry, cell-to-cell virus transmission, and fusion of infected cells. In addition, it is an important target of both humoral and cellular immune responses (5, 12, 18, 36). CMV gB is expressed as a precursor molecule that is glycosylated and then cleaved at codon 461 to form a disulfide-linked complex of gp55 and gp116. Chou and Dennison (3) described a method of CMV genotyping based on the gB nucleotide sequence that encodes a variable region encompassing the protease cleavage site and demonstrated the existence of four different gB genotypes.
It has been hypothesized that the gB type may influence in vivo viral virulence. Previous studies have suggested that certain gB genotypes may be associated with different clinical manifestations or organ tropism (38, 39). Coaquette et al. reported that coinfection with multiple gB genotypes could be a critical factor leading to severe clinical symptoms in immunocompromised patients (4). However, an accurate assessment of the relationship between circulating CMV strains and subsequent clinical events has been hampered by the lack of well-defined methods to simultaneously detect and quantify mixed CMV genotypes accurately in clinical samples. In addition, some of the methods previously described are not easily applied to high-throughput monitoring. Based on Chou's classification of gB genotypes, several methods of genotyping CMV gB were adapted, including restriction fragment analysis (RFA) of PCR products (16, 20, 34), conventional multiple nested PCR (4, 28, 38), and direct DNA sequencing (24). All of these methods either lack sensitivity or are labor intensive and/or nonquantifiable. Improved quantitative assays would facilitate the CMV genotyping of large numbers of patient specimens in order to address the complex issues related to CMV infection in immunocompromised patients and may also serve as a useful tool for epidemiologic studies. In this study, a novel real-time PCR-based assay is introduced for simultaneous genotyping and quantifying of CMV gB genotypes in SOT recipients with high sensitivity and cost-effectiveness.
MATERIALS AND METHODS
gB genotyping controls.
The clinical isolate (Merlin strain) and laboratory strain AD169 were cultured, harvested, and defined as gB genotypes 1 and 2, respectively, for use as controls. Twenty-six CMV DNA samples genotyped previously by RFA and confirmed by sequencing were also used as controls, including gB1 (n = 11), gB2 (n = 3), gB3 (n = 5), gB4 (n = 1), gB1 mixed with gB4 (n = 1), gB1 mixed with gB3 (n = 2), gB2 mixed with gB3 (n = 1), and two negative samples.
Primers and probe design.
Sixteen nucleotide sequences of the CMV gB gene (UL55) were identified in GenBank (NCBI) and aligned by using BioEdit 7.0 (http://www.mbio.ncsu.edu/BioEdit/BioEdit.html) and Lasergene 6 (DNA*, Madison, WI) to determine the homologous and heterogeneous sequence regions. The sequence strains (accession number) used included Towne (M22343), C327A (M60929), C325A (M60927), AD169 (X04606), C194A (M60926), C336A (M60931), C076A (M85228), C178A (M60925), C082A (M85229), C128A (M60924), C338A (M60932), C359A (M60934), C354A (M60933), C116 (M60923), Berline (X99847), and III86 (U52133). Representative sequence strains C327A (M60929), C336A (M60931), C076A (M85228), and C194A (M60926) were used for designing primers and probes for gB1, gB2, gB3, and gB4, respectively (28, 38). The specific primers and probes were selected from two variable-sequence regions of the CMV gB gene and designed by using Primer Express version 2.0 (Applied Biosystems). Subsequently, all sequences of the primer pairs and probes were blasted by using the search engine from GenBank (NCBI) for further confirmation of the specificity, as well as broad homology with all of the strains within the same genotype. A summary of the sequences of the primers and probes used is shown in Table 1 and Fig. 1. In order to enhance the specificity and sensitivity of the genotyping probes, gB 1, gB2, gB3, and gB4 probes were designed as 3′ minor-groove binder (MGB) probes (11, 42) and labeled with TaqMan dyes FAM, VIC, NED, and Cy5, respectively.
TABLE 1.
Primers and probes for gB genotypes
| Genotype, accession no., and polaritya | Sequence (5′-3′) | Position (nt)b | Amplicon size (bp) |
|---|---|---|---|
| gB1 (HS5GLYBG), M60929 | |||
| F+ | CATACGACGTCTGCTGCTCACT | 22 (121-142) | 72 |
| R− | GCTGACCGTTTGGGAAGAAG | 20 (144-168) | |
| Probe+ | TCGATCCGGTTCAGTCTCT | 19 (173-192) | |
| gB2 (HS5GLXBI), M60931 | |||
| F+ | TCTTTGGTGGAATTGGAACGT | 21 (1312-1332) | 79 |
| R− | TGTCACTCGTACTTCTTCTGGTCCTA | 26 (1365-1390) | |
| Probe+ | ATCCAGTCTGAATATCA | 17 (1334-1363) | |
| gB3 (HS5GLYBM), M85228 | |||
| F+ | TGTTGGAACTGGAACGTTTGG | 21 (1316-1336) | 72 |
| R− | TGCCCGTACTTCTCTTGGTTCT | 22 (1366-1387) | |
| Probe+ | CGGTGTGAACTCCA | 14 (1340-1364) | |
| gB4 (HS5GLYBM), M60926 | |||
| F+ | AAACGTGTCCGTCTTCGAAACT | 22 (1242-1263) | 75 |
| R− | TCCACCAGAGATTTTTGCTTGA | 22 (1295-1316) | |
| Probe+ | CCGGCGGACTAGTAGT | 16 (1265-1290) |
F, forward; R, reverse.
nt, nucleotide.
FIG. 1.
Multiple alignment of nucleotide sequences in the CMV gB gene for genotypes 1 to 4. The gB genotypes, accession numbers, and sequence positions are shown beside the sequences. The dots in the alignment indicate consensus nucleotide sequences. (A) The shaded sequences indicate the locations of gB1 primers and probes. (B) The shaded sequences of gB2, gB3, and gB4 are inserted below each genotype alignment.
Real-time genotyping and quantitative PCR (RT-GQ-PCR).
Double-stranded DNA of CMV was extracted from 200 μl of plasma with a Qiagen DNA mini kit according to the manufacturer's protocol (Qiagen Inc., Ontario, Canada) and eluted from the column with 50 μl of distilled H2O. Extracted DNA was aliquoted and stored at −20°C. RT-GQ-PCR was carried out in a closed-tube system with the ABI PRISM 7500 Sequence Detection System (Applied Biosystems). The reaction was performed in a 25-μl volume containing 12.5 μl of Universal DNA Master Mix (Applied Biosystems), 5 μl of DNA, 400 nM each primer, and 200 nM probe for gB1, gB2, gB3, or gB4 in separate tubes (or wells of a plate). After an initial incubation at 50°C for 2 min to activate uracil N-glycosylase and then at 95°C for 10 min for denaturing, PCR amplification was performed with 45 thermal cycles of 94°C for 20 s and 60°C for 1 min. Amplification data were collected and analyzed with Sequence Detection Software version 1.0 (Applied Biosystems).
To prepare a stock of the quantitative control, a 1,500-bp DNA fragment was amplified from CMV strain AD169 with forward primer 5′-TCTTTGGTGGAATTGGAACGT-3′ and reverse primer 5′-TGTCACTCGTACTTCTTCTGGTCCTA-3′. The product was analyzed by electrophoresis on a 1% agarose gel and further purified with a Qiagen PCR purification kit. The purified product was quantified by Ultrospec 3000 spectrophotometry (Pharmacia Biotech, Cambridge, England), dispensed in aliquots containing 6.0 log10 genome copies per tube, and stored at −70°C. The aliquots were used to prepare serial log dilutions (6.0 to 1.0 log10 genome copies) immediately after thawing in order to establish the external standard curves for the quantitation of each gB genotype run.
Evaluation of the sensitivity, specificity, precision, and efficiency of the RT-GQ-PCR assay.
The sensitivity of RT-GQ-PCR for quantification was compared with that of a LightCycler (LC) real-time quantitative PCR (RT-QPCR) assay (22) used in the routine diagnostic laboratory with 10-fold serial dilutions of four samples with known genotypes (gB1 to gB4). In order to run all genotyping reactions in one RT-GQ-PCR efficiently, the slopes of cycle threshold (CT) values from the four gB genotypes were plotted and evaluated by using diluted clinical samples of genotypes gB1 to gB4. The specificity of the assay for genotyping was evaluated by the cross-reactions between the primers/probes and target fragments of known genotypes and also against results of RFA determination. Assessment of the precision of the assay was performed with six replicates of the RT-GQ-PCRs run on 6 different days with DNA from each genotype (gB1 to gB4).
Validation of the new assay with clinical samples.
A total of 121 plasma specimens from 47 SOT recipients, including 23 kidney, 7 liver, 9 heart, and 7 lung transplant recipients and 1 kidney and pancreas transplant recipient, were examined in the validation study. All samples were CMV positive as previously determined by CMV pp65 antigenemia (1) or LC RT-QPCR assays (22). The samples had been stored at −70°C until studied.
Statistical methods.
Quantitative correlation between the gB RT-GQ-PCR and LC RT-QPCR assays was calculated by using Pearson product format correlation. Differences in viral load between single CMV gB genotypes and mixed genotypes were analyzed by one-way analysis of variance. The precision of the assay for CMV gB genotyping was expressed by the coefficient of variation of the CT value (crossing threshold). The confidence interval was set at 95%, and the statistical significance level was set at P < 0.05.
RESULTS
Evaluation of the RT-GQ-PCR assay.
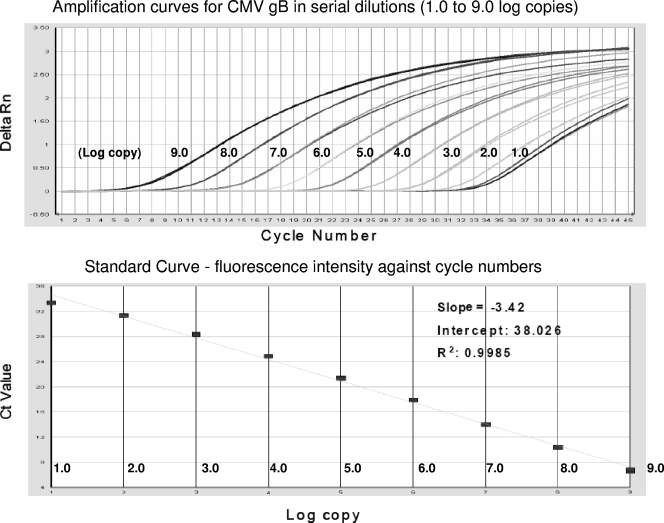
A wide dynamic range for the detection of the CMV gB gene was observed with 10-fold serial dilutions (9.0 to 1.0 log10 genomic copies) of the AD169 strain (Fig. 2). A minimum of 1 copy of target genomic DNA of CMV gB genotypes 1 through 4 in the reaction mixture was detected in one (20%) out of five repeated RT-GQ-PCR runs, and 10 copies were detected in all five RT-GQ-PCR runs (100%) against known standard DNA copy numbers. Since DNA from 20 μl of plasma was used as the starting material for the PCR, the dynamic range for the gB genotype quantitation was 2.7 to 10.7 log10 copies/ml plasma. A copy number between 50 and 499 could be detectable but was not quantifiable. Furthermore, the new RT-GQ-PCR demonstrated a sensitivity equal to that of the LC RT-QPCR for the detection of four gB genotypes in four individual reaction mixtures (Table 2).
FIG. 2.
Amplification and standard curves for CMV gB quantification with the RT-GQ-PCR. CMV DNA in serial dilutions ranging from 1.0E + 01 to 1.0E + 09 copies was amplified in the RT-GQ-PCR. Fluorescence intensity (Rn) is plotted against the number of cycles. Slope = −3.42; intercept = 38.026; R2 = 0.9985.
TABLE 2.
Comparison of sensitivity between LC RT-QPCR and RT-GQ-PCR
| Genotype (clinical sample name) and 10-fold dilution | Resulta (CT) obtained with:
|
|
|---|---|---|
| LC RT-QPCR (routine diagnostic assay) | RT-GQ-PCR (present study)a | |
| gB1 (AH13) | ||
| Undiluted | Pos (24.95) | Pos (26.31) |
| 10−1 | Pos (27.72) | Pos (29.73) |
| 10−2 | Pos (31.71) | Pos (33.75) |
| 10−3 | Pos (33.81) | Pos (38.94) |
| 10−4 | Pos (34.32) | Pos (39.37) |
| gB2 (AH27) | ||
| Undiluted | Pos (27.51) | Pos (30.33) |
| 10−1 | Pos (30.9) | Pos (34.10) |
| 10−2 | Pos (33.17) | Pos (37.25) |
| 10−3 | Neg | Neg |
| 10−4 | Neg | Neg |
| gB3 (AH14) | ||
| Undiluted | Pos (26.93) | Pos (28.30) |
| 10−1 | Pos (30.25) | Pos (31.81) |
| 10−2 | Pos (33.28) | Pos (35.52) |
| 10−3 | Neg | Pos (39.11) |
| 10−4 | Neg | Neg |
| gB4 (AH1) | ||
| Undiluted | Pos (31.44) | Pos (27.99) |
| 10−1 | Pos (31.88) | Pos (32.11) |
| 10−2 | Pos (34.25) | Pos (35.06) |
| 10−3 | Neg | Neg |
| 10−4 | Neg | Neg |
Pos, positive; Neg, negative.
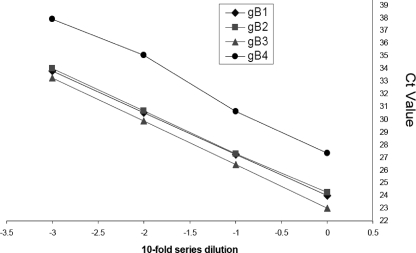
The correlation between the noise band crossing points and a serial 10-fold dilution revealed a good negative linear relationship and similar slopes (−3.28 to −3.42) in the amplifications for all four genotypes, indicating a high efficiency (>90%) for quantifying all of the genotypes in one reaction mixture (Fig. 3). Very good precision was observed in the RT-GQ-PCR assay for CMV genotyping. The coefficients of variation of the CT for each genotype (gB1 to gB4) were 1.97% (range, 1.34 to 1.27%), 1.1% (range, 0.57 to 1.37%), 1.77% (range, 0.64 to 3.22%), and 2.22% (range, 0.96 to 3.8%), respectively.
FIG. 3.
CT values of four gB genotypes under identical RT-GQ-PCR conditions were assessed by using 10-fold serial dilutions of clinical samples. The correlation between the noise band crossing points and a serial 10-fold dilution revealed a good negative linear relationship for all four genotypes. Diamonds: gB1, slope = −3.280, intercept = 23.982, R2 = 0.999. Squares: gB2, slope = −3.283, intercept = 24.153, R2 = 0.999. Triangles: gB3, slope = −3.424, intercept = 23.016, R2 = 0.999. Circles: gB4, slope = −3.424, intercept = 22.926, R2 = 0.989.
All of the primers and probes used for the four CMV genotypes tested in the RT-GQ-PCR assay were specific for each corresponding target sequence, and no cross-reactions were observed. The clinical isolate Merlin strain and laboratory strain AD169 were confirmed as gB1 and gB2, respectively. The genotyping concordance between RT-GQ-PCR and RFA was 100% with 24 samples. However, RT-GQ-PCR was able to identify more cases of CMV infections with mixed genotypes than RFA was. Among 24 samples, 8 (33%) single-genotype samples determined by RFA were identified as mixed-genotype samples by the RT-GQ-PCR assay and 1 sample with dual mixed genotypes determined by RFA was detected as three mixed genotypes by RT-GQ-PCR. A comparison of the genotyping results of the RT-GQ-PCR assay and RFA is shown in Table 3.
TABLE 3.
Comparison of gB genotypes determined by RFA and RT-GQ-PCR assay
| Genotype(s) determined by RT-GQ-PCRa | No. of samples genotyped by RFAa as:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 4 + 1 | 2 + 3 | 1 + 3 | 3 + 1 | Negb | |
| 1 | 8 | ||||||||
| 2 | 1 | ||||||||
| 3 | 2 | ||||||||
| 4 | 1 | ||||||||
| 4 + 1 | 1 | ||||||||
| 2 + 3 | 1 | ||||||||
| 1 + 3 | 1 | 1 | |||||||
| 3 + 1 | 1 | 1 | |||||||
| Neg | 2 | ||||||||
| 1 + 4 | 1 | ||||||||
| 1 + 2 | 1 | ||||||||
| 2 + 1 | 2 | ||||||||
| 3 + 1 + 2 | 1 | ||||||||
| 1 + 3 + 2 | 1 | ||||||||
In the mixed genotypes, the genotype numbers are in descending order of CMV viral load levels.
Neg, negative.
Detection and quantitation of gB genotypes in clinical samples.
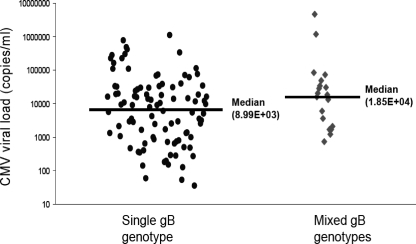
In 121 plasma specimens from 47 SOT recipients, a single-genotype infection with gB1, gB2, gB3, or gB4 was detected in 61 (50.4%), 26 (21.0%), 11 (9.1%), and 3 (2.5%) samples, respectively. A mixed-genotype infection was detected in 20 (17%) samples. Among these 20 mixed-genotype infections, all were dual gB genotype infections and included gB3 and gB1 in 10 (50%), gB2 and gB1 in 5 (25%), gB4 and gB1 in 3 (15%), and gB4 and gB3 in 2 (10%) samples. There was no association between mixed- versus single-genotype infections and transplant type (data not shown). The median (range) of CMV viral DNA quantitation (expressed as log10 copies per milliliter of plasma) in the 121 specimens was 4.03 (1.76 to 5.88) for gB1, 3.12 (1.72 to 6.04) for gB2, 4.07 (3.49 to 4.88) for gB3, 4.08 (3.98 to 4.20) for gB4, and 4.27 (2.86 to 6.67) for the mixed genotypes. Overall, the viral load was significantly higher in patients with mixed-genotype infections versus those with a single-genotype infection (3.95 versus 4.27 log10 copies/ml, P = 0.019) (Fig. 4).
FIG. 4.
CMV viral loads in single genotypes and mixed genotypes in 121 plasma samples from SOT recipients. Lines indicate the medians of the groups. The difference between the mean viral loads of the two groups was statistically significant (one-way analysis of variance, P = 0.019).
DISCUSSION
In the present study, we describe a newly developed real-time PCR assay for simultaneous genotyping and quantitation of the CMV gB gene. The CMV gB genotype is potentially an important determinant of viral virulence and pathogenesis. The viral load is also known to be an important prognostic and therapeutic indicator. Therefore, simultaneous identification of CMV gB genotypes and accurate measurement of viral loads in blood samples can undoubtedly provide important information in a clinical and research context for immunocompromised patients with CMV viremia (4, 28, 34). In addition, this type of assay may be a useful tool in studies assessing viral transmission and in other epidemiologic research. One of the major disadvantages of existing gB genotyping methods, such as RFA of PCR products, multiple nested PCR, and direct DNA sequencing, is the inability to determine the corresponding CMV viral load of the gB genotypes. Real-time PCR, with significant advantages such as speed, sensitivity, simplicity of use, and less possibility of contamination, has been increasingly used for monitoring and quantifying CMV viral loads in diagnostic and research laboratories (19, 22, 31, 35, 41). However, to our knowledge, this advanced technology has not been further explored for its potential application for typing and quantifying CMV gB genotypes.
A technical challenge for assay development was to design primers and probes for genotyping the gB gene. This is because all four genotypes still share significant sequence homology (17, 42). To solve this problem, we utilized MGB DNA probes, which offer great sensitivity in detecting a single base pair mismatch. Chemically modified fluorogenic 3′ MGB probes have a short length of oligodeoxynucleotide conjugates and a higher melting temperature, which results in increased specificity (11). MGB probes have also been used to identify single nucleotide polymorphisms (40). With specifically designed MGB probes, we demonstrated not only that this novel real-time PCR-based assay is specific for each gB gene genotype but that it also possesses a superior sensitivity for the detection of mixed genotypes in comparison with RFA. Improvement in the detection of mixed gB genotypes becomes an important feature of this assay and provides a valuable tool to study CMV infection in different clinical and research settings. For example, recent studies have shown that SOT recipients with mixed CMV gB genotypes have significant differences in clinical outcomes and higher viral loads (4, 28). It may be that simultaneous infection with multiple CMV genotypes, regardless of reactivation and/or reinfection, could be a critical determinant of the severity of disease and a poor prognosis for immunocompromised patients (4, 28, 34). The combined use of simultaneous genotyping and viral load determination may provide a powerful tool to investigate and improve our understanding of CMV transmission, reactivation/reinfection, and interaction of multiple genotypes during CMV infection. In the latter group, monitoring the relative increase or decline in specific genotypes may provide insight into in vivo fitness differences between viral strains.
As part of the validation process, we applied our assay to clinical samples. In a cohort of SOT patients with CMV viremia, CMV gB1 was the predominant genotype, followed by gB2, mixed types and gB3, and then gB4. This distribution of CMV gB genotypes was similar to but slightly different from that in previously published studies. For example, in a study of 50 SOT recipients, CMV gB1 to gB4 were detected in 38%, 18%, 24%, and 4% of the specimens examined, respectively, and mixed types were found in 16% of the specimens examined (9). In another study with SOT, gB1 to gB4 were detected in 28.9%, 19.6%, 23.7%, and 2.0%, respectively, and mixed types were found in 25.8% of the specimens studied (4). A high percentage of CMV infections with mixed genotypes in serum samples was also observed in advanced AIDS patients (40%) (38). This was second only to single-genotype infection with gB3 (42.5%), indicating that mixed-genotype infection may be common in multiple different clinical settings (38). The differences in the distribution of gB genotypes may be due to geographical factors and/or varied serostatus between the donors and recipients. We also observed higher viral load in patients with mixed-genotype infections versus single-genotype infections. This is in keeping with previous studies that have suggested that infection with mixed genotypes increases the risk of progression to CMV disease, although a correlation with CMV viral loads has not been well defined (4, 28, 34, 38).
In conclusion, very good precision, sensitivity, and specificity of CMV gB genotyping and viral load quantification were achieved with our in-house RT-GQ-PCR assay. This assay provides a reliable, efficient, and cost-effective method for sequential monitoring of alterations of CMV gB genotypes and fluctuations of corresponding viral load levels in SOT recipients over time.
Acknowledgments
We acknowledge Ran Zhou and Min Cao for their technical support in this study. We also thank Stephanie Yanow for manuscript review and comments.
Footnotes
Published ahead of print on 29 October 2008.
REFERENCES
- 1.Boeckh, M., R. A. Bowden, J. M. Goodrich, M. Pettinger, and J. D. Meyers. 1992. Cytomegalovirus antigen detection in peripheral blood leukocytes after allogeneic marrow transplantation. Blood 801358-1364. [PubMed] [Google Scholar]
- 2.Chandler, S. H., H. H. Handsfield, and J. K. McDougall. 1987. Isolation of multiple strains of cytomegalovirus from women attending a clinic for sexually transmitted disease. J. Infect. Dis. 155655-660. [DOI] [PubMed] [Google Scholar]
- 3.Chou, S. W., and K. M. Dennison. 1991. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J. Infect. Dis. 1631229-1234. [DOI] [PubMed] [Google Scholar]
- 4.Coaquette, A., A. Bourgeois, C. Dirand, A. Varin, W. Chen, and G. Herbein. 2004. Mixed cytomegalovirus glycoprotein B genotypes in immunocompromised patients. Clin. Infect. Dis. 39155-161. [DOI] [PubMed] [Google Scholar]
- 5.Cranage, M. P., T. Kouzarides, A. T. Bankier, S. Satchwell, K. Weston, P. Tomlinson, B. Barrell, H. Hart, S. E. Bell, A. C. Minson, and G. L. Smith. 1986. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 53057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drew, W. L., E. S. Sweet, R. C. Miner, and E. S. Mocarski. 1984. Multiple infections by cytomegalovirus in patients with acquired immunodeficiency syndrome: documentation by Southern blot hybridization. J. Infect. Dis. 150952-953. [DOI] [PubMed] [Google Scholar]
- 7.Grundy, J. E., S. F. Lui, M. Super, N. J. Berry, P. Sweny, O. N. Fernando, J. Moorhead, and P. D. Griffiths. 1988. Symptomatic cytomegalovirus infection in seropositive kidney recipients: reinfection with donor virus rather than reactivation of recipient virus. Lancet 2132-135. [DOI] [PubMed] [Google Scholar]
- 8.Hodson, E. M., C. A. Jones, A. C. Webster, G. F. Strippoli, P. G. Barclay, K. Kable, D. Vimalachandra, and J. C. Craig. 2005. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet 3652105-2115. [DOI] [PubMed] [Google Scholar]
- 9.Humar, A., D. Kumar, C. Gilbert, and G. Boivin. 2003. Cytomegalovirus (CMV) glycoprotein B genotypes and response to antiviral therapy, in solid-organ-transplant recipients with CMV disease. J. Infect. Dis. 188581-584. [DOI] [PubMed] [Google Scholar]
- 10.Klein, M., K. Schoppel, N. Amvrossiadis, and M. Mach. 1999. Strain-specific neutralization of human cytomegalovirus isolates by human sera. J. Virol. 73878-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutyavin, I. V., I. A. Afonina, A. Mills, V. V. Gorn, E. A. Lukhtanov, E. S. Belousov, M. J. Singer, D. K. Walburger, S. G. Lokhov, A. A. Gall, R. Dempcy, M. W. Reed, R. B. Meyer, and J. Hedgpeth. 2000. 3′-Minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Res. 28655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantto, J., J. M. Fletcher, and M. Ohlin. 2003. Binding characteristics determine the neutralizing potential of antibody fragments specific for antigenic domain 2 on glycoprotein B of human cytomegalovirus. Virology 305201-209. [DOI] [PubMed] [Google Scholar]
- 13.Limaye, A. P., L. Corey, D. M. Koelle, C. L. Davis, and M. Boeckh. 2000. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 356645-649. [DOI] [PubMed] [Google Scholar]
- 14.Mattick, C., D. Dewin, S. Polly, E. Sevilla-Reyes, S. Pignatelli, W. Rawlinson, G. Wilkinson, P. Dal Monte, and U. A. Gompels. 2004. Linkage of human cytomegalovirus glycoprotein gO variant groups identified from worldwide clinical isolates with gN genotypes, implications for disease associations and evidence for N-terminal sites of positive selection. Virology 318582-597. [DOI] [PubMed] [Google Scholar]
- 15.Meyer-König, U., K. Ebert, B. Schrage, S. Pollak, and F. T. Hufert. 1998. Simultaneous infection of healthy people with multiple human cytomegalovirus strains. Lancet 3521280-1281. [DOI] [PubMed] [Google Scholar]
- 16.Mhiri, L., J. F. Meritet, P. Lebon, and A. Slim. 2008. Different human cytomegalovirus glycoprotein B (gB) genotype distribution. Pathol. Biol. (Paris) 5639-42. [DOI] [PubMed] [Google Scholar]
- 17.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 10014976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro, D., P. Paz, S. Tugizov, K. Topp, J. La Vail, and L. Pereira. 1993. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology 197143-158. [DOI] [PubMed] [Google Scholar]
- 19.Nitsche, A., N. Steuer, C. A. Schmidt, O. Landt, and W. Siegert. 1999. Different real-time PCR formats compared for the quantitative detection of human cytomegalovirus DNA. Clin. Chem. 451932-1937. [PubMed] [Google Scholar]
- 20.Novak, Z., S. A. Ross, R. K. Patro, S. K. Pati, R. A. Kumbla, S. Brice, and S. B. Boppana. 2008. Cytomegalovirus strain diversity in seropositive women. J. Clin. Microbiol. 46882-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Numazaki, K., M. Ikehata, and S. Chiba. 2000. Subtyping of cytomegalovirus strains obtained from immunocompetent children. In Vivo 14745-746. [PubMed] [Google Scholar]
- 22.Pang, X. L., L. Chui, J. Fenton, B. Leblanc, and J. Preiksaitis. 2003. Comparison of LightCycler PCR, Cobas Amplicor CMV Monitor, and pp65 antigenemia assays for quantitative cytomegalovirus viral loads in peripheral blood from patients after solid organ transplantation. J. Clin. Microbiol. 413167-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paya, C. V., J. A. Wilson, M. J. Espy, I. G. Sia, M. J. DeBernardi, T. F. Smith, R. Patel, G. Jenkins, W. S. Harmsen, D. J. Vanness, and R. H. Wiesner. 2002. Preemptive use of oral ganciclovir to prevent cytomegalovirus infection in liver transplant patients: a randomized, placebo-controlled trial. J. Infect. Dis. 185854-860. [DOI] [PubMed] [Google Scholar]
- 24.Picone, O., J.-M. Costa, M. Leruez-Ville, P. Ernault, M. Olivi, and Y. Ville. 2004. Cytomegalovirus (CMV) glycoprotein B genotype and CMV DNA load in the amniotic fluid of infected fetuses. Prenatal Diagn. 241001-1006. [DOI] [PubMed] [Google Scholar]
- 25.Pignatelli, S., P. Dal Monte, G. Rossini, S. Chou, T. Gojobori, K. Hanada, J. J. Guo, W. Rawlinson, W. Britt, M. Mach, and M. P. Landini. 2003. Human cytomegalovirus glycoprotein N (gpUL73-gN) genomic variants: identification of a novel subgroup, geographical distribution and evidence of positive selective pressure. J. Gen. Virol. 84647-655. [DOI] [PubMed] [Google Scholar]
- 26.Plotkin, S. A., S. E. Starr, H. M. Friedman, E. Gönczöl, and R. E. Weibel. 1989. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J. Infect. Dis. 159860-865. [DOI] [PubMed] [Google Scholar]
- 27.Preiksaitis, J. K., D. C. Brennan, J. Fishman, and U. Allen. 2005. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am. J. Transplant. 5218-227. [DOI] [PubMed] [Google Scholar]
- 28.Puchhammer-Stöckl, E., I. Görzer, A. Zoufaly, P. Jaksch, C. C. Bauer, W. Klepetko, and T. Popow-Kraupp. 2006. Emergence of multiple cytomegalovirus strains in blood and lung of lung transplant recipients. Transplantation 81187-194. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen, L., A. Geissler, and M. Winters. 2003. Inter- and intragenic variations complicate the molecular epidemiology of human cytomegalovirus. J. Infect. Dis. 187809-819. [DOI] [PubMed] [Google Scholar]
- 30.Razonable, R. R., A. Rivero, A. Rodriguez, J. Wilson, J. Daniels, G. Jenkins, T. Larson, W. C. Hellinger, J. R. Spivey, and C. V. Paya. 2001. Allograft rejection predicts the occurrence of late-onset cytomegalovirus (CMV) disease among CMV-mismatched solid organ transplant patients receiving prophylaxis with oral ganciclovir. J. Infect. Dis. 841461-1464. [DOI] [PubMed] [Google Scholar]
- 31.Razonable, R. R., C. V. Paya, and T. F. Smith. 2002. Role of the laboratory in diagnosis and management of cytomegalovirus infection in hematopoietic stem cell and solid-organ transplant recipients. J. Clin. Microbiol. 40746-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razonable, R. R., and C. V. Paya. 2003. Herpesvirus infections in transplant recipients: current challenges in the clinical management of cytomegalovirus and Epstein-Barr virus infections. Herpes 1060-65. [PubMed] [Google Scholar]
- 33.Razonable, R. R. 2005. Epidemiology of cytomegalovirus disease in solid organ and hematopoietic stem cell transplant recipients. Am. J. Health Syst. Pharm. 62S7-S13. [DOI] [PubMed] [Google Scholar]
- 34.Sarcinella, L., T. Mazzulli, B. Willey, and A. Humar. 2002. Cytomegalovirus glycoprotein B genotype does not correlate with outcomes in liver transplant patients. J. Clin. Virol. 2499-105. [DOI] [PubMed] [Google Scholar]
- 35.Schaade, L., P. Kockelkorn, K. Ritter, and M. Kleines. 2000. Detection of cytomegalovirus DNA in human specimens by LightCycler PCR. J. Clin. Microbiol. 384006-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smuda, C., E. Bogner, and K. Radsak. 1997. The human cytomegalovirus glycoprotein B gene (ORF UL55) is expressed early in the infectious cycle. J. Gen. Virol. 781981-1992. [DOI] [PubMed] [Google Scholar]
- 37.Strippoli, G. F., E. M. Hodson, C. Jones, and J. C. Craig. 2006. Preemptive treatment for cytomegalovirus viremia to prevent cytomegalovirus disease in solid organ transplant recipients. Transplantation 81139-145. [DOI] [PubMed] [Google Scholar]
- 38.Tarragó, D., C. Quereda, and A. Tenorio. 2003. Different cytomegalovirus glycoprotein B genotype distribution in serum and cerebrospinal fluid specimens determined by a novel multiplex nested PCR. J. Clin. Microbiol. 412872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torok-Storb, B., M. Boeckh, C. Hoy, W. Leisenring, D. Myerson, and T. Gooley. 1997. Association of specific cytomegalovirus genotypes with death from myelosuppression after marrow transplantation. Blood 902097-2102. [PubMed] [Google Scholar]
- 40.Whiley, D. M., and T. P. Sloots. 2006. Sequence variation can affect the performance of minor groove binder TaqMan probes in viral diagnostic assays. J. Clin. Virol. 3581-83. [DOI] [PubMed] [Google Scholar]
- 41.Yun, Z., I. Lewensohn-Fuchs, P. Ljungman, and A. Vahlne. 2000. Real-time monitoring of cytomegalovirus infections after stem cell transplantation using the TaqMan polymerase chain reaction assays. Transplantation 691733-1736. [DOI] [PubMed] [Google Scholar]
- 42.Zweygberg Wirgart, B. Z., M. Brytting, A. Linde, B. Wahren, and L. Grillner. 1998. Sequence variation within three important cytomegalovirus gene regions in isolates from four different patient populations. J. Clin. Microbiol. 363662-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]