Abstract
Infection of mice with murine gammaherpesvirus 68 (MHV-68) robustly activates CD8 T cells, but only six class I major histocompatibility complex (MHC)-restricted epitopes have been described to date for the widely used H-2b haplotype mice. To explore the specificity and kinetics of the cytotoxic T-lymphocyte response in MHV-68-infected C57BL/6 mice, we screened for H-2Kb- and H-2Db-restricted epitopes using a set of 384 candidate epitopes in an MHC tetramer-based approach and identified 19 new epitopes in 16 different open reading frames. Of the six known H-2Kb- and H-2Db-restricted epitopes, we confirmed a response against three and did not detect CD8 T-cell-specific responses for the remaining three. The peak of the CD8 T-cell response to most peptides occurs between 6 and 10 days postinfection. The respective MHC tetramer-positive CD8 T cells display an activated/effector phenotype (CD62Llo and CD44hi) and produce gamma interferon upon peptide stimulation ex vivo. MHV-68 infection in vivo elicits a response to multiple viral epitopes, derived from both early and late viral antigens, illustrating a far broader T-cell repertoire and more-rapid activation than those previously recorded.
The immune response against persistent infections caused by herpesviruses involves all known aspects of innate and adaptive immunity, and yet our understanding of the exact measure of protection by antibodies, T cells, and other immune effectors is far from complete. Members of the herpesvirus family avoid recognition by cytotoxic T cells, as inferred from their ability to downregulate the expression of class I major histocompatibility complex (MHC) molecules (13, 15, 25). Given the complexity of herpesvirus genomes, it is surprising how few CD8 T-cell epitopes have been identified. We thus have only very limited knowledge of the gene products recognized at different phases of the cytotoxic T-lymphocyte (CTL) response against these persistent viruses. A case in point is the murine gammaherpesvirus 68 (MHV-68). For the widely used C57BL/6 mouse model, only six class I MHC-restricted epitopes have been described (17, 23). With this modest number of epitopes, it is difficult to characterize more fully the temporal aspects of the anti-MHV-68 CD8 T-cell response, nor is it clear which gene products contribute to class I MHC-restricted epitopes and when: is it relative abundance, time of expression, or the cell type infected that contributes in some combination?
MHV-68 is a natural pathogen of wild rodents (4). The genome of MHV-68 has been sequenced and shows a close relationship with Kaposi's sarcoma herpesvirus and Epstein-Barr virus (EBV) (34). Since the functions of some of the MHV-68 gene products are similar to those of the corresponding gene products of human gammaherpesviruses, MHV-68 is widely used as a model for the pathogenesis of gammaherpesviruses. In acute MHV-68 infection, the virus is generally cleared by 10 to 15 days postinfection (dpi), followed by a latent phase (27, 36). The primary cells that are infected in vivo are B cells (36), and these are also the cells in which the virus establishes latency (28); however, removing the latent reservoir of B cells increases the levels of latency in non-B cells (37). Furthermore, B cells also play an important role in regulating reactivation and controlling chronic MHV-68 infection (37). A number of other, different host mechanisms are involved in clearing MHV-68 during acute infection and in the control of chronic infection and latency, among which a functional CD8 response is crucial (8, 33, 36). Nevertheless, by itself the CD8 response is unable to control virus replication, and the absence of CD4 T cells is ultimately fatal (6). In order to better understand the CD8-specific T-cell response, to capture its breadth as well as its kinetics in the course of a natural infection, we undertook a recently developed screening approach (10, 12, 32) to identify H-2Kb- and H-2Db-restricted epitopes from MHV-68.
We here report the identification of 19 additional T-cell epitopes restricted by H-2Kb and H-2Db, using arrays of MHC tetramers to screen for epitope-specific primary CD8 T cells. We characterize the kinetics of the anti-MHV-68 response and show that the CD8 T-cell response is skewed toward H-2Kb-restricted epitopes. Based on the sample times, we have categorized the CTL responses into two groups, in which one set of epitopes is recognized by T cells whose frequency peaks at 6 dpi while a second pattern of reactivity is characterized by a peak response at day 10. This asynchronous response most likely represents a continuum in kinetics. These results reveal an unappreciated aspect of the T-cell response against MHV-68, emphasizing a far broader T-cell repertoire and more-rapid activation than those previously recorded.
MATERIALS AND METHODS
Experimental animals and viruses.
C57BL/6J mice were purchased from Jackson Laboratory and housed in accordance with institutional guidelines. Mice aged 6 to 10 weeks were infected intraperitoneally (i.p.) with 1 × 106 PFU of MHV-68. MHV-68 was obtained from the American Type Culture Collection (VR1465). Virus stocks of MHV-68 were prepared in 3T12 cells. 3T12 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM glutamine at 37°C. Titers of virus stocks were determined by plaque assays as described previously (6). All animal experiments were conducted in accordance with protocols approved by the MIT Committee on Animal Care.
Epitope prediction and synthesis.
The sequence data for 81 open reading frames (ORFs) from MHV-68 were obtained from the National Center for Biotechnology Information (NCBI), accession code NC_001826. The consensus epitope prediction program (CEPP) (19), which was reconstructed and made available on http://jura.wi.mit.edu/bioc/grotenbreg, was used to identify 192 octameric and 192 nonameric candidate epitopes for H-2Kb and H-2Db, respectively (see Tables S1 to S3 in the supplemental material). The peptides selected for screening were produced by Fmoc-based solid-phase peptide synthesis by the MIT Center for Cancer Research (Cambridge, MA) biopolymers facility. All peptides were dissolved in dimethyl sulfoxide (10 mg/ml) and stored at −20°C until further use.
MHC tetramer production and peptide exchange.
Recombinant protein expression, refolding of the H-2Kb and H-2Db complexes with the SV9-P7* conditional ligand (12), and their subsequent tetramerization were accomplished by following established protocols (2, 11). The peptide exchange reaction was initiated by UV irradiation (360 nm), and the resulting MHC tetramers were used directly to stain freshly prepared splenocytes as described previously (10, 12).
Cell preparation and surface marker staining.
Single-cell suspensions were prepared from spleens by mechanical disruption in ice-cold RPMI 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) containing 10% fetal calf serum. Erythrocytes were lysed with red blood cell lysis buffer (Sigma, St. Louis, MO). For the MHC-tetramer screens, the CD8 T cells were further purified with the CD8a+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA). Live-versus-dead-cell staining was accomplished with ethidium monoazide (Invitrogen) prior to surface staining of the splenocytes with saturating amounts of MHC tetramers and antibodies against CD8α, CD44, CD62L, and Vβ4+ CD8 (all from BD Pharmingen, San Diego, CA). The cells were washed with phosphate-buffered saline, directly fixed with 0.5% formaldehyde in phosphate-buffered saline, and analyzed on a FACSCalibur and LSRII flow cytometer (BD Pharmingen). Data were analyzed with FlowJo Software (Tree Star).
Intracellular IFN-γ detection assay.
Splenocytes from infected mice were seeded at 4 × 106 cells per well and restimulated for 6 h with 10 μg/ml of peptide. Cells restimulated with ionomycin and phorbol myristate acetate (Calbiochem, San Diego, CA) were treated at concentrations of 1 μM and 50 ng/ml, respectively. Cells were treated for 3 h with 10 μg/ml brefeldin A (Sigma-Aldrich), stained with ethidium monoazide, and then labeled with MHC tetramers and anti-CD8 monoclonal antibody as described above. Staining with anti-gamma interferon (anti-IFN-γ) monoclonal antibody (BD Pharmingen) was achieved using the BD Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) following the manufacturer's specifications.
Measurement of viral load by quantitative real-time PCR.
Viral load in the blood of infected mice was quantified by real-time PCR using the ABI 7900 real-time PCR system (Applied Biosystems, Foster City, CA). DNA was extracted from blood using the QIAamp DNA blood minikit (Qiagen, Hilden, Germany). The Taq-Man Universal PCR Master Mix and universal cycling conditions (Applied Biosystems) were used for amplification of a 70-bp region of the MHV-68 gB gene using the primers and probe described in reference 38. A standard curve was generated using known amounts of a plasmid containing the gB gene. The murine ribosomal protein L8 (rpl8) was amplified in parallel, using a primer and probe set described in reference 30, and used to normalize for input DNA between samples. A standard curve for rpl8 was constructed by serial dilution of a plasmid containing rpl8 (Open Biosystems clone 5684024; Open Biosystems, Huntsville, AL). The data were analyzed using the SDS 2.2.3 program and are presented as viral genome copy number relative to the copy number of rpl8 with background measurements from uninfected samples subtracted.
RESULTS
Screening for MHV-68-specific class I MHC epitopes.
Class I MHC tetramer reagents are uniquely able to stain CD8 T cells in an antigen-specific fashion (2). Their application in epitope discovery efforts has nevertheless been limited, as these reagents are cumbersome to produce. A recently developed strategy temporarily inserts a photocleavable peptide into class I MHC molecules; the peptide fragments during exposure to long-wave UV irradiation, thus evacuating the peptide binding groove (32). When the emptied MHC molecule is subsequently supplied with an epitope of interest, a novel peptide-MHC complex of defined specificity is generated in a single step from a common precursor (32). This peptide exchange approach has successfully been implemented for a variety of murine (10, 12) and human (3, 32) MHC products to rapidly produce arrays of MHC tetramers. These arrays can then be employed to screen for the presence of antigen-specific CD8 T-cell populations in infected mice in high-throughput fashion, as we have demonstrated for Chlamydia trachomatis (12) and Toxoplasma gondii (10). To identify H-2Kb- and H-2Db-restricted MHV-68-derived peptide epitopes, all of the MHV-68 ORFs were analyzed by CEPP, which combines four independent predictive algorithms (12, 19). The 384 highest-scoring epitopes (see Table S1 in the supplemental material) were generated as synthetic peptides for use in the production of MHC tetramers.
First, we confirmed that we could use this approach to generate tetramers from MHV-68-derived epitopes identified by more conventional means: the dominant p56/H-2Db and p79/H-2Kb epitopes (23), the subdominant gB604-612/H-2Kb epitope (17), and the minor epitopes from ORF9, ORF61, and ORF44 (23). As expected, the tetramers made with the p56/H-2Db epitope, the p79/H-2Kb epitope, and the gB604-612/H-2Kb epitope all stained the corresponding subsets of CD8 T cells from MHV-68-infected animals, but both H-2Kb and H-2Db tetramers made with the minor epitopes from ORF9, ORF61, and ORF44 did not stain the CD8 T-cell population from the infected animals (data not shown). The reasons for our failure to detect these epitopes were not explored further. We next performed a screen to identify additional epitopes for MHV-68. Splenic CD8 T cells from uninfected and MHV-68-infected C57BL/6 mice were collected and purified at 9 and 42 days after infection and stained with freshly generated H-2Kb and H-2Db tetramers. We identified 16 H-2Kb epitopes and three H-2Db epitopes, mapped to 16 different ORFs (Table 1; Fig. 1). At day 42 we did not detect a response against epitopes not already seen at day 9 in the CD8 T-cell response.
TABLE 1.
Characterization of epitopes
| ORF | Amino acid positions | Peptide sequence | Proposed function | Restriction element | Response type | Gene expression | % CD8+/MHC-PE+ at day:
|
|||
|---|---|---|---|---|---|---|---|---|---|---|
| 6
|
10
|
|||||||||
| Mean | SD | Mean | SD | |||||||
| ORF75c | 940-947 | KSLTYYKL | Tegument protein/FGARATc | H-2Kb | E | L | 4.513 | 1.311 | 3.673 | 0.691 |
| ORF39 | 167-174 | FVYLFHFM | Virion membrane glycoprotein M | H-2Kb | E | L | 1.927 | 0.121 | 1.530 | 1.530 |
| ORF48 | 148-155 | TNYKFSLV | Hypothetical protein | H-2Kb | E | E | 1.103 | 0.166 | 1.027 | 0.130 |
| ORF56 | 108-115 | SCLDYSHL | Component of herpesvirus helicase-primase complex | H-2Kb | E | L | 0.520 | 0.325 | 0.260 | 0.060 |
| ORF62 | 272-279 | RSYIYYAL | Capsid assembly and DNA maturation protein | H-2Kb | E | E-L | 0.443 | 0.021 | 0.223 | 0.085 |
| ORF26 | 132-139 | VNLVFPSV | Capsid protein | H-2Kb | E | L | 0.400 | 0.036 | 0.203 | 0.068 |
| ORF7 | 491-498 | LGSVYYKL | DNA packaging and transport protein | H-2Kb | E | E | 0.310 | 0.191 | 0.223 | 0.045 |
| ORF75c | 935-942 | AILKFKSL | Tegument protein/FGARATc | H-2Kb | E | L | 0.283 | 0.075 | 0.128 | 0.020 |
| ORF9 | 682-689 | SVYGFTGV | DNA polymerase family B | H-2Kb | E | E | 0.233 | 0.104 | 0.200 | 0.066 |
| ORF58 | 124-131 | CNRIYARL | Hypothetical protein | H-2Kb | E | E-L | 0.173 | 0.042 | 0.165 | 0.010 |
| ORF61 | 524-531 | TSINFVKI (p79) | Ribonucleotide reductase, large subunit | H-2Kb | L | E | 1.640 | 0.308 | 8.953 | 2.673 |
| ORF8 | 604-612 | KNYIFEEKL | Virion membrane glycoprotein B | H-2Kb | L | L | 0.650 | 0.125 | 2.410 | 0.596 |
| ORF61 | 164-171 | ITYFFEMI | Ribonucleotide reductase, large subunit | H-2Kb | L | E | 1.127 | 0.204 | 1.937 | 0.049 |
| ORF54 | 253-260 | AVVQFIRV | dUTPase | H-2Kb | L | E | 0.663 | 0.572 | 1.700 | 0.729 |
| ORF25 | 524-531 | LSPPMAHL | Herpesvirus major capsid protein | H-2Kb | L | L | 0.237 | 0.031 | 1.353 | 0.942 |
| ORF6 | 15-22 | AGYIYYQL | Major ssDNAa binding protein | H-2Kb | L | E | 0.240 | 0.053 | 0.333 | 0.241 |
| ORF21 | 304-311 | TGFRYSYM | Herpesvirus thymidine kinase | H-2Kb | L | E | 0.142 | 0.041 | 0.193 | 0.071 |
| ORF59 | 74-81 | AIYSFRNA | DNA polymerase, processivity subunit | H-2Kb | L | E | 0.180 | 0.060 | 0.137 | 0.141 |
| ORF75c | 176-184 | SAIENYETF | Tegument protein/FGARATc | H-2Db | E | L | 0.870 | 0.421 | 0.343 | 0.083 |
| ORF6 | 487-495 | AGPHNDMEI (p56) | Major ssDNA binding protein | H-2Db | L | E | 2.020 | 0.567 | 3.710 | 1.141 |
| ORF17 | 308-316 | SAITNHAAF | Capsid protein | H-2Db | L | L | 0.473 | 0.207 | 0.933 | 0.435 |
| ORF6 | 164-172 | SAPMKTVTI | Major ssDNA binding protein | H-2Db | L | E | 0.197 | 0.074 | 0.203 | 0.057 |
| Total % | 18.344 | 29.837 | ||||||||
ssDNA, single-stranded DNA.
FIG. 1.
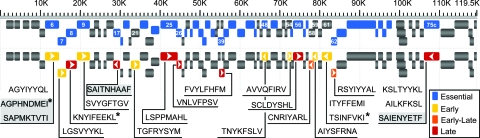
Mapping of CD8 T-cell epitopes in the MHV-68 genome. Sequences of the identified epitopes are mapped to their respective positions in the MHV-68 genome. The upper row depicts ORFs labeled as essential (blue) or nonessential (gray) genes, with the identity of the respective ORF from which the epitopes derive shown (22). In the lower row, the arrows indicate the transcriptional profile of the respective ORFs (1, 7, 18). Early-Late (E-L) refers to transcriptional classes in which both E and L gene expression has been demonstrated (1, 18). Sequences for previously identified epitopes are denoted with an asterisk, and H-2Db epitopes are shaded in gray.
We further classified the possible epitopes as derived from immediate-early (IE), early (E), and late (L) gene products (1, 7, 18) (Table 1; Fig. 1). We did not detect any tetramer-positive cells that recognized epitopes derived from IE viral proteins. About half of the viral proteins that gave rise to CD8 T-cell epitopes in this screen were derived from L proteins and included structural proteins (capsid, tegument, and envelope proteins). Other epitopes were derived from E gene products (ORF6, ORF9, ORF21, ORF54, ORF59, and ORF61) that participate in DNA replication (34). We also classified epitopes as being derived from essential and nonessential ORFs for in vitro growth (according to the division made through a transposon mutagenesis screening of MHV-68) (22). While half of the ORFs are essential, three-quarters of the CD8 T-cell epitopes are derived from essential ORFs (Fig. 1).
Post hoc analysis of the epitope prediction performance.
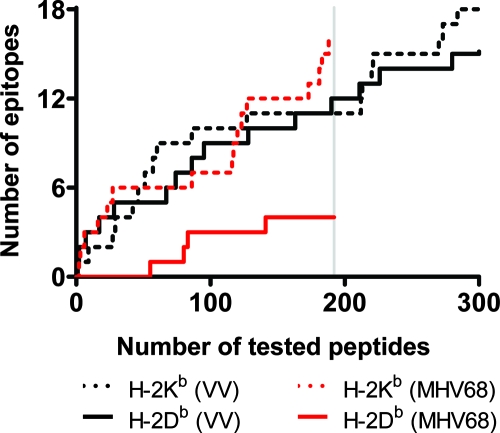
Vaccinia virus encodes 250 ORFs that yield approximately 56,400 peptides when the entire proteome is subjected to the CEPP (19). From the candidate peptides, 49 vaccinia virus epitopes were identified, of which 18 were H-2Kb-restricted octamers and 18 were H-2Db-restricted nonamers (19). For both restriction elements, the majority of these epitopes were shown to fall within the top 300 (0.5%) of the highest-ranking peptides (19). Our CEPP was constructed based on the algorithms reported by reference 19), and thus their prediction success rate for vaccinia virus could be replicated (Fig. 2). Using this CEPP, the MHV-68 genome (81 ORFs) yielded ∼37,500 possible peptides and led us to screen the highest-ranked 192 (0.5%) H-2Kb octamers and 192 (0.5%) H-2Db nonamers. We detected a CD8 T-cell response toward 16 H-2Kb epitopes but to only three H-2Db epitopes (Fig. 2). Is the preferential occurrence of H-2Kb epitopes an idiosyncratic property of MHV-68 itself? Based on the equal distribution of vaccinia virus epitopes over the H-2Kb and H-2Db restriction elements and taking into account the difference in genome size between vaccinia virus and MHV-68, we calculated the expected number of H-2Kb and H-2Db epitopes in MHV-68. Assuming that the list of vaccinia virus epitopes represents an unbiased sampling of the possible epitopes by the immune system of the C57BL/6 mouse, the number of MHV-68-derived H-2Kb epitopes does not significantly deviate from the expected value, whereas a significant underrepresentation (P = 0.034) of H-2Db-restricted epitopes is observed.
FIG. 2.
Post hoc analysis of the CEPP performance. The top-ranking 0.5% of candidate peptides derived from MHV-68 and vaccinia virus (VV), as assigned by the CEPP, are plotted (x axis) against those epitopes that were confirmed to give a CD8 T-cell response (y axis). Each confirmed epitope is treated as an individual and discrete event. The vertical line depicts the 0.5% cutoff value of the top-ranking epitopes of the MHV-68 screen. Based on the results obtained with vaccinia virus, there are significantly (P = 0.034) fewer H-2Db-restricted MHV-68 epitopes than expected for that restriction element.
The CD8 T-cell response in the course of MHV-68 infection shows differential kinetics.
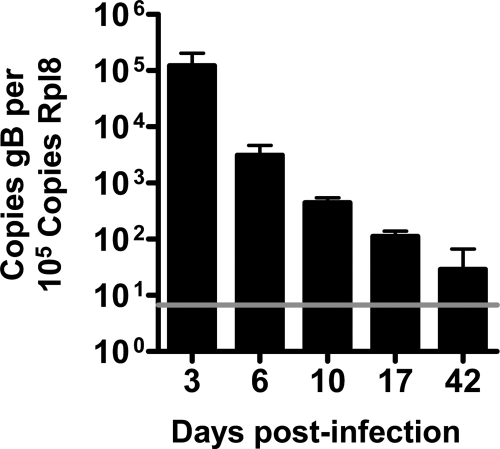
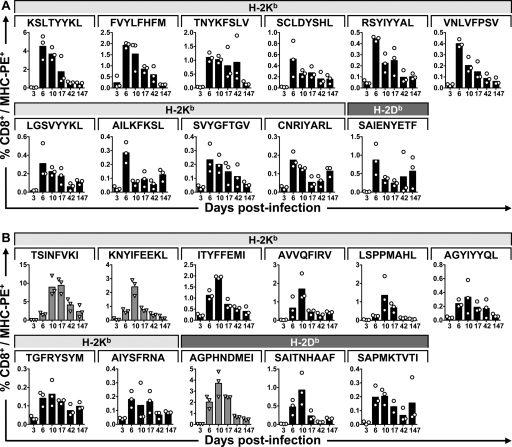
MHV-68 is characterized by an acute phase during in vivo infection. Depending on the route of infection, the virus is generally cleared by 10 to 15 days (6, 27, 36), after which it establishes early latency, and then—after some 6 weeks—it enters a late latent phase (31). We related the kinetics of the CD8 T-cell response to viral clearance. Mice were infected with MHV-68, and after 3, 6, 10, 17, 42, and 147 days, splenocytes were collected for tetramer and surface marker staining. Whole blood was also collected for real-time PCR analysis of MHV genome copy number. We measured clearance of MHV-68 by real-time PCR and detected a steady decline in viral gB copy number from day 3 onwards, approaching the limit of detection by day 42, a time at which latency has usually been established (31, 36) (Fig. 3). We found a CD8 T-cell response in which some of the epitopes appeared to peak as early as 6 dpi, whereas others peaked at 10 dpi (Fig. 4 and 5). Most epitopes were still present at days 42 and 147, even if the levels were low or, for some epitopes, nearly undetectable (Fig. 5). The p79/H-2Kb-derived epitope peaked at around 10 to 17 dpi, in accordance with previous data (23).
FIG. 3.
Real-time PCR of MHV-68 gB at various dpi. Real-time PCR was performed on whole blood from MHV-68-infected mice at different times pi. The data are presented as viral genome copy number relative to the copy number of rpl8 with background measurements from uninfected samples subtracted from each sample. Each time point represents blood samples from three animals. The horizontal line denotes the detection limit of the assay.
FIG. 4.
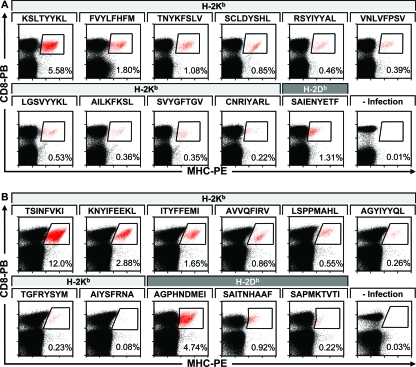
Screening for MHV-68-specific CD8 T-cell epitopes with MHC tetramers. Staining of splenocytes with H-2Kb and H-2Db tetramers with the indicated peptides (A) at day 6 pi for the epitopes against which the CD8 T-cell response peaks earlier and (B) at day 10 pi for epitopes against which the CD8 T-cell response peaks later reveals the antigen-specific CD8 T-cell subpopulations following MHV-68 infection. Representative stains from one of three mice. PE, phycoerythrin; PB, Pacific Blue.
FIG. 5.
CD8 T-cell response to MHV-68. Cell surface staining with the respective H-2Kb and H-2Db tetramers at different time points after infection. The panels show the epitopes according to the division of the respective CD8 T-cell responses into an earlier response with a peak at 6 dpi (A) and a later response with the peak at 10 dpi (B). Data shown represent three individual mice per time point. PE, phycoerythrin.
High coverage of the CD8 T-cell response by the 21 known MHV-68 epitopes.
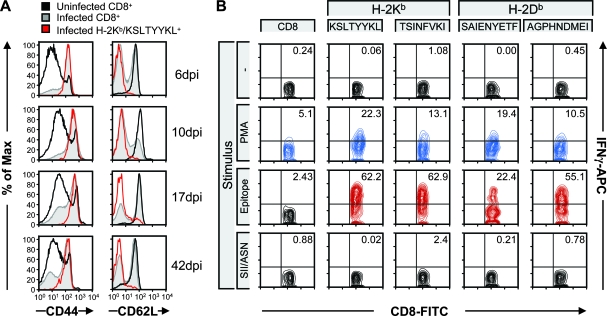
As seen for other virus infections also, the entire virus-specific CD8 T-cell pool expands dramatically when the acute phase of infection is being resolved (5, 21). Summation of the individual percentages of all MHV-68-specific CD8 T-cell responses yielded a value of 18% of the total CD8 pool in the spleen at day 6 and 30% at day 10 (Table 1), not taking into account possible cross-reactions among individual tetramer-positive CD8 T-cell populations. The tetramer-positive CD8 T cells showed upregulation of expression of CD44, consistent with persistent activation of these cells (Fig. 6A). Already at early time points we found uniform downregulation of CD62L on tetramer-positive cells, compared with the total CD8 T-cell pool in MHV-68-infected mice and in uninfected animals, consistent with T-cell receptor (TCR) stimulation. MHV-68 has been shown to elicit a persistent activation of CD8 T cells through stimulation by the MHV-68-encoded M1 protein of TCR Vβ4+ cells (9). Interestingly, when staining for Vβ4+ on CD8 T cells at day 42 pi using a pool of MHC tetramers presenting the 10 most abundant epitopes at this time, we found lower expression of Vβ4+ in the epitope-positive CD8 T cells than in the whole CD8 T-cell population (data not shown). Furthermore, the tetramer-positive cells showed a robust IFN-γ response upon restimulation in vitro with the corresponding peptide (Fig. 6B).
FIG. 6.
Epitope-positive CD8 T cells are activated. (A) Splenocytes from uninfected and infected mice were surface stained with CD44 and CD62L at 6, 10, 17, and 42 dpi. Representative stains are shown for each time point for the total CD8 T-cell population and for one of the epitope-positive populations (H-2Kb/KSLTYYKL). (B) In vitro stimulation of splenocytes from MHV-68-infected mice with no peptide (−), phorbol ester (phorbol myristate acetate [PMA]) and ionomycin, selected MHV-68 epitopes (the early KSLTYYKL/H-2Kb epitope, the late TSINFVKI/H-2Kb epitope, the early SAIENYETF/H-2Db epitope, and the late AGPHNDMEI/H-2Db epitope), or the known H-2Kb binding peptide SIINFEKL (SII) or known H-2Db binding peptide ASNENMDAM (ASN) demonstrates that CD8 T cells produce IFN-γ in an epitope-specific fashion. The data shown are representative of independent experiments. APC, allophycocyanin; FITC, fluorescein isothiocyanate.
DISCUSSION
Relatively little is known about the CTL response to MHV-68 in H-2b mice. So far, only six H-2b-restricted CD8 T-cell epitopes have been described (17, 23). It is to be expected that there are a significant number of unknown epitopes in highly complex viruses such as herpesviruses. Limitations in the techniques previously used for epitope identification have surely contributed to this shortfall.
For example, greater than 70% of the CTL response in C57BL/6 mice against herpes simplex virus type 1 (HSV-1) is directed against a single epitope derived from glycoprotein B (gB498-505) (35). Upon mutation of the anchor residue in gB498-505, only a modest reduction of the HSV-specific CTL response was seen, and this compensation was not accounted for by the already-known subdominant epitope (RR822-829) (26). This suggests that a yet-unknown epitope(s) to HSV-1 exists and can elicit a strong response in the absence of the dominant epitope (26). A genomic approach that identified 24 H-2b-restricted murine cytomegalovirus (MCMV) epitopes from 18 different viral proteins (among which 10 were H-2Db restricted and 14 were H-2Kb restricted) (21) likewise testifies to the highly diverse CD8 T-cell response against a herpesvirus in its natural host. Here we identify 19 new epitopes using a class I MHC tetramer-based screen, applied to the commonly used MHV-68 infectious model in C57BL/6 mice. We found that the CD8 T-cell response to MHV-68 is far broader than previously appreciated.
Which are the viral proteins most likely to elicit a CTL response, and what is the number of CD8 T cells expected to respond to a single pathogen? The primary response to EBV involves up to 30% of the CD8 T cells in some cases and is dominated by clonal populations of CD8 T cells specific for one or a few epitopes, but multiple smaller clones consisting of subdominant responses participate as well (as reviewed in reference 5). This response is directed at both lytic IE and E proteins and from proteins expressed in the latent phase. In HLA-A2-positive individuals, the acute anti-EBV HLA-A2-restricted CD8 T-cell response includes as many as 12% of total CD8 T cells and targets the early lytic cycle proteins BMLF1 and BMRF1, whereas the response toward latent EBV proteins is much lower: the frequency of HLA-B8-restricted EBNA3A-specific CD8 T cells is <2.5% and sometimes even undetectable at the first sampling point (5). For a population that covers most common class I MHC alleles (15 HLA-A, 26 HLA-B, and 13 HLA-C alleles represented), a total of 107 human cytomegalovirus (HCMV) ORFs contribute to the CD8 T-cell response (29). In the aggregate, then, we should expect the CD8 T-cell response against a typical herpesvirus in its natural host to be highly complex.
The ORFs recognized by the HCMV-specific CD8 T cells span all kinetic and functional categories, and ORF immunogenicity was influenced only modestly by expression kinetics and function (29). IE gene products were recognized threefold more frequently than their numerical representation in the genome, compared to other classes, which all were recognized in proportion to the coding space occupied. The total HCMV-specific CD8 T-cell response comprises on average around 10% of the CD8 T-cell memory compartment in blood (29). Acute MCMV infection yields a broad CD8 T-cell response comprising at least 24 epitopes that account for about 50% of total CD8 T cells at 7 dpi and are derived mostly from E genes (21). For MHV-68 the various transcriptional classes have not all been verified experimentally and not all published data are in agreement (1, 7, 18). Around half of the epitopes identified in the present study are encoded by E genes and half by L genes, with no epitopes derived from IE genes (Fig. 1).
For MCMV, the CD8 T-cell response to some epitopes continues to increase with time (14) and, in chronically infected mice, is dominated by only 3 of the 24 epitopes identified (20, 21). Two additional epitopes encoded by the IE gene IE3 were recognized by CD8 T cells only during chronic infection (20). We performed our epitope screen at day 9 and day 42 pi and did not observe the emergence of new epitopes at day 42. However, to observe shifts similar to the one seen for MCMV, longer observation periods may be required (20). In the case of MCMV, the chronic phase can last 1.5 years, during which memory cells could expand preferentially in response to select peptides that are generated during the attempts of the virus to reactivate.
In our infectious model we found that the CD8 T-cell response kinetics is characterized by some epitopes that tend to peak earlier than others. These peaks could broadly be categorized into two groups, with the earlier response peaking around 6 dpi and a slightly later response peaking around 10 dpi. The asynchronous response kinetics probably describes a continuum that stems from differences in viral gene or protein expression, antigen processing and presentation, and the induced strength of the CD8 T-cell response. The MHV-68 lytic cycle epitopes p79/H-2Kb and p56/H-2Db reported earlier (23) are generated in two distinct phases during infection, with an initial prominent response to p56/H-2Db and a slower p79/H-2Kb response, with a maximum after 15 to 20 days (23). By 30 to 40 days, all peptide-specific CTLs had declined in numbers (23). Experiments on the p79/H-2Kb, p56/H-2Db, and gB604-612/H-2Kb epitopes yielded two patterns, assayed with antigen-specific hybridomas: a peak at day 6 in the mediastinal lymph nodes and a peak at day 18 in spleen (16). Again, none of the lytic antigens were detected after 30 days of infection. We find that the response against all epitopes declines with time, but most responses are still detectable at days 42 and 147. Noteworthy is that in both the above-mentioned studies the animals were infected intranasally, which might well approximate the natural route of infection, and not i.p. as in our study. The different route of infection, as well as the viral dose, could affect the composition and the kinetics of the immune response. However, both intranasal and i.p. infections are commonly used for the MHV-68 model, and it has been shown that for the establishment and maintenance of latency, neither the dose nor the route of infection is important (31). Only at very low viral doses were the acute-phase replication and viral clearance shown to be delayed (31). Therefore, the likelihood of major differences in the viral clearance and the immune response in the different infectious systems is small.
MHV-68 elicits a persistent activation of CD8 T cells through stimulation by the MHV-68-encoded M1 protein of TCR Vβ4+ cells (9). These cells exhibit memory T-cell characteristics and appear to suppress virus reactivation from peritoneal cells by means of IFN-γ production. The activation of these Vβ4+ CD8 T cells is independent of class I MHC expression, and instead M1 may signal through a nonclassical MHC class Ib molecule or manipulate TCR or CD8 ligation (9). It is not known at present whether these M1-reactive cells are specific for MHV-68 or whether they are T cells that simply respond to the M1 protein through ligation of a specific receptor for M1. The expansion of Vβ4+ CD8 T cells begins 18 to 20 dpi (9) and has also been shown to expand after the epitope-specific T cells already have declined (23). We found lower percentages of Vβ4+ CD8 T-cell populations in the tetramer-positive CD8 T cells than in the general CD8 T-cell populations at 42 dpi, indicating that for the identified epitope-specific T cells, Vβ4 usage by the corresponding TCRs is not higher than that for the general population of CD8 T cells, but rather lower. These data are in accordance with the previously reported findings that Vβ4+ CD8 T cells did not respond to any of the epitopes tested in CTL assays (23), as well as the finding that sorted Vβ4+ CD8 T cells from MHV-68-infected mice showed specific CTL precursor frequencies significantly lower than those for all sorted CD8 T cells (23).
The epitope-specific populations described in this study were uniformly CD62Llo and CD44hi over the 42 days, consistent with their being an effector subset (39), whereas the CD8 T-cell population as a whole contained CD8 T cells that were both CD62Lhi and CD62Llo; the CD44 expression declined gradually, but not to the level seen for CD8 T cells from uninfected mice for at least 42 dpi.
Do MHV-68-infected cells preferentially present H-2Kb-restricted epitopes? One could argue that there is a bias toward the prediction of H-2Kb epitopes in the prediction programs that were used, but we do not think that this is the case. Using very similar algorithms, Sette and coworkers examined the H-2b-restricted CD8 T-cell response against vaccinia virus (19). They identified a total of some 50 epitopes, equally distributed over H-2Kb and H-2Db restriction elements, which represent the vast majority of the total vaccinia virus CD8 T-cell response in C57BL/6 mice. As described in Results, we find a striking underrepresentation of H-2Db-restricted epitopes. Although we cannot establish what could account for the bias against H-2Db-restricted epitopes, immune selection could certainly be a contributing factor.
MHV-68 mutants that—through mutation in the course of coevolution with their host—lose CD8 T-cell epitopes would be expected to have a selective advantage, and over time several such mutations may have become fixed in the currently prevalent strains of MHV-68. Also, the ability of MHV-68 to persist in a latent state for the lifetime of the host is possibly relevant for immune control exerted by CD8 T cells and a further target for immune selection against class I MHC-restricted epitopes. Whether the skewing of the CD8 T-cell repertoire against MHV-68 toward H-2Kb-restricted epitopes and the avoidance of H-2Db as a restriction element could be the evolutionary results of CD8-driven processes is open to debate. However, the MHV-68 K3 product targets the H-2Kb, H-2Db, and H-2Ld molecules both in lytically infected fibroblasts and in vivo for destruction and would thus thwart their use as restriction elements (24, 25). Downregulation of H-2Kb and H-2Db in vivo is difficult to measure, especially since we cannot directly access the antigen-presenting cells responsible for priming the CD8 T-cell response. Even low levels of H-2Kb and/or H-2Db will contribute to presenting peptides to CD8 T cells. Perhaps the MHV-68-encoded K3 is more efficient at downregulating H-2Db, and epitopes possibly restricted by H-2Db would end up being underrepresented.
In any case, the increased number of epitopes now available for MHV-68 will make it possible to further dissect the host response to this complex pathogen and map specific T-cell responses, as well as define more accurately what is required to generate a protective T-cell response.
Supplementary Material
Acknowledgments
This study was supported by grants from the NIH (to H.L.P.). S.G.-R. is supported by a postdoctoral grant from the Wenner-Gren Foundations (Sweden).
We thank Eli Papa for the statistical analysis and helpful discussions.
Footnotes
Published ahead of print on 15 October 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Ahn, J. W., K. L. Powell, P. Kellam, and D. G. Alber. 2002. Gammaherpesvirus lytic gene expression as characterized by DNA array. J. Virol. 766244-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 27494-96. [DOI] [PubMed] [Google Scholar]
- 3.Bakker, A. H., R. Hoppes, C. Linnemann, M. Toebes, B. Rodenko, C. R. Berkers, S. R. Hadrup, W. J. van Esch, M. H. Heemskerk, H. Ovaa, and T. N. Schumacher. 2008. Conditional MHC class I ligands and peptide exchange technology for the human MHC gene products HLA-A1, -A3, -A11, and -B7. Proc. Natl. Acad. Sci. USA 1053825-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasdell, K., C. McCracken, A. Morris, A. A. Nash, M. Begon, M. Bennett, and J. P. Stewart. 2003. The wood mouse is a natural host for murid herpesvirus 4. J. Gen. Virol. 84111-113. [DOI] [PubMed] [Google Scholar]
- 5.Callan, M. F. 2003. The evolution of antigen-specific CD8+ T cell responses after natural primary infection of humans with Epstein-Barr virus. Viral Immunol. 163-16. [DOI] [PubMed] [Google Scholar]
- 6.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebrahimi, B., B. M. Dutia, K. L. Roberts, J. J. Garcia-Ramirez, P. Dickinson, J. P. Stewart, P. Ghazal, D. J. Roy, and A. A. Nash. 2003. Transcriptome profile of murine gammaherpesvirus-68 lytic infection. J. Gen. Virol. 8499-109. [DOI] [PubMed] [Google Scholar]
- 8.Ehtisham, S., N. P. Sunil-Chandra, and A. A. Nash. 1993. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J. Virol. 675247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans, A. G., J. M. Moser, L. T. Krug, V. Pozharskaya, A. L. Mora, and S. H. Speck. 2008. A gammaherpesvirus-secreted activator of Vbeta4+ CD8+ T cells regulates chronic infection and immunopathology. J. Exp. Med. 205669-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frickel, E.-M., N. Sahoo, J. Hopp, M.-J. Gubbels, M. P. J. Craver, L. J. Knoll, H. L. Ploegh, and G. M. Grotenbreg. 15 October 2008. Parasite stage-specific recognition of endogenous Toxoplasma gondii derived CD8+ T cell epitopes. J. Infect. Dis. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 11.Garboczi, D. N., D. T. Hung, and D. C. Wiley. 1992. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc. Natl. Acad. Sci. USA 893429-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grotenbreg, G. M., N. R. Roan, E. Guillen, R. Meijers, J. H. Wang, G. W. Bell, M. N. Starnbach, and H. L. Ploegh. 2008. Discovery of CD8+ T cell epitopes in Chlamydia trachomatis infection through use of caged class I MHC tetramers. Proc. Natl. Acad. Sci. USA 1053831-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewitt, E. W. 2003. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology 110163-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karrer, U., S. Sierro, M. Wagner, A. Oxenius, H. Hengel, U. H. Koszinowski, R. E. Phillips, and P. Klenerman. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 1702022-2029. [DOI] [PubMed] [Google Scholar]
- 15.Krmpotic, A., M. Messerle, I. Crnkovic-Mertens, B. Polic, S. Jonjic, and U. H. Koszinowski. 1999. The immunoevasive function encoded by the mouse cytomegalovirus gene m152 protects the virus against T cell control in vivo. J. Exp. Med. 1901285-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, L., E. Flano, E. J. Usherwood, S. Surman, M. A. Blackman, and D. L. Woodland. 1999. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J. Immunol. 163868-874. [PubMed] [Google Scholar]
- 17.Liu, L., E. J. Usherwood, M. A. Blackman, and D. L. Woodland. 1999. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J. Virol. 739849-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Guzman, D., T. Rickabaugh, T. T. Wu, H. Brown, S. Cole, M. J. Song, L. Tong, and R. Sun. 2003. Transcription program of murine gammaherpesvirus 68. J. Virol. 7710488-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moutaftsi, M., B. Peters, V. Pasquetto, D. C. Tscharke, J. Sidney, H. H. Bui, H. Grey, and A. Sette. 2006. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat. Biotechnol. 24817-819. [DOI] [PubMed] [Google Scholar]
- 20.Munks, M. W., K. S. Cho, A. K. Pinto, S. Sierro, P. Klenerman, and A. B. Hill. 2006. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 177450-458. [DOI] [PubMed] [Google Scholar]
- 21.Munks, M. W., M. C. Gold, A. L. Zajac, C. M. Doom, C. S. Morello, D. H. Spector, and A. B. Hill. 2006. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J. Immunol. 1763760-3766. [DOI] [PubMed] [Google Scholar]
- 22.Song, M. J., S. Hwang, W. H. Wong, T. T. Wu, S. Lee, H. I. Liao, and R. Sun. 2005. Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis. Proc. Natl. Acad. Sci. USA 1023805-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1999. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur. J. Immunol. 291059-1067. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson, P. G., S. Efstathiou, P. C. Doherty, and P. J. Lehner. 2000. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc. Natl. Acad. Sci. USA 978455-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson, P. G., J. S. May, X. G. Smith, S. Marques, H. Adler, U. H. Koszinowski, J. P. Simas, and S. Efstathiou. 2002. K3-mediated evasion of CD8(+) T cells aids amplification of a latent gamma-herpesvirus. Nat. Immunol. 3733-740. [DOI] [PubMed] [Google Scholar]
- 26.Stock, A. T., C. M. Jones, W. R. Heath, and F. R. Carbone. 2006. CTL response compensation for the loss of an immunodominant class I-restricted HSV-1 determinant. Immunol. Cell Biol. 84543-550. [DOI] [PubMed] [Google Scholar]
- 27.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 732347-2356. [DOI] [PubMed] [Google Scholar]
- 28.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 733275-3279. [DOI] [PubMed] [Google Scholar]
- 29.Sylwester, A. W., B. L. Mitchell, J. B. Edgar, C. Taormina, C. Pelte, F. Ruchti, P. R. Sleath, K. H. Grabstein, N. A. Hosken, F. Kern, J. A. Nelson, and L. J. Picker. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakur, N. N., S. El-Gogo, B. Steer, K. Freimuller, A. Waha, and H. Adler. 2007. A gammaherpesviral internal repeat contributes to latency amplification. PLoS ONE 2e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tibbetts, S. A., J. Loh, V. van Berkel, J. S. McClellan, M. A. Jacoby, S. B. Kapadia, S. H. Speck, and H. W. Virgin IV. 2003. Establishment and maintenance of gammaherpesvirus latency are independent of infective dose and route of infection. J. Virol. 777696-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toebes, M., M. Coccoris, A. Bins, B. Rodenko, R. Gomez, N. J. Nieuwkoop, W. van de Kasteele, G. F. Rimmelzwaan, J. B. Haanen, H. Ovaa, and T. N. Schumacher. 2006. Design and use of conditional MHC class I ligands. Nat. Med. 12246-251. [DOI] [PubMed] [Google Scholar]
- 33.Usherwood, E. J., D. J. Roy, K. Ward, S. L. Surman, B. M. Dutia, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2000. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T cells. J. Exp. Med. 192943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 715894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace, M. E., R. Keating, W. R. Heath, and F. R. Carbone. 1999. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J. Virol. 737619-7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. I. Virgin. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 706775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 734651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg, J. B., M. L. Lutzke, R. Alfinito, and R. Rochford. 2004. Mouse strain differences in the chemokine response to acute lung infection with a murine gammaherpesvirus. Viral Immunol. 1769-77. [DOI] [PubMed] [Google Scholar]
- 39.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 785535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.