Abstract
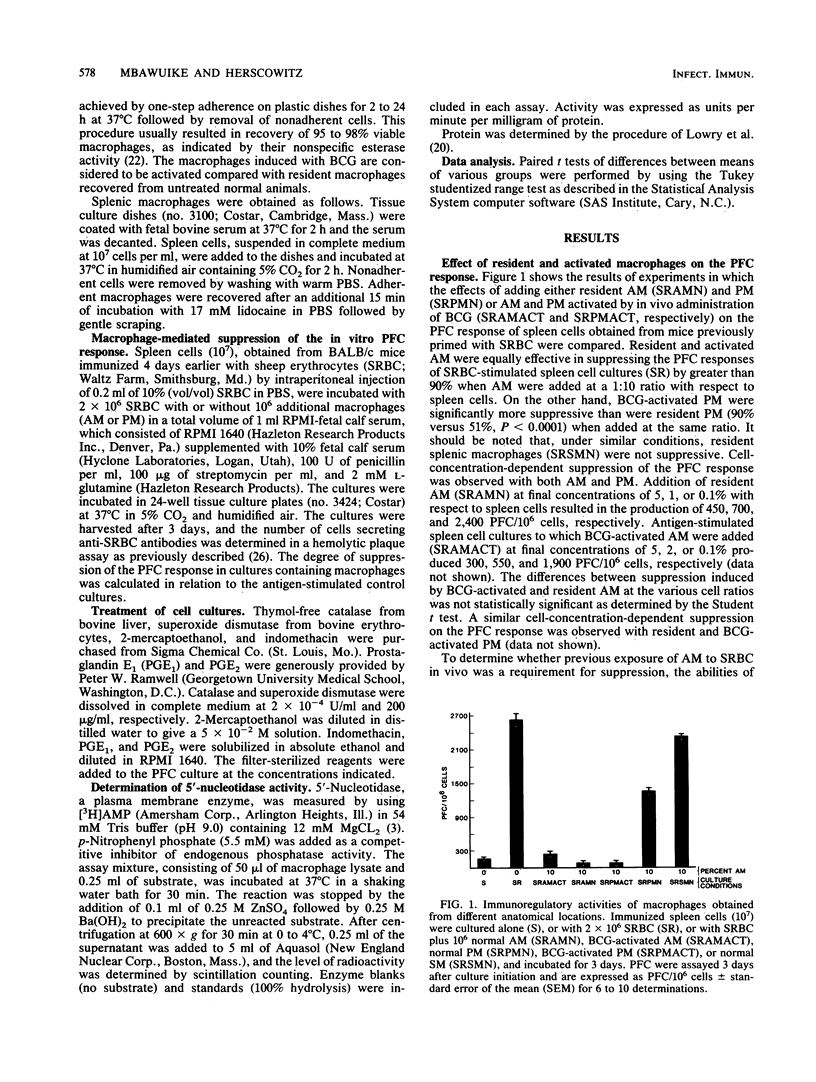
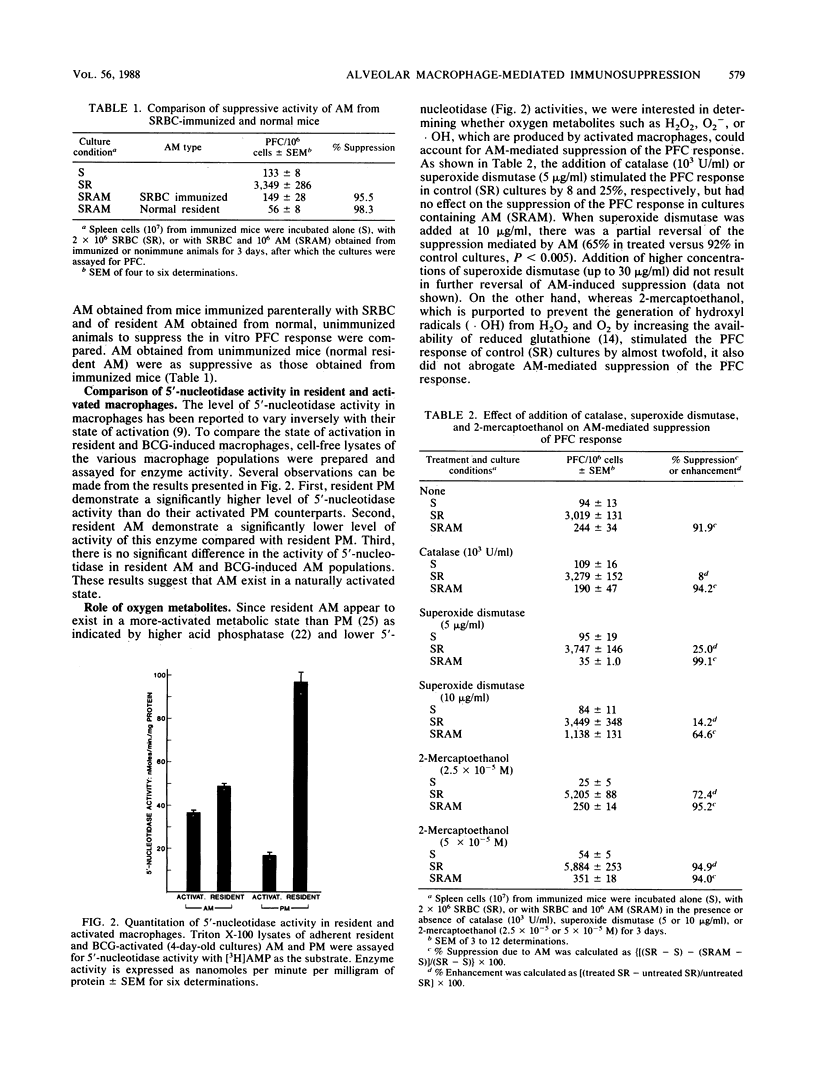
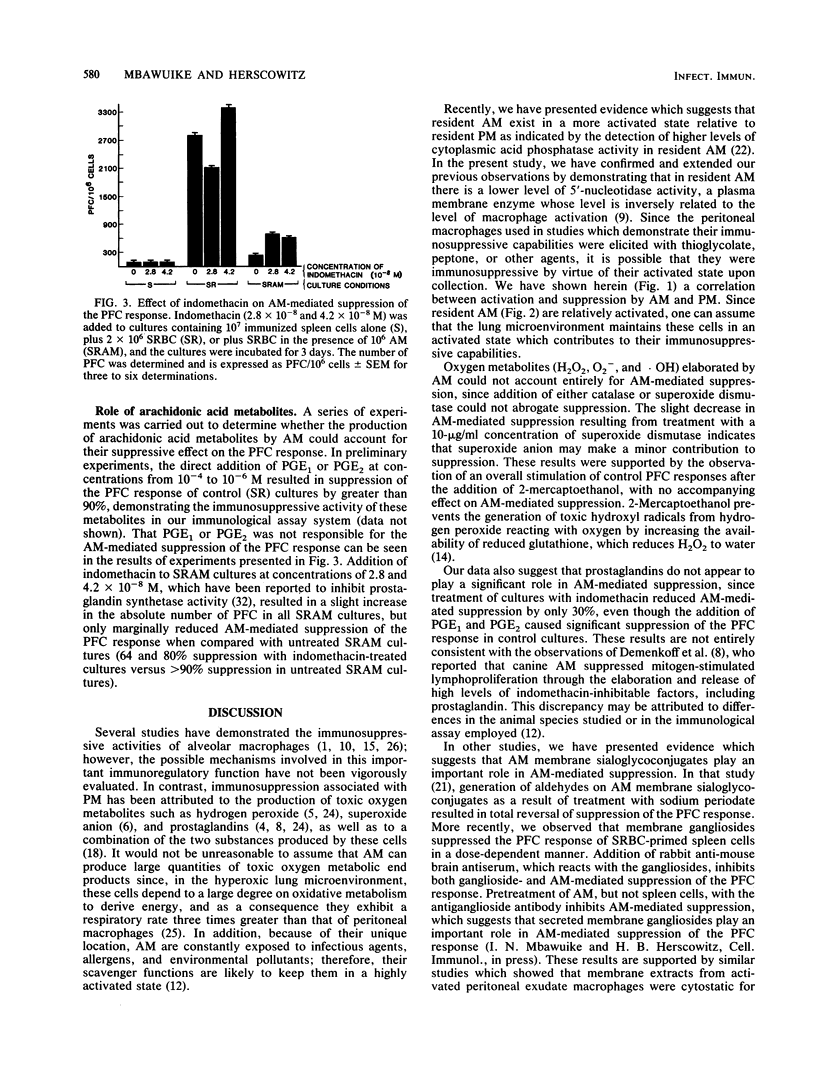
Alveolar macrophages (AM) are highly suppressive of the in vitro plaque-forming cell (PFC) response of spleen cells obtained from mice primed with sheep erythrocytes. Comparison of macrophage populations obtained from disparate anatomical sites revealed that although in both cases there was a cell-concentration-dependent suppression of the PFC response, resident AM or AM activated as a result of intravenous injection of Mycobacterium bovis BCG were equally suppressive at the doses examined. Although there was a similar dose-dependent suppression with peritoneal macrophages, BCG-activated cells were more suppressive of the PFC response than were resident cells. In contrast, splenic macrophages at comparable concentrations were not at all suppressive. Resident AM exhibited significantly lower levels of 5'-nucleotidase activity than did resident peritoneal macrophages. Macrophage-mediated suppression of the in vitro PFC response could not be attributed to the release of toxic oxygen metabolites (H2O2, O2- ,and .OH) or prostaglandins, since the addition of catalase, superoxide dismutase, 2-mercaptoethanol, or indomethacin did not completely reverse suppression. These results suggest that the lung microenvironment may maintain AM in an activated state which contributes to their potential immunoregulatory functions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansfield M. J., Kaltreider H. B., Caldwell J. L., Herskowitz F. N. Hyporesponsiveness of canine bronchoalveolar lymphocytes to mitogens: inhibition of lymphocyte proliferation by alveolar macrophages. J Immunol. 1979 Feb;122(2):542–548. [PubMed] [Google Scholar]
- Aune T. M., Pierce C. W. Mechanism of action of macrophage-derived suppressor factor produced by soluble immune response suppressor-treated macrophages. J Immunol. 1981 Jul;127(1):368–372. [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Censini S., Tagliabue A. Interferon-gamma reduces macrophage-suppressive activity by inhibiting prostaglandin E2 release and inducing interleukin 1 production. J Immunol. 1984 Aug;133(2):764–768. [PubMed] [Google Scholar]
- Boraschi D., Ghezzi P., Pasqualetto E., Salmona M., Nencioni L., Soldateschi D., Villa L., Tagliabue A. Interferon decreases production of hydrogen peroxide by macrophages: correlation with reduction of suppressive capacity and of anti-microbial activity. Immunology. 1983 Nov;50(3):359–368. [PMC free article] [PubMed] [Google Scholar]
- Boraschi D., Ghezzi P., Salmona M., Tagliabue A. IFN-beta-induced reduction of superoxide anion generation by macrophages. Immunology. 1982 Apr;45(4):621–628. [PMC free article] [PubMed] [Google Scholar]
- Cohen A. B., Gold W. M. Defense mechanisms of the lungs. Annu Rev Physiol. 1975;37:325–350. doi: 10.1146/annurev.ph.37.030175.001545. [DOI] [PubMed] [Google Scholar]
- Demenkoff J. H., Ansfield M. J., Kaltreider H. B., Adam E. Alveolar macrophage suppression of canine bronchoalveolar lymphocytes: the role of prostaglandin E2 in the inhibition of mitogen-responses. J Immunol. 1980 Mar;124(3):1365–1370. [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. 5'-Nucleotidase activity of mouse peritoneal macrophages. I. Synthesis and degradation in resident and inflammatory populations. J Exp Med. 1976 Dec 1;144(6):1581–1595. doi: 10.1084/jem.144.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M., Touaty E., Gerber F., Medrano G., Pariente R. Suppression of bronchoalveolar lymphocyte antibody secretion by alveolar macrophage: an in vitro study in the rabbit. Bull Eur Physiopathol Respir. 1984 May-Jun;20(3):229–235. [PubMed] [Google Scholar]
- Gorenberg D. J., Daniele R. P. The alveolar macrophage: its capacity to act as an accessory cell in mitogen-stimulated proliferation of guinea pig lymphocytes. Cell Immunol. 1978 Mar 1;36(1):115–127. doi: 10.1016/0008-8749(78)90255-1. [DOI] [PubMed] [Google Scholar]
- Herscowitz H. B. In defense of the lung: paradoxical role of the pulmonary alveolar macrophage. Ann Allergy. 1985 Nov;55(5):634–650. [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med. 1979 Sep 13;301(11):580–587. doi: 10.1056/NEJM197909133011104. [DOI] [PubMed] [Google Scholar]
- Hoffeld J. T., Oppenheim J. J. Enhancement of the primary antibody response by 2-mercaptoethanol is mediated by its action on glutathione in the serum. Eur J Immunol. 1980 May;10(5):391–395. doi: 10.1002/eji.1830100514. [DOI] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. II. Inhibition of lymphocyte proliferation by purified macrophages from rat lung. Immunology. 1979 Jun;37(2):429–436. [PMC free article] [PubMed] [Google Scholar]
- Kaltreider H. B. Alveolar macrophages. Enhancers or suppressors of pulmonary immune reactivity? Chest. 1982 Sep;82(3):261–262. doi: 10.1378/chest.82.3.261. [DOI] [PubMed] [Google Scholar]
- Koretzky G. A., Elias J. A., Kay S. L., Rossman M. D., Nowell P. C., Daniele R. P. Spontaneous production of interleukin-1 by human alveolar macrophages. Clin Immunol Immunopathol. 1983 Dec;29(3):443–450. doi: 10.1016/0090-1229(83)90047-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laughter A. H., Martin R. R., Twomey J. J. Lymphoproliferative responses to antigens mediated by human pulmonary alveolar macrophages. J Lab Clin Med. 1977 Jun;89(6):1326–1332. [PubMed] [Google Scholar]
- Lipscomb M. F., Toews G. B., Lyons C. R., Uhr J. W. Antigen presentation by guinea pig alveolar macrophages. J Immunol. 1981 Jan;126(1):286–291. [PubMed] [Google Scholar]
- Mbawuike I. N., Luhr J. E., Herscowitz H. B. Enhanced recovery of murine alveolar macrophages: morphological and functional characteristics following intravenous injection of heat-killed Mycobacterium bovis BCG. Infect Immun. 1986 Feb;51(2):483–489. doi: 10.1128/iai.51.2.483-489.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbawuike I. N., Luhr J. E., Herscowitz H. B. Reversal of murine alveolar macrophage-mediated suppression of plaque-forming cell response by sodium periodate. Cell Immunol. 1986 Apr 15;99(1):300–307. doi: 10.1016/0008-8749(86)90238-8. [DOI] [PubMed] [Google Scholar]
- McCarron R. M., Goroff D. K., Luhr J. E., Murphy M. A., Herscowitz H. B. Methods for the collection of peritoneal and alveolar macrophages. Methods Enzymol. 1984;108:274–284. doi: 10.1016/s0076-6879(84)08092-7. [DOI] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Pennline K. J., Herscowitz H. B. Dual role for alveolar macrophages in humoral and cell-mediated immune responses: evidence for suppressor and enhancing functions. J Reticuloendothel Soc. 1981 Sep;30(3):205–217. [PubMed] [Google Scholar]
- Ritter K., Härtl R., Bandlow G., Thomssen R. Cytostatic effect of gangliosides present in the membrane of macrophages. Cell Immunol. 1986 Feb;97(2):248–256. doi: 10.1016/0008-8749(86)90395-3. [DOI] [PubMed] [Google Scholar]
- Shellito J., Caldwell J. L., Kaltreider H. B. Immune functions of murine alveolar macrophages: binding of lymphocytes and support of lymphocyte proliferation. Exp Lung Res. 1983 Feb;4(2):93–107. doi: 10.3109/01902148309055007. [DOI] [PubMed] [Google Scholar]
- Sweeney T. D., Castranova V., Bowman L., Miles P. R. Factors which affect superoxide anion release from rat alveolar macrophages. Exp Lung Res. 1981 May;2(2):85–96. doi: 10.3109/01902148109052305. [DOI] [PubMed] [Google Scholar]
- Ullrich S. E., Herscowitz H. B. Immunological function of alveolar macrophages: interaction with a soluble protein antigen and the immunogenicity of alveolar macrophage-associated antigen. J Reticuloendothel Soc. 1980 Aug;28(2):111–127. [PubMed] [Google Scholar]
- Ward P. A., Duque R. E., Sulavik M. C., Johnson K. J. In vitro and in vivo stimulation of rat neutrophils and alveolar macrophages by immune complexes. Production of O-2 and H2O2. Am J Pathol. 1983 Mar;110(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- Webb D. R., Nowowiejski I. The role of prostaglandins in the control of the primary 19S immune response to sRBC. Cell Immunol. 1977 Sep;33(1):1–10. doi: 10.1016/0008-8749(77)90129-0. [DOI] [PubMed] [Google Scholar]
- Weinberg D. S., Unanue E. R. Antigen-presenting function of alveolar macrophages: uptake and presentation of Listeria monocytogenes. J Immunol. 1981 Feb;126(2):794–799. [PubMed] [Google Scholar]


