Abstract
Hepatitis C virus (HCV) infection is dependent on at least three coreceptors: CD81, scavenger receptor BI (SR-BI), and claudin-1. The mechanism of how these molecules coordinate HCV entry is unknown. In this study we demonstrate that a cell culture-adapted JFH-1 mutant, with an amino acid change in E2 at position 451 (G451R), has a reduced dependency on SR-BI. This altered receptor dependency is accompanied by an increased sensitivity to neutralization by soluble CD81 and enhanced binding of recombinant E2 to cell surface-expressed and soluble CD81. Fractionation of HCV by density gradient centrifugation allows the analysis of particle-lipoprotein associations. The cell culture-adapted mutation alters the relationship between particle density and infectivity, with the peak infectivity occurring at higher density than the parental virus. No association was observed between particle density and SR-BI or CD81 coreceptor dependence. JFH-1 G451R is highly sensitive to neutralization by gp-specific antibodies, suggesting increased epitope exposure at the virion surface. Finally, an association was observed between JFH-1 particle density and sensitivity to neutralizing antibodies (NAbs), suggesting that lipoprotein association reduces the sensitivity of particles to NAbs. In summary, mutation of E2 at position 451 alters the relationship between particle density and infectivity, disrupts coreceptor dependence, and increases virion sensitivity to receptor mimics and NAbs. Our data suggest that a balanced interplay between HCV particles, lipoprotein components, and viral receptors allows the evasion of host immune responses.
Hepatitis C virus (HCV), the sole member of the Hepacivirus genus within the Flaviviridae, poses a global health burden, with an estimated 170 million infected individuals (according to the WHO). The majority of patients suffer a chronic infection that is associated with a progressive liver disease (1). HCV has a short positive-sense RNA genome encoding three structural (core protein, E1, and E2) glycoproteins (gps) and seven nonstructural proteins (p7 and NS2 to NS5) (40). The E1 and E2 gps interact with cell surface receptors to facilitate particle entry via low-pH and clathrin-dependent endocytosis (9, 15, 29, 47, 71). The recent discovery that the JFH-1 strain of HCV can replicate and assemble infectious particles in cultured cells (HCVcc) has allowed investigation into the viral life cycle for the first time since its identification almost 20 years ago (41, 75, 79).
Early studies with truncated soluble HCV E2 (sE2) identified interactions with the tetraspanin CD81 and scavenger receptor class B type I (SR-BI) (56, 62). The recent availability of HCVcc and HCV pseudoparticles (HCVpp) provided the tools to validate receptor candidates. HCV entry is thought to require at least three cellular receptors: CD81, SR-BI, and the tight junction protein claudin-1 (reviewed in references 21 and 74). Other candidate components include glycosaminoglycans (5, 6, 51), low-density lipoprotein receptor (49, 77), and the C-type lectins DC-SIGN (dendritic cell-specific ICAM-3-grabbing nonintegrin) and L-SIGN (liver/lymph node-specific ICAM-3-grabbing nonintegrin) (37, 42, 43, 58). HCVpp demonstrate a restricted entry into human cells of liver origin (7, 29), suggesting that gp-receptor interaction(s) may in part define HCV tropism for the liver.
CD81, a tetraspanin, is expressed throughout the body; it facilitates the formation of highly ordered protein complexes at the plasma membrane and regulates multiple signaling pathways important for cell-cell adhesion, migration, activation, and proliferation (reviewed in reference 39). Expression of CD81 in CD81-negative human liver cells allows HCV infection, demonstrating a critical role for CD81 in viral entry (29, 38, 41). Viral entry is dependent on the large extracellular loop (LEL) of CD81, and antibodies that perturb this interaction neutralize viral infectivity (24, 25, 29, 33, 34, 54).
SR-BI, also known as CLA-1, is expressed predominantly in the liver and steroidogenic tissue, where it mediates the selective uptake of cholesterol from ligands such as high-density lipoprotein (HDL) (reviewed in references 36 and 64). HCV E2 interaction with SR-BI is believed to occur via hypervariable region 1 located within the N-terminal region of the E2 glycoprotein (8, 62). Native lipoprotein ligands, HDL and oxidized low-density lipoprotein, enhance and inhibit HCV infection, respectively, suggesting a complex interplay between SR-BI, lipoproteins, and HCV (8, 72, 73). Transduction of Huh-7.5 cells to overexpress SR-BI enhanced HCV entry, suggesting that SR-BI levels limit viral entry (27). Anti-SR-BI antibodies and small interfering RNA silencing of SR-BI expression perturb HCV infection (12, 27), and recent data demonstrate that antibodies specific for SR-BI and CD81 inhibit JFH-1 infectivity in a synergistic manner, suggesting cooperativity between the receptors (31, 78).
There has been a steady accumulation of evidence to suggest that lipoproteins play a key role in the HCV life cycle. Early observations with plasma from infected individuals reported that antibodies specific for the apoprotein component of very-low-density lipoprotein (VLDL) could precipitate HCV RNA, suggesting an association of viral particles with host lipoproteins (2, 52, 53, 67, 68). Recent studies with HCVcc have reported virion assembly to be intrinsically linked to VLDL synthesis, suggesting that HCV may be incorporated into lipo-viro-particles (LVPs) during assembly or release (13, 26, 30). Several reports demonstrate that anti-apoprotein antibodies inhibit HCV infection, lending further support for a role of lipoproteins in HCV LVP entry (3, 13).
Several groups have characterized the adaptation of JFH-1 and intergenotypic chimeras, resulting in the selection of viruses with enhanced replicative potential (10, 32, 60, 80). Zhong and colleagues reported that a glycine-to-arginine mutation at position 451 (G451R) in E2 promoted JFH-1 infectivity (80). Our studies with JFH-1 G451R demonstrate a reduced dependency on SR-BI and increased binding to CD81. Analysis of HCV buoyant density as a measure of lipoprotein association demonstrated an altered relationship between particle density and infectivity of the mutant virus, with the peak JFH-1 G451R infectivity occurring at higher density than parental virus. The mutation also increased the sensitivity of particles to neutralization by gp-specific antibodies, suggesting an increased availability of epitopes on the mutant particle. An association was noted between JFH-1 density and sensitivity to neutralizing immunoglobulin G (IgG), suggesting that lipoproteins reduce the sensitivity of particles to neutralizing antibodies (NAbs). These data support a model where mutation of E2 at position 451 alters gp structure, modulating viral interaction with coreceptors and NAbs and disrupting the relationship between particle density and infectivity.
MATERIALS AND METHODS
Cells and antibodies.
293T and Chinese hamster ovary (CHO) cells were obtained from the American Type Culture Collection and propagated according to the supplier's recommendations. Huh-7.5 cells (provided by Charles Rice, The Rockefeller University, New York, NY) (10) were propagated in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% nonessential amino acids. All cells were grown at 37°C in 5% CO2. CHO cells stably expressing SR-BI were generated and propagated as previously described (44). CHO cells stably expressing CD81 were generated by transfection with a pCDNA3.1 plasmid vector encoding human CD81. Anti-SR-BI serum was generated as previously reported (44), and anti-CLA-1 monoclonal antibody (MAb) was obtained from BD biosciences. Anti-CD81 MAbs (2s131, 1s201, and 2s139) were generated by immunizing mice with full-length CD81. Soluble human CD81 (hCD81) LEL was generated as reported by Drummer and coworkers (19, 20). Polyclonal IgG was isolated from healthy donors and chronically HCV-infected patient serum using protein G-conjugated Sepharose beads (GE Healthcare, United Kingdom). MAbs 3/11 and 10/76b were generated as previously described (24, 63).
Transduction of cells to overexpress SR-BI.
TRIP lentiviruses expressing SR-BI were generated in 293T cells as previously reported (27). Briefly, Huh-7.5 cells were seeded at 8 × 105 cells per well of a six-well plate and infected 24 h later with the packaged lentivirus diluted in DMEM supplemented with 3% FBS. After 12 h, cells were washed, trypsinized, and seeded into appropriate plates for HCV infection. Flow-cytometric analysis demonstrated that Huh-7.5 TRIP-SR-BI cells expressed twofold more SR-BI than parental cells.
HCVcc genesis, infection, and neutralization assays.
JFH-1 and G451R viruses were generated as previously described (40). Briefly, RNA was transcribed in vitro from full-length genomes using a Megascript T7 kit (Ambion, Austin, TX) and electroporated into Huh-7.5 cells. Virus was harvested from cells within 10 days postelectroporation. Huh-7.5 cells were seeded at 1.5 × 104 cells per well in 48-well plates and infected the following day with JFH-1 wild-type (wt) or G451R particles diluted in 3% FBS-DMEM. At 48 h postinoculation infection was detected by methanol fixation and staining for NS5A antigen with anti-NS5A MAb 9E10 and AlexaFluor 488-conjugated anti-mouse IgG (Invitrogen, CA) (40). Infections were quantified by enumerating the total number of infected cells per well, and the results were used to calculate the number of infectious units per ml (IU/ml) of virus inoculum. For neutralization and receptor blocking assays, virus or cells were preincubated at 37°C for 1 h with the appropriate inhibitory or control antibody prior to infection. In each case the initial virus inoculum was diluted to approximately 2,000 IU/ml. The percent neutralization of test treatments was expressed relative to control irrelevant antibody-treated infections. Typical JFH-1 wt and G451R infectivities were 2,500 and 10,000 IU/ml, respectively, corresponding to an infected-cell count of 100 to 200 cells/well at the dilutions tested.
Quantification of HCV particle buoyant density.
HCVcc virus was concentrated 50-fold using a Vivaspin 20 column with a 100-kDa cut off (Sartorius, Germany). Linear iodixanol (Axis-Shield, United Kingdom) gradients were prepared using a two-chamber gradient maker (Jencons, United Kingdom) with light (6%) and dense (56%) iodixanol solutions (53). Gradients were used immediately after preparation, and 0.4 ml of concentrated virus was loaded onto each gradient. Samples were centrifuged at 100,000 × g for 21 h at 4°C in an L80-M ultracentrifuge (Beckman, United Kingdom); fractions were harvested, and their densities were determined with a digital refractometer (Atago, Japan).
Quantification of HCV RNA.
RNA was extracted using an RNeasy Mini Kit (Qiagen, Germany) or QIAamp MinElute virus kit (Qiagen, Germany), according to manufacturer's instructions. The amplification efficiency of cell-free HCV RNA preparations was assessed by the addition of a small quantity of exogenous HeLa RNA (10 pg) to the reverse transcription-PCR (RT-PCR) mixture. HCV amplification was performed using a modification of a previously described method (16, 66) in accordance with the manufacturer's guidelines (CellsDirect kit, Invitrogen, CA). Fluorescence was monitored in a 7900 HT real-time PCR machine (ABI, CA) (46). In all reactions the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase was included as an internal endogenous control for amplification efficiency and RNA quantification (primer-limited endogenous control; ABI).
Immunofluorescent microscopy.
CHO cells expressing SR-BI or CD81 were fixed in ice-cold methanol for 5 min, blocked with 5% bovine serum albumin-phosphate-buffered saline (PBS), and stained with 0.1 μg/ml anti-CLA-1 (SR-BI) or 2s139 (CD81); bound antibody was detected with an AlexaFluor 488-conjugated anti-mouse IgG (Invitrogen, CA). Images were taken at a magnification of ×200 with a TE2000-S microscope using a Hamamatsu C4742-65 camera (Nikon, Japan).
Expression of soluble truncated HCV E2.
The nucleotide sequence encoding amino acids 384 to 661 of the HCV polyprotein was amplified from a cDNA clone of JFH-1 or G451R, and the products were cloned into pcDNA3.1 downstream of the tissue plasminogen activator leader sequence. The reverse primer encoded a C-terminal human immunodeficiency virus (HIV) gp120 epitope tag, recognized by MAb 10/76b. Plasmids were introduced into 293T cells with ProFection (Promega, WI), and the sE2 was harvested from cells propagated in DMEM supplemented with 3% delipidated FBS at 48 and 72 h posttransfection. The relative concentrations of sE2 preparations were quantified by enzyme immunoassay using MAb 10/76b, as previously reported (45).
Quantification of sE2 coreceptor interactions.
Soluble JFH-1 wt and G451R E2 (sE2) were diluted to the same relative concentrations and assayed for their ability to bind SR-BI and CD81, as previously reported (24, 73). Briefly, sE2 was incubated at 37°C with parental CHO cells or with cells expressing SR-BI/CD81 for 1 h, and bound E2 was detected with MAb 10/76b and AlexaFluor 488-conjugated anti-rat IgG (Invitrogen, CA). Samples were quantified on a FACSCalibur flow cytometer (BD Biosciences), and the data were analyzed with FlowJo software (Tree Star, San Carlos, CA). sE2 interaction with hCD81 LEL was quantified by enzyme-linked immunosorbent assay, as previously reported (20, 24). Briefly, Immulon 2HB enzyme-linked immunosorbent assay plates (Thermo, MA) were coated overnight with hCD81 LEL at 0.5 μg/well, blocked with 5% bovine serum albumin-PBS, and incubated with sE2 for 4 h at 37°C. Bound E2 was detected with MAb 10/76b and horseradish peroxidase-conjugated donkey anti-rat IgG (Jackson Immunoresearch, United Kingdom). Bound horseradish peroxidase conjugates were detected colorimetrically after reaction with a TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution (Biofix, MD).
RESULTS
JFH-1 G451R has a reduced dependence on SR-BI.
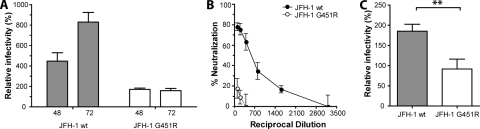
We previously reported that overexpression of SR-BI in Huh-7.5 cells enhanced JFH-1 entry (27). To investigate whether the adaptive mutation affects JFH-1 interaction(s) with SR-BI, parental Huh-7.5 cells and cells transduced to overexpress SR-BI were infected with JFH-1 wt and G451R. We previously reported that HCV can infect hepatoma cells via cell-free and cell-cell routes (69). To discriminate between cell-free particle primary infection and secondary transmission events, infection was allowed to proceed for 48 and 72 h, respectively (69). Overexpression of SR-BI enhanced the infectivity of JFH-1 fourfold at 48 h postinfection, and this increased to eightfold by 72 h (Fig. 1A). At 72 h the increased infectivity was associated with a doubling in the number of infected cells per focus (data not shown), suggesting increased cell-cell transfer of infection. In contrast, JFH-1 G451R showed a 1.5-fold increase in infectivity at both time points with no change in focal size (Fig. 1A).
FIG. 1.
JFH-1 G451R has an altered dependence on SR-BI. (A) Huh-7.5 cells overexpressing SR-BI were incubated with JFH-1 wt or JFH-1 G451R for 8 h. Cells were fixed after 48 and 72 h and stained for NS5A, and the mean number of infected cells per well was determined. Infectivity is expressed relative to parental Huh-7.5 cells. (B) Huh-7.5 cells were incubated with a serial dilution of rabbit anti-SR-BI serum for 1 h prior to challenge with JFH-1 wt or JFH-1 G451R. The data are expressed as percent neutralization relative to infection of Huh-7.5 cells treated with control rabbit serum. (C) Huh-7.5 cells were inoculated with JFH-1 wt or JFH-1 G451R in the presence of 10 μg/ml HDL. Infection is expressed relative to infection in the absence of HDL. Error bars indicate standard deviation from the mean (n = 3; P = 0.0069, unpaired t test).
To further investigate JFH-1 G451R interaction(s) with SR-BI, we employed a neutralizing rabbit anti-SR-BI serum capable of inhibiting HCV infectivity and preventing sE2 interaction with SR-BI (27, 44). Huh-7.5 cells were preincubated with the anti-SR-BI serum prior to challenge with JFH-1 wt or G451R. While the infectivity of both viruses was inhibited, JFH-1 G451R is less sensitive to neutralization; a 1/100 dilution of antiserum reduced JFH-1 G451R infectivity by 20%, compared to 80% for wt virus (Fig. 1B).
It is widely reported that HDL enhances HCV infection via an SR-BI-dependent mechanism requiring the transfer of lipids from HDL (8, 17, 72). To ascertain whether the altered relationship of G451R with SR-BI extends to lipoprotein enhancement, JFH-1 wt and G451R infections were supplemented with 10 μg/ml HDL. HDL promoted JFH-1 wt infectivity twofold, consistent with previous reports; however, G451R infectivity was unaltered (Fig. 1C). Taken together, these data suggest that cell culture adaptation reduces the requirement for SR-BI during virus entry.
JFH-1 G451R has an increased sensitivity to neutralization by soluble CD81.
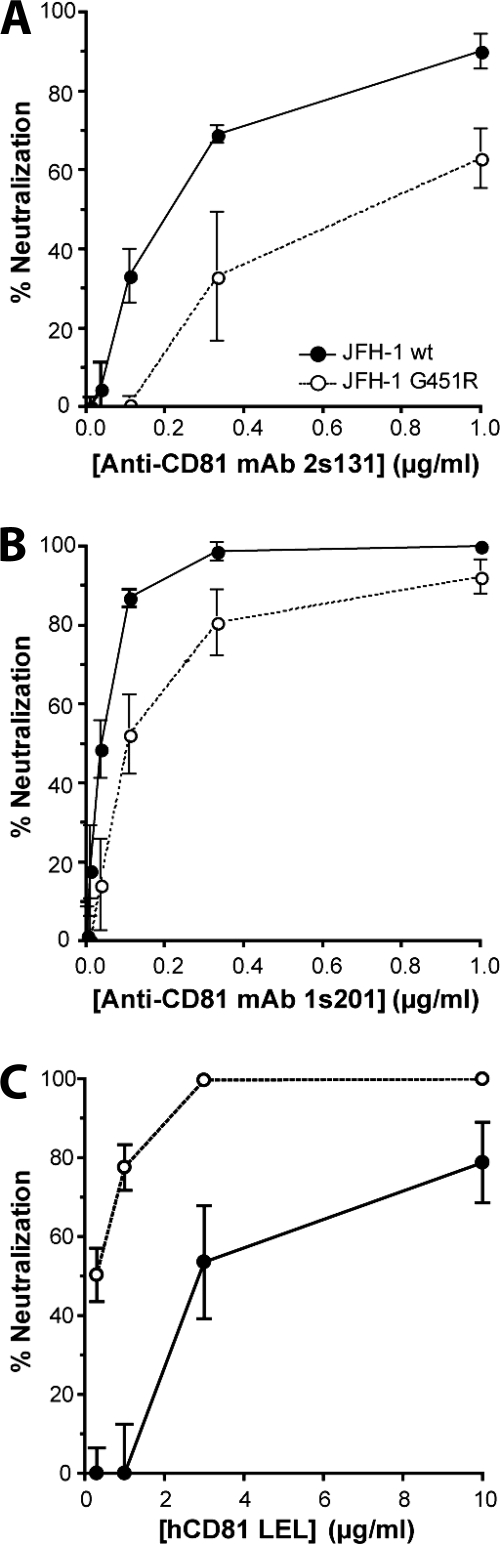
Having established that the G451R mutation alters the relationship between HCV and SR-BI, we wanted to investigate the effects of this mutation on CD81-dependent routes of entry. Huh-7.5 cells were incubated with anti-CD81 MAbs prior to infection with JFH-1 wt or G451R. The infectivity of both viruses was reduced by the anti-CD81 MAbs; however, JFH-1 G451R was less sensitive to treatment by anti-CD81 MAbs (Fig. 2A and B). We along with other investigators have reported that a soluble form of hCD81 LEL interacts with the viral gps and inhibits HCV infectivity (7, 25, 29, 40, 50). JFH-1 G451R demonstrated increased sensitivity to neutralization by hCD81 LEL, with 10-fold less protein required to reduce infectivity by 50% (Fig. 2C), suggesting an increased exposure or affinity of CD81 binding residues on the mutant viral gps.
FIG. 2.
CD81 dependence of JFH-1 wt and G451R infection. Huh-7.5 cells were incubated with a serial dilution of 2s131 (A) or 1s201 (B) mouse anti-CD81 MAb for 1 h prior to challenge with JFH-1 wt (filled circles) or JFH-1 G451R (open circles). (C) JFH-1 wt or G451R viruses were incubated with human CD81 LEL for 1 h prior to infection of Huh-7.5 cells. The data are expressed as percent neutralization relative to viral infection in the presence of an irrelevant mouse IgG or nonactive mouse CD81 LEL, respectively. Error bars indicate standard deviation from the mean (n = 3).
JFH-1 G451R sE2 demonstrates increased binding to CD81.
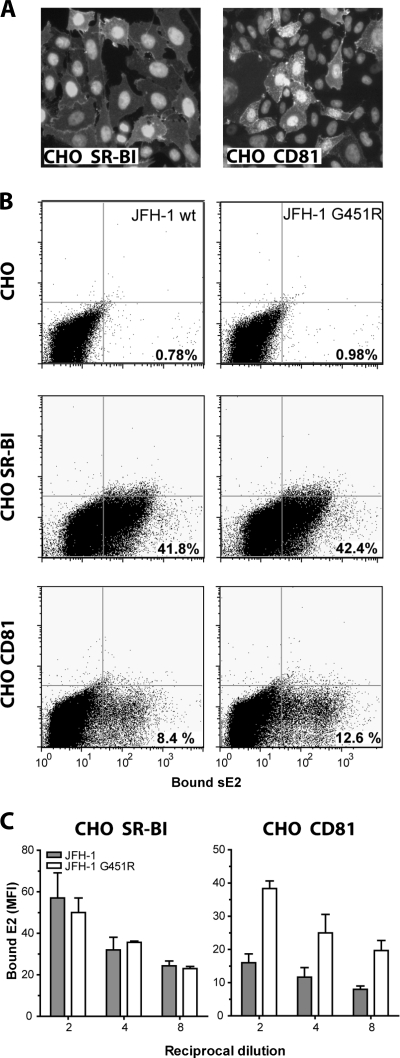
To investigate whether the G451R mutation modulates E2 binding to SR-BI or CD81, soluble forms of JFH-1 wt and G451R E2 were expressed in 293T cells and used to quantify receptor interactions. sE2 was harvested from the medium of cells supplemented with 3% delipidated FBS to eliminate the possibility of lipoprotein association(s). CHO cells expressing either human SR-BI (CHO-SR-BI) or CD81 (CHO-CD81) (Fig. 3A) were incubated with equal amounts of JFH-1 wt or G451R sE2; the bound gps were detected via a C-terminal tag recognized by MAb 10/76b and quantified by flow cytometry (Fig. 3B). JFH-1 wt sE2 bound specifically to CHO cells expressing either SR-BI or CD81, as previously reported for genotype 1 sE2 (24, 27, 62). JFH-1 wt and G451R sE2 bound to CHO-SR-BI cells with comparable staining intensities; however, the mutant protein showed enhanced binding to CD81, with 50% more CHO-CD81 cells binding G451R sE2 than JFH-1 wt (Fig. 3B and C). These data are consistent with the increased sensitivity of mutant virus to hCD81 LEL neutralization.
FIG. 3.
Interaction of JFH-1 wt and G451R sE2 with CHO cells expressing SR-BI and CD81. (A) CHO-SR-BI cells (>80% positive) or CHO-CD81 cells (20 to 30% positive) were methanol fixed and stained with MAb anti-CLA1 or anti-CD81 2s139. (B) Binding of recombinant JFH-1 wt and G451R sE2 to parental CHO cells and CHO-SR-BI and CHO-CD81 cells. Bound E2 was detected with MAb 10/76b. (C) The mean fluorescence intensity (MFI) of JFH-1 wt and G451R sE2 bound to CHO-SR-BI and CHO-CD81 cells is shown; the signal from the sE2-CHO cell interaction was subtracted. Error bars indicate standard deviation from the mean (n = 3).
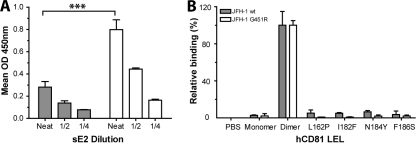
To study further the interaction of wt and G451R sE2 with CD81, we followed the binding of sE2 with hCD81 LEL by enzyme immunoassay. Previous studies have reported that E2 interaction with CD81 is dependent on the dimeric status of CD81 (19, 20). CD81 dimers bound approximately threefold more JFH-1 G451R than wt sE2 (Fig. 4A), confirming our earlier studies with CHO cell-expressed CD81. hCD81 LEL monomers failed to interact with wt or mutant sE2 (Fig. 4B). To further characterize the interaction of G451R sE2 with hCD81 LEL, we compared mutant and wt sE2 interactions with a panel of CD81 variants with substitutions at amino acid residues reported to be critical for interacting with E2 (20). All mutations abrogated CD81 interaction with both wt and G451R sE2 proteins, suggesting that the G451R mutation does not alter the nature of the E2-CD81 interaction (Fig. 4B).
FIG. 4.
Interaction JFH-1 wt and G451R sE2 with recombinant CD81. (A) Dose-dependent binding of JFH-1 wt (gray bars) or G451R (white bars) sE2 with hCD81 LEL dimer. Data are represented as the mean optical density (OD) at 450 nm. (B) JFH-1 wt or G451R sE2 association with PBS, monomeric and dimeric hCD81 LEL, and mutants of hCD81 LEL that abrogate CD81 interaction with E2. All mutants were characterized for their effects on CD81 oligomerization and were shown to have minimal effect on dimerization. E2 binding to the mutants is expressed relative to CD81 LEL dimer. Error bars indicate standard deviation from the mean (n = 3).
Relationship between JFH-1 and G451R particle density, infectivity, and coreceptor interactions.
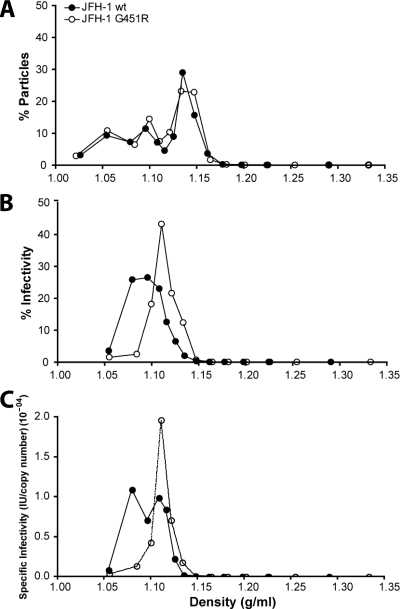
HCV particles associate with lipoproteins to form LVPs that can be fractionated according to their buoyant density. (2, 40, 41, 48, 53, 67). Initial experiments examined the distribution of wt and mutant particles across iodixanol gradients by quantifying genome copy numbers in each fraction. wt and mutant viruses demonstrated a similar range of particle densities, suggesting comparable physical properties (Fig. 5A). Low-density particles are reported to have the highest specific infectivity (40, 41); in support of this we observed peak JFH-1 wt infectivity at a density of 1.09 g/ml (Fig. 5B). In contrast, the majority of JFH-1 G451R infectivity resided in the higher-density fractions at 1.12 g/ml (Fig. 5B), suggesting an altered relationship between particle density and infectivity. The RNA copy number and infectivity data were used to calculate the specific infectivities of particles within each fraction; the G451R adaptation appears to have increased the infectivity of higher-density particles while perturbing the infectivity of low-density particles (Fig. 5C).
FIG. 5.
Analysis of JFH-1 wt and G451R buoyant density. Concentrated JFH-1 wt and G451R were separated on an iodixanol gradient. (A) The number of HCV particles per fraction was assessed by quantifying HCV RNA genomes by RT-PCR. The particle number in each fraction is expressed as a percentage of the total for either virus. (B) The infectivity per fraction was assessed by inoculating Huh-7.5 cells. The infectivity within each fraction is expressed as a percentage of the total for either virus. Error bars indicate standard deviations from the mean (n = 3). (C) The number of infectious units per RNA genome copy were calculated to analyze the specific infectivity of particles within each fraction.
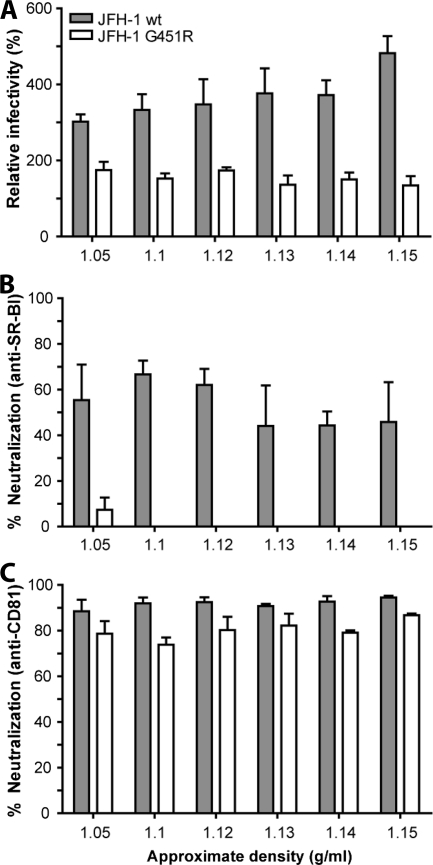
To study the relationship between particle density and receptor-dependent infection, JFH-1 wt and G451R iodixanol gradient fractions were screened for infection of parental and Huh-7.5 cells transduced to overexpress SR-BI and for their sensitivity to neutralization by SR-BI- and CD81-specific antibodies (Fig. 6). We failed to observe any association between wt or mutant virus density and SR-BI dependence, with all fractions showing comparable levels of infectivity under the respective conditions (Fig. 6A and B). JFH-1 G451R demonstrated a lower dependence on SR-BI, with minimal inhibition observed with the anti-SR-BI antibody (Fig. 6B). Similar results were observed with the anti-CD81 MAb, with all fractions demonstrating a similar sensitivity to neutralization (Fig. 6C). The lack of correlation between JFH-1 wt and G451R particle density and sensitivity to antireceptor antibodies suggests that the mutant phenotype of reduced SR-BI dependence and increased sensitivity to hCD81 LEL neutralization may be largely attributable to an altered affinity or interaction of the viral gps with CD81 (Fig. 3 and 4).
FIG. 6.
Relationship between particle density and coreceptor dependency. The density fractions containing infectious JFH-1 wt or JFH-1 G451R were used to assess the relationship between particle density and SR-BI and CD81 interaction(s). The approximate densities of each fraction are indicated on the x axis. (A) Huh-7.5 cells overexpressing SR-BI were inoculated with JFH-1 wt and G451R virus, and infectivity is expressed relative to parental Huh-7.5 cells. Huh-7.5 cells were incubated with either anti-SR-BI serum at 1/300 (B) or anti-CD81 1s201 at 0.1 μg/ml (C) prior to challenge with infectious fractions of JFH-1 wt and G451R. Error bars indicate standard deviations from the mean (n = 3).
JFH-1 G451R demonstrates an increased sensitivity to NAbs.
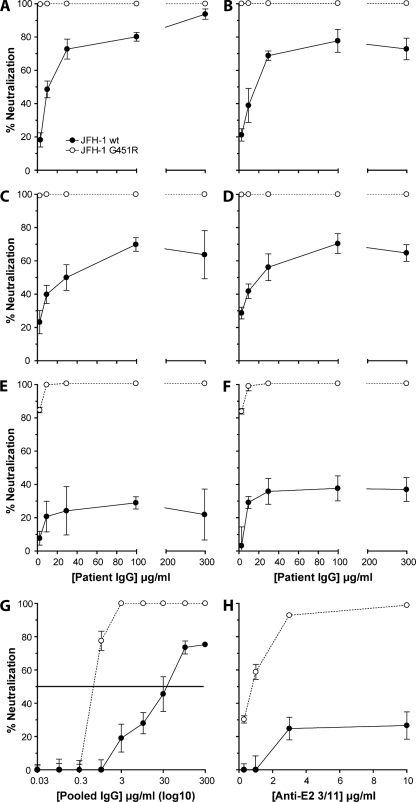
Several reports have suggested that antibodies specific for the HCV gps neutralize viral infectivity by inhibiting HCV interaction(s) with CD81 (33, 34, 55) (reviewed in reference 65). Given the increased binding of G451R E2 to CD81, we were interested to investigate the effects of this mutation on particle sensitivity to NAbs. We screened the sensitivity of JFH-1 wt and G451R virus to neutralization by IgG purified from the sera of six HCV-infected individuals. In each case G451R demonstrated an increased sensitivity to inhibition by patient IgG (Fig. 7A to F). JFH-1 wt was inhibited to various degrees by the patient IgG, and in five of six cases the percent neutralization reached a plateau below 80%, suggesting that a population of particles was resistant to neutralization. To quantify the increased sensitivity of G451R to antibody-dependent neutralization, we determined the concentration of pooled HCV-positive(HCV+) patient IgG required to inhibit 50% of infectivity (50% inhibitory concentration [IC50]). The IC50s for the wt and mutant viruses are 40 μg/ml and 0.75 μg/ml, respectively, indicating that G451R is 50-fold more sensitive to neutralization (Fig. 7G). Patient-derived IgG is polyclonal in nature and likely to target multiple conformation-dependent epitopes. To study virus neutralization via a defined epitope, we screened the sensitivity of both viruses to MAb 3/11, specific for E2 amino acids 412 to 423. G451R showed an increased sensitivity to MAb 3/11 neutralization (Fig. 7H). These data are consistent with an increased sensitivity of G451R particles to NAbs targeting diverse epitopes, suggesting an increased availability of epitopes on G451R compared to the parental virus. However, we failed to detect any difference in HCV+ patient IgG or MAb 3/11 binding to immobilized JFH-1 wt or G451R sE2 or to infected cells by flow cytometry, suggesting that differential epitope presentation may occur in the context of a virus particle.
FIG. 7.
JFH-1 G451R demonstrates an increased sensitivity to neutralization by gp-specific antibodies. JFH-1 wt (closed circles) or G451R (open circles) was incubated with IgG purified from the sera of six HCV-infected subjects (A to F) or anti-E2 MAb 3/11 (H) prior to infection of Huh-7.5 cells. Percent neutralization was calculated by quantifying viral infectivity in the presence of anti-HCV-specific antibodies relative to HCV-negative IgG or irrelevant MAb, respectively. (G) To determine the concentration of IgG required to neutralize 50% of JFH-1 and G451R infectivity (IC50), both viruses were incubated with a pool of the six-patient-derived IgG. The IC50 is depicted as a horizontal line. Error bars indicate standard deviations from the mean (n = 3).
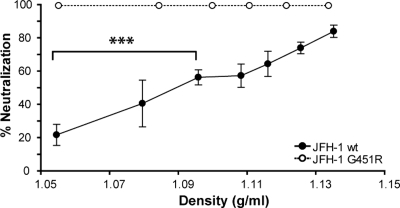
To investigate whether the increased sensitivity of G451R to NAbs was attributable to alterations in particle density, the iodixanol gradient fractions of wt and mutant viruses were normalized for infectivity and incubated with 10 μg/ml pooled HCV+ patient IgG. For JFH-1 wt, the sensitivity to neutralization increased with particle density such that the lower-density fraction was neutralized by 20% and the higher-density fraction by 80% (Fig. 8). In contrast, all G451R fractions were neutralized by 100%. Control experiments established that iodixanol concentration had minimal effects on JFH-1 infectivity and sensitivity to IgG neutralization (data not shown). These data provide the first evidence that lipoprotein association of JFH-1 reduces the sensitivity of particles to NAbs; however, the increased infectivity of high-density G451R viruses does not explain their heightened sensitivity to antibody-dependent neutralization.
FIG. 8.
Association between JFH-1 particle density and sensitivity to neutralizing antibodies. JFH-1 wt and G451R were separated on an iodixanol gradient as detailed in the legend of Fig. 5, and the fractions were tested for their sensitivity to neutralization by pooled HCV-infected patient IgG (10 μg/ml). Data are expressed as percent neutralization calculated by comparing infectivity in the presence of HCV-negative IgG. A positive correlation was observed between JFH-1 particle density and neutralization by pooled patient IgG (P < 0.0001, unpaired t test). Error bars indicate standard deviations from the mean (n = 4).
DISCUSSION
We have demonstrated that a cell culture-adaptive mutation in E2 has pleiotropic effects on HCV interaction(s) with SR-BI, CD81, and NAbs. JFH-1 G451R infectivity was not enhanced in Huh-7.5 cells transduced to overexpress SR-BI and was insensitive to anti-SR-BI and HDL treatments (Fig. 1), suggesting a reduced requirement for SR-BI during entry. Definitive evidence of SR-BI independence is hampered by the lack of SR-BI-negative permissive cell lines. We failed to detect any effect(s) of the G451R mutation on sE2 interaction with CHO cells expressing SR-BI (Fig. 3B and C); however, soluble forms of E2 may not recapitulate the interaction of virus particles with SR-BI.
CD81 is a critical coreceptor for HCV particle entry (40, 45). The mutant virus demonstrated a 10-fold increase in the sensitivity to neutralization by hCD81 LEL, suggesting an increased affinity of the gps for CD81 (Fig. 2C). Indeed, G451R sE2 demonstrated increased binding to CHO-CD81 cells and hCD81 LEL, supporting this conclusion (Fig. 3 and 4). The observation that G451R demonstrated reduced sensitivity to neutralization by anti-CD81 MAbs relative to wt virus is consistent with an increased affinity of G451R glycoproteins that are able to more effectively compete with subsaturating levels of MAbs than wt for cell surface-expressed CD81 (Fig. 2A and B). Mutations in CD81 reported to prevent interaction with E2 (20) abolished the binding of both JFH-1 wt and G451R sE2 (Fig. 4B), suggesting that the interface between JFH-1 G451R E2 and CD81 is unaltered and that the viral phenotype may simply reflect a greater affinity of the gp with the coreceptor. Studies with HCVpp suggest that the CD81 binding site on E2 involves three discontinuous regions (18, 55, 59), and G451R is located immediately downstream of one such region, G436WLAGLFY (18). Thus, the adaptive mutation may directly modulate E2 affinity for CD81. Our data suggest an important role for position 451 in JFH-1 E2 coordination of particle interaction with SR-BI and CD81 and highlight the importance of studying mutant gp association with multiple viral coreceptors.
Numerous reports have used density gradient centrifugation to study the association of plasma or serum-derived HCV with the major VLDL apoproteins (ApoB-100 and ApoE) (2, 53, 68). Recent data implicate VLDL synthesis in HCV particle assembly and/or release, suggesting that HCVcc particles interact with lipoproteins in an analogous manner to blood-derived virus (13, 26, 30, 41). Nielsen et al. reported that iodixanol density gradient centrifugation preserved HCV VLDL interactions (53), and we employed this technique to investigate the relationship between JFH-1 particle density and infectivity. Zhong et al. reported an altered relationship between JFH-1 G451R buoyant density and infectivity, with high-density mutant virus demonstrating greater infectivity than wt (80). Similar data were observed with iodixanol density gradient separation (Fig. 5B). Importantly, analysis of particle number(s) by quantitative RT-PCR demonstrated a comparable distribution of wt and mutant viruses (Fig. 5A), suggesting that both viruses associate with lipoproteins in a comparable manner. Analysis of specific infectivity throughout the density gradient suggests that the G451R mutation increases the infectivity of higher-density particles while reducing that of low-density particles (Fig. 5C). Determining how the cell culture adaptation alters the relationship between particle density and infectivity will require a better understanding of the contribution that lipoprotein components make to particle infectivity.
Lipoproteins have been implicated in HCV entry and particle interaction(s) with SR-BI (3, 13, 44). However, we failed to observe any association between infectious particle density and responsiveness to SR-BI overexpression or receptor “neutralization” for JFH-1 wt or G451R (Fig. 6). These data do not discount the role of lipoproteins in the primary engagement between virus and receptors; however, it suggests that the “functional outcome,” i.e., entry, is principally driven by the viral gps. HCVpp assembly and entry are less dependent on host lipoproteins and offer a tool to dissect the role of the adaptive G451R mutation in viral entry. However, E1E2 gps with the G451R mutation failed to generate infectious HCVpp (data not shown).
The heightened sensitivity of JFH-1 G451R to neutralization is not confined to hCD81 LEL; IgG from six HCV-infected individuals inhibited JFH-1 G451R 50-fold more effectively than wt (Fig. 7A to G). The polyclonal IgG was isolated from patients infected with HCV genotypes 1 or 3 and most likely reflects a mixture of antibodies specific for diverse conformation-dependent epitopes. In addition, G451R was >10-fold more sensitive to neutralization by anti-E2 MAb 3/11 (Fig. 7H), which is specific for amino acids 412 to 423 (29, 55). Experiments to assess the binding of polyclonal HCV+ patient IgG and MAb 3/11 to immobilized JFH-1 wt and G451R sE2 by enzyme immunoassay found no differences (data not shown). However, sE2 may not be an accurate mimic of epitope availability in a native virus particle (14, 25, 35).
We hypothesized that virus association with lipoproteins reduces the efficacy of NAbs. Taking particle density as a measure of lipoprotein interaction(s), we demonstrate that low-density JFH-1 has reduced sensitivity to HCV+ patient IgG neutralization (Fig. 8), showing a range of neutralization values from 20 to 80% between the densities of 1.04 to 1.14 g/ml. The data support a model where lipoproteins obscure critical epitopes from NAbs, and, since the majority of infectious JFH-1 particles are of low density, this may explain their insensitivity to NAbs (Fig. 7). The mechanism by which this occurs is unknown; however, lipoproteins may restrict the access of antibodies to E2 or promote the stabilization of virus particles. Our findings are consistent with observations with serum-derived HCV (2, 41, 67) and with the findings of Thommsen et al., who reported that ApoB-100-associated virus failed to precipitate with polyclonal IgG (68). Similarly, Molina et al. reported that serum virus infectivity was neutralized with anti-CD81 antibodies targeting the host cell whereas infectivity was resistant to hCD81 LEL, suggesting reduced exposure of CD81 binding epitopes on circulating particles (50).
The majority of patient-derived HCV strains have proven difficult to propagate in vitro. In this regard, the JFH-1 strain is atypical, and understanding whether it represents the global HCV population is an important area for future research. In this light, studying cell culture-adapted mutations may be viewed as having limited potential; however, similar studies with HIV variants demonstrated the value of such work in defining the mechanisms of viral entry.
Primary attachment of HIV to CD4+ lymphoid cells promotes a conformational change in the viral envelope protein gp120, which exposes epitopes that are critical for later stages of entry (61, 70, 76). CD4 expression levels limit HIV entry, and cell culture adaptation leads to viruses with reduced dependence on CD4 and a heightened sensitivity to NAbs (4, 11, 22, 28, 57). SR-BI may fulfill a role similar to that of CD4; indeed, Evans et al. reported that HCVcc virions bound to CHO cells expressing SR-BI but not CD81, suggesting that SR-BI binding residues are exposed on the particle surface (23). However, the analogy with HIV only extends so far; HCV association with host lipoproteins adds another level of complexity, and it is becoming increasingly clear that both viral and host components contribute to infectivity. We demonstrate that low-density HCV particles are less sensitive to antibody neutralization, suggesting that lipoproteins provide sanctuary from the immune system. The G451R cell culture-adapted mutant modulates virion interaction(s) with viral coreceptors and NAbs, suggesting altered epitope presentation at the virion surface. In addition, the mutation perturbed the relationship between particle density and specific infectivity. These studies highlight the interplay between particle association with lipoproteins, coreceptors, and NAbs, and further studies to define how a single amino acid change can exert such pleiotropic effects will require a greater understanding of HCV LVP genesis and entry into naïve target cells.
Acknowledgments
We thank Takaji Wakita for JFH-1, Charles Rice for Huh-7.5 cells and anti-NS5A 9E10 MAb, Roslyn Bill for purified full-length CD81, Ke Hu and Margaret Goodall for anti-CD81 MAbs 2s131 and 1s201, David Adams and David Mutimer for access to normal and HCV+ patient sera, and Thierry Huby for anti-SR-BI sera and CHO-SR-BI cells. We thank Zania Stamataki for critical reading of the manuscript.
This work was supported by PHS grants AI50798 and AI40034-14, the MRC, and the Wellcome Trust.
Footnotes
Published ahead of print on 1 October 2008.
REFERENCES
- 1.Alberti, A., L. Chemello, and L. Benvegnu. 1999. Natural history of hepatitis C. J. Hepatol. 31(Suppl. 1)17-24. [DOI] [PubMed] [Google Scholar]
- 2.Andre, P., F. Komurian-Pradel, S. Deforges, M. Perret, J. L. Berland, M. Sodoyer, S. Pol, C. Brechot, G. Paranhos-Baccala, and V. Lotteau. 2002. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J. Virol. 766919-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreo, U., P. Maillard, O. Kalinina, M. Walic, E. Meurs, M. Martinot, P. Marcellin, and A. Budkowska. 2007. Lipoprotein lipase mediates hepatitis C virus (HCV) cell entry and inhibits HCV infection. Cell Microbiol. 92445-2456. [DOI] [PubMed] [Google Scholar]
- 4.Bannert, N., D. Schenten, S. Craig, and J. Sodroski. 2000. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J. Virol. 7410984-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth, H., C. Schafer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 27841003-41012. [DOI] [PubMed] [Google Scholar]
- 6.Barth, H., E. K. Schnober, F. Zhang, R. J. Linhardt, E. Depla, B. Boson, F. L. Cosset, A. H. Patel, H. E. Blum, and T. F. Baumert. 2006. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J. Virol. 8010579-10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartosch, B., G. Verney, M. Dreux, P. Donot, Y. Morice, F. Penin, J. M. Pawlotsky, D. Lavillette, and F. L. Cosset. 2005. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J. Virol. 798217-8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanchard, E., S. Belouzard, L. Goueslain, T. Wakita, J. Dubuisson, C. Wychowski, and Y. Rouille. 2006. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J. Virol. 806964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blight, K. J., J. A. McKeating, and C. M. Rice. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 7613001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blish, C. A., M. A. Nguyen, and J. Overbaugh. 2008. Enhancing exposure of HIV-1 neutralization epitopes through mutations in gp41. PLoS Med. 5e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catanese, M. T., R. Graziani, T. von Hahn, M. Moreau, T. Huby, G. Paonessa, C. Santini, A. Luzzago, C. M. Rice, R. Cortese, A. Vitelli, and A. Nicosia. 2007. High-avidity monoclonal antibodies against the human scavenger class B type I receptor efficiently block hepatitis C virus infection in the presence of high-density lipoprotein. J. Virol. 818063-8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, K. S., J. Jiang, Z. Cai, and G. Luo. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J. Virol. 8113783-13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton, R. F., A. Owsianka, J. Aitken, S. Graham, D. Bhella, and A. H. Patel. 2002. Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles. J. Virol. 767672-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Codran, A., C. Royer, D. Jaeck, M. Bastien-Valle, T. F. Baumert, M. P. Kieny, C. A. Pereira, and J. P. Martin. 2006. Entry of hepatitis C virus pseudotypes into primary human hepatocytes by clathrin-dependent endocytosis. J. Gen. Virol. 872583-2593. [DOI] [PubMed] [Google Scholar]
- 16.Cook, L., K. W. Ng, A. Bagabag, L. Corey, and K. R. Jerome. 2004. Use of the MagNA pure LC automated nucleic acid extraction system followed by real-time reverse transcription-PCR for ultrasensitive quantitation of hepatitis C virus RNA. J. Clin. Microbiol. 424130-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreux, M., T. Pietschmann, C. Granier, C. Voisset, S. Ricard-Blum, P. E. Mangeot, Z. Keck, S. Foung, N. Vu-Dac, J. Dubuisson, R. Bartenschlager, D. Lavillette, and F. L. Cosset. 2006. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J. Biol. Chem. 28118285-18295. [DOI] [PubMed] [Google Scholar]
- 18.Drummer, H. E., I. Boo, A. L. Maerz, and P. Poumbourios. 2006. A conserved Gly436-Trp-Leu-Ala-Gly-Leu-Phe-Tyr motif in hepatitis C virus glycoprotein E2 is a determinant of CD81 binding and viral entry. J. Virol. 807844-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummer, H. E., K. A. Wilson, and P. Poumbourios. 2005. Determinants of CD81 dimerization and interaction with hepatitis C virus glycoprotein E2. Biochem. Biophys. Res. Commun. 328251-257. [DOI] [PubMed] [Google Scholar]
- 20.Drummer, H. E., K. A. Wilson, and P. Poumbourios. 2002. Identification of the hepatitis C virus E2 glycoprotein binding site on the large extracellular loop of CD81. J. Virol. 7611143-11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubuisson, J., F. Helle, and L. Cocquerel. 2008. Early steps of the hepatitis C virus life cycle. Cell Microbiol. 10821-827. [DOI] [PubMed] [Google Scholar]
- 22.Dumonceaux, J., C. Chanel, S. Valente, L. Quivet, P. Briand, and U. Hazan. 1999. Mutations in the env gene of human immunodeficiency virus type 1 NDK isolates and the use of African green monkey CXCR4 as a co-receptor in COS-7 cells. J. Gen. Virol. 801975-1982. [DOI] [PubMed] [Google Scholar]
- 23.Evans, M. J., T. von Hahn, D. M. Tscherne, A. J. Syder, M. Panis, B. Wolk, T. Hatziioannou, J. A. McKeating, P. D. Bieniasz, and C. M. Rice. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446801-805. [DOI] [PubMed] [Google Scholar]
- 24.Flint, M., C. Maidens, L. D. Loomis-Price, C. Shotton, J. Dubuisson, P. Monk, A. Higginbottom, S. Levy, and J. A. McKeating. 1999. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 736235-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flint, M., T. von Hahn, J. Zhang, M. Farquhar, C. T. Jones, P. Balfe, C. M. Rice, and J. A. McKeating. 2006. Diverse CD81 proteins support hepatitis C virus infection. J. Virol. [DOI] [PMC free article] [PubMed]
- 26.Gastaminza, P., G. Cheng, S. Wieland, J. Zhong, W. Liao, and F. V. Chisari. 2008. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J. Virol. 822120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grove, J., T. Huby, Z. Stamataki, T. Vanwolleghem, P. Meuleman, M. Farquhar, A. Schwarz, M. Moreau, J. S. Owen, G. Leroux-Roels, P. Balfe, and J. A. McKeating. 2007. Scavenger receptor BI and BII expression levels modulate hepatitis C virus infectivity. J. Virol. 813162-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman, T. L., C. C. LaBranche, W. Zhang, G. Canziani, J. Robinson, I. Chaiken, J. A. Hoxie, and R. W. Doms. 1999. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc. Natl. Acad. Sci. USA 966359-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 1007271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, H., F. Sun, D. M. Owen, W. Li, Y. Chen, M. Gale, Jr., and J. Ye. 2007. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. USA 1045848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapadia, S. B., H. Barth, T. Baumert, J. A. McKeating, and F. V. Chisari. 2007. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J. Virol. 81374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaul, A., I. Woerz, P. Meuleman, G. Leroux-Roels, and R. Bartenschlager. 2007. Cell culture adaptation of hepatitis C virus and in vivo viability of an adapted variant. J. Virol. 8113168-13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keck, Z. Y., T. K. Li, J. Xia, M. Gal-Tanamy, O. Olson, S. H. Li, A. H. Patel, J. K. Ball, S. M. Lemon, and S. K. Foung. 2008. Definition of a conserved immunodominant domain on hepatitis C virus E2 glycoprotein by neutralizing human monoclonal antibodies. J. Virol. 826061-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keck, Z. Y., O. Olson, M. Gal-Tanamy, J. Xia, A. H. Patel, M. Dreux, F. L. Cosset, S. M. Lemon, and S. K. Foung. 2008. A point mutation leading to hepatitis C virus escape from neutralization by a monoclonal antibody to a conserved conformational epitope. J. Virol. 826067-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keck, Z. Y., J. Xia, Z. Cai, T. K. Li, A. M. Owsianka, A. H. Patel, G. Luo, and S. K. Foung. 2007. Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J. Virol. 811043-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krieger, M. 2001. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J. Clin. Investig. 108793-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai, W. K., P. J. Sun, J. Zhang, A. Jennings, P. F. Lalor, S. Hubscher, J. A. McKeating, and D. H. Adams. 2006. Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium: a role for capturing hepatitis C virus particles. Am. J. Pathol. 169200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavillette, D., A. W. Tarr, C. Voisset, P. Donot, B. Bartosch, C. Bain, A. H. Patel, J. Dubuisson, J. K. Ball, and F. L. Cosset. 2005. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology 41265-274. [DOI] [PubMed] [Google Scholar]
- 39.Levy, S., S. C. Todd, and H. T. Maecker. 1998. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu. Rev. Immunol. 1689-109. [DOI] [PubMed] [Google Scholar]
- 40.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309623-626. [DOI] [PubMed] [Google Scholar]
- 41.Lindenbach, B. D., P. Meuleman, A. Ploss, T. Vanwolleghem, A. J. Syder, J. A. McKeating, R. E. Lanford, S. M. Feinstone, M. E. Major, G. Leroux-Roels, and C. M. Rice. 2006. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc. Natl. Acad. Sci. USA 1033805-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lozach, P. Y., A. Amara, B. Bartosch, J. L. Virelizier, F. Arenzana-Seisdedos, F. L. Cosset, and R. Altmeyer. 2004. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J. Biol. Chem. 27932035-32045. [DOI] [PubMed] [Google Scholar]
- 43.Lozach, P. Y., H. Lortat-Jacob, A. de Lacroix de Lavalette, I. Staropoli, S. Foung, A. Amara, C. Houles, F. Fieschi, O. Schwartz, J. L. Virelizier, F. Arenzana-Seisdedos, and R. Altmeyer. 2003. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem. 27820358-20366. [DOI] [PubMed] [Google Scholar]
- 44.Maillard, P., T. Huby, U. Andreo, M. Moreau, J. Chapman, and A. Budkowska. 2006. The interaction of natural hepatitis C virus with human scavenger receptor SR-BI/Cla1 is mediated by ApoB-containing lipoproteins. FASEB J. 20735-737. [DOI] [PubMed] [Google Scholar]
- 45.McKeating, J. A., L. Q. Zhang, C. Logvinoff, M. Flint, J. Zhang, J. Yu, D. Butera, D. D. Ho, L. B. Dustin, C. M. Rice, and P. Balfe. 2004. Diverse hepatitis C virus glycoproteins mediate viral infection in a CD81-dependent manner. J. Virol. 788496-8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mee, C. J., J. Grove, H. J. Harris, K. Hu, P. Balfe, and J. A. McKeating. 2008. Effect of cell polarization on hepatitis C virus entry. J. Virol. 82461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meertens, L., C. Bertaux, and T. Dragic. 2006. Hepatitis C Virus entry requires a critical post-internalization step and delivery to early endosomes via clathrin coated vesicles. J. Virol. 8011571-11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyanari, Y., K. Atsuzawa, N. Usuda, K. Watashi, T. Hishiki, M. Zayas, R. Bartenschlager, T. Wakita, M. Hijikata, and K. Shimotohno. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 91089-1097. [DOI] [PubMed] [Google Scholar]
- 49.Molina, S., V. Castet, C. Fournier-Wirth, L. Pichard-Garcia, R. Avner, D. Harats, J. Roitelman, R. Barbaras, P. Graber, P. Ghersa, M. Smolarsky, A. Funaro, F. Malavasi, D. Larrey, J. Coste, J. M. Fabre, A. Sa-Cunha, and P. Maurel. 2007. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J. Hepatol. 46411-419. [DOI] [PubMed] [Google Scholar]
- 50.Molina, S., V. Castet, L. Pichard-Garcia, C. Wychowski, E. Meurs, J. M. Pascussi, C. Sureau, J. M. Fabre, A. Sacunha, D. Larrey, J. Dubuisson, J. Coste, J. McKeating, P. Maurel, and C. Fournier-Wirth. 2008. Serum-derived hepatitis C virus infection of primary human hepatocytes is tetraspanin CD81 dependent. J. Virol. 82569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morikawa, K., Z. Zhao, T. Date, M. Miyamoto, A. Murayama, D. Akazawa, J. Tanabe, S. Sone, and T. Wakita. 2007. The roles of CD81 and glycosaminoglycans in the adsorption and uptake of infectious HCV particles. J. Med. Virol. 79714-723. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen, S. U., M. F. Bassendine, A. D. Burt, D. J. Bevitt, and G. L. Toms. 2004. Characterization of the genome and structural proteins of hepatitis C virus resolved from infected human liver. J. Gen. Virol. 851497-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen, S. U., M. F. Bassendine, A. D. Burt, C. Martin, W. Pumeechockchai, and G. L. Toms. 2006. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J. Virol. 802418-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owsianka, A. M., A. W. Tarr, Z. Y. Keck, T. K. Li, J. Witteveldt, R. Adair, S. K. Foung, J. K. Ball, and A. H. Patel. 2008. Broadly neutralizing human monoclonal antibodies to the hepatitis C virus E2 glycoprotein. J. Gen. Virol. 89653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owsianka, A. M., J. M. Timms, A. W. Tarr, R. J. Brown, T. P. Hickling, A. Szwejk, K. Bienkowska-Szewczyk, B. J. Thomson, A. H. Patel, and J. K. Ball. 2006. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J. Virol. 808695-8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282938-941. [DOI] [PubMed] [Google Scholar]
- 57.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 722855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pohlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 774070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothwangl, K. B., B. Manicassamy, S. L. Uprichard, and L. Rong. 2008. Dissecting the role of putative CD81 binding regions of E2 in mediating HCV entry: putative CD81 binding region 1 is not involved in CD81 binding. Virol. J. 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell, R. S., J. C. Meunier, S. Takikawa, K. Faulk, R. E. Engle, J. Bukh, R. H. Purcell, and S. U. Emerson. 2008. Advantages of a single-cycle production assay to study cell culture-adaptive mutations of hepatitis C virus. Proc. Natl. Acad. Sci. USA 1054370-4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sattentau, Q. J., J. P. Moore, F. Vignaux, F. Traincard, and P. Poignard. 1993. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J. Virol. 677383-7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scarselli, E., H. Ansuini, R. Cerino, R. M. Roccasecca, S. Acali, G. Filocamo, C. Traboni, A. Nicosia, R. Cortese, and A. Vitelli. 2002. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 215017-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shotton, C., C. Arnold, Q. Sattentau, J. Sodroski, and J. A. McKeating. 1995. Identification and characterization of monoclonal antibodies specific for polymorphic antigenic determinants within the V2 region of the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 69222-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silver, D. L. 2004. SR-BI and protein-protein interactions in hepatic high density lipoprotein metabolism. Rev. Endocr. Metab. Disord. 5327-333. [DOI] [PubMed] [Google Scholar]
- 65.Stamataki, Z., J. Grove, P. Balfe, and J. A. McKeating. 2008. Hepatitis C virus entry and neutralization. Clin. Liver Dis. 12693-712. [DOI] [PubMed] [Google Scholar]
- 66.Tang, Y., Q. Wang, and Y. M. Saif. 2005. Development of a ssRNA internal control template reagent for a multiplex RT-PCR to detect turkey astroviruses. J. Virol. Methods 12681-86. [DOI] [PubMed] [Google Scholar]
- 67.Thomssen, R., S. Bonk, C. Propfe, K. H. Heermann, H. G. Kochel, and A. Uy. 1992. Association of hepatitis C virus in human sera with beta-lipoprotein. Med. Microbiol. Immunol. 181293-300. [DOI] [PubMed] [Google Scholar]
- 68.Thomssen, R., S. Bonk, and A. Thiele. 1993. Density heterogeneities of hepatitis C virus in human sera due to the binding of beta-lipoproteins and immunoglobulins. Med. Microbiol. Immunol. 182329-334. [DOI] [PubMed] [Google Scholar]
- 69.Timpe, J. M., Z. Stamataki, A. Jennings, K. Hu, M. J. Farquhar, H. J. Harris, A. Schwarz, I. Desombere, G. L. Roels, P. Balfe, and J. A. McKeating. 2008. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology 4717-24. [DOI] [PubMed] [Google Scholar]
- 70.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384184-187. [DOI] [PubMed] [Google Scholar]
- 71.Tscherne, D. M., C. T. Jones, M. J. Evans, B. D. Lindenbach, J. A. McKeating, and C. M. Rice. 2006. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J. Virol. 801734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voisset, C., N. Callens, E. Blanchard, A. Op De Beeck, J. Dubuisson, and N. Vu-Dac. 2005. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J. Biol. Chem. 2807793-7799. [DOI] [PubMed] [Google Scholar]
- 73.von Hahn, T., B. D. Lindenbach, A. Boullier, O. Quehenberger, M. Paulson, C. M. Rice, and J. A. McKeating. 2006. Oxidized low-density lipoprotein inhibits hepatitis C virus cell entry in human hepatoma cells. Hepatology 43932-942. [DOI] [PubMed] [Google Scholar]
- 74.von Hahn, T., and C. M. Rice. 2008. Hepatitis C virus entry. J. Biol. Chem. 2833689-3693. [DOI] [PubMed] [Google Scholar]
- 75.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384179-183. [DOI] [PubMed] [Google Scholar]
- 77.Wunschmann, S., J. D. Medh, D. Klinzmann, W. N. Schmidt, and J. T. Stapleton. 2000. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J. Virol. 7410055-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeisel, M. B., G. Koutsoudakis, E. K. Schnober, A. Haberstroh, H. E. Blum, F. L. Cosset, T. Wakita, D. Jaeck, M. Doffoel, C. Royer, E. Soulier, E. Schvoerer, C. Schuster, F. Stoll-Keller, R. Bartenschlager, T. Pietschmann, H. Barth, and T. F. Baumert. 2007. Scavenger receptor class B type I is a key host factor for hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology 461722-1731. [DOI] [PubMed] [Google Scholar]
- 79.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 1029294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhong, J., P. Gastaminza, J. Chung, Z. Stamataki, M. Isogawa, G. Cheng, J. A. McKeating, and F. V. Chisari. 2006. Persistent hepatitis C virus infection in vitro: coevolution of virus and host. J. Virol. 8011082-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]