Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) and its murine homolog, murine gammaherpesvirus 68 (MHV68), are lymphotropic viruses that establish latent infection in their host. Surprisingly, while B cells are the main viral reservoir in vivo, B-cell lines are poorly permissive to infection by either MHV68 or KSHV. Here, we report that most B-cell lines express very little to no cell surface heparan sulfate (HS), a glycosaminoglycan that is essential for infection by these viruses. We found that Ext1, a key enzyme in the biosynthesis of HS, was expressed at a low level in these cells. Transfection of B-cell lines with Ext1 restored high HS expression at the cell surface. Overexpression of Ext1 in murine A20 and M12 B-cell lines increased MHV68 surface binding and enhanced the efficiency of infection. Finally, although it was not sufficient to allow efficient infection, the expression of HS on BJAB cells promoted KSHV binding at the cell surface. Thus, our results indicate that MHV68 and KSHV cycles are blocked in B-cell lines at the binding step due to a lack of surface HS.
One of the characteristics of gammaherpesviruses is their tropism for B lymphocytes, where they establish latency (i.e., limited viral gene expression) and persist during the whole life of their host. Kaposi's sarcoma-associated herpesvirus (KSHV, also known as human herpesvirus 8) is a gammaherpesvirus associated with both lymphoid and nonlymphoid cell tumors in humans, mostly in immunodeficient patients. KSHV is the etiologic agent of Kaposi's sarcoma, an AIDS-associated skin cancer, as well as B-cell lymphoproliferative disorders such as primary effusion lymphoma and Castleman disease (9, 10, 39). Studies of KSHV are limited by the lack of cell lines able to support productive infection as well as the strict restriction in host range. Murine gammaherpesvirus 68 (MHV68) is phylogenetically related to KSHV (13, 48). MHV68 infects mice, where it establishes latency mostly in B cells (15, 16, 42), and has been associated with lymphoproliferative diseases in long-term-infected mice (41) or immunodeficent mice (44). Moreover, unlike KSHV, MHV68 replicates efficiently in vitro in different fibroblast and epithelial cell lines. Thus, MHV68 provides a small-animal model for the analysis of gammaherpesvirus pathogenesis both in vitro and in vivo (37, 40, 47).
Researchers in the field have been puzzled by the fact that while B cells are the main viral reservoir in vivo, B-cell lines are mostly resistant to infection by KSHV and MHV68. Even though KSHV does not replicate efficiently in cell lines, it can establish latent infection in a variety of adherent cell lines (4). However, B-cell lines appear to be among the most resistant cell lines (4, 8, 24, 35). Even more striking, whereas numerous cell lines are highly permissive for the MHV68 productive cycle, B-cell lines are poorly infected. MHV68 viral transcript (orf73) could be detected by reverse transcription (RT)-PCR (17) or real-time RT-PCR (unpublished observations) after infection of the A20 murine B-cell line. However, we were not able to detect significant green fluorescent protein (GFP) expression after infection of A20 or M12 B-cell lines with an MHV68 virus that encodes GFP under the control of a cytomegalovirus promoter (unpublished observations), indicating that the level of infection was very low. So far, the B-cell line systems available to study MHV68 pathogenesis are (i) an MHV68-infected tumor cell line (S11) isolated from an infected mouse (45) and (ii) a latently infected A20 cell line obtained after infection with a recombinant MHV68 that encodes hygromycin and selection for hygromycin resistance (17). Although these systems are of unquestionable value in determining the events involved in the maintenance of latency and reactivation, they preclude the study of most early events, such as viral entry. The reasons for the inability of KSHV and MHV68 to efficiently infect B-cell lines are not understood. However, there are indications that, at least in the case of KSHV, there might be a block at the level of viral entry. Indeed, B-lymphoma cell lines were resistant in KSHV glycoprotein-mediated cell fusion and viral entry assays (24). Moreover, the transfection of the KSHV genome into B-lymphoma BJAB cells led to the establishment of latency (11). These studies suggest that B-cell lines might lack a major determinant for KSHV entry.
Heparan sulfate (HS) is a sulfated polysaccharide that is found on the surfaces of most cells as part of proteoglycans (6). HS binds to numerous ligands, such as growth factors, cytokines, and chemokines, as well as microorganisms. For many viruses, binding to cell surface HS is a critical step in the invasion of a cell (28). HS provides initial docking sites thought to facilitate the subsequent interaction with a more-specific receptor. In many cases, deficiency in HS expression dramatically affects the efficiency of infection, as shown for several herpesviruses (36), in particular KSHV (3, 7) and MHV68 (12, 23). Both of these viruses encode several glycoproteins that interact with HS (1, 7, 19, 20, 49). The binding of KSHV to different cell lines is inhibited by the enzymatic removal of HS from the cell surface or by competition with soluble heparin (3, 7). CHO cells deficient for HS expression are resistant to KSHV binding or MHV68 infection (3, 12, 23).
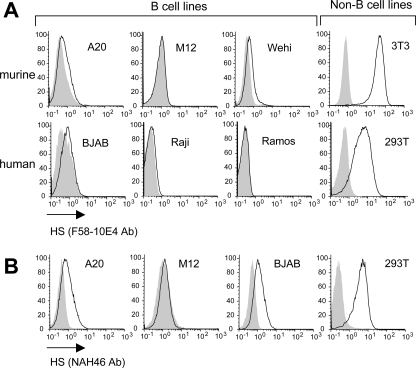
Given the critical role of HS in KSHV and MHV68 entry, we decided to examine the status of HS expression at the surfaces of B-cell lines. Murine B-cell lines (A20, M12, Wehi) and human B-cell lines (BJAB, Ramos, Raji) were surface stained with F58-10E4 antibody (Seikagaku Corporation), the most commonly used anti-HS antibody. As shown in Fig. 1A, whereas control non-B cells (murine NIH 3T3 and human 293T) expressed high levels of HS, the signal was either very low or undetectable in all the B-cell lines that we tested. HS is synthesized by the stepwise addition of glucuronic acid (GlcA) alternating with N-acetylglucosamine (GlcNAc) attached to a proteoglycan core protein. The chains are subsequently modified through several reactions which transform the polymeric heparan precursor to HS. In particular, 40 to 50% of the polymer is subjected to N-deacetylation followed by N-sulfation of GlcNAc (14, 18, 22). The final and functional HS product displays a domain-type arrangement of more- or less-modified saccharide sequences, where the sulfated domains are the main regions involved in ligand binding. The F58-10E4 antibody reacts with an epitope that includes N-sulfated glucosamine residues on HS chains. Such residues are critical for the reactivity of the F58-10E4 antibody; therefore, this antibody will not recognize the unmodified heparan precursor. On the other hand, a newly available anti-HS antibody, NAH46 (Seikagaku Corporation), is specific for GlcNAc residues and can thus recognize unmodified heparan precursors (as well as HS, since the deacetylation modification is not complete along the HS polymer). Using this antibody, we could detect a signal at the surfaces of some of the B-cell lines, albeit at much lower levels than those detected for non-B-cell lines (Fig. 1B). Since HS refers to modified polymers, containing in particular N-sulfated residues recognized by F58-10E4, we conclude that B-cell lines are defective for HS expression. The low level of expression detected using the NAH46 antibody could be due to the presence of nonsulfated HS precursors or abnormally short HS chains. This will be discussed elsewhere in this report.
FIG. 1.
B-cell lines were stained with the F58-10E4 anti-HS antibody (1:100) that recognizes only functional modified HS polymers (A) or with the NAH46 antibody (1:100) (B), followed by a fluorochrome-conjugated anti-mouse IgM antibody. Non-B-cell lines are shown as a positive control (mouse NIH 3T3 cells and human 293T cells). The shaded histograms correspond to staining with an isotope control (mouse IgMkappa, TEPC183; Sigma).
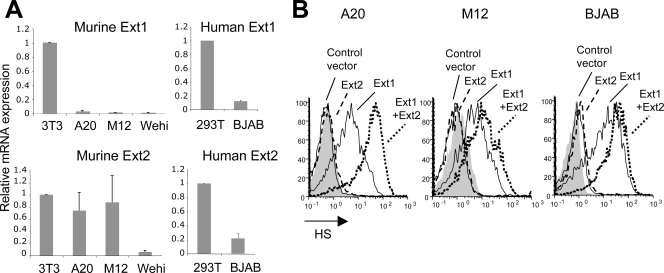
A critical step in HS biosynthesis is the polymerization of alternating GlcA and GlcNAc residues, catalyzed by the synergistic action of Ext1 and Ext2 enzymes (27, 29, 30). We examined the level of Ext1 and Ext2 mRNAs in B-cell lines by real-time RT-PCR and found that these cells expressed low levels of Ext1. In contrast, the closely related Ext2 mRNA was, on average, expressed at higher levels, although it varied depending on the cell line (Fig. 2A). We next transfected B-cell lines with vectors encoding Ext1 and Ext2 (29), either separately or together. Cells were transfected with a vector encoding Ext1 fused to GFP (pExt1-GFP), a vector encoding Ext2 fused to GFP (mExt2-GFP), or pExt1-GFP together with a vector encoding Ext2 (mExt2). As a control, a vector encoding GFP alone was used. Twenty-four hours after transfection, cells were stained with the F58-10E4 anti-HS antibody followed by a phycoerythrin (PE)-conjugated anti-immunoglobulin M (IgM) antibody and analyzed by flow cytometry. As shown in Fig. 2B, Ext1-transfected cells expressed high levels of HS (shown for A20, M12, and BJAB cells), whereas Ext2 alone had no effect on HS expression. Similar results were obtained for the other two B-cell lines that we tested (Wehi and Raji; data not shown). In most cases, the transfection of Ext1 together with Ext2 (Ext1+2) increased HS expression compared to results for Ext1 alone, consistent with the cooperative function of these two enzymes (29). The NAH46 antibody also gave rise to a dramatic signal increase in Ext1-transfected cells (data not shown). Thus, the defect in HS expression at the surfaces of B-cell lines can be overcome by the overexpression of Ext1.
FIG. 2.
(A) RNA was extracted from B-cell lines or from non-B-cell lines (NIH 3T3 and 293T) as a control and subjected to RT- and real-time PCR using primers specific for murine or human Ext1 and Ext2 cDNA. Results were normalized to the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase), and the comparative threshold cycle (CT) method was used for quantification. (B) A20, M12, and BJAB cells were transfected with a vector encoding Ext1-GFP, in the presence or absence of Ext2. Samples transfected with a vector encoding Ext2-GFP were included, as were cells transfected with a control vector expressing GFP only. After 24 h, cells were stained with the F58-10E4 anti-HS antibody followed by a PE-conjugated secondary antibody. HS staining of the GFP-positive cells is shown (shaded histogram, mouse IgMkappa isotype control).
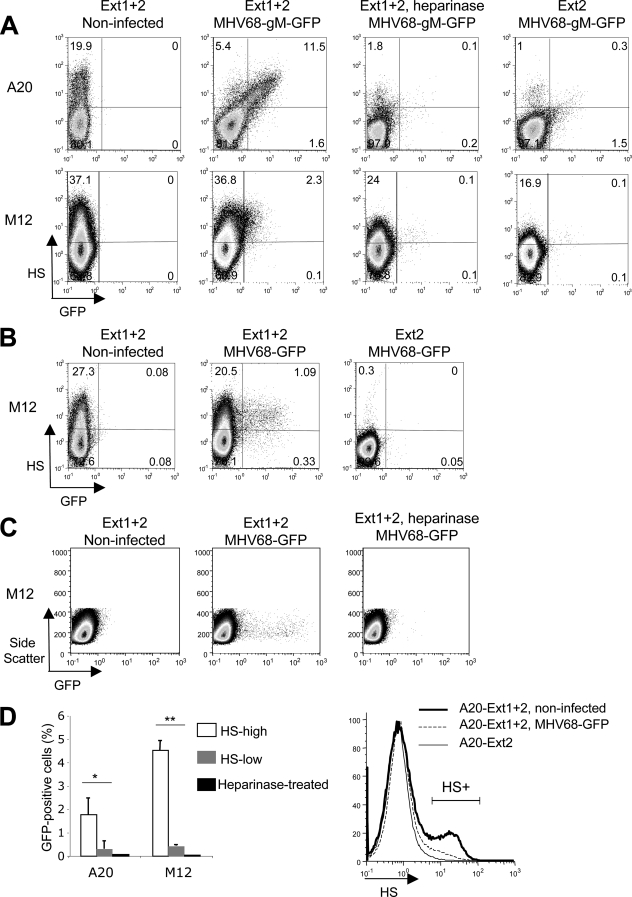
Fibroblast and epithelial cell lines are readily susceptible to MHV68 infection. MHV68 critically depends on surface HS to infect CHO cells (12, 23) as well as bone marrow-derived dendritic cells (38). To test if the poor susceptibility of B-cell lines to MHV68 infection is due to their defect in HS expression, we transfected A20 and M12 murine B cells with Ext1+2 to achieve a high level of surface HS. As a negative control, cells were transfected with Ext2 alone. After 24 h, MHV68 surface binding was examined using MHV68 virions carrying an enhanced GFP tag on the endogenous gM C terminus (MHV68 gM-GFP) (21). Cells were incubated with the virus for 90 min on ice and then washed and stained for surface HS. Cells were fixed in paraformaldehyde and analyzed by flow cytometry. As shown in Fig. 3A, there was a correlation between the GFP signal in A20 cells and the amount of HS at the cell surface, indicating that the efficiency of viral binding was dependent on HS expression. The same trend was observed for M12 cells, albeit to a lesser extent. In both cases, the pretreatment of Ext1+2-transfected cells with heparinase III, an enzyme that cleaves HS at the cell surface, drastically decreased the extent of GFP signal, to a level comparable to that seen for Ext2-transfected cells. Altogether, our data indicate that MHV68 binding on Ext1-expressing cells is mediated by surface HS.
FIG. 3.
(A) A20 and M12 cells were transfected with Ext1+2 or Ext2 alone. Twenty-four hours after transfection, a fraction of Ext1+2-transfected cells were treated with heparinase III for 3 h (1 U/ml) at 37°C. Cells were then incubated on ice in the presence of MHV68 gM-GFP at a multiplicity of infection of 5. After 90 min, cells were washed and stained with the NAH46 anti-HS antibody, followed by a PE-conjugated secondary antibody. Cells were fixed and analyzed by flow cytometry. (B) Cells were transfected with Ext1+2 or Ext2 alone, as described for panel A, and infected 24 h later with MHV68-GFP at a multiplicity of infection of 3. Eighteen hours after infection, the expression of GFP and HS (NAH46 staining, as in panel A) was analyzed. (C) Cells were transfected as described above. A fraction of Ext1+2-transfected cells was treated for 5 h with heparinase III (10 U/ml) before infection with MHV68-GFP. Heparinase was left in the medium during and after incubation with the virus. Eighteen hours after infection, GFP expression was examined by flow cytometry. (D) Left, the percentage of GFP-positive cells in HS-low or HS-high cells was determined in three independent experiments performed as for panel B. Dot plots were divided in quadrants as shown in panel B. *, P = 0.03; **, P < 0.0001. Right, A20 cells transiently transfected with Ext1+2 were infected with MHV68-GFP or were mock infected. After 18 h, cells were stained for HS expression as before. Ext2-transfected cells are shown as a control. HS+, percentage of HS-positive cells.
We next asked whether the increased viral binding upon HS expression was physiologically relevant and could promote viral entry. Twenty-four hours after transfection with Ext1+2 as described above, or Ext2 alone as a negative control, A20 and M12 cells were infected with an MHV68 that encodes GFP under a cytomegalovirus promoter (MHV68-GFP). Unlike the virus used in the previous experiment, the virions are not GFP tagged; thus, GFP expression is strictly dependent on viral access to the nucleus. Eighteen hours after infection, cells were examined for HS and GFP expression by flow cytometry. Results of two representative experiments are shown for M12 cells (Fig. 3B and C), as is quantification from three independent experiments (A20 and M12 cells) (Fig. 3D, left). GFP expression could be detected for both A20 and M12 HS-positive cells, to a significantly higher level than that detected for the HS-negative population. Importantly, cells treated with heparinase did not support significant infection (Fig. 3C and D). The quantification shown in Fig. 3D (left) was done by determining the percentage of GFP-positive cells among the HS-positive cells, or among the HS-negative cells, in samples transfected with Ext1+2, with the assumption that HS expression was not modified between the time of infection and the HS analysis 18 h later. However, we observed a significant decrease in the percentage of HS-positive cells in A20-infected samples (HS+; 6%) compared to that in the noninfected control (HS+; 20%) (Fig. 3D, right), suggesting that infected cells might have undergone HS downregulation. Thus, in the analysis of A20 infection, we are probably underestimating the fraction of infected cells that were HS positive at the time of infection. Surprisingly, however, the decrease in HS-positive cells following MHV68 infection is greater than the percentage of infected cells. It could be that MHV68 binding at the surface (Fig. 3A) promotes viral endocytosis (and thus downregulation of HS from the cell surface), with only a fraction of it leading to efficient infection.
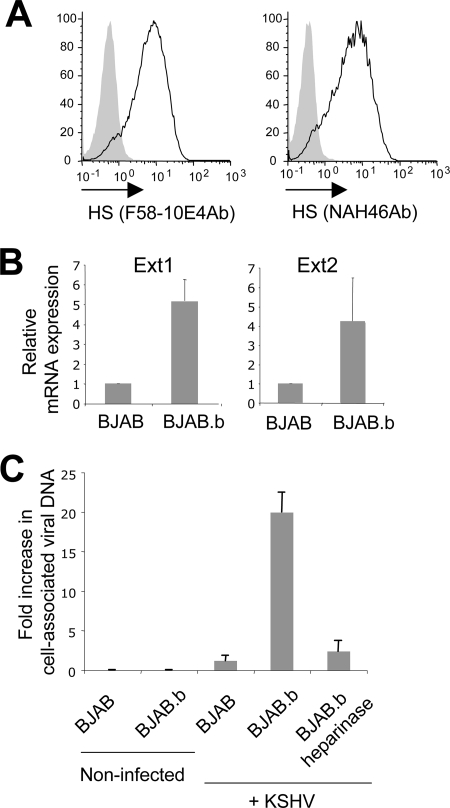
While several studies found that BJAB cells are resistant to KSHV infection (4, 8, 35), Chandran et al. reported that these cells could bind KSHV, and internalized viral particles were detected by electron microscopy (3). Interestingly, in that study, KSHV binding at the cell surface was blocked by heparin, indicating a role for HS. These observations prompted us to examine whether BJAB cells from the laboratory of B. Chandran (referred here as BJAB.b cells) naturally expressed HS, unlike the ones from the laboratory of L. Coscoy (Fig. 1 and 2). As shown in Fig. 4A, we found that BJAB.b cells expressed a high level of HS, as detected by both F58-10E4 and NAH46 anti-HS antibodies. We measured the level of Ext1 and Ext2 mRNAs by real-time RT-PCR as before and found that BJAB.b cells exhibited higher levels of both Ext1 and Ext2 mRNAs than those exhibited by HS-negative BJAB cells (Fig. 4B). We next examined the efficiency of KSHV binding at the cell surface in the HS-negative and -positive BJAB cells. BJAB.b cells were pretreated or not with heparinase III. HS removal from the cell surface in the heparinase-treated sample was verified by flow cytometry (data not shown). Cells were incubated in the presence of KSHV for 90 min on ice and then washed extensively and subjected to DNA extraction (DNeasy kit; Qiagen). The presence of KSHV genome was detected by real-time PCR. As shown in Fig. 4C, KSHV surface binding was significantly higher in BJAB.b cells than in HS-negative BJAB cells. Heparinase treatment inhibited KSHV binding on BJAB.b cells, consistent with a previous report by Chandran et al. (3). We then infected BJAB.b cells with KSHV and examined viral expression after 36 h. The latent protein Lana and the lytic gene product orf59 were not detected by immunofluorescence (data not shown). Real-time RT-PCR for the corresponding mRNAs was also negative at 24 to 36 h (data not shown). An extremely low signal for orf59 mRNA was observed repeatedly for BJAB.b cells, but not HS-negative BJAB cells, 8 h after infection (data not shown). However, the signal was very close to background and was absent after 12 h, suggesting a possible abortive infection. Alternatively, orf59 mRNA detection at 8 h postinfection could come from mRNA trapped in the virus particle.
FIG. 4.
(A) BJAB.b cells were surface stained with anti-HS antibodies (Ab) (shaded histograms, mouse IgMkappa isotype control). (B) RNA was extracted from BJAB and BJAB.b cells and subjected to RT- and real-time PCR using primers specific for human Ext1 and Ext2 cDNA. Results were normalized using the housekeeping gene GAPDH, and the comparative CT method was used for quantification. Ext1, P < 0.00003; Ext2, P = 0.019. (C) To measure KSHV surface binding, BJAB or BJAB.b cells were incubated with the virus for 90 min on ice and subjected to DNA isolation and real-time PCR using primers specific for the KSHV genome. Results were normalized using the housekeeping gene GAPDH, and the comparative CT method was used. Results are expressed as the increase (n-fold) over the signal obtained with HS-negative BJAB cells incubated with KSHV (set at 1).
Altogether, our results show that B-cell lines are defective for HS expression and, as a consequence, are resistant to MHV68 and KSHV binding. We found that B-cell lines express a low level of Ext1 and, consequently, very little HS at their surfaces. Interestingly, fibroblast cells with a hypomorphic gene trap mutation in Ext1 produce HS chains that are short but have a normal sulfation pattern (50). This might explain why we are able to detect a small signal using the NAH46 antibody in some of the B-cell lines. Indeed, due to their low Ext1 level, B-cell lines might produce short sulfated HS chains, providing enough epitopes for detection with the NAH46 antibody, but too few epitopes for detection with the F58-10E4 antibody. Overexpression of Ext1 might cause HS polymers to dramatically lengthen, resulting in a high signal with both NAH46 and F58-10E4 antibodies. Alternatively, we cannot completely exclude the possibility that B cells might also have a defect in sulfation (explaining the absence of F58-10E4 staining) and that overexpression of Ext1 overcomes that defect. However, we think this is unlikely, since Presto et al. showed that overexpression of Ext2, but not Ext1, indirectly increased HS sulfation (32).
We show that HS is critical for MHV68 binding and infection of A20 and M12 cells. To our knowledge, this is the first evidence that HS is critical for infection of B cells by MHV68. Some viral binding was detected in the HS-low A20 cells (Fig. 3A), probably due to the small endogenous level of HS, as detected with the NAH46 antibody. This modest level of HS expression probably explains the low level of infection that we could detect by real-time RT-PCR (data not shown) and that was also reported in a recent study (17). Transfection of syndecan 1, a carrier of HS, was used in an earlier study to enhance A20 permissivity to MHV68 (5). In that context, overexpression of the carrier protein probably leads to an increase in the number of short HS chains presented at the cell surface and thus might compensate for the lack of long HS polymers. Likewise, overexpression of syndecan 1 in Raji cells increased HS expression at the cell surface (26).
The efficiency of infection of HS-positive A20 and M12 cells remains relatively low (Fig. 3B to D). Interestingly, a massive viral binding could be detected in HS-positive A20 cells (Fig. 3A). However, these cells are infected to a lower extent than M12 cells that do not bind virus as efficiently. Thus, the level of infection does not strictly correlate with the amount of bound virus, indicating that there are additional blocks downstream of MHV68 binding to HS. It would be interesting to examine the nature of the proteoglycan to which HS is attached to determine whether it is important for MHV68 entry.
Consistent with earlier reports (3), we found that BJAB cells expressing HS (BJAB.b) could promote KSHV binding in an HS-dependent manner, while HS-negative BJAB cells supported very little binding. However, we were not able to effectively infect these HS-positive BJAB cells with KSHV. Interestingly, the introduction of KSHV DNA into BJAB cells led to infection (11). Altogether, this suggests that BJAB cells might lack a specific KSHV surface receptor. Indeed, whereas HS is required for the binding of numerous viruses to cell surfaces, it is generally not sufficient to promote viral entry. Alternatively, intracellular factors involved in the transport of the nucleocapsid to the nuclear membrane, viral DNA delivery into the nucleus, or DNA circularization might be missing in BJAB cells.
Several KSHV surface receptors have been identified (2, 25, 34). A recent study reported that transfection of DC-SIGN (dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin) into the Raji B-cell line could render these cells susceptible to KSHV infection (33). It would be interesting to determine if those Raji cells express HS, since variations between cell lines from different laboratories have been observed, both in HS expression (present study) and in receptor expression, such as that of cystine transporter xCT (25, 33).
Our study provides evidence that both MHV68 and KSHV require HS to infect cells of the B lineage in vitro. In contrast, Epstein-Barr virus, a closely related human gammaherpesvirus, does not appear to depend on HS for viral binding and entry. Instead, primary attachment to B cells is mediated by binding to complement receptor 2 (CD21) (31, 43). Importantly, the mechanism of B-cell infection in vivo by KSHV and MHV68 still remains to be elucidated, since naïve B cells express very little to no HS (46; unpublished data).
Acknowledgments
Many thanks to Philip G. Stevenson and Herbert W. “Skip” Virgin for providing MHV68 gM-GFP and MHV68-GFP viruses, respectively. Ext1- and Ext2-encoding vectors were kindly provided by Craig McCormick. We acknowledge the CHPS Mouse/Virus core for providing viral stocks. We thank Harshita Satija for excellent technical assistance and Maria Tokuyama for proofreading the manuscript.
This work was supported by grants from the Cancer Research Coordinating Committee and from the NIH.
Footnotes
Published ahead of print on 8 October 2008.
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284235-249. [DOI] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108407-419. [DOI] [PubMed] [Google Scholar]
- 3.Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282245-255. [DOI] [PubMed] [Google Scholar]
- 4.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 776474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett, N. J., J. S. May, and P. G. Stevenson. 2005. Gamma-herpesvirus latency requires T cell evasion during episome maintenance. PLoS Biol. 3e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68729-777. [DOI] [PubMed] [Google Scholar]
- 7.Birkmann, A., K. Mahr, A. Ensser, S. Yağuboğlu, F. Titgemeyer, B. Fleckenstein, and F. Neipel. 2001. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J. Virol. 7511583-11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackbourn, D. J., E. Lennette, B. Klencke, A. Moses, B. Chandran, M. Weinstein, R. G. Glogau, M. H. Witte, D. L. Way, T. Kutzkey, B. Herndier, and J. A. Levy. 2000. The restricted cellular host range of human herpesvirus 8. AIDS 141123-1133. [DOI] [PubMed] [Google Scholar]
- 9.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 3321186-1191. [DOI] [PubMed] [Google Scholar]
- 10.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 2661865-1869. [DOI] [PubMed] [Google Scholar]
- 11.Chen, L., and M. Lagunoff. 2005. Establishment and maintenance of Kaposi's sarcoma-associated herpesvirus latency in B cells. J. Virol. 7914383-14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lima, B. D., J. S. May, and P. G. Stevenson. 2004. Murine gammaherpesvirus 68 lacking gp150 shows defective virion release but establishes normal latency in vivo. J. Virol. 785103-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efstathiou, S., Y. M. Ho, S. Hall, C. J. Styles, S. D. Scott, and U. A. Gompels. 1990. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J. Gen. Virol. 711365-1372. [DOI] [PubMed] [Google Scholar]
- 14.Esko, J. D., and U. Lindahl. 2001. Molecular diversity of heparan sulfate. J. Clin. Investig. 108169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaño, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 1651074-1081. [DOI] [PubMed] [Google Scholar]
- 16.Flaño, E., I. J. Kim, D. L. Woodland, and M. A. Blackman. 2002. Gamma-herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells. J. Exp. Med. 1961363-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrest, J. C., and S. H. Speck. 2008. Establishment of B-cell lines latently infected with reactivation-competent murine gammaherpesvirus 68 provides evidence for viral alteration of a DNA damage-signaling cascade. J. Virol. 827688-7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher, J. T. 2001. Heparan sulfate: growth control with a restricted sequence menu. J. Clin. Investig. 108357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillet, L., H. Adler, and P. G. Stevenson. 2007. Glycosaminoglycan interactions in murine gammaherpesvirus-68 infection. PLoS ONE 2e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillet, L., S. Colaco, and P. G. Stevenson. 2008. The murid herpesvirus-4 gH/gL binds to glycosaminoglycans. PLoS ONE 3e1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillet, L., M. B. Gill, S. Colaco, C. M. Smith, and P. G. Stevenson. 2006. Murine gammaherpesvirus-68 glycoprotein B presents a difficult neutralization target to monoclonal antibodies derived from infected mice. J. Gen. Virol. 873515-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorsi, B., and S. E. Stringer. 2007. Tinkering with heparan sulfate sulfation to steer development. Trends Cell Biol. 17173-177. [DOI] [PubMed] [Google Scholar]
- 23.Jarousse, N., and L. Coscoy. 2008. Selection of mutant CHO clones resistant to murine gammaherpesvirus 68 infection. Virology 373376-386. [DOI] [PubMed] [Google Scholar]
- 24.Kaleeba, J. A., and E. A. Berger. 2006. Broad target cell selectivity of Kaposi's sarcoma-associated herpesvirus glycoprotein-mediated cell fusion and virion entry. Virology 3547-14. [DOI] [PubMed] [Google Scholar]
- 25.Kaleeba, J. A., and E. A. Berger. 2006. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 3111921-1924. [DOI] [PubMed] [Google Scholar]
- 26.Lebakken, C. S., and A. C. Rapraeger. 1996. Syndecan-1 mediates cell spreading in transfected human lymphoblastoid (Raji) cells. J. Cell Biol. 1321209-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind, T., F. Tufaro, C. McCormick, U. Lindahl, and K. Lidholt. 1998. The putative tumor suppressors EXT1 and EXT2 are glycosyltransferases required for the biosynthesis of heparan sulfate. J. Biol. Chem. 27326265-26268. [DOI] [PubMed] [Google Scholar]
- 28.Liu, J., and S. C. Thorp. 2002. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 221-25. [DOI] [PubMed] [Google Scholar]
- 29.McCormick, C., G. Duncan, K. T. Goutsos, and F. Tufaro. 2000. The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc. Natl. Acad. Sci. USA 97668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormick, C., Y. Leduc, D. Martindale, K. Mattison, L. E. Esford, A. P. Dyer, and F. Tufaro. 1998. The putative tumour suppressor EXT1 alters the expression of cell-surface heparan sulfate. Nat. Genet. 19158-161. [DOI] [PubMed] [Google Scholar]
- 31.Nemerow, G. R., C. Mold, V. K. Schwend, V. Tollefson, and N. R. Cooper. 1987. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J. Virol. 611416-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Presto, J., M. Thuveson, P. Carlsson, M. Busse, M. Wilen, I. Eriksson, M. Kusche-Gullberg, and L. Kjellen. 2008. Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST1 expression and heparan sulfate sulfation. Proc. Natl. Acad. Sci. USA 1054751-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rappocciolo, G., H. R. Hensler, M. Jais, T. A. Reinhart, A. Pegu, F. J. Jenkins, and C. R. Rinaldo. 2008. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J. Virol. 824793-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rappocciolo, G., F. J. Jenkins, H. R. Hensler, P. Piazza, M. Jais, L. Borowski, S. C. Watkins, and C. R. Rinaldo, Jr. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J. Immunol. 1761741-1749. [DOI] [PubMed] [Google Scholar]
- 35.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 725182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shukla, D., and P. G. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simas, J. P., and S. Efstathiou. 1998. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 6276-282. [DOI] [PubMed] [Google Scholar]
- 38.Smith, C. M., M. B. Gill, J. S. May, and P. G. Stevenson. 2007. Murine gammaherpesvirus-68 inhibits antigen presentation by dendritic cells. PLoS ONE 2e1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 861276-1280. [PubMed] [Google Scholar]
- 40.Speck, S. H., and H. W. Virgin. 1999. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr. Opin. Microbiol. 2403-409. [DOI] [PubMed] [Google Scholar]
- 41.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145818-826. [PMC free article] [PubMed] [Google Scholar]
- 42.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 733275-3279. [DOI] [PubMed] [Google Scholar]
- 43.Tanner, J., J. Weis, D. Fearon, Y. Whang, and E. Kieff. 1987. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell 50203-213. [DOI] [PubMed] [Google Scholar]
- 44.Tarakanova, V. L., F. Suarez, S. A. Tibbetts, M. A. Jacoby, K. E. Weck, J. L. Hess, S. H. Speck, and H. W. Virgin IV. 2005. Murine gammaherpesvirus 68 infection is associated with lymphoproliferative disease and lymphoma in BALB β2 microglobulin-deficient mice. J. Virol. 7914668-14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usherwood, E. J., J. P. Stewart, and A. A. Nash. 1996. Characterization of tumor cell lines derived from murine gammaherpesvirus-68-infected mice. J. Virol. 706516-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Voort, R., R. M. Keehnen, E. A. Beuling, M. Spaargaren, and S. T. Pals. 2000. Regulation of cytokine signaling by B cell antigen receptor and CD40-controlled expression of heparan sulfate proteoglycans. J. Exp. Med. 1921115-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Virgin, H. W., and S. H. Speck. 1999. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr. Opin. Immunol. 11371-379. [DOI] [PubMed] [Google Scholar]
- 48.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 715894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, F. Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J. Virol. 757517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamada, S., M. Busse, M. Ueno, O. G. Kelly, W. C. Skarnes, K. Sugahara, and M. Kusche-Gullberg. 2004. Embryonic fibroblasts with a gene trap mutation in Ext1 produce short heparan sulfate chains. J. Biol. Chem. 27932134-32141. [DOI] [PubMed] [Google Scholar]