Abstract
Borna disease virus (BDV) is one of the infectious agents that causes diseases of the central nervous system in a wide range of vertebrate species and, perhaps, in humans. The phosphoprotein (P) of BDV, an essential cofactor of virus RNA-dependent RNA polymerase, is required for virus replication. In this study, we identified the gamma-aminobutyric acid receptor-associated protein (GABARAP) with functions in neurobiology as one of the viral P protein-interacting cellular factors by using an approach of phage display-based protein-protein interaction analysis. Direct binding between GABARAP and P protein was confirmed by coimmunoprecipitation, protein pull-down, and mammalian two-hybrid analyses. GABARAP originally was identified as a linker between the gamma-aminobutyric acid receptor (GABAR) and the microtubule to regulate receptor trafficking and plays important roles in the regulation of the inhibitory neural transmitter gamma-aminobutyric acid (GABA). We showed that GABARAP colocalizes with P protein in the cells infected with BDV or transfected with the P gene, which resulted in shifting the localization of GABARAP from the cytosol to the nucleus. We further demonstrated that P protein blocks the trafficking of GABAR, a principal GABA-gated ion channel that plays important roles in neural transmission, to the surface of cells infected with BDV or transfected with the P gene. We proposed that during BDV infection, P protein binds to GABARAP, shifts the distribution of GABARAP from the cytoplasm to the nucleus, and disrupts the trafficking of GABARs to the cell membranes, which may result in the inhibition of GABA-induced currents and in the enhancement of hyperactivity and anxiety.
Borna disease virus (BDV), a nonsegmental negative-strand RNA virus, belongs to the Bornaviridae family and is characterized by low productivity, neurotropism, and the nuclear localization of transcription and replication (12, 33). BDV was reported to cause diseases of the central nervous system (CNS) in sheep and horses originally and then in a wide range of other vertebrate species (25). Epidemiological studies have shown that a higher prevalence of BDV infection was found in psychiatric patients than in controls, indicating that BDV is a potential human pathogen related to psychiatric diseases (1, 19). In contrast, some reports suggested that BDV does not play significant roles in human health (9, 36).
One of the most prominent features of BDV infection is the heavy inflammatory reaction in the CNS and the rare degeneration of neurons in naturally infected hosts (25). In experimentally infected rats, the histopathology of the CNS is dependent on the immune status of the host at the time of inoculation, the genetic background, and the route of infection (33). The inoculation of immunocompetent adult rats with BDV results in marked immune-mediated meningoencephalitis consistent with the classical Borna disease. In the process of the persistent infection of BDV in adult rats, the degeneration of the neurons is observed. In contrast, immunoincompetent rats such as neonates show a tolerance to the infection of BDV without signs of Borna disease or encephalitis. Neonatal infection with the virus causes significant alterations in the development of the CNS (23). These studies provided evidence for direct effects of BDV infection on cellular functions in the absence of immunopathological degeneration.
The phosphoprotein (P) of BDV, an essential cofactor of virus RNA-dependent RNA polymerase, is regulated by protein kinase Cɛ and casein kinase II (26) and binds to the N, X, and L proteins of BDV to modulate the active viral polymerase complex (24, 27, 28). Our recent study showed that BDV and its P protein could inhibit the expression of inducible nitric oxide synthase (iNOS) in astrocytes, suggesting that BDV establishes a persistent infection at least in part due to its inhibition of iNOS to impair immune responses (22). This viral protein also can counteract the expression of TBK-1-dependent beta interferon, and thus it can establish an antiviral state (34). Moreover, P protein binds directly to a multifunctional protein (HMGB-1) and represses p53-mediated transcriptional activity (14, 38). Finally, the expression of BDV P protein was demonstrated to induce behavioral and neurological abnormalities in transgenic mice (13).
Gamma-aminobutyric acid (GABA) exists predominantly in the brain, with glycine as one of the two inhibitory amino acid neural transmitters (20). Its receptor, GABA type A receptor (GABAA-R), is the principal GABA-gated ion channel (17) and plays important roles in pharmacology. GABAA-R acts as the target of benzodiazepine-related drugs, such as diazepam, triazolam, and alprazolam, which are widely used for the treatment of anxiety and insomnia. GABAA-R-associated protein (GABARAP) originally was identified as a linker between GABAA-Rs and microtubules. Recent study has demonstrated that GABARAP can interact with the γ2 subunit of GABAA-R and has a functional effect on the regulation of GABA activities by disrupting the trafficking of GABAA-Rs to the cell membranes (4, 5).
In this study, we demonstrated that BDV P protein can bind directly to GABARAP both in vitro and in vivo by using different approaches. Biological effects of the interaction of P protein with GABARAP and the molecular mechanisms underlying the effects of P protein on the regulation of GABARAP and GABAA-Rs also were investigated and discussed.
MATERIALS AND METHODS
Cell culture and virus propagation.
293T cells, HeLa cells, COS-7 cells, and OL cells were obtained from the ATCC and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS). The BDV viral strain H1766 used for the infection of COS-7 and 293T cells in this study and a BDV persistent infecting cell line (BDV/OL cells) were gifts from K. Ikuta of Osaka University. In general, adherent cells were infected at a multiplicity of infection of 0.1 in DMEM containing 2% FBS and then cultured for 5 to 7 days (3). All cell cultures were maintained at 37°C in 5% CO2.
Plasmid construction.
The plasmid pcDNA-P was kindly provided by Thorsten Wolff and Peter Staeheli. Plasmids pCMV-α1, pCMV-β2, and pRK5-γ2L-GFPN (15), expressing the α1, β2, and γ2 subunits of the GABAA-R, respectively, were kindly provided by Bill Wisden and Erwin Sigel. The P gene of BDV was amplified from pcDNA-P by PCR using primers 5′-TTTGGATCCATGGCAACGCGACCATCGAG-3′ and 5′-TTTCTCGAGTGGTATGATGTCCCACTCATC-3′. The resulting PCR product then was subcloned into pET-28a to yield plasmid pET-28a-P. To generate pVP16-P, the P gene was amplified from pcDNA-P by PCR using primers 5′-CGCGGATCCCCATGGCAACGCGACCATC-3′ and 5′-CCCAAGCTTTTATGGTATGATGTCCCAC-3′, and the PCR product was subcloned into pVP16. To generate pEGFP-P, the P gene was amplified from pcDNA-P using primers 5′-CCCAAGCTTATGGCAACGCGACCATCG-3′ and 5′-CGCGGATCCTTATGGTATGATGTCCCAC-3′, and the PCR product then was subcloned into pEGFP-C3.
The GABARAP cDNA was amplified by PCR using primers 5′-CGCGGATCCGGATGAAGTTCGTGTACA-3′ and 5′-CCCAAGCTTTCACAGACCGTAGACACT-3′ to introduce BamHI and HindIII cloning sites and then was subcloned into appropriate restriction sites of plasmids pM, pET-28a, and pCMV-Tag2C to generate the plasmids pM-GABARAP, pET-28a-GABARAP, and pCMV-Tag2C-GABARAP, respectively. The GABARAP cDNA was amplified by PCR using primers 5′-CCCAAGCTTGGATGAAGTTCGTGTACA-3′ and 5′-CGCGGATCCTCACAGACCGTAGACACT-3′ and then subcloned into pAsRed2C1 to yield plasmid pAsRed2C1-GABARAP.
The mutant forms of the GABARAP and P genes in the expression plasmids were generated from the wild-type plasmid by using PCR and recloning techniques. The primers used in PCR to create the mutant plasmids are available on request. The introduction of the correct mutations for each mutant was confirmed by DNA sequencing.
Recombinant P protein purification and anti-P antibody generation.
pET-28a-P, carrying the P gene fused in frame with an N-terminal hexahistidine tag, was transformed into Escherichia coli strain BL21, grown at 37°C to an optical density at 600 nm of 0.5, induced with 1 mM isopropyl-ß-d-thiogalactopyranoside (IPTG), and cultured for another 2 to 4 h at 37°C. After harvest, the cells were resuspended in buffer C (50 mM Tris-HCl, pH 8.0, 500 mM NaCl, 5 mM MgCl2, 5% glycerol, 0.5% NP-40, 2 mM imidazole, and 7 mM β-mercaptoethanol), lysed by sonication, and centrifuged for 20 min at 8,500 × g and 4°C. Supernatants then were incubated at 4°C for 2 h with equilibrated Ni+-NTA agarose beads (Qiagen). The beads were washed with buffer C containing 20 mM imidazole, and recombinant proteins were eluted in buffer D (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 5 mM MgCl2, 20% glycerol, 0.1% NP-40, 7 mM β-mercaptoethanol, and 250 mM imidazole). Protein concentrations were determined using a Bio-Rad protein assay. Purified recombinant protein was analyzed on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel by staining with Coomassie brilliant blue.
The recombinant P protein was used as an immunogen. Rabbits (4 weeks old) were immunized with the recombinant P protein with complete Freund's adjuvant. The polyclonal anti-BDV-P antibody was prepared from immunized rabbits' hyperimmune sera by ammonium sulfate precipitation.
T7Select biopanning.
A premade T7Select lung cDNA library (no. 70646-3; Novagen) was screened with the T7Select biopanning kit (Novagen) according to the manufacturer's instructions. The P protein expressed from bacteria and purified by affinity chromatography was used as the bait. After five rounds of selection, 96 randomly selected clones were picked up from each library, and the sequences of each of the clones were determined by DNA sequence analysis and searched for homology to known sequences with the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST).
Mammalian two-hybrid analysis.
293T cells or HeLa cells were transfected with luciferase reporter plasmid pG5luc (Promega) and test plasmids, respectively, using Lipofectamine 2000 transfection reagent in 24-well culture plates. Forty-eight hours after transfection, cells were lysed in 100 μl of lysis buffer for 60 min with shaking at room temperature. After centrifugation at 12,000 × g for 5 min at 4°C, the cell extracts were assayed for luciferase activity using the dual-luciferase reporter assay system (Promega) according to the manufacturer's recommendations.
Immunoprecipitation assay.
293T cells were cotransfected with plasmids pCMV-GABARAP expressing Flag-tagged GABARAP and pCDNA-P expressing P protein. Forty-eight hours posttransfection, transfected cells were lysed by freeze-thaw cycling in a buffer containing 10 mM Tris, pH 7.6, 150 mM NaCl, 0.5% NP-40, and 1.0 mM phenylmethylsulfonyl fluoride. After centrifugation, the soluble fraction was immunoprecipitated with anti-P antibody for 2 h at 4°C, and the precipitates then were recovered by incubation with protein A/G-agarose beads (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 2 h at 4°C. After being thoroughly washed, proteins bound to the agarose beads were separated by SDS-PAGE and electrotransferred onto a polyvinylidene difluoride membrane, which then was blocked with 5% skimmed milk in phosphate-buffered saline (PBS)-0.05% Tween 20 (PBS-T) overnight at 4°C. The membrane was reacted with anti-Flag mouse monoclonal antibody in PBS-T containing 5% skimmed milk for 1 h at room temperature. After being washed, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) for 1 h at room temperature and visualized by the enhanced chemiluminescence system (Pierce).
To verify the interaction between BDV P protein and endogenous GABARAP, 293T cells were infected with BDV. Protein extracts prepared from infected or noninfected cells were immunoprecipitated with anti-GABARAP antibody (sc-28938; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and detected by anti-P antibody.
Protein pull-down assays.
Purified hexahistidine-tagged recombinant proteins (40 μg) were incubated with 300 μl Ni+-NTA agarose beads in 1 ml binding buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 0.5% NP-40, and 1 mM dithiothreitol [DTT]) for 2 h at 4°C. After two washes with 1 ml binding buffer, some of the hybridized beads were analyzed by SDS-PAGE, and the rest of the beads were incubated with extracts of BDV-infected (OL/BDV) or uninfected (OL) cells in 1 ml of hybridization buffer (10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 0.5 mM EDTA, 0.1% NP-40, 1 mM DTT, and 10 mM imidazole) for 1 h at 4°C. Beads then were washed four times with hybridization buffer, and bound proteins were eluted in Laemmli buffer and determined by Western blot analysis.
Laser-scanning confocal microscopy analysis.
Recombinant plasmids pAsRed2C1-GABARAP and/or pEGFP-P, pAsRed2C1-GABARAP, pEGFP-P134, and 1.2 μl Lipofectamine 2000 were mixed in 30 μl DMEM for 20 min and then cotransfected into COS-7 cells. Transfected cells were cultured on coverslips in a 24-well plate. Twenty-four to 36 h posttransfection, cells were fixed with 4% paraformaldehyde for 15 min, washed three times with 1× PBS, counterstained with Hoechst 33258 for 10 min, and then washed three times with 1× PBS. Fluorescence was detected by using a confocal laser-scanning microscope (LSM 510 META; Carl Zeiss). For the indirect immunofluorescence assay, BDV-infected COS-7 cells were transfected with plasmid pAsRed2C1-GABARAP and fixed with 4% paraformaldehyde prior to treatment with 0.4% Triton X-100. After a reaction with the optimal anti-P antibody (1:1,000) as the first antibody, cells were stained with secondary anti-rabbit IgG antibodies labeled with fluorescein isothiocyanate. AsRed2 fusion proteins were visualized with red fluorescence. Fluorescence was detected by using a confocal laser-scanning microscope (LSM 510 META). The overlays were processed with Photoshop 7.
Flow cytometry analysis.
293T cells and BDV-infected 293T cells were grown in 6-well plates until 50% confluence and then were transfected with pCMV-α1, pCMV-β2, and PRK5-γ2L-GFPN using Lipofectamine reagent (Invitrogen) to express the GABAA-R. The ratio of the mixture of α1-β2-γ2-P-GABARAP was 1:1:2:2:2. α1-β2-γ2, α1-β2-γ2-GABARAP, and α1-β2-γ2-P were used as controls in the same ratio and were normalized with empty vector. For BDV-infected 293T cells, the ratio of the mixture of α1-β2-γ2-GABARAP was 1:1:2:2. α1-β2-γ2 was used as the control in the same ratio. α1-β2-γ2 and α1-β2-γ2-GABARAP were used in 293T cells as additional controls in the same ratio and were normalized with empty vector.
Forty-eight hours posttransfection, cells were dislodged from the plates with PBS and 50 mM EDTA, placed on ice, spun down at 4°C, resuspended in antibody incubation buffer (PBS, 0.2% bovine serum albumin, and 0.01% sodium azide) containing rabbit anti-green fluorescent protein (GFP) antibody (1:500; Santa Cruz Biotechnology), and incubated on ice for 30 min. Treated cells then were washed three times with cold PBS, resuspended in antibody incubation buffer containing R-PE-labeled goat anti-rabbit secondary antibody (1:100; PTGLAB), and incubated for 30 min, followed by three washes with PBS. Cells then were fixed in PBS and 4% paraformaldehyde for 5 min, washed three times with ice-cold PBS, and held at 4°C until analysis by flow cytometry. Flow cytometry analysis was performed using a flow cytometer (Cytomics Fc500; Beckman Coulter). Fluorochromes excited from the argon laser were observed at 510 nm for GFP and 570 nm for R-PE. A total of 10,000 cells were randomly selected for analysis.
RESULTS
BDV P protein is purified and its antibody is generated.
To study the function of BDV P protein, the P gene was amplified from plasmid pcDNA-P and cloned into expression plasmid pET-28a to yield pET-28a-P, which then was transformed into Escherichia coli strain BL21. Results from SDS-PAGE analysis showed that P protein was expressed as a fusion protein with hexahistidine tagged to its N terminus under the induction of IPTG (Fig. 1A, lane 2). The expressed fusion protein with an expected molecular mass of 25.5 kDa was purified by Ni+-NTA agarose beads and analyzed on an SDS-PAGE gel (Fig. 1B, lanes 1 and 2). Purified P protein then was used as an antigen to immunize rabbits. Polyclonal antibody to BDV P was prepared from immunized rabbits, and its specificity to P protein OL/BDV expressed in (Fig. 1C, lane 1) and to P protein expressed in 243T cells transfected with pcDNA-P (Fig. 1C, lane 3) was determined by Western blot analysis.
FIG. 1.
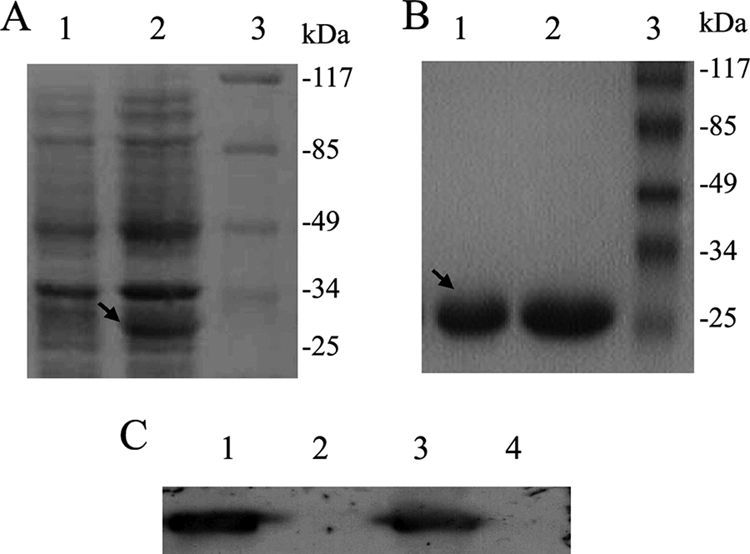
P protein expression, purification, and antibody preparation. (A) SDS-PAGE of the induction and expression of His-P protein in E. coli BL21. The gels were stained with Coomassie brilliant blue R-250. Lane 1, proteins expressed in BL21 cells transformed with pET-P without IPTG induction; lane 2, proteins expressed in BL21 cells transformed with pET-P and induced with 1 mM IPTG; lane 3, prestained protein markers 0431. (B) SDS-PAGE of purified His-P protein. Lanes 1 and 2, purified His-P protein; lane 3, prestained protein markers 0431. (C) Western blot of antibody-recognized P protein. Lane 1, proteins prepared from OL BDV-infected (OL/BDV) cells detected by Western blotting using polyclonal antibody to P protein; lane 2, protein preparation of uninfected (OL) cells detected by Western blotting using polyclonal antibody to P protein; lane 3, protein preparation of 293T cells transfected with pcDNA-P detected by Western blotting using polyclonal antibody to P protein; lane 4, protein preparation of 293T cells transfected with pCNDA-3 detected by Western blotting using polyclonal antibody to P protein.
P protein of BDV interacts with GABAA-R-associated protein.
To identify P protein-interacting cellular partners, we employed an approach of phage display using purified full-length P protein as a bait to screen a human lung cDNA library. After five rounds of biopanning and phage amplification to enrich for specific interactions, we identified several phages containing cDNA fragments as positive candidates. These phages were isolated, and then the nucleotide sequences of their inserted cDNA fragments were determined. The sequence analysis of one of the cDNA fragments (Fig. 2A) revealed that it encodes a peptide of 117 amino acids (Fig. 2B). Further analysis from the BLAST search showed that this peptide is 100% homologous to the GABAA-R-associated protein (GABARAP) (Fig. 2C), suggesting that the P protein of BDV interacts with the cellular protein GABARAP.
FIG. 2.
Identification and sequencing of protein interactions with P protein. (A) The nucleotide sequences of a cDNA fragment isolated from a P protein-reactive phage in a phage display-based protein interaction screen. GAATTC and AAGCTT are sequences of restriction sites. Underlined are the nucleotide sequences with homology to the GABARAP gene. (B) Sequence of 117 amino acids translated from the underlined nucleotide sequences of panel A. (C) Results from a BLAST search of the GenBank databases at the NCBI website, which indicated that the sequences of protein expressed from the phage display that interacted with the P protein were 100% homologous to the cellular protein GABARAP.
BDV P protein binds to GABARAP both in vivo and in vitro.
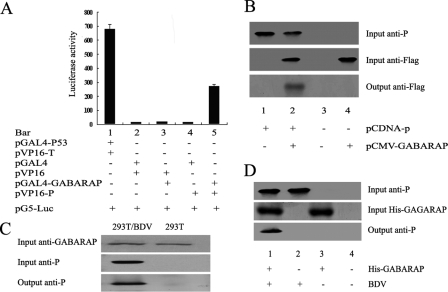
The binding of BDV P protein to GABARAP in vivo was evaluated by using a GAL4/VP16-based mammalian two-hybrid system in 293T cells. GABARAP was fused in frame with the GAL4 DNA-binding domain in plasmid pGAL4-GABARAP. The P protein was fused in frame with the VP16 transactivating domain in plasmid pVP16-P. 293T cells were cotransfected with each of the two individual plasmid constructs or both and a luciferase reporter plasmid. Forty-eight hours posttransfection, luciferase activity was measured in cell extracts as an indication of protein-protein interaction. Results showed that the levels of luciferase activity were very high in cells cotransfected with pGAL4-p53 and pVP16-T (Fig. 3A, bar 1) as expected, high in cells cotransfected with pVP16-P and pGAL4-GABARAP (Fig. 3A, bar 5), but barely detected in cells cotransfected with pGAL4 and pVP16 (Fig. 3A, bar 2), pVP16 and pGAL4-GABARAP (Fig. 3A, bar 3), or pGAL4 and pVP16-P (Fig. 3A, bar 4). These results suggested that BDV P protein interacts with GABARAP in mammalian cells.
FIG. 3.
Determination of the interaction between BDV P protein and GABARAP in vivo. (A) Determination of the protein-protein interaction in 293T cells by mammalian two-hybrid analysis. 293T cells were cotransfected with pG5luc (a luciferase reporter plasmid), pVP16-P, and pGAL4-GABARAP or with control plasmids. Binding between proteins was monitored by luciferase activity, which was measured 48 h after transfection using the dual-luciferase reporter assay system. Values are expressed as the means plus standard errors of the means (three values per sample). (B) Determination of the protein-protein interaction in 293T cells by in vivo immunoprecipitations. 293T cells were cotransfected with pcDNA-P (expressing P protein) and pCMV-GABARAP (expressing Flag-tagged GABARAP). The immunoprecipitation was performed with anti-P antibody, and the Western blotting was performed with anti-Flag antibody. Lane 1, P protein alone; lane 2, P protein plus GABARAP; lane 3, extracts of untransfected 293T cells; lane 4, GABARAP alone. (C) Determination of the interaction between BDV P protein and endogenous GABARAP in 293T cells by in vivo immunoprecipitations. 293T cells were infected with BDV, and the interaction between proteins was analyzed by immunoprecipitation. In vivo immunoprecipitation was performed with anti-GABARAP antibody or anti-P antibody, and Western blotting was performed with anti-P antibody. Lane 1, 293T cells infected with BDV; lane 2, uninfected cells. (D) Determination of the interaction between BDV P protein and GABARAP in vitro. His-tagged GABARAP was bound to His-bound Sepharose beads. Some of the beads were detected by SDS-PAGE, and the rest were incubated with the extracts of BDV-infected (OL/BDV) or uninfected (OL) cells. After being washed, bound proteins were eluted with elution buffer, separated by SDS-PAGE, and probed with antibodies to P protein or anti-His antibody. Western blotting was performed with anti-P antibody. Lane 1, extracts of BDV-infected (OL/BDV) cells treated with His-GABARAP-bound agarose beads; lane 2, extracts of BDV-infected (OL/BDV) cells treated with unbound agarose beads; lane 3, extracts of uninfected (OL) cells treated with His-GABARAP-bound agarose beads; lane 4, extracts of uninfected (OL) cells treated with unbound agarose beads.
To further confirm the binding of P protein to GABARAP protein, in vivo immunoprecipitation analysis was performed. 293T cells were cotransfected with plasmids pcDNA-P and pCMV-GABARAP, expressing P protein and flag-tagged GABARAP, respectively. Protein extracts prepared from transfected cells were immunoprecipitated with anti-P antibody and detected by anti-flag antibody. Results showed that GABARAP protein was detected in the presence of the P protein, indicating that GABARAP coimmunoprecipitated with P protein (Fig. 3B). In addition, 293T cells were infected with BDV or were left uninfected. Protein extracts prepared from infected or noninfected cells were immunoprecipitated with anti-GABARAP antibody and detected by anti-P antibody. Results showed that GABARAP protein was detected in the presence of the P protein, indicating that GABARAP coimmunoprecipitated with P protein (Fig. 3C). This result further demonstrates that BDV P protein binds to endogenous GABARAP in mammalian cells.
The interaction between GABARAP and P protein in vitro also was determined by using protein pull-down analysis. His-tagged GABARAP was generated and immobilized on Ni+-NTA agarose beads. Cell extracts were prepared from OL cells infected with BDV or left uninfected and used in protein pull-down analysis. Results indicated that His-tagged GABARAP protein could specifically pull down the viral P protein (Fig. 3D), as detected by Western blot analysis probing with polyclonal antibodies to the P protein of BDV. Thus, these results demonstrate that BDV P protein interacts with GABARAP in vitro.
C-terminal 27 amino acids of the P protein are essential for its binding to GABARAP.
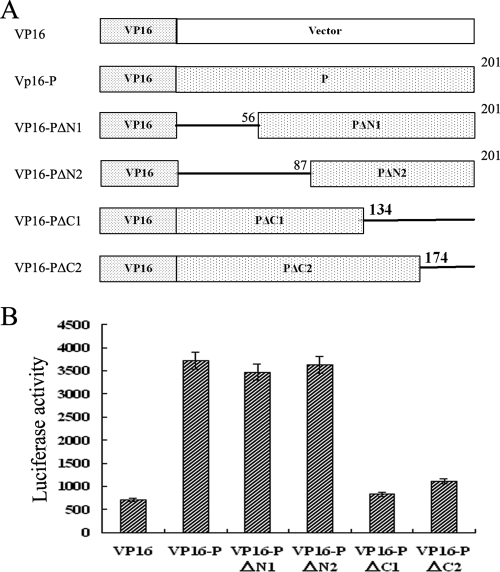
To dissect the sequences of P protein required for its binding to GABARAP, several P gene deletions encoding the truncated proteins VP16-PΔN1, VP16-PΔN2, VP16-PΔC1, and VP16-PΔC2 were generated. Full-length and truncated P genes were fused in frame with the VP16 transactivating domain to generate plasmids pVP16-P, pVP16-PΔN1, pVP16-PΔN2, pVP16-PΔC1, and pVP16-PΔC2, which express corresponding peptides (Fig. 4A). The GABARAP gene was fused in frame with the GAL4 DNA-binding domain to create plasmid pGAL4-GABARAP, which expresses the GAL4-GABARAP fusion protein (Fig. 5A). HeLa cells were cotransfected with pGAL4-GABARAP and pVP16-P, pVP16-PΔN1, pVP16-PΔN2, pVP16-PΔC1, or pVP16-PΔC2 and tested for the binding of GABARAP with P protein using a GAL4-VP16-based mammalian two-hybrid system.
FIG. 4.
Functional analysis of P protein in its binding to GABARAP by the mammalian two-hybrid system. (A) Schematic representations of P protein deletions and its fusion proteins. The numbers indicate the amino acid positions of BDV P protein. VP16, transactivating domain of VP16. (B) Functional analysis of P protein in its binding to GABARAP by the mammalian two-hybrid system. HeLa cells were cotransfected with the luciferase reporter plasmid pG5luc along with pGAL4-GABARAP, pVP16, pVP16-P, pVP16-PΔN1, pVP16-PΔN2, pVP16-PΔC1, and pVP16-PΔC2 as indicated. Forty-eight hours posttransfection, cell extracts were prepared and assayed for luciferase activity. The values are expressed as means plus standard errors of the means.
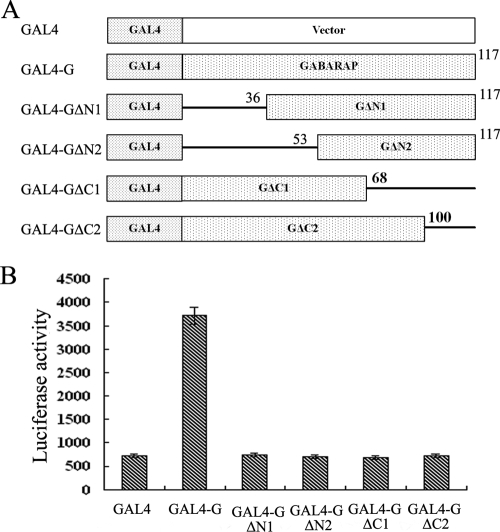
FIG. 5.
Functional analysis of GABARAP in its binding to P protein by the mammalian two-hybrid system. (A) Schematic representation of GABARAP deletions and GAL4-GABARAP fusion proteins. The numbers indicate amino acid positions in GABARAP. GAL4, the DNA-binding domain of GAL4. (B) Functional analysis of GABARAP in the binding of P protein by the mammalian two-hybrid system. HeLa cells were cotransfected with luciferase reporter plasmid pG5luc along with pVP16-P, pGAL4-G, pGAL4-GΔN1, pGAL4-GΔN2, pGAL4-GΔC1, and pGAL4-GΔC2 as indicated. Forty-eight hours posttransfection, cell extracts were prepared and assayed for luciferase activity. The values are expressed as means plus standard errors of the means.
Results showed that the levels of luciferase activity were high in cells cotransfected with pGAL4-G and pVP16-P, pVP16-PΔN1, or pVP16-PΔN2, but they were very low in cells cotransfected with pGAL4-G and pVP6, pVP16-PΔC1, or pVP16-PΔC2 (Fig. 4B). These results indicated that GAL4-GABARAP could interact with VP16-P, VP16-PΔN1, and VP16-PΔN2 but failed to bind to VP16-PΔC1 and VP16-PΔC2. Since both VP16-PΔC1 and VP16-PΔC2 lacked amino acids 174 to 201 of the P protein, the C-terminal 27 amino acids are essential for the binding of P protein to GABARAP. On the other hand, since VP16-PΔN2 contains the C-terminal sequences from amino acids 87 to 201 of the P protein, the C-terminal 114 amino acids are sufficient for the binding of P protein to GABARAP.
Intact GABARAP protein is required for its binding to the viral P protein.
We used a similar strategy to investigate the sequences of GABARAP that are required for their binding to P protein by constructing several deletions of the GABARAP gene encoding the truncated GABARAP proteins GAL4-G, GAL4-GΔN1, GAL4-GΔN2, GAL4-GΔC1, and GAL4-GΔC2 (Fig. 5A). Full-length and truncated GABARAP proteins were fused in frame with the GAL4 DNA-binding domain to generate plasmids pGAL4-G, pGAL4-GΔN1, pGAL4-GΔN2, pGAL4-GΔC1, and pGAL4-GΔC2. The P protein was fused in frame with the VP16 transactivating domain to yield pVP16-P (Fig. 4A). HeLa cells were cotransfected with pVP16-P and pGAL4-G, pGAL4-GΔN1, pGAL4-GΔN2, pGAL4-GΔC1, and pGAL4-GΔC2, respectively, and analyzed for the binding of the P protein with the GABARAP proteins using a GAL4-VP16-based mammalian two-hybrid system. Results indicated that the viral P protein could interact only with the wild-type protein GAL4-G and failed to bind to the four GABARAP mutants GAL4-GΔN1, GAL4-GΔN2, GAL4-GΔC1, and GAL4-GΔC2 (Fig. 5B). These results suggested that GABARAP with an intact structure is essential for its binding to BDV P protein.
BDV P protein stimulates the translocation of GABARAP from the cytosol to nuclei.
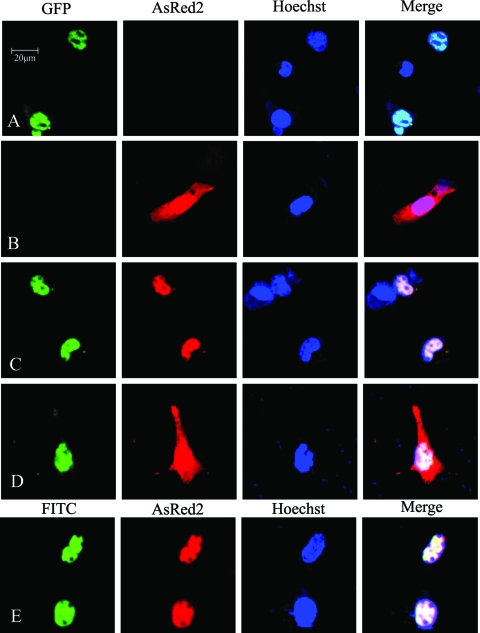
Subcellular localizations of P protein and GABARAP were investigated by examining COS-7 cells transfected with plasmids expressing enhanced GFP-P (EGFP-P) or AsRed2-GABARAP fusion proteins, respectively. The cellular localization of the fusion proteins was visualized by laser-scanning confocal microscopy. Results showed that EGFP-P was localized mainly in nuclei (Fig. 6A) and that AsRed2-GABARAP was uniformly distributed in the cytosol (Fig. 6B). To show the locations of the nuclei, cells were stained with Hoechst.
FIG. 6.
Determination of the subcellular distribution and colocalization of GABARAP and BDV P protein. (A and B) pEGFP-P or pAsRed2C1-GABARAP was transfected individually into COS-7 cells. The expression and distribution of fusion proteins GFP-P (A) and AsRed2-GABARAP (B) in transfected cells were examined by laser-scanning confocal microscopy. (C and D) pAsRed2C1-GABARAP and pEGFP-P or pEGFP-PΔC1 were cotransfected into COS-7 cells. The colocalization of fusion proteins GFP-P with AsRed2-GABARAP (C) and GFP-PΔC1 with AsRed2-GABARAP (D) in transfected cells was examined by laser-scanning confocal microscopy with appropriate filters. (E) For investigating the colocalization of P and GABARAP in BDV-infected COS-7 cells, cells were infected with BDV and then transfected with pAsRed2C1-GABARAP. Anti-P antibody was used as the first antibody, and anti-rabbit IgG antibody labeled with fluorescein isothiocyanate (FITC) was used as the second antibody. Proteins were examined by laser-scanning confocal microscopy. To show the location of the nuclei, cells were stained with Hoechst 33258. Scale bar, 20 μm.
The biological relevance of the interaction between BDV P protein and GABARAP was determined by the examination of the colocalization of the two proteins. COS-7 cells were cotransfected with plasmids expressing EGFP-P and AsRed2-GABARAP fusion proteins. The distributions of the fusion proteins were examined by laser-scanning confocal microscopy. Results showed that the majority of the EGFP-P and AsRed2-GABARAP fusion proteins were localized in nuclei (Fig. 6C). These results demonstrated that BDV P protein colocalized with GABARAP resulted in the translocation of GABARAP from the cytosol to the nuclei of the cells.
To evaluate the effects of P protein on the localization of GABARAP, one of the P protein mutants, PΔC1 (or P134), was included in the study. In the presence of PΔC1, the majority of GABARAP remained in the cytosol of the cells (Fig. 6D). This result was consistent with previous finding that the PΔC1 protein failed to interact with GABARAP (Fig. 4). This result also suggested that the translocation of GABARAP from the cytosol to the nuclei in the presence of P protein was due to its interaction with a functional P protein.
To further investigate the effects of P protein on the cellular distribution of GABARAP in infected cells, COS-7 cells were infected with BDV and then transfected with plasmid expressing AsRed2-GABARAP fusion protein. The subcellular distribution of the fusion protein was examined by laser-scanning confocal microscopy. Results showed that the viral P protein also could interact with the fusion protein by shifting the subcellular distributions of AsRed2-GABARAP from the cytosol to the nuclei in BDV-infected COS-7 cells (Fig. 6E).
BDV P protein inhibits the translocation of GABAA-Rs to the cell surface.
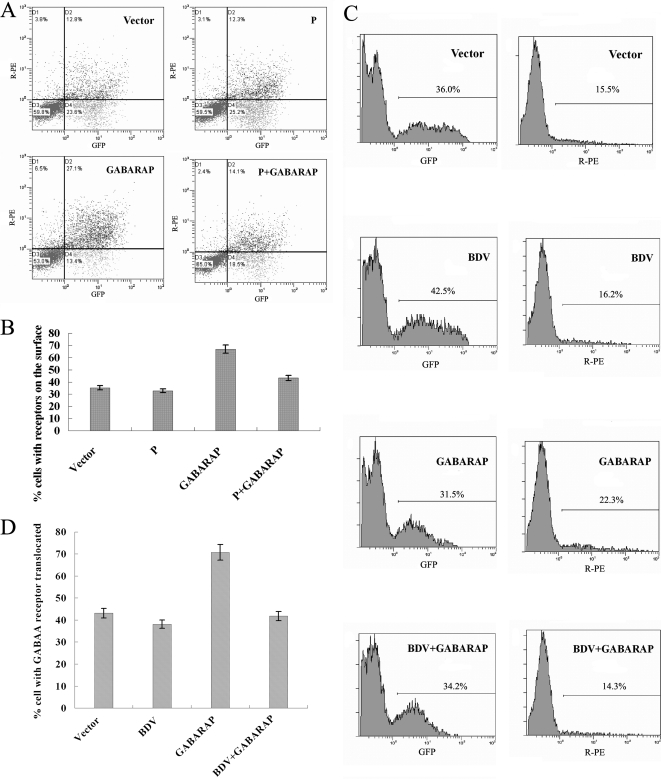
The fact that GABARAP interacts with GABAA-Rs and enhances the translocation receptors to the cell membrane prompted us to determine the effect of the P protein on the translocation of GABAA-Rs to the cell surface regulated by GABARAP. 293T cells were cotransfected with plasmids expressing the three subunits of GABAA-Rs (α, β, and GFP-γ), GABARAP, and P protein. The numbers of cells with expressed GFP-γ and cells with expressed GFP-γ on the membrane were measured by flow cytometry analyses. Results showed that the percentage of cells with GABAA-Rs translocated to the cell surface was 35.2% (in the presence of vector), 32.0% (in the presence of P protein), 66.9% (in the presence of GABARAP), and 43.2% (in the presence of GABARAP and P protein) (Fig. 7A and B). These results demonstrated that GABARAP stimulates the translocation of GABAA-Rs to the cell surface as expected, while the BDV P protein inhibits the translocation of GABAA-Rs to the cell surface by blocking the function of GABARAP.
FIG. 7.
Determination of the effect of P protein on GABARAP in the trafficking of GABAA-Rs. (A) 293T cells were cotransfected with plasmids pCMV-α1 (expressing receptor subunit α1), pCMV-β2 (expressing receptor subunit β2), pRK5-γ2L-GFPN (expressing receptor subunit γ2 and GFP fusion protein γ2-GFP), pCMV-GABARAP, and pCMV-P. Forty-eight hours posttransfection, cells were harvested and the receptors translocated on the cell surface were measured as described in Materials and Methods. Data are expressed as percentages of GFP-positive and R-PE-positive cells and as percentages of GFP-positive and R-PE-negative cells. (B) Flow cytometry data were analyzed, and the percentages of GFP, R-PE double-positive cells in GFP single-positive cells were illustrated. A total of 10,000 cells were randomly selected for analysis. (C) BDV-infected 293T cells or uninfected 293T cells were cotransfected with pCMV-α1, pCMV-β2, pRK5-γ2L-GFPN, and pCMV-GABARAP. Forty-eight hours posttreatment, cells were harvested and the receptors translocated on the cell surface were measured according to the method described in Materials and Methods. Data are expressed as percentages of GFP-positive and R-PE-positive cells. (D) Flow cytometry data were analyzed, and the percentages of R-PE-positive cells and GFP-positive cells were illustrated. A total of 10,000 cells were randomly selected for analysis.
To further evaluate the effects of the P protein of BDV on the trafficking of GABAA-Rs in infected cells, 293T cells were infected with BDV and then cotransfected with plasmids expressing the three subunits of GABAA-Rs (α, β, and GFP-γ) and GABARAP. Cells with expressed GFP-γ and cells with expressed GFP-γ on the membrane were measured by flow cytometry analyses. Results showed that the percentage of cells with GABAA-Rs translocated to the cell surface was 43.1% (in the presence of vector), 38.1% (infected with BDV), 70.7% (in the presence of GABARAP), and 41.8% (in the presence of BDV and GABARAP), respectively (Fig. 7C and D). These results further demonstrated that GABARAP stimulates the translocation of GABAA-Rs to the cell surface as expected, while BDV inhibits the translocation of GABAA-Rs to the cell surface through the viral P protein blocking the function of GABARAP.
DISCUSSION
BDV is an infectious agent that causes CNS diseases in a wide range of vertebrate species and perhaps in humans (1, 19, 25). The P protein of BDV, which is abundantly expressed in infected cells and animal brains, plays important roles in the regulation of the polymerase complex of BDV by binding to the viral proteins N, X, and L (16, 24, 27, 28). In addition to its role in viral replication, P protein plays a role in the induction of behavioral and neurological abnormalities in transgenic mice (13). This viral protein can counteract TBK-1-dependent beta interferon expression in cells to form an antiviral state (34). Our recent study showed that P protein can inhibit iNOS expression in astrocytes, which perhaps results in the establishment of a persistent infection (22).
To explore the neurological functions of P protein, we used purified P protein as the bait to identify its cellular targets by screening a human cDNA library using a phage display approach. Using this technique, we identified several cellular factors, including HMGB-1 (data not shown) and GABARAP. Previous results from far-Western blotting assays showed that P protein interacts directly with HMGB-1 and inhibits its functions in cultured neural cells (14, 38). GABARAP, a 14-kDa polypeptide, originally was identified as an intracellular protein with sequence similarity to light chain 3 of microtubule-associated proteins 1A and 1B (18). GABARAP can interact with the γ2 subunits of GABAA-Rs (35) and facilitate the surface presentation of GABAA-Rs (2, 4).
The direct binding of P protein to GABARAP was confirmed by several independent approaches, such as mammalian two-hybrid assay, coimmunoprecipitation, and protein pull-down analyses. All results were consistent and demonstrated that P protein interacts directly with GABARAP both in vivo and in vitro, indicating that P protein is involved in the regulation of the function of GABAA-Rs.
The C terminus of P protein has been reported to have several functions. Residues 135 to 182 are responsible for its interacting with the viral polymerase (28), and residues 194 to 201 are responsible for its interacting with BDV N protein (30). All the findings demonstrated that the C terminus of P protein is critical for protein-protein interaction. Our data in this study were consistent with these results, which indicated that amino acids 175 to 201 of the P protein are important, and the deletion of this region results in the loss of P protein function in its interaction with GABARAP.
In the process of dissecting functional domains that are responsible for the binding of GABARAP to P protein, we found that the interaction of the viral protein requires an intact full-length GABARAP. It was reported that GABARAP contains two domains, a larger C-terminal domain (residues 27 to 117) that mediates GABAA-R binding (4, 35) and a smaller N-terminal one (residues 1 to 26) that contains a novel tubulin-binding motif (7, 32, 35). Glycine 116 is required for the C-terminal processing of GABARAP, the localization of the protein, and its functions as a trafficking protein (5).
The cellular colocalization of GABARAP and P protein and the status of their interaction were explored in mammalian cells in this study. When the two proteins were expressed separately, BDV P protein was rich in the nucleus, while GABARAP was distributed predominantly in the cytosol, which was consistent with previous reports (29, 31, 37). However, when both proteins were present in the same cells, P protein could bind to GABARAP and recruit GABARAP to P protein-rich loci (in the nucleus). In contrast, the coexpression of the GABARAP protein and the truncated P protein (PΔN1) resulted in the failure of PΔN1 to bind to GABARAP and in an inhibitory effect on the recruitment of GABARAP from the cytosol to the nucleus. In addition, in BDV-infected COS-7 cells, GABARAP also was distributed in the nuclei of the cells, which is predominantly where the viral P protein was found. These results demonstrated that the viral P protein may play a role in the regulation of the neurological functions of GABARAP.
Previous studies have demonstrated that GABARAP is important for interactions with several cellular transcription factors, including the γ2 subunit, tubulin, gephyrin, NSF, ULK1, p130, and transferin GABAA-Rs, resulting in shipping GABAA-Rs to the cell surface, organizing them into postsynaptic clusters, and regulating the steady-state receptor density (4, 6). Our findings raise the possibility that the viral P protein and its cellular partners competitively interfere with each other in binding to GABARAP. Among these cellular factors, GABAA-R subunit γ2 is a particularly interesting one, due to the facts that it plays a direct role in neurological functions and that BDV infects a lot of big neurons, including GABA-ergic neurons (11).
Interestingly, in this study we demonstrated that the P protein of BDV inhibits the function of GABARAP in promoting the translocation of GABAA-Rs to the cell surface by using heterologous expression systems. Thus, we predict that BDV P protein inhibits GABA-induced currents based on the previous literature (4, 10), although further studies have to be done in order to confirm this hypothesis. Nevertheless, our results suggested that the BDV P protein plays an important role in the regulation of the neurological functions of the cells, since it has been reported that decreased GABAA-R translocation results in enhanced anxiety and a bias for threat cues (8).
Although the clinical course and histopathology of Borna disease vary with the age of the animal at the time of infection, our results may explain the neurological effects (hyperactivity and enhanced anxiety) in some cases. For example, many experimental data on BDV infection have been obtained in rat models in which the infection of adult immunocompetent rats with BDV causes acute disease characterized by hyperactivity and aggressiveness, followed by tremors, paralysis, and often death (21). Neonatal BDV brain infection results in selective developmental damage to the hippocampal dentate gyrus and the cerebellum. When mature, neonatally BDV-infected rats show extreme locomotor hyperactivity (23). The possible interpretation of these behavioral abnormalities suggests decreased GABAA-R translocation in infected rats compared to that in normal animals.
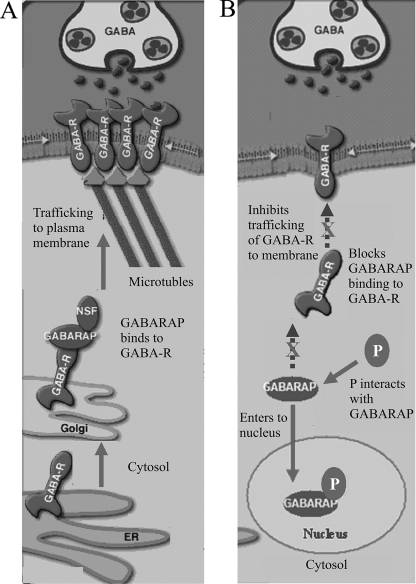
In summary, we demonstrated that BDV P protein interacts directly with the cellular protein GABARAP, shifts the distribution of GABARAP from the cytoplasm to the nucleus, and inhibits the GABARAP trafficking of GABAA-Rs to the cell membrane (Fig. 8), which may lead to the inhibition of GABA-induced currents and the pathogenesis of neural abnormality caused by BDV infection.
FIG. 8.
Model for the effect of BDV P protein on GABARAP in the trafficking of GABAA-Rs. (A) In the absence of BDV P protein, GABAA-R is the principal GABA-gated ion channel and plays important roles in neural transmission (17). GABARAP distributes in the cytosol of the cells, interacts with the γ2 subunit of GABAA-Rs, links GABAA-Rs to microtubules, facilitates the trafficking of GABAA-Rs to the plasma membrane (4, 5), and thus enhances GABA-induced currents (10). ER, endoplasmic reticulum. (B) In the presence of BDV P protein (P) or in BDV-infected cells, P protein binds to GABARAP, shifts the distribution of GABARAP from the cytoplasm to the nucleus, and blocks the trafficking of GABAA-Rs to the cell surface by inhibiting GABARAP translocation, which may result in neural disorder.
Acknowledgments
We thank Bill Wisden and Erwin Sigel for kindly providing plasmids pCMV-α1, pCMV-β2, and pRK5-γ2L-GFPN; Thorsten Wolff and Peter Staeheli for kindly providing plasmid pcDNA-P; and K. Ikuta for the gift of BDV strain H1766.
This work was supported by research grants from the Major State Basic Research Development Program of China (973 project nos. 2005CB522901 and 2005CB522905), the National Natural Science Foundation of China (nos. 30570070 and 30730001), the Program for Changjiang Scholars and Innovative Research Team in Universities (no. IRT0745), and the Department of Science Technology of Hubei Province (no. 2005ABC003) to J. Wu.
Footnotes
Published ahead of print on 24 September 2008.
REFERENCES
- 1.Bode, L., and H. Ludwig. 2003. Borna disease virus infection, a human mental-health risk. Clin. Microbiol. Rev. 16534-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boileau, A. J., R. A. Pearce, and C. Czajkowski. 2005. Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. J. Neurosci. 2511219-11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourteele, S., K. Oesterle, S. Pleschka, G. Unterstab, C. Ehrhardt, T. Wolff, S. Ludwig, and O. Planz. 2005. Constitutive activation of the transcription factor NF-κB results in impaired Borna disease virus replication. J. Virol. 796043-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, L., H. Wang, S. Vicini, and R. W. Olsen. 2000. The gamma-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc. Natl. Acad. Sci. USA 9711557-11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Z. W., C. S. Chang, T. A. Leil, and R. W. Olsen. 2007. C-terminal modification is required for GABARAP-mediated GABA(A) receptor trafficking. J. Neurosci. 276655-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyle, J. E., and D. B. Nikolov. 2003. GABARAP: lessons for synaptogenesis. Neuroscientist 9205-216. [DOI] [PubMed] [Google Scholar]
- 7.Coyle, J. E., S. Qamar, K. R. Rajashankar, and D. B. Nikolov. 2002. Structure of GABARAP in two conformations: implications for GABA(A) receptor localization and tubulin binding. Neuron 3363-74. [DOI] [PubMed] [Google Scholar]
- 8.Crestani, F., M. Lorez, K. Baer, C. Essrich, D. Benke, J. P. Laurent, C. Belzung, J. M. Fritschy, B. Luscher, and H. Mohler. 1999. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 2833-839. [DOI] [PubMed] [Google Scholar]
- 9.Dürrwald, R., J. Kolodziejek, S. Herzog, and N. Nowotny. 2007. Meta-analysis of putative human bornavirus sequences fails to provide evidence implicating Borna disease virus in mental illness. Rev. Med. Virol. 17181-203. [DOI] [PubMed] [Google Scholar]
- 10.Everitt, A. B., T. Luu, B. Cromer, M. L. Tierney, B. Birnir, R. W. Olsen, and P. W. Gage. 2004. Conductance of recombinant GABA (A) channels is increased in cells coexpressing GABA(A) receptor-associated protein. J. Biol. Chem. 27921701-21706. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Dunia, D., M. Watanabe, S. Syan, M. Mallory, E. Masliah, and J. C. De La Torre. 2000. Synaptic pathology in Borna disease virus persistent infection. J. Virol. 743441-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan, I., and W. I. Lipkin. 2001. Borna disease virus. Rev. Med. Virol. 1137-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamitani, W., E. Ono, S. Yoshino, T. Kobayashi, S. Taharaguchi, B. J. Lee, M. Yamashita, M. Okamoto, H. Taniyama, K. Tomonaga, and K. Ikuta. 2003. Glial expression of Borna disease virus phosphoprotein induces behavioral and neurological abnormalities in transgenic mice. Proc. Natl. Acad. Sci. USA 1008969-8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamitani, W., Y. Shoya, T. Kobayashi, M. Watanabe, B. J. Lee, G. Zhang, K. Tomonaga, and K. Ikuta. 2001. Borna disease virus phosphoprotein binds a neurite outgrowth factor, amphoterin/HMG-1. J. Virol. 758742-8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kittler, J. T., J. Wang, C. N. Connolly, S. Vicini, T. G. Smart, and S. J. Moss. 2000. Analysis of GABAA receptor assembly in mammalian cell lines and hippocampal neurons using gamma 2 subunit green fluorescent protein chimeras. Mol. Cell Neurosci. 16440-452. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, T., G. Zhang, B. J. Lee, S. Baba, M. Yamashita, W. Kamitani, H. Yanai, K. Tomonaga, and K. Ikuta. 2003. Modulation of Borna disease virus phosphoprotein nuclear localization by the viral protein X encoded in the overlapping open reading frame. J. Virol. 778099-8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macdonald, R. L., and R. W. Olsen. 1994. GABAA receptor channels. Annu. Rev. Neurosci. 17569-602. [DOI] [PubMed] [Google Scholar]
- 18.Mann, S. S., and J. A. Hammarback. 1994. Molecular characterization of light chain 3. A microtubule binding subunit of MAP1A and MAP1B. J. Biol. Chem. 26911492-11497. [PubMed] [Google Scholar]
- 19.Miranda, H. C., S. O. Nunes, E. S. Calvo, S. Suzart, E. N. Itano, and M. A. Watanabe. 2006. Detection of Borna disease virus p24 RNA in peripheral blood cells from Brazilian mood and psychotic disorder patients. J. Affect Disord. 9043-47. [DOI] [PubMed] [Google Scholar]
- 20.Moss, S. J., and T. G. Smart. 2001. Constructing inhibitory synapses. Nat. Rev. Neurosci. 2240-250. [DOI] [PubMed] [Google Scholar]
- 21.Narayan, O., S. Herzog, K. Frese, H. Scheffers, and R. Rott. 1983. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J. Infect. Dis. 148305-315. [DOI] [PubMed] [Google Scholar]
- 22.Peng, G., F. Zhang, Q. Zhang, K. Wu, F. Zhu, and J. Wu. 2007. Borna disease virus P protein inhibits nitric oxide synthase gene expression in astrocytes. Virology 366446-452. [DOI] [PubMed] [Google Scholar]
- 23.Pletnikov, M. V., S. A. Rubin, G. J. Schwartz, T. H. Moran, T. J. Sobotka, and K. M. Carbone. 1999. Persistent neonatal Borna disease virus (BDV) infection of the brain causes chronic emotional abnormalities in adult rats. Physiol. Behav. 66823-831. [DOI] [PubMed] [Google Scholar]
- 24.Poenisch, M., G. Unterstab, T. Wolff, P. Staeheli, and U. Schneider. 2004. The X protein of Borna disease virus regulates viral polymerase activity through interaction with the P protein. J. Gen. Virol. 851895-1898. [DOI] [PubMed] [Google Scholar]
- 25.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 19017-30. [DOI] [PubMed] [Google Scholar]
- 26.Schmid, S., D. Mayer, U. Schneider, and M. Schwemmle. 2007. Functional characterization of the major and minor phosphorylation sites of the P protein of Borna disease virus. J. Virol. 815497-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider, U. 2005. Novel insights into the regulation of the viral polymerase complex of neurotropic Borna disease virus. Virus Res. 111148-160. [DOI] [PubMed] [Google Scholar]
- 28.Schneider, U., K. Blechschmidt, M. Schwemmle, and P. Staeheli. 2004. Overlap of interaction domains indicates a central role of the P protein in assembly and regulation of the Borna disease virus polymerase complex. J. Biol. Chem. 27955290-55296. [DOI] [PubMed] [Google Scholar]
- 29.Schwemmle, M., C. Jehle, T. Shoemaker, and W. I. Lipkin. 1999. Characterization of the major nuclear localization signal of the Borna disease virus phosphoprotein. J. Gen. Virol. 8097-100. [DOI] [PubMed] [Google Scholar]
- 30.Schwemmle, M., M. Salvatore, L. Shi, J. Richt, C. H. Lee, and W. I. Lipkin. 1998. Interactions of the borna disease virus P, N, and X proteins and their functional implications. J. Biol. Chem. 2739007-9012. [DOI] [PubMed] [Google Scholar]
- 31.Shoya, Y., T. Kobayashi, T. Koda, K. Ikuta, M. Kakinuma, and M. Kishi. 1998. Two proline-rich nuclear localization signals in the amino- and carboxyl-terminal regions of the Borna disease virus phosphoprotein. J. Virol. 729755-9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stangler, T., L. M. Mayr, and D. Willbold. 2002. Solution structure of human GABA(A) receptor-associated protein GABARAP: implications for biolgoical funcrion and its regulation. J. Biol. Chem. 27713363-13366. [DOI] [PubMed] [Google Scholar]
- 33.Tomonaga, K., T. Kobayashi, and K. Ikuta. 2002. Molecular and cellular biology of Borna disease virus infection. Microbes Infect. 4491-500. [DOI] [PubMed] [Google Scholar]
- 34.Unterstab, G., S. Ludwig, A. Anton, O. Planz, B. Dauber, D. Krappmann, G. Heins, C. Ehrhardt, and T. Wolff. 2005. Viral targeting of the interferon-β-inducing Traf family member-associated NF-κB activator (TANK)-binding kinase-1. Proc. Natl. Acad. Sci. USA 10213640-13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, H., F. K. Bedford, N. J. Brandon, S. J. Moss, and R. W. Olsen. 1999. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature 39769-72. [DOI] [PubMed] [Google Scholar]
- 36.Wolff, T., G. Heins, G. Pauli, R. Burger, and R. Kurth. 2006. Failure to detect Borna disease virus antigen and RNA in human blood. J. Clin. Virol. 36309-311. [DOI] [PubMed] [Google Scholar]
- 37.Wu, M., G. Yin, X. Zhao, C. Ji, S. Gu, R. Tang, H. Dong, Y. Xie, and Y. Mao. 2006. Human RAB24, interestingly and predominantly distributed in the nuclei of COS-7 cells, is colocalized with cyclophilin A and GABARAP. Int. J. Mol. Med. 17749-754. [PubMed] [Google Scholar]
- 38.Zhang, G., T. Kobayashi, W. Kamitani, S. Komoto, M. Yamashita, S. Baba, H. Yanai, K. Ikuta, and K. Tomonaga. 2003. Borna disease virus phosphoprotein represses p53-mediated transcriptional activity by interference with HMGB1. J. Virol. 7712243-12251. [DOI] [PMC free article] [PubMed] [Google Scholar]