Abstract
The acyclic nucleoside phosphonate (ANP) family of drugs shows promise as therapeutics for treating poxvirus infections. However, it has been questioned whether the utility of these compounds could be compromised through the intentional genetic modification of viral sequences by bioterrorists or the selection of drug resistance viruses during the course of antiviral therapy. To address these concerns, vaccinia virus (strain Lederle) was passaged 40 times in medium containing an escalating dose of (S)-1-[3-hydroxy-2-(phosphonomethoxypropyl)-2,6-diaminopurine [(S)-HPMPDAP], which selected for mutant viruses exhibiting a ∼15-fold-increased resistance to the drug. (S)-HPMPDAP-resistant viruses were generated because this compound was shown to be one of the most highly selective and effective ANPs for the treatment of poxvirus infections. DNA sequence analysis revealed that these viruses encoded mutations in the E9L (DNA polymerase) gene, and marker rescue studies showed that the phenotype was produced by a combination of two (A684V and S851Y) substitution mutations. The effects of these mutations on drug resistance were tested against various ANPs, both separately and collectively, and compared with E9L A314T and A684V mutations previously isolated using selection for resistance to cidofovir, i.e., (S)-1-[3-hydroxy-2-(phosphonomethoxypropyl)cytosine]. These studies demonstrated a complex pattern of resistance, although as a general rule, the double-mutant viruses exhibited greater resistance to the deoxyadenosine than to deoxycytidine nucleotide analogs. The S851Y mutant virus exhibited a low level of resistance to dCMP analogues but high-level resistance to dAMP analogues and to 6-[3-hydroxy-2-(phosphonomethoxy)propoxy]-2,4-diaminopyrimidine, which is considered to mimic the purine ring system. Notably, (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]-3-deazaadenine retained marked activity against most of these mutant viruses. In vitro studies showed that the A684V mutation partially suppressed a virus growth defect and mutator phenotype created by the S851Y mutation, but all of the mutant viruses still exhibited a variable degree of reduced virulence in a mouse intranasal challenge model. Infections caused by these drug-resistant viruses in mice were still treatable with higher concentrations of the ANPs. These studies have identified a novel mechanism for the development of mutator DNA polymerases and provide further evidence that antipoxviral therapeutic strategies would not readily be undermined by selection for resistance to ANP drugs.
Poxviruses are large enveloped DNA viruses that cause a variety of diseases of veterinary and medical importance. Humans can be infected by viruses belonging to the genera Orthopoxvirus, Molluscipoxvirus, Parapoxvirus, and Yatapoxvirus, but it is the orthopoxviruses variola virus and monkeypox virus that are of primary concern. Variola virus causes smallpox, which was eradicated in the 1970s using ring containment methods and immunization with vaccinia virus (VACV) (24, 25). Human cases of monkeypox still occur in parts of central Africa, where monkeypox virus infects a rodent reservoir, and have been exported to North America by the exotic pet trade (21, 32). Although uncommon, the rare zoonotic infections with cowpox virus can pose a serious health risk to human beings, particularly among young children and immunocompromised persons (55). The only other poxvirus disease commonly seen in humans is that caused by molluscum contagiosum virus (MCV). MCV infections are rarely serious in healthy persons, although the disease can be troublesome when immunity is compromised (8, 53).
Most large-scale smallpox vaccination campaigns were discontinued about 30 years ago. This has raised concerns among public health authorities because the decline in herd immunity renders human populations at risk of reinfection from the accidental (or malicious) release of archived or unknown stocks of variola virus (31, 57). Consequently, considerable efforts to find safe, effective, and rapidly deliverable new vaccines and treatment options for smallpox have been made in recent years (12, 31). Vaccines provide the preferred tool for protecting populations threatened by renascent smallpox but cannot be used to treat disease, given that their efficacy in people already exposed to variola virus appears to be very weak. A recent report by Stittelaar et al. comparing the efficacy of postexposure vaccination to antiviral therapy of monkeypox-infected nonhuman primates showed that antiviral therapy with cidofovir, (S)-1-[3-hydroxy-2-(phosphonylmethoxypropyl)cytosine] [(S)-HPMPC], or a related compound, 6-[3-hydroxy-2-(phosphonomethoxy)propoxy]-2,4-diaminopyrimidine [(R)-HPMPO-DAPy], was more effective than smallpox vaccination (50). Consequently, antiviral drugs appear to be essential for the treatment of severe poxviral disease. The development of new antipoxvirus agents not only would provide health authorities with a means of containing smallpox but could also be used to treat MCV infections as well as the rarer infections caused by monkeypox virus, cowpox virus, and Orf virus (6, 41, 45). Such drugs could also prove very useful for treating adverse responses to vaccines, and indeed, the usefulness of antiviral agents in the treatment of severe eczema vaccinatum in a household contact of a smallpox vaccinee was recently reported (54). Two antiviral treatments (i.e., cidofovir and ST-246), each with different mechanisms of action, were used for the first time together with VACV immune globulin to successfully treat a pediatric patient suffering from eczema vaccinatum (54). Effective antiviral compounds may also be useful for limiting possible side effects occurring with the therapeutic use of poxviruses in cancer therapy. Poxviruses are currently being investigated for use in cancer therapy in order to deliver therapeutic genes to tumor cells, stimulate antitumor immunological responses, and/or simply cause the lysis of tumor cells from the replication of the viral agent (29, 44).
Many elements of the orthopoxvirus life cycle are potentially susceptible to drug interference. One such pathway is virion assembly and dissemination, which appears to be the target of the new drug ST-246 (22, 58). This orally available antiviral drug has been shown to be highly efficacious against orthopoxvirus in vitro and in vivo and is currently under development. However, the enzymes catalyzing poxvirus DNA synthesis also offer promising targets, and drugs that target the viral DNA polymerase do indeed inhibit orthopoxvirus replication in vitro and in vivo (14, 47). One of these compounds is (S)-HPMPC, which is also known as cidofovir and marketed as Vistide (Fig. 1). Cidofovir is authorized for use in treating cytomegalovirus-induced retinitis but has been used off label to successfully treat other DNA virus infections including those caused by MCV and Orf virus (13, 15, 19). The mode of drug action is well established. (S)-HPMPC is taken up by cells and converted to the diphosphoryl derivative [(S)-HPMPCpp], which is a dCTP analogue and a substrate for the virus DNA polymerase. In vitro, (S)-HPMPCpp inhibits primer extension catalyzed by purified VACV DNA polymerase and, because it can still be incorporated into DNA, also blocks “translesion” synthesis across drug molecules incorporated into the template strand (38, 39).
FIG. 1.
Chemical structures of the different ANPs evaluated in the present study. (S)-HPMPDAP was used to select for ANP-resistant viruses in this study.
The finding that the DNA polymerase is the drug target is shown by the fact that exposing VACV to HPMPC in vitro selects for mutations in the E9L (DNA polymerase) gene that likely affect both the 3′-to-5′ exonuclease and the 5′-to-3′ polymerase activities (2, 5, 33). Interestingly, three separate studies found that the passage of VACV in the presence of HPMPC leads to the selection of virus encoding either an alanine-to-threonine (2) or alanine-to-valine substitution at position 314 in the viral DNA polymerase (5, 33). Our previous study clearly showed through marker rescue experiments that the A314T substitution could confer an approximately fivefold increase in HPMPC resistance in VACV compared to wild-type virus (2). Due to this residue's location in the putative exonuclease domain of the viral polymerase, we hypothesized that this substitution may alter the ability of the enzyme to remove HPMPC residues from viral DNA. Kornbluth et al. also previously found several other exonuclease domain substitutions in the DNA polymerase genes of their HPMPC-resistant (HPMPCR) VACV, but the roles of these mutations in drug resistance are unclear, as viruses encoding these individual mutations were not isolated (33). A recent study by Becker et al. has shown that VACVs encoding only the A314V substitution are sevenfold more resistant to HPMPC than wild-type VACV; however, these viruses grow poorly in culture (5), unlike our VACV encoding the A314T substitution, which replicated as well as wild-type virus in culture (2). These results suggest that while both substitutions confer similar levels of resistance, viruses encoding the A314T substitution are likely more fit for replication in culture, although a consistent finding among these studies is that HPMPCR viruses were highly attenuated in their virulence in mouse models (2, 5). Our previous study also identified an alanine-to-valine substitution at position 684 in the putative polymerase domain of our HPMPCR VACV DNA polymerase genes (2). Marker rescue experiments were able to show that this A684V substitution could also confer HPMPC resistance independently of the A314T substitution. We hypothesized that this substitution may affect drug residue recognition of the viral enzyme due to the presumptive location of the A684 residue near the nucleotide-binding pocket of the enzyme (2). Interestingly, both Kornbluth et al. and Becker et al. also found amino acid substitutions in the putative polymerase domain of their HPMPCR isolates (5, 33), suggesting that both exonuclease and polymerase domain substitutions contribute to HPMPC resistance, although it should be noted that in those studies, recombinant viruses encoding only the polymerase domain substitutions were not constructed, and it is therefore difficult to deduce the specific contribution of these substitutions to drug resistance. What is clear from these studies is that resistance to acyclic nucleoside phosphonates (ANPs) can result from a genetic mutation at the E9L locus in VACV, similar to the development of resistance to other DNA polymerase inhibitors such as cytosine arabinoside (AraC) and phosophonoacetic acid (PAA) (51, 52).
(S)-HPMPC belongs to the class of antiviral drugs known as ANPs (Fig. 1). The first generation of ANPs can be classified into three subcategories according to chemical structure, with a clear structure-activity relationship (16, 17). First are the 3-hydroxy-2-phosphonomethoxypropyl (HPMP) derivatives, which target many different DNA viruses. (S)-HPMPC is an example of this class. Second are the (2-phosphonomethoxyethyl) (PME) derivatives, which target DNA viruses as well as hepadnaviruses and retroviruses. 9-[2-(Phosphonomethoxy)ethyl]adenine (PMEA) (adefovir) represents the prototypic example of this class of drugs. Thirdly are the 2-phosphonomethoxypropyl compounds, which exhibit little or no activity against most DNA viruses but are very effective against retroviruses and hepatitis B virus. Tenofovir is the prototype of this third class. Oral formulations of both PMEA and tenofovir have been licensed for treating hepatitis B virus and human immunodeficiency virus infections, respectively; however, the use of (S)-HPMPC has been limited by its poor oral bioavailability and renal toxicity. Despite these limitations, (S)-HPMPC remains one of the most promising candidates for the treatment of human poxvirus infections. More recently, the alkoxyalkyl esters of (S)-HPMPC and cyclic (S)-HPMPC have been shown to exhibit improved oral uptake and absorption and an increased antiviral activity compared to those of the parent compounds (10, 42, 43). The hexadecyloxypropyl ester of (S)-HPMPC (CMX001) has been demonstrated to have a good balance between high efficacy and low toxicity both in vitro and in vivo and is currently under development for the treatment of smallpox in case of a reemergence of variola virus (40).
Following the success of these drugs, two new classes of ANPs that are often referred to as “second-generation” and “third-generation” ANPs have been described. The second generation of these compounds includes the open-ring or O-linked ANPs [containing 6-(2-phosphonomethoxyalkoxy)-2,4-diaminopyrimidines] and target a broad range of DNA viruses and retroviruses (4, 18, 30). Two examples of these second-generation compounds are (R)-HPMPO-DAPy and 6-[2-(phosphonomethoxy)ethoxy]-2,4-diaminopyrimidine] (PMEO-DAPy) (Fig. 1). The third generation of ANPs encompasses the 5-aza derivatives of (S)-HPMP that display broad-spectrum anti-DNA virus activity (Fig. 1) (34-36). The availability of these new ANPs creates an opportunity to test whether different classes of virus mutants might arise under selection exerted by these compounds, versus (S)-HPMPC, tested previously (2). Of special interest is (S)-1-[3-hydroxy-2-(phosphonomethoxypropyl)-2, 6-diaminopurine [(S)-HPMPDAP] (Fig. 1), which is one of the most potent of the ANP-based antipoxvirus agents. We have previously shown that this compound was among the most effective ANPs in the treatment of VACV infections, and it was also highly effective in the inhibition of camelpox virus and Orf virus replication in organotypic epithelial raft cultures (11, 23, 48). (S)-HPMPDAP has also been proven to be active in different models of VACV infection in mice, and several prodrugs of (S)-HPMPDAP are currently under development (our unpublished data). Although (S)-HPMPDAP appears to be a promising candidate for antipoxviral treatment, the properties of (S)-HPMPDAP-resistant (HPMPDAPR) poxviruses have not been described. Thus, it was important to determine if HPMPDAPR viruses could be produced and, if so, whether these strains would exhibit cross-resistance to other ANPs. Furthermore, it was of importance to determine whether infection with HPMPDAPR poxviruses would still cause disease and whether this potential disease would respond to antiviral treatment. We show here that one can select for poxviruses exhibiting resistance to HPMPDAP and that these viruses share some similarities, as well as important differences, with HPMPCR viruses. Perhaps most importantly, the viruses selected for resistance using this strategy are, like HPMPCR viruses, highly attenuated in vivo. This study further supports the hypothesis that ANP-based antipoxviral drug therapies would not be easily circumvented by either the development of drug-resistant viruses during the course of treatment or the intentional production of drug-resistant viruses through bioterrorism.
MATERIALS AND METHODS
Cell and virus culture.
The cells, viruses, and culture methods used to select, identify, and characterize mutant viruses were described in detail previously (2). VACV strains Lederle and Western Reserve (WR) were cultured on human embryonic lung (HEL) fibroblasts or BSC-40 cells.
Materials.
The sources of the compounds were as follows: AraC (cytosine β-d-arabinofuranoside) and aphidicolin were obtained from Sigma (St. Louis, MO); (S)-HPMPC, cyclic (S)-HPMPC, and PMEA were obtained from Gilead Sciences (Foster City, CA); (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine [(S)-HPMPA], cyclic (S)-HPMPA, (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]-3-deazaadenine [3-deaza-(S)-HPMPA], cyclic 3-deaza-(S)-HPMPA, (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]-3-deazaadenine (7-deaza-HPMPA), cyclic-7-deaza-(S)-HPMPA, (S)-HPMPDAP, 9-[2-(phosphonomethoxy)ethyl]-2,6-diaminopurine and cyclic (S)-HPMPDAP, (R)-HPMPO-DAPy and cyclic (R)-HPMPO-DAPy, PMEO-DAPy, 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]-5-azacytosine [(S)-HPMP-5-azaC], and cyclic (S)-HPMP-5-azaC were obtained from A. Holý and M. Krečmerova (Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Prague, Czechoslovakia); and PAA and isatin-β-thiosemicarbazone (IBT) were obtained from Pfaltz & Bauer (Waterbury, CT).
Selection and purification of HPMPDAPR viruses.
Vaccinia virus (strain Lederle) was passaged repeatedly in HEL cells in the presence of increasing amounts of (S)-HPMPDAP. The starting concentration was 0.25 μg/ml, and it was increased twofold with each subsequent passage. Periodically, virus growth in drug-free medium was done to restore the virus titer. The viruses that replicated in the presence of a final dose of 50 μg/ml were cultured once more in drug-free medium and then plaque purified on HEL cells. Nine HPMPDAPR clones were selected for further analysis. Five wild-type clones were previously isolated from a virus stock passaged in drug-free medium (2).
Growth, CPE, virus yield, and plaque reduction assays.
Cytopathic effect (CPE) and plaque reduction assays were described elsewhere previously (2). CPE assays used viruses grown on HEL cells for 2 to 3 days, and CPE was recorded using a scale of 0 to 5. The 50% effective concentration (EC50) for CPE (EC50,CPE) was defined as the compound concentration that reduced the CPE by 50%. The reported values are means obtained from four or more independent experiments.
Plaque reduction assays were performed, in triplicate, using BSC-40 cells freeze-thawed to release virus. The EC50 is defined as the drug concentration affording 50% inhibition of plaque formation compared to drug-free plates. To measure virus growth rates, we infected BSC-40 cells with virus at a multiplicity of infection of 0.03, harvested the cells at different time points, and determined the yield by plaque assay.
To determine the effects of the different compounds on virus production, virus yield reduction assays were carried out at 72 h postinfection. HEL cells were grown in 24-well microtiter plates and infected with ∼100 PFU of each recombinant virus or wild-type strain WR. After 2 h at 37°C, the cells were washed, and medium containing different concentrations of the test compounds was added. Following 3 days of incubation, the viruses were released by freeze-thawing and then titrated by plaque assay in HEL cells. The EC90 and EC99 are defined as the drug concentrations causing a 90 and 99% reduction, respectively, in virus production as measured following viral titration by plaque assay.
DNA cloning, sequencing, and marker rescue.
Sequencing of the DNA polymerase genes of HPMPDAPR clones was done as previously described (2). DNA was extracted from virus-infected HEL cells, and the entire E9L gene was PCR amplified as two overlapping amplicons. The PCR products were purified and sequenced with the use of a cycle sequencing kit (Amersham Biosciences), 20 primers targeting both strands of the E9L gene, and a capillary DNA sequencing system (Amersham Biosciences). The data were assembled and compared to the DNA sequences obtained from the wild-type clones using Sequencher (Gene Codes Corporation) software. Molecular genetic analyses were also performed as previously described (2). Expand high-fidelity DNA polymerase (Roche Applied Science, Indianapolis, IN) was used to PCR amplify the E9L gene, which was then sequenced with primers targeting both gene strands. Portions of the E9L gene were cloned from HPMPDAPR (or wild-type) virus using PCR and primers that amplified 2.1-kb (primers VVE9L-P3F and VVE9L-P2R) or 0.9-kb (primers VVE9L-P7AF and VVE9L-P2R) fragments of the right end of the gene (see Fig. 3). (The 2.1-kb amplicon spanned both A684V and S851Y mutations, while the 0.9-kb fragment encoded just the S851Y mutation.) The PCR products were cloned into pCR2.1-TOPO (Invitrogen) and sequenced. These mutant E9L alleles were then introduced into a wild-type VACV background by marker rescue. BSC-40 cells were infected with VACV (strain WR), transfected with E9L-carrying plasmid DNAs using Lipofectamine (Invitrogen), and cultured overnight. Recombinants encoding each drug-resistant allele were then isolated by repeated passage and plaque purification in the presence of 100 to 300 μM (S)-HPMPC. Five independent isolates of each virus were retained for further analysis. For purposes of sequence comparison, Orthopoxvirus DNA polymerase gene sequences were obtained from the VOCS database (7) and aligned using Clustal W.
FIG. 3.
Map positions of mutations conferring resistance to HPMPDAP. (Top) The E9L gene and the locations of the two mutations found at positions 684 and 851 in HPMPDAPR virus. Also shown on the map are highly conserved DNA polymerase motifs, a previously mapped mutation found at position 314 in HPMPCR virus, mutations conferring resistance to other polymerase inhibitors, and the PCR amplicons used in marker rescue studies. (Bottom) Alignment of the region surrounding the S851Y mutation in vaccinia virus (VV) with corresponding portions of other B-family DNA polymerases encoded by HSV-1 (HV) and Sulfolobus solfataricus (SS). These alignments were generated by BLAST searches and show conserved residues in boldface type. An 18-amino-acid (aa) insertion found only in HSV-1 DNA polymerase has been omitted for clarity. Aph, aphidicolin.
Animal studies.
Adult NMRI mice were inoculated (or mock inoculated) with 40 to 4,000 PFU of virus diluted in 20 μl of phosphate-buffered saline. Five animals were used per viral dose delivered intranasally. The body weights were recorded over the next 30 days or until the animals had to be euthanized because of more than a 30% loss in body weight. Where indicated, 10 to 50 mg/kg of body weight/day of (S)-HPMPC, (S)-HPMP-5-azaC, or (S)-HPMPDAP was injected subcutaneously over 3 days, starting on the day of infection. All animal procedures were approved by the K. U. Leuven Animal Care Committee. To determine the extent of viral replication in the lungs from mice inoculated with VACV treated or not with the indicated doses of compound, animals were euthanized on day 7 postinfection, and lungs were aseptically removed, weighed, homogenized in minimal essential medium, and frozen at −80°C until samples were titrated on HEL cells.
Statistical analyses.
Mean EC50,CPE values from at least three independent experiments were obtained for each compound listed in Fig. 2 and 5 for the indicated viruses. Unpaired t tests were used to compare mean EC50,CPE values between wild-type and HPMPDAPR viruses or between the wild type and one of the indicated recombinant viruses. Mean EC50,CPE values and the results of these statistical tests are summarized in Tables S1 and S2 in the supplemental material. Viral strains were classified as possessing low levels of resistance if their mean EC50,CPE values were at least 2.5-fold higher than wild-type values and were significantly different (P < 0.05) from wild-type mean EC50,CPE values as assessed by unpaired t tests. If the mutant strain had an EC50,CPE value more than sevenfold higher than that of the wild type, it was classified as having a high level of resistance to the particular compound. Strains were classified as being hypersensitive to a particular compound if their mean EC50,CPE values were at least 2.0-fold lower than wild-type mean EC50,CPE values and were also statistically different from the wild type. In some cases, the mean increase (from at least three independent experiments) in EC50,CPE values were compared between two groups using unpaired t tests.
FIG. 2.

Resistance properties of plaque-purified HPMPDAPR VACV (strain Lederle). HEL cells were grown to confluence in 96-well dishes, infected with 50 PFU of each cloned virus (nine HPMPDAPR [○] and five wild-type [•] viruses), and incubated for 3 to 4 days with serial dilutions of each test compound. At least four independent experiments were performed for each test compound. The data are presented as the EC50,CPE for the HPMPDAPR clones versus the EC50,CPE for the parent VACV clones (strain Lederle). Unpaired t tests were used to compare mean EC50,CPE values of the HPMPDAPR and wild-type clones for each compound as described in Materials and Methods. P values of <0.01 were considered to be highly significant (**), P values of <0.05 were considered to be significant (*), and P values of >0.05 were considered to not be significant. The changes in mean EC50,CPE values between wild-type and HPMPDAPR strains are also shown. Low levels of resistance (change in the range of 2.5- to 7.0-fold and with a P value of <0.05) are indicated by “L,” and high levels of resistance (change of more than sevenfold and a P value of <0.05) are indicated by an “H.” “∼wt” indicates that drug sensitivity was not significantly difference from that of the wild type. The EC50,CPE values, P values, and changes in EC50,CPE values are summarized in Table S1 in the supplemental material.
FIG. 5.
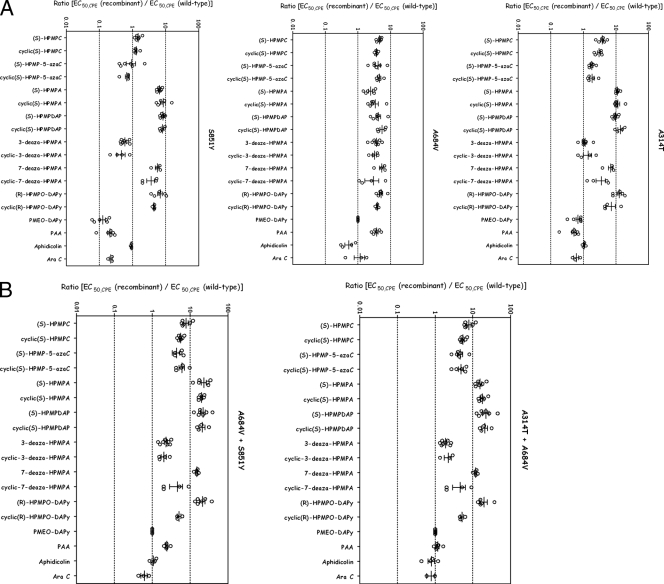
Drug resistance properties of different recombinant viruses bearing single-amino-acid (A) or double-amino-acid (B) substitutions as determined using a CPE reduction assay with HEL cells. The effects of different drugs on viruses encoding the indicated mutations are depicted. The data are presented as the ratio of the EC50,CPE for the recombinant virus to the EC50,CPE for the parent VACV strain (strain WR) for each independent experiment on a logarithmic scale. This method of presentation facilitates comparisons of the changes in resistance to different drugs where the absolute EC50,CPE values vary greatly. Mean EC50,CPE values for each compound, P values, and changes in EC50,CPE are summarized in Table S2 in the supplemental material.
Spontaneous mutation frequencies of wild-type and recombinant virus populations were analyzed by an IBT resistance assay (2). Median IBT-resistant (IBTR) plaque numbers for wild-type and recombinant virus populations were the results of four independent experiments and were compared with results from Mann-Whitney U tests (2).
To compare growth rates of wild-type and S851Y or A684V-S851Y recombinant viruses, a method described previously by Wang and Bushman was used, with minor modifications (56). Briefly, “best-fit” curves representing the exponential phase of replication (3 to 48 h postinfection) were fit by linear regression for each of three independent growth experiments using the natural logarithm (ln) of raw virus titer values. The resulting slopes of these curves, representing viral growth rates in the units “ln[(PFU/ml)/h],” of the wild type and either recombinant virus were then compared by an unpaired t test. Mean total virus titers from the 72-h time point in each of the three experiments were also used as a measure of total virus production for wild-type and recombinant viruses. These total virus titers were also compared by unpaired t tests.
For animal experiments, mortality rates were analyzed by Fisher's exact test, and a P value of ≤0.05 was considered to be significant. Viral titers in lung tissue were analyzed by Mann-Whitney U tests. All statistical analyses were performed using GraphPad prism, version 4.0, software (San Diego, CA).
RESULTS
Isolation and characterization of HPMPDAPR virus.
HPMPDAPR VACVs were obtained over the course of 1 year after 40 rounds of serial virus passage in the presence of increasing amounts of drug. Nine clones were plaque purified from this mixed stock, and the EC50,CPE values were then calculated for each of the nine HPMPDAPR isolates using different ANPs plus the unrelated reference compounds PAA and AraC. We also determined EC50,CPE values for five different plaque-purified wild-type VACV stocks. These viruses were isolated previously using an identical passage strategy but with drug selection omitted (2). Figure 2 shows the results of this analysis, and mean EC50,CPE values and the results of statistical tests are summarized in Table S1 in the supplemental material. The HPMPDAPR clones exhibited high levels of resistance to (S)-HPMPDAP and (S)-HPMPC, with EC50,CPE values ∼15- and 10-fold higher, respectively, than those of wild-type clones. These viruses also exhibited a low level of resistance (four- to sevenfold) to most of the other HPMP derivatives tested. An exception was 3-deaza-(S)-HPMPA, which remained active against both wild-type and HPMPDAPR viruses. The mutant viruses also exhibited a high level (∼10-fold increase over the wild type) of cross-resistance to (R)-HPMPO-DAPy but no alteration in their sensitivity to PMEO-DAPy, PAA, or AraC.
(S)-HPMPDAP treatment is expected to create a selection for mutations in the virus DNA polymerase. We therefore PCR amplified and sequenced the E9L genes from each of the nine HPMPDAPR clones and compared these sequences to E9L sequences from wild-type viruses (Table 1). While four amino acid changes (i.e., Q246R, L420S, A684V, and S851Y) were identified in all HPMPDAPR clones when the DNA polymerase sequences were compared to that of parental VACV strain Lederle, some amino acid substitutions were found in some but not all clones. Thus, three of the HPMPDAPR isolates each encoded a different clone-specific mutation (T513S, V545L, and R713Q). However, these viruses exhibited a pattern of resistance identical to those of the other isolates, suggesting that these mutations are idiosyncratic mutations that do not contribute to the phenotype. In addition, in all three isolates, the mutations led to rather conservative amino acid substitutions (T to S, V to L, and R to Q). All nine HPMPDAPR viruses encoded Q246 and L420 sequence variants, but these sites are polymorphic in VACV (Lederle) stocks as well as being encoded by many wild-type (i.e., drug-sensitive) orthopoxviruses. Thus, these substitutions were most likely not responsible for drug resistance. Ultimately, only two substitution mutations were found in all nine clones and were reasonable candidates for mutations causing (S)-HPMPDAP resistance. Both mutations are located in the C-terminal DNA polymerase domain (Fig. 3), and one, A684V, was previously isolated in an independent screen for viruses that are resistant to (S)-HPMPC (2). The role of the S851Y mutation was unclear, and marker rescue studies were therefore undertaken to elucidate its function.
TABLE 1.
Mutations in the E9L gene of HPMPDAPR VACV strain Lederle
| Virus | Amino acid at position(s)a:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 246 | 420 | 513 | 545 | 684 | 713 | 851 | 936-938 | |
| HPMPDAPR clones | ||||||||
| 1, 3, 5, 6, 8, 9 | Q | L | T | V | V | R | Y | ANV |
| 2 | Q | L | T | L | V | R | Y | ANV |
| 4 | Q | L | T | V | V | Q | Y | ANV |
| 7 | Q | L | S | V | V | R | Y | ANV |
| VACV consensusb | ||||||||
| VACV strain Lederle | Q/R | L/S | T | V | A | R | S | NΔG/ANV |
| VACV (all others)c | R | L/S | T | V | A | R | S | NΔG/ANV |
| Other orthopoxvirus consensusb | ||||||||
| Camelpox virus | Q | S | T | V | A | R | S | ANV |
| Cowpox virus | Q/R | S | T | V | A | R | S | NΔG/ANV |
| Ectromelia virus | Q | S | T | V | A | R | S | ANE |
| Monkeypox virus | Q | S | T | V | A | R | S | ANV |
| Variola virus | Q | S | T | V | A | R | S | ANV |
The numbering is derived from that of the vaccinia virus DNA polymerase gene.
Sequence data were accessed from the VOCS database April 2008.
Includes rabbitpox and horsepox viruses.
Marker rescue and growth analysis.
Portions of the E9L gene spanning either the S851Y mutation alone or both the A684V and S851Y mutations (Fig. 3) were cloned, sequenced again, and transfected into cells infected with VACV (strain WR). Recombinant viruses were readily isolated based upon a selection for growth in 100 to 300 μM (S)-HPMPC. These viruses were plaque purified three times (twice with and once without drug selection), the recombinant genes were PCR amplified and resequenced to confirm that no other mutations had arisen during virus isolation, and the virus was further characterized as explained below.
Growth curves were obtained for each of the new recombinant viruses, along with parental VACV (strain WR), which was cultured in parallel (Fig. 4A). Linear regression analysis was used to generate best-fit curves (Fig. 4B) from which growth rates [ln(PFU/ml)/h] for the exponential phase of viral replication were extracted as described in Materials and Methods. The mean growth rate (±standard error [SE]) for the parental (wild-type) strain (0.24 ± 0.01) was significantly higher (P < 0.05) than that for the S851Y recombinant (0.20 ± 0.003). Furthermore, the mean log10 total virus yield of the parental strain (±SE) (8.0 ± 0.1) was significantly higher (P < 0.01) than the yield of the S851Y recombinant (7.0 ± 0.1) after 72 h of replication. In contrast, the virus encoding both A684V and S851Y mutations had a mean growth rate (0.24 ± 0.02) and total yield (7.6 ± 0.3) indistinguishable from those of the parental strain (growth rate of 0.24 ± 0.02 and total yield of 7.9 ± 0.04). The single S851Y mutation was linked to a growth defect since the virus encoding this allele grew slightly slower, giving a ∼10-fold-lower titer than that of the wild-type strain. However, viruses encoding both A684V and S851Y mutations grew normally, suggesting that the A684V allele can suppress the S851Y-linked growth defect. Viruses encoding only the A684V allele were previously shown to exhibit a normal growth pattern (2).
FIG. 4.
Growth properties of recombinant VACVs. (A) BSC-40 cells were infected with the indicated viruses, in parallel, at a multiplicity of infection of 0.03. The viruses were harvested at the indicated time points, and titers were determined using BSC-40 cells. Each point represents an average of three independent determinants of titer from three independent experiments, and SE bars are shown, although they were often the size of the symbols used. (B) Analysis of rates of viral replication of indicated viruses from A. Shown are “best-fit” lines resulting from linear regression analysis of hours 3 to 48 postinfection (representing the exponential phase of replication) that were determined as described in Materials and Methods. The mean slopes (growth rate) {units = ln[(PFU/ml)/h]} for wild-type and S851Y (or A684V plus S851Y) virus regression lines were compared by an unpaired t test. Statistically significant differences (P < 0.05) between growth rates are indicated by an asterisk. Viruses encoding the S851Y mutation replicated more slowly than did wild-type virus, and this effect appears to be suppressed by the A684V mutation.
Drug susceptibility profiles of recombinant HPMPDAPR viruses.
Susceptibility profiles were determined for each of the five recombinant viruses and wild-type strain WR using a CPE reduction assay, and resistance and/or hypersusceptibility to each compound was analyzed according to the criteria described in Materials and Methods. We compared the resistance profiles of wild-type VACV (strain WR) with those of the two new recombinant viruses (encoding the S851Y or A684V-S851Y alterations) and with those of viruses previously isolated using selection for HPMPCR virus (encoding A314T, A314T-A684V, or A684V changes) (2). The viruses were cultured on HEL cells in the presence of different drug concentrations, and the EC50,CPE values were calculated for each virus-drug combination. Figure 5 shows the results of this analysis, where we have plotted the logarithm of the ratio of EC50,CPE for the mutant to EC50,CPE for the wild type. The mean EC50,CPE values for each recombinant virus with each test compound are summarized in Table S2 in the supplemental material, along with the results of statistical analysis comparing wild-type and recombinant strains.
When tested against (S)-HPMPDAP, the new recombinant viruses encoding both the A684V and S851Y changes were also highly resistant to (S)-HPMPDAP and (S)-HPMPC (Fig. 5), much like the original HPMPDAPR Lederle clones (Fig. 2). In fact, these double-mutant recombinant viruses possessed a drug susceptibility profile that is nearly identical to that observed with the original HPMPDAPR Lederle isolates, strongly arguing that resistance is linked solely to mutations in the viral DNA polymerase gene. The A684V-S851Y recombinant viruses along with the other double-mutant recombinant virus (A314T plus A684V) generally displayed the highest levels of resistance to ANPs. A singular exception to this rule is 3-deaza-(S)-HPMPA (and its cyclic form), which is discussed in greater detail below. A second general observation is that the virus encoding the A684V and S851Y changes exhibited greater (P < 0.01 by unpaired t test) increases in mean EC50,CPE values for deoxyadenosine nucleotide analogues [i.e., (S)-HPMPA, (S)-HPMPDAP, and their cyclic forms and 7-deaza-(S)-HPMPA] (mean increase ± standard deviation [SD] in EC50,CPE of 20.5-fold ± 0.4-fold over wild-type values) compared to related deoxycytidine nucleotide analogues [i.e., (S)-HPMPC and (S)-HPMP-5-azaC and their cyclic derivatives] (mean increase ± SD in EC50,CPE of 6.1-fold ± 0.4-fold over wild-type values). Viruses encoding A314T-A684V substitutions also had significantly higher (P < 0.01 by unpaired t test) increases in EC50,CPE values for deoxyadenosine analogues (mean increase ± SD in EC50,CPE of 18.7-fold ± 1.8-fold over wild-type values) than for deoxycytidine analogues (mean increase ± SD in EC50,CPE of 5.4-fold ± 0.7-fold over wild-type values). These results suggest that this bias is not dependent upon whether (S)-HPMPC or (S)-HPMPDAP is used as a method of selection for resistant strains. Double-mutant viruses also exhibited a high level of resistance to (R)-HPMPO-DAPy and cyclic (R)-HPMPO-DAPy. Wild-type VACV is naturally not inhibited by PMEO-DAPy (Fig. 2), and this phenotype is unaffected by combinations of either A314T-A684V or A684V-S851Y mutations. Another interesting observation is that the double-mutant virus showed lower levels of resistance to the 5-aza derivatives of (S)-HPMPC and cyclic (S)-HPMPC than did the parent compounds.
When one analyzes the phenotypes of viruses encoding individual mutations, it is seen that each change contributes to the resistance properties of double-mutant viruses in a complex way (Fig. 5). The A684V mutation seemed to confer a similar (about three- to fivefold) increase in EC50,CPE values across all classes of the ANPs tested. The A314T mutant showed high levels of resistance to purine-based ANPs compared to pyrimidine-based drugs, although this strain did not demonstrate significant levels of resistance to the 5-aza derivatives of (S)-HPMPC and cyclic (S)-HPMPC. The S851Y mutant viruses exhibited an even more biased phenotype. Viruses encoding only the S851Y mutation still exhibited resistance to most ANP purine analogues [i.e., (S)-HPMPA, (S)-HPMPDAP, 7-deaza-(S)-HPMPA, and their cyclic derivatives] as well as (R)-HPMPO-DAPy and cyclic (R)-HPMPO-DAPy. However, the S851Y virus exhibited little or no resistance to (S)-HPMPC and (S)-HPMP-5-azaC. These data suggest that (R)-HPMPO-DAPy and its cyclic derivative may be recognized by VACV DNA polymerase in a manner similar to that of HPMP purine derivatives.
A closer inspection of these data suggests that there are some special exceptions to these rules. Viruses encoding A314T or S851Y mutations displayed enhanced sensitivity to PMEO-DAPy (mean EC50,CPE ± SE of 34.3 ± 11.06 μg/ml for the A314T virus and mean EC50,CPE of 6.33 ± 2.83 μg/ml compared to an EC50,CPE value of >50 μg/ml for the wild-type virus), whereas the A684V mutation was neutral in this regard. Interestingly, this hypersensitivity to PMEO-DAPy appeared to correlate with hypersensitivity to PAA. Oddly, combining A314T and A684V or A684V and S851Y mutations seemed to suppress the effects of individual A314T and S851Y mutations. It is also notable that 3-deaza-HPMPA and its cyclic derivative retained good activity against most of these viruses, with the only resistant strain, encoding the A684V mutation, exhibiting a low (∼3.6-fold) increase in EC50,CPE values. Moreover, the small advantage conferred by the A684V mutation is counteracted by the A314T and S851Y mutations, as both double-mutant recombinants did not display resistance to the 3-deaza-HPMPA compounds (Fig. 5). In contrast, all of the recombinants demonstrated significant resistance to 7-deaza-(S)-HPMPA and/or its cyclic form. The PAA hypersensitivity observed with the S851Y mutant likely contributed to the significant reduction (P < 0.05 by unpaired t test) in mean increases in EC50,CPE values for PAA in the A684V-S851Y recombinant virus if one compares the mean increase over wild-type values (± SE) for the A684V mutant virus (3.8-fold ± 0.4-fold) to that observed for the A684V-S851Y recombinant strain (2.4-fold ± 0.2-fold). The hypersensitivities of the S851Y mutant were likely not simply the result of its reduced replicative ability (Fig. 4) because this recombinant virus did not display hypersensitivity to other DNA polymerase inhibitors such as aphidicolin and AraC (Fig. 5).
Effects of selected antipoxvirus drugs on VACV growth.
Virus yield reduction assays were also performed to confirm the data obtained using CPE reduction assays. The results are shown in Table 2. In Fig. S1 in the supplemental material, the dose-response curves of each drug for the different recombinant viruses and the wild-type virus (WR) are depicted. In agreement with the results shown in Fig. 5, the recombinant virus encoding the A684V and S851Y mutations exhibited both the highest proportional increase in resistance to (S)-HPMPDAP (∼26-fold) as well the highest absolute level of resistance to this compound (EC90 and EC99 of 16 and 20 μg/ml, respectively, versus 0.4 and1.6 μg/ml for wild-type virus). We also noted the same trend detected earlier in that both the A314T-A684V and the A684V-S851Y mutations generated proportionally greater resistance to purine analogues [i.e., (S)-HPMPA, (S)-HPMPDAP, 7-deaza-(S)-HPMPA, and their cyclic derivatives] than to pyrimidine analogues [i.e., (S)-HPMPC and (S)-HPMP-5-azaC, and their cyclic derivatives]. As noted above, both double-mutant viruses also exhibited a high level of resistance to (R)-HPMPO-DAPy in virus yield reduction assays.
TABLE 2.
Effects of VACV DNA polymerase mutations on drug resistance calculated using a virus yield reduction assay
| Drug | Drug concn (μg/ml)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild type
|
A314T
|
A684V
|
S851Y
|
A314T + A684V
|
A684V + S851Y
|
|||||||
| EC90 | EC99 | EC90 | EC99 | EC90 | EC99 | EC90 | EC99 | EC90 | EC99 | EC90 | EC99 | |
| (S)-HPMPC | 1.7 | 3.7 | 10 | 19 | 15 | 19 | 3.5 | 10 | 18 | 45 | 27 | 47 |
| Cyclic (S)-HPMPC | 1.6 | 3.7 | 14 | 19 | 16 | 20 | 2.0 | 10 | 11 | 32 | 27 | 50 |
| (S)-HPMP-5-azaC | 1.5 | 2.0 | 1.9 | 5.0 | 15 | 20 | 1.5 | 2.0 | 5.7 | 18 | 6.9 | 18 |
| Cyclic (S)-HPMP-5-azaC | 1.4 | 1.9 | 1.7 | 3.7 | 7.9 | 18 | 1.5 | 1.9 | 2.2 | 13 | 4.7 | 16 |
| (R)-HPMPO-DAPy | 1.6 | 2.0 | 4.2 | 14 | 2.7 | 17 | 8.8 | 18 | 17 | 37 | 12 | 37 |
| PMEO-DAPy | >200 | >200 | 200 | >200 | >200 | >200 | 20 | 200 | >200 | >200 | >200 | >200 |
| (S)-HPMPA | 0.1 | 0.5 | 2.0 | 4.5 | 0.4 | 1.3 | 0.5 | 3.2 | 1.3 | 5.0 | 0.6 | 15 |
| Cyclic (S)-HPMPA | 0.3 | 0.5 | 3.1 | 4.8 | 0.5 | 1.7 | 1.1 | 4.0 | 3.4 | 13 | 4.9 | 17 |
| (S)-HPMPDAP | 0.4 | 1.6 | 4.5 | 17 | 1.5 | 3.7 | 2.7 | 4.7 | 11 | 20 | 16 | 20 |
| 3-Deaza-(S)-HPMPA | 0.4 | 1.6 | 0.7 | 1.8 | 1.2 | 3.2 | 0.3 | 1.3 | 0.3 | 1.4 | 0.4 | 2.0 |
| PAA | 34 | 50 | 17 | 42 | 170 | 200 | 4.3 | 20 | 22 | 46 | 48 | 160 |
When one analyzes the phenotype of virus encoding individual point mutations, the data obtained using the virus yield reduction assay again reproduced that obtained using CPE reduction assays. The A684V mutation conferred a modest (2.5- to 10-fold) increase in EC90 and EC99 values across all classes of the compounds tested, with somewhat higher EC90 and EC99 measurements for pyrimidine-based ANPs. The A314T and S851Y mutants exhibited the reciprocal phenotype in that they again exhibited relatively greater increases in resistance to purine-based ANPs than to the pyrimidine-based drugs, with the S851Y mutant viruses exhibiting a slightly more biased phenotype. An interesting feature of (S)-HPMP-5-azaC [and cyclic (S)-HPMP-5-azaC] is that the N5 substitution in the pyrimidine ring counteracts the HPMPC resistance conferred by the A314T and S851Y mutations. The chemical substitution had little effect on virus encoding the A684V mutation. As noted in Table 2, except for the A684V mutant virus, all other recombinant viruses presented lower EC90 and EC99 values for the 5-aza-HPMPC derivatives than did the parent compounds. Furthermore, similarly to the results obtained by CPE reduction assay, the pattern of resistance to (R)-HPMPO-DAPy resembled that found for the different HPMP purine derivatives.
As predicted by the CPE reduction assay, 3-deaza-(S)-HPMPA remained active against all five different recombinant viruses. Although statistical analysis was not performed for these experiments, the trends were similar to those for the CPE reduction assays in that the virus harboring the A684V substitution exhibited the greatest resistance to 3-deaza-(S)-HPMPA (EC90 and EC99 of 1.2 and 3.2 μg/ml, respectively), although this was still only two- to threefold higher than values for wild-type virus (EC90 and EC99 of 0.4 and 1.6 μg/ml, respectively), which is similar to the A684V recombinant's 3.6-fold increase in mean EC50,CPE values for 3-deaza(S)-HPMPA (see Table S2 in the supplemental material). Similarly, although viruses encoding A314T-A684V and A684V-S851Y mutations exhibit high levels of resistance to most ANPs, they exhibit wild-type sensitivity to 3-deaza-(S)-HPMPA. These virus yield reduction assays also confirmed that PMEO-DAPy does not exhibit significant activity against wild-type VACV. However, the S851Y and A314T mutations made mutant viruses hypersensitive to PMEO-DAPy as well as PAA, and this suggests that the compound may interact with VACV DNA polymerase in a manner resembling that of PAA.
Pathogenicity of VACV encoding the S851Y or the A684V-S851Y mutations in mice.
We previously used a mouse intranasal infection model to show that any combination of the mutations creating HPMPCR (A314T and/or A684V substitutions) decreases VACV virulence (2). This raised the question of whether the mutations responsible for HPMPDAPR are also linked to reduced virulence. Therefore, groups of five NMRI mice were challenged with 10-fold serial dilutions of wild-type and mutant viruses and monitored for morbidity (body weight) and mortality over the next 20 days. Figure 6 shows the percentage of change in body weight for a typical pathogenicity experiment. A dose of 4,000 PFU of wild-type virus resulted in 100% mortality, while 40% mortality was seen in mice inoculated with 400 PFU. Viruses encoding the S851Y mutation were essentially avirulent, with even the highest dose of 4,000 PFU having no effect on mortality and having hardly any effect on morbidity. The A684V virus retained some pathogenicity, although it was much reduced compared to that of the wild-type strain. The highest dose of A684V virus tested (4,000 PFU) caused the death of three out of five mice, whereas lower does were not lethal. The A684V-S851Y virus exhibited an intermediate phenotype. It caused no deaths even at the highest doses tested, but mice exposed to higher doses of the double-mutant VACV did exhibit a transient morbidity. When the mortality data from different experiments performed with a virus dose of 4,000 PFU/mouse were analyzed, all recombinant viruses exhibited a significant degree of attenuation compared to the wild-type virus (P < 0.01). Thus, a challenge of 4,000 PFU resulted in 100% mortality of wild-type virus (25/25) compared to 30% (3/10) for the A684V virus; 13.3% (2/15) for the A314T-S851Y virus; and 0% for the A314T (0/5), S851Y (0/5), and A684V-S851Y (0/10) viruses. The finding that recombinant viruses are attenuated in vivo was confirmed later by quantification of the viral titers in lungs. As shown in Table 3, statistically significant differences in lung titers between the wild type and all of the mutants with the exception of the A314T single mutant were observed, providing strong evidence for the attenuation of these viruses.
FIG. 6.
Pathogenicity of recombinant viruses in mice following intranasal inoculation. Groups of five NMRI mice were infected with the indicated doses of different viruses or mock infected with saline. Each cohort was then monitored for changes in weight over the next 3 weeks. The figure shows the percentage of the change in average weight for each group of mice. The experiment could not be monitored beyond the first week for mice exposed to 4,000 PFU of wild-type virus due to mortality on the days indicated (†, dead animal).
TABLE 3.
Pathogenicity of wild-type and drug-resistant viruses in NMRI mice
| Virus | Mean virus titer (log10 PFU/g of lung tissue) ± SEa |
|---|---|
| Wild type | 6.5 ± 0.2 |
| A314T | 5.7 ± 0.2 |
| A684V | 4.4 ± 1.0b |
| S851Y | 2.7 ± 0.7b |
| A314T + A684V | 4.7 ± 0.6b |
| A684V + S851Y | 4.1 ± 0.7b |
Virus titers are expressed as mean log10 PFU/g of tissue ± SE obtained from at least four mice.
Significantly different (P < 0.05) from the wild type.
The dramatic reduction in virulence of HPMPDAPR viruses may be related to another novel phenotype, which is that these viruses exhibit a high frequency of spontaneous mutations. To test this property, we picked six plaques for each of three different viruses, expanded the 18 separate stocks with two rounds of passage in drug-free medium, and then measured the proportion of virus progeny exhibiting resistance to IBT. The results are presented in Fig. 7. Viruses encoding only the S851Y mutation generated a remarkable proportion of IBTR virus, more than 10-fold higher than that seen in stocks of wild-type virus (140 ± 20 versus 12 ± 2 IBTR plaques per 1,000 PFU plated). The mutant frequency was reduced when the virus carried both the A684V and S851Y alleles but was still significantly higher (89 ± 23 PFU IBTR virus per 1,000 PFU) than that of the wild-type virus. It is difficult to convert numbers based upon these forward mutation rates into error rates per base pair per generation, since at least three genes are known IBT targets, and the number of rounds of replication is uncertain. However, one can estimate that these stocks of S851Y VACVs might exhibit about a 4% (0.14/3 × 100%) chance of any given gene encoding a mutation.
FIG. 7.
HPMPDAPR viruses exhibit a mutator phenotype. Six different plaques were selected for each of the indicated viruses, and the amount of virus was then expanded in each stock by two further rounds of passage in the absence of selection. The titer of each stock was then determined, with and without 60 μM IBT, to determine the proportion of IBTR virus. (Top) Proportion of IBTR virus in each separate stock of virus. (Bottom) “Box-and-whisker” plot of the same data. About 14% of the virus in the S851Y stock exhibited resistance to IBT in this forward mutation assay after only two rounds of passage. This is 10-fold higher than that is seen in stocks of the wild-type virus. Asterisks indicate significant (P < 0.05) differences in median IBTR plaque numbers compared to those of wild-type controls for four independent experiments as determined by Mann-Whitney U test.
Efficacy of (S)-HPMPC, (S)-HPMP-5-azaC, and (S)-HPMPDAP in treating drug-resistant viruses.
It seems unlikely that one could select for ANP-resistant viruses during the course of drug therapy because of the high genetic stability of poxviruses and the many rounds of passage needed to isolate mutants. Considering the acute nature of poxvirus disease, which, unlike longer, chronic infections such as those associated with human immunodeficiency virus or hepatitis B virus, would require treatment over shorter time periods, the opportunity for the evolution of resistant poxviruses appears to be low. However, concerns exist regarding the possibility of mutant viruses being isolated prior to their deliberate release through bioterrorism. Could one still use ANPs to treat such drug-resistant viruses? For this purpose, we have evaluated the activities of (S)-HPMPC, (S)-HPMP-5-azaC, and (S)-HPMPDAP against the two double-mutant recombinant viruses that displayed the highest levels of resistance. NMRI mice were infected intranasally with 4,000 PFU of either mutant (A314T-A684V or A684V-S851Y) or wild-type viruses and then treated with different drugs using a subcutaneous route for 3 days starting on the day of infection. We tested doses of 10 and 50 mg/kg/day and used body weight as a quantitative measure of morbidity. As shown in Fig. 8, mice infected with the wild-type virus responded well to all of the treatment regimens; all of the mice survived the infections, and few showed much evidence of morbidity. In contrast, all five of the infected but untreated mice died. The mice challenged with 4,000 PFU of the A314T-A684V recombinant virus still responded well to treatment with 50 mg/kg/day of (S)-HPMP-5-azaC and to the higher (50-mg/kg/day) dose of (S)-HPMPC. (S)-HPMPDAP and the lower (10-mg/kg/day) dose of (S)-HPMPC were not as efficacious, although they did appear to improve the rate of recovery. Similarly, mice infected with the A684V-S851Y recombinant virus responded well to (S)-HPMPC or (S)-HPMP-5-azaC when given at 50 mg/kg/day, whereas mice treated with HPMPDAP or 10 mg/kg/day (S)-HPMPC still experienced morbidity. In conclusion, all of these drug and dose regimens offer protection against wild-type VACV infection. Moreover, (S)-HPMPC or (S)-HPMP-5-azaC administered at 50 mg/kg/dose for three consecutive days can still also afford significant protection against even drug-resistant viruses. Viral titers in the lungs of mice infected with the double recombinant viruses or the wild-type VACV strain treated with a dose of 50 mg/kg of compound were determined at 7 days postinfection. The results in Table 4 clearly show that drug treatment of wild-type infections significantly reduces viral titers. No statistically significant differences between treated and untreated animals infected with the double mutants were noted. This is likely due to the fact that the mutants already grow to lower titers than does the wild type even in the absence of drug treatment (Table 3), and therefore, the differences between untreated and treated mice are expected to be smaller than what would be found with wild-type infections.
FIG. 8.

Effect of ANP treatment on disease progression in mice. Groups of five NMRI mice were infected with 4,000 PFU of the indicated viruses or mock infected with saline. Each cohort was then subjected to the indicated daily treatment regimen (or mock treated with phosphate-buffered saline), starting on the first day of infection, and weight changes were monitored over the next 3 weeks. The figure shows the percentage of change in average weight for each group of mice. +, dead animal.
TABLE 4.
Lung titers of NMRI mice after 3 days of infection with 4,000 PFU of wild-type or drug-resistant viruses
| Virus | Mean virus titer (log10 PFU/g of lung tissue) ± SEa
|
|||
|---|---|---|---|---|
| Untreated | (S)-HPMPC | (S)-HPMPDAP | (S)-HPMP-5-azaC | |
| Wild type | 6.5 ± 0.2 | 1.9 ± 0.03b | 2.5 ± 0.4b | 2.0 ± 0.1b |
| A314T + A684V | 4.4 ± 0.6c | 2.3 ± 0.2c | 2.8 ± 0.8 | 2.1 ± 0.1 |
| A684V + S851Y | 4.1 ± 0.7c | 2.6 ± 0.6c | 4.5 ± 0.9 | 2.9 ± 0.7 |
Virus titers are expressed as mean log10 PFU/g of tissue ± SE obtained from at least four mice. Doses of compounds were 50 mg/kg/day.
Significantly different (P < 0.05) from untreated mice within the same virus group.
Significantly different (P < 0.05) from the wild type within the same treatment group.
DISCUSSION
The need for effective antipoxvirus therapies has grown with recent concerns over the potential for variola virus to be used as an agent of bioterrorism coupled with an increasing incidence of other poxvirus infections in humans. Although there are currently no clinically approved drug regimens for treating poxvirus infections, previous in vitro and in vivo evidence suggests that ANPs represent a class of drugs that holds great promise for the treatment of poxvirus infections. The nephrotoxicity and limited oral bioavailability of (S)-HPMPC have led to the development of new ANP-related compounds in order to overcome these obstacles. Despite their efficacy against a number of DNA viruses, the mechanism(s) by which these drugs inhibit viral replication and how resistance to these drugs develops remain poorly defined.
Study of how drug resistance develops provides one of the most useful approaches to an understanding of the mechanism of action of a particular antiviral compound. Our previous study began to address this question by isolating and characterizing HPMPCR VACV and demonstrating that the E9L gene represented the locus of resistance to this compound. The present study expands and complements our previous study by characterizing drug-resistant VACV strains isolated after repeated passage in the presence of another ANP, (S)-HPMPDAP, which, as described previously, has great promise for the treatment of poxvirus infections (20, 22, 48). (S)-HPMPDAP, the 2,6-diaminopurine derivative of (S)-HPMPA, was previously shown to have effective, broad-spectrum activity against a range of DNA viruses. However, the properties of viruses selected for resistance to (S)-HPMPDAP had not been described. Prolonged culture of VACV in the presence of (S)-HPMPDAP gave rise to the appearance of drug-resistant mutant viruses. These viruses exhibited a 16-fold increase (as measured by a CPE reduction assay) in resistance to (S)-HPMPDAP. Marker rescue analysis showed that the increased resistance reflects the contributions of two independently acting mutations, i.e., A684V and S851Y, both located in the C-terminal DNA polymerase domain of VACV DNA polymerase. One of these mutations, the A684V substitution, was isolated previously in an independent selection for HPMPCR virus and conferred an approximately threefold increase in resistance to nearly all of the ANPs tested, including (S)-HPMPDAP (2). The second mutation was located closer to the C terminus of the protein (S851Y) and was not previously shown to confer ANP resistance. The presence of the S851Y mutation in combination with the A684V change increased the resistance to (S)-HPMPDAP and other purine-based ANPs about fivefold, but viruses encoding only the S851Y substitution exhibited near-wild-type sensitivity to pyrimidine ANPs such as (S)-HPMPC and (S)-HPMP-5-azaC. The “purine-specific” properties of the S851Y mutation may account for why it was recovered using (S)-HPMPDAP as a selective agent and was not previously recovered using selection for HPMPC resistance. Furthermore, the “purine specificity” of the S851Y mutant virus coupled with the acquisition of resistance to (R)-HMPO-DAPy and its cyclic form provide evidence for the recognition of these compounds by the viral DNA polymerase as purine derivatives. Due to the fact that the aliphatic phosphonate chain is linked to C-6 (and not to N-1) of the pyrimidine ring (Fig. 1), this group of pyrimidine ANPs may mimic an incomplete purine ring system (4, 18).
It should be noted that although both the A684V and S851Y changes occur in the putative polymerase domain (Fig. 3), they likely alter the interaction of the DNA polymerase with the drugs in an independent manner. This hypothesis is further supported by the finding that A684V single mutant viruses are resistant to PAA, while S851Y single mutant viruses are hypersensitive to this compound (Fig. 5 and Table 2). Interestingly, the A314T substitution in the exonuclease domain also confers PAA hypersensitivity. Further parallels between the S851Y and A314T changes can be seen with respect to PMEO-DAPy. Although the PME subclass of ANP compounds is typically ineffective against poxviruses (20, 48), both the S851Y and A314T single mutant recombinants displayed hypersensitivity to PMEO-DAPy, while the highest doses tested (200 μg/ml) did not impede wild-type virus replication (Table 2). On the other hand, the presence of the A684V substitution, which confers resistance to PAA, in combination with either the A314T or S851Y change abrogates the effect of these mutations (Fig. 5 and Table 2). We have previously shown that during selective pressure with (S)-HPMPC, the A314T mutation is selected prior the A684V mutation (2). However, in the case of selection with (S)-HPMPDAP, when viruses recovered from different intermediate passages were evaluated for the presence of changes at positions 684 and 851 of the DNA polymerase, changes at both positions were noted (data not shown). This phenomenon can be explained by the reduced fitness observed both in vitro and in vivo for the S851Y mutant virus (Fig. 4 and 6), which may impair the appearance of the S851Y mutant virus alone under conditions of natural selection.
Since PME-containing compounds lack an extendable hydroxyl group (Fig. 1), they likely act as chain terminators (19). In contrast, our recent studies have suggested that HPMP-related compounds such as (S)-HPMPC and (S)-HPMPA can be faithfully incorporated into primer strands by VACV DNA polymerase without a complete inhibition of further chain elongation, although the incorporation of consecutive (S)-HPMPC [but not (S)-HPMPA] residues into the primer strand does impede elongation rates (38, 39). Furthermore, we have shown that primers containing (S)-HPMPC and (S)-HPMPA in the penultimate position are refractory to removal by VACV DNA polymerase's 3′-to-5′ proofreading activity, and when these compounds are in the template strand, they create a lesion that further blocks elongation by the VACV polymerase (38). Thus, the mechanism by which PME and HPMP compounds inhibit viral replication may substantially differ, and this might explain why cross-resistance to these compounds is not observed. Why exactly PAA hypersensitivity corresponds to PMEO-DAPy hypersensitivity is unclear since PAA is a pyrophosphate analogue and is not incorporated into DNA. On the other hand, a parallel between susceptibility and/or resistance to PME derivatives and PMEO-DAPy with PAA has also been observed in drug-resistant herpes simplex virus (HSV) and human cytomegaloviruses, showing that ANPs carrying a phosphonomethyl group may interact with the viral DNA polymerases in a manner similar to that of pyrophosphate analogues such as PAA or phosphonoformic acid (foscarnet) (1, 3, 9, 27, 28, 46).
The results obtained using CPE reduction assays were validated using virus yield reduction assays. One of the most interesting features of these studies was the manner in which 3-deaza-(S)-HPMPA exhibited activity against all five of the recombinant viruses. The mutant viruses exhibited a 3- to 18-fold increase in resistance to (S)-HPMPA, while the same viruses exhibited only a 0.8- to 2.5-fold increase in resistance to 3-deaza-(S)-HPMPA relative to wild-type VACV. Most importantly, the highly resistant double-mutant viruses exhibited essentially wild-type sensitivity to this drug. For example, when tested against 3-deaza-(S)-HPMPA, we measured an EC99 of 1.4 μg/ml for the A314T-A684V virus, versus 1.6 μg/ml for the wild-type strain. Similarly, the EC99 was 2.0 μg/ml for the A684V-S851Y virus. Another important observation was the finding that except for the A684V mutant virus, (S)-HPMP-5-azaC compares favorably to (S)-HPMPC in its activity against VACV recombinants (Fig. 5 and Table 2).
When tested in a mouse intranasal infection model, the new virus again displayed an attenuated phenotype. Virulence followed the order wild type > A684V > A684V plus S851Y > S851Y, with the S851Y virus being essentially avirulent (Fig. 6 and Table 3). This phenotype was anticipated from the growth curves, where the wild-type virus was seen to replicate more rapidly and to ∼10-fold-higher titers than viruses encoding the S851Y mutation (Fig. 4). Viruses encoding the A684V and S851Y alleles did not exhibit an obvious growth defect in vitro, but these viruses still exhibited reduced virulence in vivo (Fig. 4 and 6 and Table 3). Why these mutations should reduce yield and virulence is not immediately obvious. However, the degree of attenuation also correlated with an increase in the spontaneous mutation rates (Fig. 7). We estimate that stocks of VACV encoding the S851Y mutation might exhibit about a 4% chance of any given gene encoding a mutation after only two rounds of passage, a frequency sufficiently high that it could well reduce yields and virulence due to the large proportion of mutant viruses in such populations.
How these mutations affect polymerase function can be guessed from a consideration of their locations in the E9L gene. Based upon sequence alignments and structural modeling, we previously speculated that the A684V mutation might create drug resistance by reducing the use of diphosphorylated ANPs as substrates or by promoting trans-lesion DNA synthesis, leading to DNA replication across template-encoded drug molecules that normally act as replication-blocking “lesions” (2, 26). Such a mutation could also enhance spontaneous mutation rates by promoting the misincorporation of deoxynucleoside triphosphates or by favoring DNA synthesis across damaged templates. It is less clear how the S851Y mutation could so dramatically affect the fidelity of DNA synthesis. Sequence alignments (Fig. 3) suggest that S851 resides in an α-helix located at the base of the “thumb” in homologous DNA polymerase structures (49). Figure 9 illustrates this region of the protein using HSV-1 DNA polymerase as a model (37). These three conserved helices (Fig. 10, blue) provide structure to the thumb domain and are connected to the N terminus of the protein by an extended peptide that associates with the minor groove of the DNA in the RB69 polymerizing structure (26). Inserting a bulky aromatic ring between this α-helix and the β-sheet that comprises part of the “palm” domain might be expected to alter the position of the thumb and thus the interaction with duplex DNA. This could conceivably create resistance by altering the manner in which the DNA polymerase interacts with template-encoded drug molecules or by affecting DNA switching between polymerase and exonuclease domains. Interestingly, we previously identified several amino acid substitutions in the thumb subdomain of HSV-1 DNA polymerase that are also associated with resistance to HPMP (i.e., K960R, W998L, L1007M, and I1028T) and PME (i.e., R959H and D1070N) derivatives (1). This region of the protein is conserved in herpesvirus DNA polymerases (37), and it is likely that some of these mutations generate resistance by perturbing the interaction of the herpesviral polymerases with duplex DNA in a manner similar to that of the VACV S851Y mutation.
FIG. 9.
Proposed structural context of the S851Y mutation. The structure of HSV-1 DNA polymerase (37) was color coded to illustrate the putative location of the S851Y mutation and the sequences surrounding it. The region of interest lies at the base of the “thumb” domain near the C terminus of the enzyme and is shown here looking along the DNA-binding groove toward the proofreading exonuclease domain at the top-back of the enzyme. The three conserved α-helices found in this and other B-family DNA polymerases are shown as blue tubes, the surrounding amino acids are shown in red and purple (with red tags marking conserved residues), and the putative homolog of residue S851 is shown as a yellow side chain. This is phenylalanine in HSV-1 DNA polymerase. (Bottom) A closer view that illustrates how residue F978 packs closely into the β-sheet comprising part of the “palm” of the enzyme. Also shown in the sequence alignment are the positions of two mutations previously shown to confer resistance to PME (R959H) and HPMP (K960R) derivatives in HSV-1 DNA polymerase (1). Images were generated using Cn3D (v4.1).
Finally, the ability to select for ANP-resistant viruses creates concern as to whether the proposed strategies for treating renascent smallpox or monkeypox might be undermined by selection for drug-resistant viruses. Our studies show that this new ANP-resistant poxvirus, like those that were described previously (2, 5, 33), is still attenuated in mice and also still sensitive to drug therapy. None of these mutant viruses exhibit a high level of resistance, and these infections can still be treated using higher doses of (S)-HPMPC, (S)-HPMPDAP, or (S)-HPMP-5-azaC (Fig. 8 and Table 4). Even if it is difficult to extrapolate to the human host, these findings warrant the further development of these drugs as potential antipoxvirus agents. Despite the fact that no data on PK, tissue distribution, and metabolism of the compounds are available, the model of infection that we have used to test the pathogenicity of the VACV mutants and activity of the compounds is a well-recognized model, and it is generally accepted that compounds that are active in this model of infection present great promise for the treatment of poxvirus infections. Although one can never exclude the possibility that it may be possible to generate mutant poxviruses that are both fully virulent and highly resistant to all classes of ANPs, two independent screens for HPMPCR and HPMPDAPR viruses have thus far failed to obtain such isolates. Most probably, fitness and resistance are mutually exclusive phenotypes. Hence, ANPs continue to represent an important class of antiviral compounds that will be critical, along with vaccines and infection control measures, in providing an effective smallpox containment strategy.
Supplementary Material
Acknowledgments
We thank L. Reha-Krantz for advice on the molecular genetic properties of DNA polymerases and Anita Camps and Steven Carmans for excellent technical assistance.
D.B.G. is an NSERC Canada Graduate Doctoral Scholar. This research was supported by awards from the CIHR (to D.H.E.), research project IOCB Z40550506; the Centre for New Antivirals and Antineoplastics grant 1M0508 by the Ministry of Education, Youth, and Sports of the Czech Republic; the program of targeted projects of Academy of Sciences of the Czech Republic grant 1QS400550501; Gilead Sciences and IOCB Research Centre; Fonds voor Wetenschappelijk Onderzoek (FWO-Vlaanderen) grant G.0680.08; grant AI 062540-01 from the NIH, Bethesda, MD; and the Centre of Excellence grant CE/05/015.
Footnotes
Published ahead of print on 8 October 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Andrei, G., P. Fiten, M. Froeyen, E. De Clercq, G. Opdenakker, and R. Snoeck. 2007. DNA polymerase mutations in drug-resistant herpes simplex virus mutants determine in vivo neurovirulence and drug-enzyme interactions. Antivir. Ther. 12719-732. [PubMed] [Google Scholar]
- 2.Andrei, G., D. B. Gammon, P. Fiten, E. De Clercq, G. Opdenakker, R. Snoeck, and D. H. Evans. 2006. Cidofovir resistance in vaccinia virus is linked to diminished virulence in mice. J. Virol. 809391-9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldanti, F., N. Lurain, and G. Gerna. 2004. Clinical and biologic aspects of human cytomegalovirus resistance to antiviral drugs. Hum. Immunol. 65403-409. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J., C. Pannecouque, L. Naesens, G. Andrei, R. Snoeck, E. De Clercq, D. Hockova, and A. Holy. 2004. 6-[2-Phosphonomethoxy)alkoxy]-2,4-diaminopyrimidines: a new class of acyclic pyrimidine nucleoside phosphonates with antiviral activity. Nucleosides Nucleotides Nucleic Acids 231321-1327. [DOI] [PubMed] [Google Scholar]
- 5.Becker, M. N., M. Obraztsova, E. R. Kern, D. C. Quenelle, K. A. Keith, M. N. Prichard, M. Luo, and R. W. Moyer. 2008. Isolation and characterization of cidofovir resistant vaccinia viruses. Virol. J. 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray, M. 2003. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antivir. Res. 58101-114. [DOI] [PubMed] [Google Scholar]
- 7.Brodie, R., A. J. Smith, R. L. Roper, V. Tcherepanov, and C. Upton. 2004. Base-By-Base: single nucleotide-level analysis of whole viral genome alignments. BMC Bioinformatics 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, J., C. K. Janniger, R. A. Schwartz, and N. B. Silverberg. 2006. Childhood molluscum contagiosum. Int. J. Dermatol. 4593-99. [DOI] [PubMed] [Google Scholar]
- 9.Chou, S. W. 2001. Cytomegalovirus drug resistance and clinical implications. Transpl. Infect. Dis. 3(Suppl. 2)20-24. [DOI] [PubMed] [Google Scholar]
- 10.Ciesla, S. L., J. Trahan, W. B. Wan, J. R. Beadle, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 59163-171. [DOI] [PubMed] [Google Scholar]
- 11.Dal Pozzo, F., G. Andrei, A. Holy, J. van den Oord, A. Scagliarini, E. De Clercq, and R. Snoeck. 2005. Activities of acyclic nucleoside phosphonates against Orf virus in human and ovine cell monolayers and organotypic ovine raft cultures. Antimicrob. Agents Chemother. 494843-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darling, R. G., C. L. Catlett, K. D. Huebner, and D. G. Jarrett. 2002. Threats in bioterrorism. I. CDC category A agents. Emerg. Med. Clin. N. Am. 20273-309. [DOI] [PubMed] [Google Scholar]
- 13.De Clercq, E. 1996. Therapeutic potential of cidofovir (HPMPC, Vistide) for the treatment of DNA virus (i.e. herpes-, papova-, pox- and adenovirus) infections. Verh. K. Acad. Geneeskd. Belg. 5819-47. [PubMed] [Google Scholar]
- 14.De Clercq, E. 2001. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 14382-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Clercq, E. 2002. Cidofovir in the treatment of poxvirus infections. Antivir. Res. 551-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Clercq, E. 2007. Acyclic nucleoside phosphonates: past, present and future. Bridging chemistry to HIV, HBV, HCV, HPV, adeno-, herpes-, and poxvirus infections: the phosphonate bridge. Biochem. Pharmacol. 73911-922. [DOI] [PubMed] [Google Scholar]
- 17.De Clercq, E. 2007. The acyclic nucleoside phosphonates from inception to clinical use: historical perspective. Antivir. Res. 751-13. [DOI] [PubMed] [Google Scholar]
- 18.De Clercq, E., G. Andrei, J. Balzarini, P. Leyssen, L. Naesens, J. Neyts, C. Pannecouque, R. Snoeck, C. Ying, D. Hockova, and A. Holy. 2005. Antiviral potential of a new generation of acyclic nucleoside phosphonates, the 6-[2-(phosphonomethoxy)alkoxy]-2,4-diaminopyrimidines. Nucleosides Nucleotides Nucleic Acids 24331-341. [DOI] [PubMed] [Google Scholar]
- 19.De Clercq, E., and A. Holy. 2005. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 4928-940. [DOI] [PubMed] [Google Scholar]
- 20.De Clercq, E., and J. Neyts. 2004. Therapeutic potential of nucleoside/nucleotide analogues against poxvirus infections. Rev. Med. Virol. 14289-300. [DOI] [PubMed] [Google Scholar]
- 21.Di Giulio, D. B., and P. B. Eckburg. 2004. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 415-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duraffour, S., R. Snoeck, R. de Vos, J. J. van Den Oord, J. M. Crance, D. Garin, D. E. Hruby, R. Jordan, E. De Clercq, and G. Andrei. 2007. Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures. Antivir. Ther. 121205-1216. [PubMed] [Google Scholar]
- 23.Duraffour, S., R. Snoeck, M. Krecmerova, J. van den Oord, R. de Vos, A. Holy, J. M. Crance, D. Garin, E. De Clercq, and G. Andrei. 2007. Activities of several classes of acyclic nucleoside phosphonates against camelpox virus replication in different cell culture models. Antimicrob. Agents Chemother. 514410-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenner, F. 1982. A successful eradication campaign. Global eradication of smallpox. Rev. Infect. Dis. 4916-930. [DOI] [PubMed] [Google Scholar]
- 25.Fenner, F. 1993. Smallpox: emergence, global spread, and eradication. Hist. Philos. Life Sci. 15397-420. [PubMed] [Google Scholar]
- 26.Franklin, M. C., J. Wang, and T. A. Steitz. 2001. Structure of the replicating complex of a pol alpha family DNA polymerase. Cell 105657-667. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert, C., J. Bestman-Smith, and G. Boivin. 2002. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist. Updat. 588-114. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert, C., and G. Boivin. 2005. Human cytomegalovirus resistance to antiviral drugs. Antimicrob. Agents Chemother. 49873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert, P. A., and G. McFadden. 2006. Poxvirus cancer therapy. Recent patents. Anti-Infect. Drug Discov. 1309-321. [DOI] [PubMed] [Google Scholar]
- 30.Hockova, D., A. Holy, M. Masojidkova, G. Andrei, R. Snoeck, E. De Clercq, and J. Balzarini. 2003. 5-substituted-2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidines—acyclic nucleoside phosphonate analogues with antiviral activity. J. Med. Chem. 465064-5073. [DOI] [PubMed] [Google Scholar]
- 31.Jahrling, P. B., E. A. Fritz, and L. E. Hensley. 2005. Countermeasures to the bioterrorist threat of smallpox. Curr. Mol. Med. 5817-826. [DOI] [PubMed] [Google Scholar]
- 32.Kile, J. C., A. T. Fleischauer, B. Beard, M. J. Kuehnert, R. S. Kanwal, P. Pontones, H. J. Messersmith, R. Teclaw, K. L. Karem, Z. H. Braden, I. Damon, A. S. Khan, and M. Fischer. 2005. Transmission of monkeypox among persons exposed to infected prairie dogs in Indiana in 2003. Arch. Pediatr. Adolesc. Med. 1591022-1025. [DOI] [PubMed] [Google Scholar]
- 33.Kornbluth, R. S., D. F. Smee, R. W. Sidwell, V. Snarsky, D. H. Evans, and K. Y. Hostetler. 2006. Mutations in the E9L polymerase gene of cidofovir-resistant vaccinia virus strain WR are associated with the drug resistance phenotype. Antimicrob. Agents Chemother. 504038-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krecmerova, M., A. Holy, A. Piskala, M. Masojidkova, G. Andrei, L. Naesens, J. Neyts, J. Balzarini, E. De Clercq, and R. Snoeck. 2007. Antiviral activity of triazine analogues of 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine (cidofovir) and related compounds. J. Med. Chem. 501069-1077. [DOI] [PubMed] [Google Scholar]
- 35.Krecmerova, M., A. Holy, R. Pohl, M. Masojidkova, G. Andrei, L. Naesens, J. Neyts, J. Balzarini, E. De Clercq, and R. Snoeck. 2007. Ester prodrugs of cyclic 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]-5-azacytosine: synthesis and antiviral activity. J. Med. Chem. 505765-5772. [DOI] [PubMed] [Google Scholar]
- 36.Lebeau, I., G. Andrei, M. Krecmerova, E. De Clercq, A. Holy, and R. Snoeck. 2007. Inhibitory activities of three classes of acyclic nucleoside phosphonates against murine polyomavirus and primate simian virus 40 strains. Antimicrob. Agents Chemother. 512268-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, S., J. D. Knafels, J. S. Chang, G. A. Waszak, E. T. Baldwin, M. R. Deibel, Jr., D. R. Thomsen, F. L. Homa, P. A. Wells, M. C. Tory, R. A. Poorman, H. Gao, X. Qiu, and A. P. Seddon. 2006. Crystal structure of the herpes simplex virus 1 DNA polymerase. J. Biol. Chem. 28118193-18200. [DOI] [PubMed] [Google Scholar]
- 38.Magee, W. C., K. A. Aldern, K. Y. Hostetler, and D. H. Evans. 2008. Cidofovir and (S)-9-[3-hydroxy-(2-phosphonomethoxy)propyl]adenine are highly effective inhibitors of vaccinia virus DNA polymerase when incorporated into the template strand. Antimicrob. Agents Chemother. 52586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magee, W. C., K. Y. Hostetler, and D. H. Evans. 2005. Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate. Antimicrob. Agents Chemother. 493153-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker, S., E. Touchette, C. Oberle, M. Almond, A. Robertson, L. C. Trost, B. Lampert, G. Painter, and R. M. Buller. 2008. Efficacy of therapeutic intervention with an oral ether-lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antivir. Res. 7739-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prichard, M. N., and E. R. Kern. 2005. Orthopoxvirus targets for the development of antiviral therapies. Curr. Drug Targets Infect. Disord. 517-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quenelle, D. C., D. J. Collins, W. B. Wan, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2004. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiz, J. C., J. R. Beadle, K. A. Aldern, K. A. Keith, C. B. Hartline, E. R. Kern, and K. Y. Hostetler. 2007. Synthesis and antiviral evaluation of alkoxyalkyl-phosphate conjugates of cidofovir and adefovir. Antivir. Res. 7587-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen, Y., and J. Nemunaitis. 2005. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol. Ther. 11180-195. [DOI] [PubMed] [Google Scholar]
- 45.Sliva, K., and B. Schnierle. 2007. From actually toxic to highly specific—novel drugs against poxviruses. Virol. J. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snoeck, R., G. Andrei, and E. De Clercq. 1996. Patterns of resistance and sensitivity to antiviral compounds of drug-resistant strains of human cytomegalovirus selected in vitro. Eur. J. Clin. Microbiol. Infect. Dis. 15574-579. [DOI] [PubMed] [Google Scholar]
- 47.Snoeck, R., G. Andrei, and E. De Clercq. 2007. Therapy of poxvirus infections, p. 375-395. In A. A. Mercer, A. Schmidt, and O. Weber (ed.), Poxviruses. Birkhäuser Verslag, Basel, Switzerland.
- 48.Snoeck, R., A. Holy, C. Dewolf-Peeters, J. van den Oord, E. De Clercq, and G. Andrei. 2002. Antivaccinia activities of acyclic nucleoside phosphonate derivatives in epithelial cells and organotypic cultures. Antimicrob. Agents Chemother. 463356-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spicer, E. K., J. Rush, C. Fung, L. J. Reha-Krantz, J. D. Karam, and W. H. Konigsberg. 1988. Primary structure of T4 DNA polymerase. Evolutionary relatedness to eucaryotic and other procaryotic DNA polymerases. J. Biol. Chem. 2637478-7486. [PubMed] [Google Scholar]
- 50.Stittelaar, K. J., J. Neyts, L. Naesens, G. van Amerongen, R. F. van Lavieren, A. Holy, E. De Clercq, H. G. Niesters, E. Fries, C. Maas, P. G. Mulder, B. A. van der Zeijst, and A. D. Osterhaus. 2006. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature 439745-748. [DOI] [PubMed] [Google Scholar]
- 51.Taddie, J. A., and P. Traktman. 1991. Genetic characterization of the vaccinia virus DNA polymerase: identification of point mutations conferring altered drug sensitivities and reduced fidelity. J. Virol. 65869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taddie, J. A., and P. Traktman. 1993. Genetic characterization of the vaccinia virus DNA polymerase: cytosine arabinoside resistance requires a variable lesion conferring phosphonoacetate resistance in conjunction with an invariant mutation localized to the 3′-5′ exonuclease domain. J. Virol. 674323-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyring, S. K. 2003. Molluscum contagiosum: the importance of early diagnosis and treatment. Am. J. Obstet. Gynecol. 189S12-S16. [DOI] [PubMed] [Google Scholar]
- 54.Vora, S., I. Damon, V. Fulginiti, S. G. Weber, M. Kahana, S. L. Stein, S. I. Gerber, S. Garcia-Houchins, E. Lederman, D. Hruby, L. Collins, D. Scott, K. Thompson, J. V. Barson, R. Regnery, C. Hughes, R. S. Daum, Y. Li, H. Zhao, S. Smith, Z. Braden, K. Karem, V. Olson, W. Davidson, G. Trindade, T. Bolken, R. Jordan, D. Tien, and J. Marcinak. 2008. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 461555-1561. [DOI] [PubMed] [Google Scholar]
- 55.Vorou, R. M., V. G. Papavassiliou, and I. N. Pierroutsakos. 2008. Cowpox virus infection: an emerging health threat. Curr. Opin. Infect. Dis. 21153-156. [DOI] [PubMed] [Google Scholar]
- 56.Wang, G. P., and F. D. Bushman. 2006. A statistical method for comparing viral growth curves. J. Virol. Methods 135118-123. [DOI] [PubMed] [Google Scholar]
- 57.Whitley, R. J. 2003. Smallpox: a potential agent of bioterrorism. Antivir. Res. 577-12. [DOI] [PubMed] [Google Scholar]
- 58.Yang, G., D. C. Pevear, M. H. Davies, M. S. Collett, T. Bailey, S. Rippen, L. Barone, C. Burns, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, R. L. Buller, E. Touchette, K. Waller, J. Schriewer, J. Neyts, E. DeClercq, K. Jones, D. Hruby, and R. Jordan. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 7913139-13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.