Abstract
Susceptibility testing of anidulafungin (AFG) against 32 mold isolates showed an excellent correlation between disk diffusion (DD) and broth microdilution methods. Based on our data, a 2-μg disk of AFG and a 24-h reading time might represent the best parameters for AFG DD testing against filamentous fungi.
The number of invasive fungal infections, including systemic infections caused by Aspergillus species, zygomycetes, and other species of molds (Fusarium and Scedosporium) (3, 5, 11, 12, 15, 20, 23, 24, 26, 27), has risen over the last 20 years. Recently, the novel echinocandin anidulafungin (AFG) has been licensed and exhibits high antifungal activity (1, 4, 6, 7, 13, 21, 22, 28). Some investigators have explored the use of disk diffusion (DD) susceptibility testing for caspofungin and AFG against yeasts (specifically, Candida isolates) (9, 10, 14, 19), but there is not much data available for AFG DD testing against molds (2, 8). Therefore, in this study we compared the AFG inhibition zones (IZs) determined via the DD assay with the minimum effective concentrations (MECs) obtained by the broth microdilution (BD) reference method to determine the best correlation between the two methods. The study was conducted at two university centers, and the intralaboratory reproducibility and interlaboratory agreement results were evaluated. The AFG susceptibilities of 33 isolates were determined by both the BD and DD assays. All assays were performed in duplicate on two different days by each center. A total of 32 clinical mold isolates were tested (Aspergillus fumigatus, n = 8; Aspergillus flavus, n = 6; Aspergillus terreus, n = 4; Aspergillus niger, n = 3; Acremonium curvulum, n = 1; Acremonium strictum, n = 1; Fusarium oxysporum; n = 2; Fusarium dimerum, n = 2; Absidia corymbifera, n = 2; Rhizopus oryzae, n = 2; Scopulariopsis brevicaulis, n = 1). Candida krusei ATCC 6258 was used as a quality control (QC). AFG was provided as a pure powder form by Pfizer, Inc., and a stock solution was prepared in dimethyl sulfoxide.
The BD method was performed as described in the NCCLS M38-A document (16). Stock solutions for the QC yeast isolate were prepared as described in the NCCLS M27-A2 document (17). Conidial inocula and AFG were prepared by using RPMI 1640 broth medium buffered to pH 7.0 with 0.165 M morpholinopropanesulfonic acid buffer. The final concentrations of the antifungal agent in the microdilution trays ranged from 0.03 to 16 μg/ml. Growth (drug free) and sterility controls were included for each tested isolate. Microdilution trays were incubated at 35°C, and the AFG MECs, defined as the lowest drug concentrations that produced growth of small, rounded, compact colonies compared to the hyphal growth of the control well (19), were determined at 24 h for all the tested species. Similarly, QC MICs were read at 24 h. The DD assays were performed with homemade AFG disks. Blank disks that were 6.3 mm in diameter (Becton Dickinson) were impregnated with 20 μl of AFG at final concentrations of 2, 5, 10, and 25 μg/disk and allowed to dry at room temperature. A modification of the NCCLS M44-A DD method (18) for yeast testing was performed to determine the diameters of the antifungal IZs in millimeters at each center. The mold inocula were prepared at optical densities ranging from 80 to 82% and from 68 to 70% transmittance for Aspergillus species and the other species, respectively. A suspension with a 0.5 McFarland standard was utilized for the QC strain. Inoculum quantification was performed by counting the number of CFU per milliliter of diluted inoculum on Sabouraud dextrose agar plates. For the 32 mold isolates and the one QC isolate, 94.5% of the inocula were within the range of 1 × 106 to 5.0 × 106 CFU/ml, with higher inoculum densities (6.20 × 106 to 3.57 × 108) observed for eight isolates (three isolates of A. fumigatus, two of A. niger, and two of R. oryzae). Mueller-Hinton agar plates supplemented with 2% dextrose and 0.5 μl/ml methylene blue were inoculated using sterile cotton swabs, and the antifungal disks were applied to their surfaces. The plates were incubated at 35°C, and IZs were measured at 24, 48, and 72 h. The edges of the IZs were used as the points of marked decrease in fungal density.
The comparative evaluation of the DD and BD methods was performed by calculating the medians and the ranges of the IZ diameters and MECs. Pearson's correlation coefficient was used to analyze the correlation between the MECs and the DD zone diameters. The intralaboratory reproducibility and the interlaboratory agreement results were calculated as the percentages of IZs with diameters within 3 mm of each other.
Table 1 summarizes the susceptibility results of AFG against 33 fungal isolates, with MECs/MICs ranging from ≤0.03 to >16 μg/ml. In general, isolates of Aspergillus spp. proved to be highly susceptible to this new echinocandin, as shown by a median MEC of ≤0.03 μg/ml. Our data agree with those reported by Messer et al. showing that AFG MECs of 0.03 μg/ml inhibited 100% of the tested isolates (13). With the exception of S. brevicaulis, which showed a median MEC of ≤0.03 μg/ml, the other mold isolates tested, including R. oryzae, A. corymbifera, Fusarium spp., and Acremonium spp., showed median MECs of >16 μg/ml (range, 4 to >16 μg/ml).
TABLE 1.
In vitro activity of AFG against 33 fungal isolates
| Fungal isolate(s) tested (no. of isolates)a | AFG concentration (μg/disk) | IZ diam (mm) by DD method atb:
|
MEC (μg/ml) by BD
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h
|
48 h
|
72 h
|
|||||||
| Range | Median | Range | Median | Range | Median | Range | Median | ||
| Aspergillus fumigatus (8) | 2 | 13-18 | 16 | 13-17 | 14.5 | 13-17 | 14 | ≤0.03-0.06 | ≤0.03 |
| 5 | 15-19 | 17 | 11-18 | 16 | 14-18 | 15 | |||
| 10 | 17-21 | 18 | 16-21 | 17 | 15-21 | 17 | |||
| 25 | 17-22 | 19 | 13-20 | 19 | 17-22 | 19 | |||
| Aspergillus flavus (6) | 2 | 11-19 | 16 | 11-17 | 15 | 11-17 | 15 | ≤0.03 | ≤0.03 |
| 5 | 15-20 | 17 | 14-18 | 16 | 14-18 | 16 | |||
| 10 | 15-21 | 19 | 15-20 | 17.5 | 15-20 | 17 | |||
| 25 | 19-23 | 20 | 16-23 | 19.5 | 17-23 | 19 | |||
| Aspergillus terreus (4) | 2 | 14-19 | 16.5 | 13-18 | 15 | 13-18 | 15.5 | ≤0.03-0.06 | ≤0.03 |
| 5 | 16-19 | 18 | 14-18 | 17 | 14-18 | 17 | |||
| 10 | 18-22 | 20 | 16-20 | 18.5 | 16-21 | 19 | |||
| 25 | 19-23 | 21 | 17-21 | 19 | 17-22 | 20 | |||
| Aspergillus niger (3) | 2 | 13-18 | 15.5 | 13-16 | 15 | 13-16 | 14.5 | ≤0.03 | ≤0.03 |
| 5 | 15-19 | 17 | 15-17 | 16 | 15-18 | 16 | |||
| 10 | 17-20 | 19 | 15-18 | 17 | 15-19 | 17 | |||
| 25 | 19-22 | 20 | 16-20 | 18 | 16-19 | 18 | |||
| Other molds (10)c | 2 | NZD | NZD | NZD | NZD | NZD | NZD | 4.0->16 | >16 |
| 5 | NZD | NZD | NZD | NZD | NZD | NZD | |||
| 10 | NZD | NZD | NZD | NZD | NZD | NZD | |||
| 25 | NZD | NZD | NZD | NZD | NZD | NZD | |||
| Scopulariopsis brevicaulis (1) | 2 | 12-15 | 13 | 12-15 | 13.5 | 11-15 | 12 | ≤0.03 | ≤0.03 |
| 5 | 13-18 | 17 | 12-18 | 17 | 12-18 | 15 | |||
| 10 | 14-19 | 18 | 13-19 | 18 | 13-19 | 17.5 | |||
| 25 | 15-21 | 19.5 | 14-21 | 16.5 | 15-21 | 20 | |||
| Candida krusei ATCC 6258 | 2 | 9-14 | 10.5 | 6-13 | 10.5 | ND | ND | ≤0.03 | ≤0.03 |
| 5 | 11-15 | 13 | 11-14 | 12.5 | ND | ND | |||
| 10 | 12-16 | 14.5 | 12-15 | 14 | ND | ND | |||
| 25 | 15-17 | 16 | 13-16 | 15.5 | ND | ND | |||
Each isolate was tested in duplicate on two different days by each university center.
NZD, no zone diameter; ND, not determined.
Other molds included Acremonium curvulum (n = 1), A. strictum (n = 1), Fusarium oxysporum (n = 2), Fusarium dimerum (n = 2), Absidia corymbifera (n = 2), and Rhizopus oryzae (n = 2).
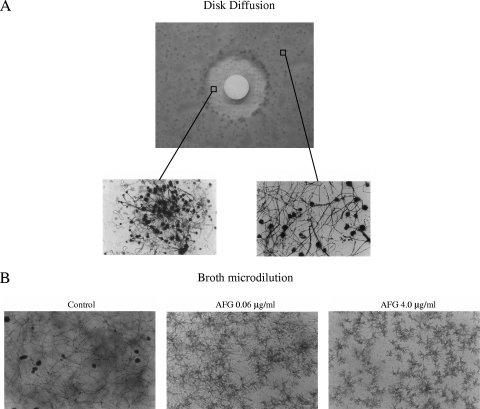
The disks embedded with AFG generated measurable IZs for all Aspergillus species and for S. brevicaulis (Table 1). Diameter sizes were distributed over a relatively narrow range (from 11 to 23 mm). In general, as the AFG concentrations increased, so did the diameters of the IZs. Despite the drug concentrations, we often observed microcolonies inside the halos. These colonies were not considered in the diameter measurements because their morphologies were similar to the short, stubby hyphal branching observed at the MEC (Fig. 1). Our data are in agreement with those reported by Arikan et al., who found consistent intrazonal growth in the halos of caspofungin disks when tested against 78 isolates of Aspergillus spp. (2).
FIG. 1.
(A) Photograph of DD assay results using a disk containing 2 μg of AFG against an A. fumigatus isolate. The two insets provide the microscopic views (Scotch tape test; magnification, ×20) of the hyphal growth within (left) and outside (right) the IZ diameter. (B) Photomicrographs of A. fumigatus isolates after 48 h of incubation with AFG at concentrations of 0.06 μg/ml and 4.0 μg/ml or with drug-free medium (control).
According to the NCCLS BD results, the other mold isolates belonging to four different genera (Rhizopus, Absidia, Fusarium, and Acremonium) did not yield any measurable zone diameters.
The Pearson's correlation coefficient between the BD and DD results was excellent, ranging from −0.928 to −0.943, regardless of either the AFG concentrations or the reading times.
We also observed reasonable intralaboratory reproducibility and interlaboratory agreement results, ranging from 92.7 to 99.2% and from 81.5 to 92.7%, respectively (Table 2).
TABLE 2.
Intralaboratory reproducibility and interlaboratory agreement results of AFG by the DD method
| Study center | Time (h) | Results at the indicated AFG concna
|
|||
|---|---|---|---|---|---|
| 2 μg/disk | 5 μg/disk | 10 μg/disk | 25 μg/disk | ||
| A | 24 | 98.4 | 95.2 | 98.4 | 95.2 |
| 48 | 99.2 | 99.2 | 96.8 | 94.4 | |
| B | 24 | 96.8 | 96.0 | 96.8 | 97.6 |
| 48 | 93.5 | 96.0 | 96.8 | 92.7 | |
| A vs B | 24 | 92.7 | 92.7 | 86.3 | 87.9 |
| 48 | 91.1 | 83.9 | 83.9 | 81.5 | |
The intralaboratory reproducibility and interlaboratory agreement results were calculated as percentages of zone diameters within 3 mm of the mean.
Therefore, based on the overall data, a 2-μg disk of AFG and a 24-h reading time might represent the best parameters for DD testing of AFG against filamentous fungi. These testing conditions are preferred in order to lower the quantity of drug necessary to perform the test and to lower the time needed to read the plates. Being less time-consuming and less labor-intensive, the DD method could be a good alternative to the BD method. Further studies which include a larger number of clinical filamentous fungi are warranted to confirm our results.
Footnotes
Published ahead of print on 15 October 2008.
REFERENCES
- 1.Arikan, S., and J. H. Rex. 2000. New agents for treatment of systemic fungal infections. Emerg. Drugs 5135-160. [DOI] [PubMed] [Google Scholar]
- 2.Arikan, S., V. Paetznick, and J. H. Rex. 2002. Comparative evaluation of disk diffusion with microdilution assay in susceptibility testing of caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 463084-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26781-803. [DOI] [PubMed] [Google Scholar]
- 4.Denning, D. W. 2002. Echinocandins: a novel class of antifungal. J. Clin. Chemother. 49889-891. [DOI] [PubMed] [Google Scholar]
- 5.De Pauw, B., T. J. Walsh, J. P. Donnelly, D. A. Stevens, J. E. Edwards, T. Calandra, P. G. Pappas, J. Maertens, O. Lortholary, C. A. Kauffman, D. W. Denning, T. F. Patterson, G. Maschmeyer, J. Bille, W. E. Dismukes, R. Herbrecht, W. W. Hope, C. C. Kibbler, B. J. Kullberg, K. A. Marr, P. Muñoz, F. C. Odds, J. R. Perfect, A. Restrepo, M. Ruhnke, B. H. Segal, J. D. Sobel, T. C. Sorrell, C. Viscoli, J. R. Wingard, T. Zaoutis, J. E. Bennett, et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 461813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new trizole SCH 56592 and the echinocandins MK-0991 (L-743,872) and LY 303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 362950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A. 2003. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam. Micol. 20121-136. [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A., B. Arthington-Skaggs, N. Iqbal, D. Ellis, M. A. Pfaller, S. Messer, M. Rinaldi, A. Fothergill, D. L. Gibbs, and A. Wang. 2007. Multicenter evaluation of a new disk agar diffusion method for susceptibility testing of filamentous fungi with voriconazole, posaconazole, itraconazole, amphotericin B, and caspofungin. J. Clin. Microbiol. 451811-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, R. N., J. T. Kirby, S. A. Messer, and D. J. Sheehan. 2007. Development of anidulafungin for disk diffusion susceptibility testing against Candida spp. Diagn. Microbiol. Infect. Dis. 58371-374. [DOI] [PubMed] [Google Scholar]
- 10.Lozano-Chiu, M., P. W. Nelson, V. L. Paetznick, and J. H. Rex. 1999. Disk diffusion method for determining susceptibilities of Candida spp. to MK-0991. J. Clin. Microbiol. 371625-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34909-917. [DOI] [PubMed] [Google Scholar]
- 12.McNeil, M. M., S. L. Nash, R. A. Hajjeh, M. A. Phelan, L. A. Conn, B. D. Plikaytis, and D. W. Warnock. 2001. Trends in mortality due to invasive mycotic disease in the United States, 1980-1997. Clin. Infect. Dis. 33641-647. [DOI] [PubMed] [Google Scholar]
- 13.Messer, S. A., J. T. Kirby, H. S. Sader, T. R. Fritsche, and R. N. Jones. 2004. Initial results from a longitudinal international surveillance programme for anidulafungin (2003). J. Antimicrob. Chemother. 541051-1056. [DOI] [PubMed] [Google Scholar]
- 14.Milici, M. E., C. M. Maida, E. Spreghini, B. Ravazzolo, S. Oliveri, G. Scalise, and F. Barchiesi. 2007. Comparison between disk diffusion and microdilution methods for determining susceptibility of clinical fungal isolates to caspofungin. J. Clin. Microbiol. 453529-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan, J., K. A. Wannemuehler, K. A. Marr, S. Hadley, D. P. Kontoyiannis, T. J. Walsh, S. K. Fridkin, P. G. Pappas, and D. W. Warnock. 2005. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med. Mycol. 43(Suppl. 1)S49-S58. [DOI] [PubMed] [Google Scholar]
- 16.NCCLS/CLSI. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. NCCLS M38-A. National Committee for Clinical Laboratory Standards. Wayne, Pa.
- 17.NCCLS/CLSI. 2002. Reference method for broth dilution susceptibility testing of yeasts. Approved standard, 2nd ed. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 18.NCCLS/CLSI. 2004. Method for antifungal disk diffusion susceptibility testing of yeasts. Approved guideline. M44-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 19.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Cantón, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdière, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Pemán, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J.-L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 423475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, and J. R. Graybill. 2000. Invasive aspergillosis: disease spectrum, treatment practices and outcomes. Medicine 79250-260. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., F. Marco, S. A. Messer, and R. N. Jones. 1998. In vitro activity of two echinocandin derivatives, LY 303366 and MK-0991 (L-743,872), against clinical isolates of Aspergillus, Fusarium, Rhizopus and other filamentous fungi. Diagn. Microbiol. Infect. Dis. 30251-255. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2005. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J. Clin. Microbiol. 435425-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribes, J. A., C. L. Vanover-Sans, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey. 2000. Practice guidelines for disease caused by Aspergillus. Clin. Infect. Dis. 30696-709. [DOI] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Walsh, T. J., A. Groll, J. Hiemenz, R. Flemming, E. Roilides, and E. Anaissie. 2004. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 1048-66. [DOI] [PubMed] [Google Scholar]
- 27.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard, and T. F. Petterson. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Disease Society of America. Clin. Infect. Dis. 46327-360. [DOI] [PubMed] [Google Scholar]
- 28.Wiederhold, N. P., L. K. Najvar, R. Bocanegra, D. Molina, M. Olivo, and J. R. Graybill. 2007. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 511616-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]