Abstract
The spindles of Anomala cuprea entomopoxvirus (AncuEPV), which are composed of glycoprotein fusolin, are known to enhance the peroral infectivity of AncuEPV itself and of nucleopolyhedroviruses. This has been demonstrated to involve the disruption of intestinal peritrophic membrane (PM), composed of chitin matrix, glycosaminoglycans, and proteins. To identify essential and nonessential regions for this enhancement activity, AncuEPV fusolin and its deletion mutants were expressed in Sf21 cells using a baculovirus system, and their enhancement abilities were analyzed. The recombinant fusolin enhanced the peroral infectivity of Bombyx mori nucleopolyhedrovirus up to 320-fold and facilitated the infection of host insect with AncuEPV. Deletion mutagenesis revealed that the N-terminal region (amino acids 1 to 253), a possible chitin-binding domain, is essential for the enhancement of infection, whereas the C-terminal region is entirely dispensable. The glycosylation-defective mutants N191Q, whose Asn191 is replaced with Gln, and ΔSIG, whose signal peptide is deleted, showed considerably reduced and abolished enhancing activities, respectively, indicating that the carbohydrate chain is important in the enhancing activity. Interestingly, the C-terminal dispensable region was digested by a serine protease(s) in insect digestive juice. Moreover, both the N-terminal conserved region and the carbohydrate chain were necessary not only for chitin binding but also for stability in digestive juice. A triple amino acid replacement mutant, IHE (Ile-His-Glu161 to Ala-Ala-Ala), was stable in digestive juice and had chitin-binding ability but did not retain its enhancing activity. These results suggest that the enhancement of infectivity involves more than the tolerance to digestive juice and chitin-binding ability.
Entomopoxviruses (EPVs) are members of the family Poxviridae that infect insects of orders such as Coleoptera, Lepidoptera, Orthoptera, and Diptera. Many EPVs produce two types of proteinaceous crystalline structures, spindles and spheroids, which are composed of fusolin and spheroidin, respectively. The spheroids are spherical in shape and embed virions, whereas the spindles are bipyramidal in shape and contain no virions. The spindles of several EPVs have been shown to strongly enhance the infectivity of several insect viruses (2, 9, 23, 25, 27, 29, 30, 44). We previously demonstrated that the peritrophic membranes (PMs) of Bombyx mori and Anomala cuprea larvae are disintegrated by peroral administration of Anomala cuprea entomopoxvirus (AncuEPV) spindles (26, 27). Thus, disintegration of the PM is thought to be a mechanism by which spindles enhance virus infectivity.
Due to the above-mentioned properties, EPV spindles may be of use as a synergistic additive to viral insecticides, and development of an efficient production system for fusolin has been attempted. Armyworm (Pseudaletia separata) larvae feeding on transgenic rice plants expressing Pseudaletia separata entomopoxvirus (PsEPV) enhancing factor (EF), which is identical to fusolin, were shown to have 260- to 360-fold increased susceptibility to Pseudaletia unipuncta nucleopolyhedrovirus compared to larvae feeding on the nontransgenic rice (15). Moreover, recombinant PsEPV EF expressed in Escherichia coli was reported to enhance P. unipuncta NPV infection up to 270-fold when analyzed with bacterial crude extract and up to 40-fold with purified recombinant protein (16).
EPV fusolins are approximately 350 to 390 amino acids in length, with numerous cysteine residues scattered along the amino acid chain. These residues have been thought to be involved in bipyramidal crystal formation, possibly through the formation of cystine dimers (1, 6, 10, 24, 45). The first ca. 20 amino acids of the N terminus form a signal peptide, which is believed to be involved in endoplasmic reticulum targeting (6, 10, 17, 45). The signal peptide is cleaved at the site immediately prior to an HGY motif that is conserved in all known fusolins. To date, all fusolins that contain one potential N-glycosylation site have been shown to be glycoproteins with the exception of the Heliothis armigera entomopoxvirus fusolin (6). The N-terminal region of the mature fusolin (ca. 230 amino acids) contains five discrete and highly conserved sequence elements, and these elements form a putative chitin-binding domain (5, 20, 28, 34, 40). Although the chitin-binding ability of the EPV fusolins has not yet been demonstrated, it is thought to be crucial for PM disintegration, since chitin is a constituent of the PM (20, 22). However, much remains to be learned about the molecular biological aspects of fusolin.
In this study, we expressed AncuEPV fusolin and its mutants using an Autographa californica multiple nucleopolyhedrovirus (AcMNPV)-based vector and investigated their enhancing activities in virus peroral infection, chitin-binding activities, and stabilities in insect digestive juice.
MATERIALS AND METHODS
Viruses and insects.
Propagation methods of AncuEPV and Bombyx mori nucleopolyhedrovirus (BmNPV) were as described by Furuta et al. (9). B. mori larvae (hybrid strain C146 × N137, maintained at NIAS) and A. cuprea larvae (F1 progeny from adult insects collected in Ibaraki, Japan) were used for peroral inoculation tests of BmNPV polyhedra (OBs) and AncuEPV spheroids, respectively.
Purification of spindles, spheroids, and BmNPV OBs.
Purification of spindles and spheroids was performed as described by Mitsuhashi et al. (26). BmNPV OBs were purified following the procedure of Mitsuhashi et al. (25). The purities and concentrations of spindles, spheroids, and BmNPV OBs were evaluated by light microscopy using a hemocytometer and/or sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis following the procedure described below.
Construction of AncuEPV fusolin expression baculovirus-based vector.
To construct an AcMNPV-based baculovirus vector expressing hexahistidine (His)-tagged AncuEPV fusolin, a Bac-to-Bac baculovirus expression system (Invitrogen, Carlsbad, CA) was used. The AncuEPV fusolin gene was amplified by PCR from a DNA clone containing a full-length fusolin gene (28) using AcFu-F/BssSal and AcFu-R/SmaHis6Hind primers (Table 1). The PCR product obtained was ligated into the BssHII-HindIII site of the pFastBac1 donor plasmid, and the resulting plasmid (pFBAcFu) was introduced into E. coli strain DH10BAC harboring a baculovirus shuttle vector called a bacmid. Recombinant bacmid DNA was isolated and transfected into Spodoptera frugiperda (Sf21) cells by using Cellfectin reagent according to the manufacturer's instructions, and a conditioned medium containing recombinant baculovirus was collected 72 h posttransfection. To generate an untagged version of the AncuEPV fusolin expression vector, an ochre codon was introduced just before a SmaI site of pFBAcFu by PCR-based site-directed mutagenesis (35) using AcFu-R/StopSma primer (Table 1), and the recombinant bacmid DNA and baculovirus vector were generated as described above. To prepare a negative control protein, a His-tagged β-glucuronidase (rGUS) expression baculovirus vector was constructed.
TABLE 1.
Primers used for construction of the AncuEPV fusolin expression vector and its derivatives
| Primer | Sequencea |
|---|---|
| AcFu-F/BssSal | 5′-CACAGTCGACGCGCGCACCATGTTAAAATTATATATATTATTAATC-3′ |
| AcFu-R/SmaHis6Hind | 5′-CACAAAGCTTAGTGATGGTGATGGTGATGCCCGGGATATACTCTATTTCTTACTGTAGACACAC-3′ |
| AcFu-R/StopSma | 5′-GGTGATGCCCGGGTTAATATACTCTATTTC-3′ |
| Sig-F/Nco | 5′-GTATACGGCCATGGATATGTAACCTTTCCTATAGC-3′ |
| AcFu-F/ΔSIG | 5′-CACAGTCGACGCGCGCACCATGCACGGATATGTAACCTTTCCTATAGC-3′ |
| AcFu-R/N191Q | 5′-CGTCTATTAACTAGTGGTACAGTACTTTGAAATACTAATTCTAATAGTGGCC-3′ |
| dn1 | 5′-CACACCATGGATATGCAGCTTATCAATATGTATTTAAC-3′ |
| dn2 | 5′-CACACCATGGATATAATGAATATGCAGCTCTCGCCGGACC-3′ |
| dn3 | 5′-CACACCATGGATATTTTGGAGATAAGACTGGTATGGATATTG-3′ |
| dn4 | 5′-CACACCATGGATATGAATTCTGTCCAACTGCAATTCATG-3′ |
| dn5 | 5′-CACACCATGGATATTTTAATAGTACTGTACCACTAG-3′ |
| dc1 | 5′-GGGAGCATATGCTATTTCCCATGCTCT-3′ |
| dc2 | 5′-GGGATATCTATTGTAATCATGCTTATG-3′ |
| dc3 | 5′-GGGAGCTGGTGGAGGAGGTATCATATC-3′ |
| dc4 | 5′-GGGTCTGTTTGCAAATACAGCATCAACAC-3′ |
| dc5 | 5′-GGGTTGTCTATATGGAACAGGAACAAT-3′ |
| dc6 | 5′-GGGTACTAATTCTAATAGTGGCCAAGT-3′ |
| dc7 | 5′-GGGTAATTCTATAGGAATAGTATTAAC-3′ |
| Ihe | 5′-TGTCCAACTGCAGCGGCCGCACCAAGTTATTTT-3′ |
| Ymf | 5′-AGTGCAGCTCAAGCGGCCGCTCAGCAAGATAAT-3′ |
Nucleotides corresponding to the hexahistidine tag are indicated in italics. Positions of base substitutions causing amino acid replacements are in boldface. Restriction sites used for cloning are underlined. BssHII, CGCGCG; HindIII, AAGCTT; SmaI, CCCGGG; NcoI, CCATGG.
Constructions of AncuEPV fusolin mutants.
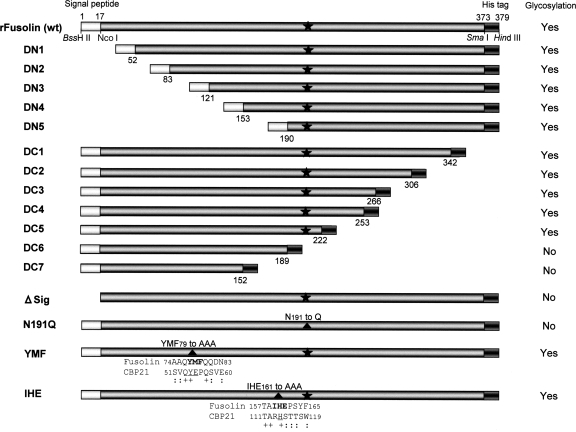
The N- and C-terminal deletion fragments of the AncuEPV fusolin gene were generated by PCR using the primer pairs dn1 and AcFu-R/Sma for DN1, dn2 and AcFu-R/Sma for DN2, dn3 and AcFu-R/Sma for DN3, dn4 and AcFu-R/Sma for DN4, dn5 and AcFu-R/Sma for DN5, Sig-F/Nco and dc1 for DC1, Sig-F/Nco and dc2 for DC2, Sig-F/Nco and dc3 for DC3, Sig-F/Nco and dc4 for DC4, Sig-F/Nco and dc5 for DC5, Sig-F/Nco and dc6 for DC6, and Sig-F/Nco and dc7 for DC7 (Table 1). These deletions were constructed downstream of the HGY motif (His-Gly-Tyr19) of the signal sequence (Fig. 1). To introduce theses deletion fragments into a pFBAcFu donor vector, thymine was substituted for cytosine at nucleotide position 51 of the fusolin gene in pFBAcFu by PCR-based site-directed mutagenesis (35) using the Sig-F/Nco primer (Table 1), and the resulting plasmid was designated pFBAcFuNS. This single nucleotide substitution created an NcoI site at the end of the signal sequence with no amino acid substitution. The NcoI-SmaI fragment of pFBAcFuNS was replaced with each deletion fragment under investigation. To construct the ΔSIG mutant, the fusolin gene was amplified by PCR from a DNA clone containing a full-length fusolin gene (28) by using AcFu-F/ΔSIG and AcFu-R/Sma primers, and the PCR product obtained was cloned into the BssHII-SmaI site of the pFBAcFuNS donor vector. Amino acid substitutions were introduced in the fusolin gene by PCR-based site-directed mutagenesis (35) using appropriate primers (AcFu-R/N191Q for N191Q, Ihe for IHE, and Ymf for YMF) (Table 1) and pFBAcFuNS as the parental plasmid. Mutated donor plasmids were introduced into E. coli strain DH10BAC, and recombinant bacmid DNA was isolated. Preparation of recombinant baculoviruses was performed as described above.
FIG. 1.
Schematic diagram of recombinant AncuEPV fusolin (rFusolin) and its derivatives. The signal peptide and the artificial hexahistidine tag are represented by light gray and dark gray boxes, respectively. The positions of restriction sites used for vector construction are indicated below the rFusolin map (pFBAcFuNS). The numbers below the boxes indicate the amino acid positions of the deletion sites. Black stars indicate the glycosylation site (Asn191), and the positions with introduced amino acid substitutions are represented by closed triangles. The results of PAS stain analysis are represented as “Yes” (glycosylated) or “No” (not glycosylated). Amino acid alignments of AncuEPV fusolin and Serratia marcescens CBP21 around triple amino acid replacement sites are indicated in the YMF and IHE mutants. Amino acids of the CBP21 involved in chitin binding are underlined, and identical and similar amino acids are indicated by + and :, respectively.
Expression and purification of recombinant proteins.
To express fusolin, recombinant baculovirus was inoculated into Sf21 cells at a multiplicity of infection of 1 to 5. Infected cells were harvested 72 h postinoculation. Preliminary Western blot analysis results using an antibody to the AncuEPV spindles (26) indicated that the recombinant fusolin was contained only in the insoluble fraction of infected cells. Therefore, protein purification was performed under a denaturing condition as described below. Infected cells were lysed in lysis/wash buffer (50 mM sodium dihydrogen phosphate, 300 mM sodium chloride, 5 M guanidine hydrochloride, 0.1% [vol/vol] Tween 20, pH 7.7), and the lysate was centrifuged at 10,000 × g for 10 min. Talon metal affinity resin (Clontech, Mountain View, CA) was added to the supernatant and shaken gently for 1 h to let the His-tagged recombinant protein adsorb onto the resin. The resin was washed three times with 10 ml of the lysis/wash buffer, and the protein was eluted with 5 ml of elution buffer (lysis/washing buffer containing 500 mM imidazole). The eluate was dialyzed in Milli-Q water overnight with three changes of the dialysate, and the aggregated protein was collected by centrifugation at 10,000 × g for 10 min. The protein pellet was washed with Milli-Q water four times and resuspended in sterile Milli-Q water. The purity and concentration of the protein were assessed by SDS-PAGE, using bovine serum albumin (BSA) as a standard.
SDS-PAGE and Western blot analysis.
Each protein suspension was mixed with an equal volume of SDS sample buffer (0.1 M sodium phosphate buffer [pH 7.8] containing 5% SDS, 0.72 M 2-mercaptoethanol, and 8 M urea) and boiled at 100°C for 10 min. SDS-PAGE was carried out using a low-bis-polyacrylamide gel according to the method of Hirano (13). Bands on the gel were detected by staining with Coomassie brilliant blue R-250 (CBB). Precision Plus all blue standard (Bio-Rad, Hercules, CA) was used as molecular mass standard. For Western blot analysis, protein samples were run on a low-bis polyacrylamide gel and blotted onto a polyvinylidene difluoride (PVDF) membrane (FluoroTrans; Pall, East Hills, NY). The membranes were soaked in a blocking solution (0.1 M maleic acid buffer [pH 7.5] containing 0.15 M sodium chloride and 1% [wt/vol] blocking reagent [Roche Diagnostics, Indianapolis, IN]) at room temperature for 30 min and incubated with 5,000-fold-diluted anti-AncuEPV spindles antibody (26) in Tris-buffered saline containing 0.05% Tween 20 at room temperature for 1 h. Binding of the antibody was probed with alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (Promega, Madison, WI) and visualized using a 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (BCIP-NBT) solution kit for alkaline phosphatase staining (Nakalai Tesque, Tokyo, Japan).
Bioassay.
For the bioassay with BmNPV, various concentrations of BmNPV OBs suspensions (101 to 107 OBs per 3 μl) either with or without recombinant fusolin (6 or 9 μg per 3 μl) were prepared as inocula. An inoculum (3 μl) was dripped onto a small piece (ca. 3 mm by 3 mm by 1 mm) of an artificial insect diet (Silkmate; Nihon-nosan, Tokyo, Japan) and fed to a second- or third-instar first-day larvae of B. mori (hybrid strain, C146 × N137) kept in a 24-well plastic plate (MS-8024R; Sumitomo Bakelite, Tokyo, Japan) under humidified conditions. The larvae that consumed inocula within 36 h were individually transferred to plastic containers (diameter, 50 mm; height, 30 mm; Mineron Kasei, Tokyo, Japan) containing a fresh diet without inoculum, and the number of dead larvae and symptoms were monitored for 7 days. The rearing of each larva, including the inoculation step, was carried out individually at 25°C (14 h light/10 h dark).
For the bioassay with AncuEPV, an inoculum (3 μl) was dripped onto a small piece (ca. 2 mm by 2 mm by 2 mm) of an artificial insect diet (Insecta LF; Nihon-nosan) and given to a second-instar first-day larvae of A. cuprea kept in a six-well plastic plate (MS-8006R; Sumitomo Bakelite) under humidified conditions. The larvae that consumed inocula within 24 h were individually transferred to plastic containers (diameter, 80 mm; height, 50 mm; Mineron Kasei) containing leaf mold and a typical symptom of AncuEPV infection, a whitish external appearance, was checked visually at 45 days posttransfer. At all stages, including the inoculation step, larvae were reared individually at 25°C in the dark.
PAS staining and lectin-binding assay.
Periodic acid-Schiff (PAS) staining was performed according to the procedure of Davine and Warren (8). Purified recombinant proteins were subjected to SDS-PAGE and electroblotted onto a PVDF membrane. The membrane was treated with 0.5% (wt/vol) periodic acid for 2 h and washed first with 5% (vol/vol) acetic acid containing 0.5% (wt/vol) sodium arsenate for 30 min and then with 5% (vol/vol) acetic acid containing 0.1% (wt/vol) sodium arsenate twice for 20 min. The membrane was soaked in 5% (vol/vol) acetic acid for 10 min and then immersed in Schiff's reagent (Nakalai Tesque) overnight. Finally, the membrane was rinsed with 0.1 M hydrochloric acid containing 0.6% (wt/vol) sodium metabisulfite several times until the rinse solution failed to turn pink after the addition of formaldehyde.
The lectin-binding assay was performed using a Lectin Set II-HRP kit (for probing with ConA, DBA, LCA, and RCA120; J-OIL Mills, Tokyo, Japan) and a DIG glycan differentiation kit (for probing with Galanthus nivalis agglutinin [GNA], Sambucus nigra agglutinin [SNA], Maackia amurensis agglutinin [MAA], peanut agglutinin [PNA], and Datura stramonium agglutinin [DSA]; Roche Diagnostics). In brief, proteins were run on a low-bis polyacrylamide gel and electroblotted onto a PVDF membrane. Incubation with each lectin and detection of binding were performed following each manufacturer's instructions. Appropriate control proteins were used in all the binding experiments (positive controls were carboxypeptidase Y for GNA, transferrin for SNA, fetuin for RCA120, MAA, and DSA, asialofetuin for DBA and PNA, and bovine immunoglobulin G for LCA; the negative control was BSA).
Chitin-binding assay.
Ten micrograms of each protein was solubilized in 100 μl of dissolving solution (50 mM sodium carbonate containing 10 mM dithiothreitol and 0.05% [vol/vol] Tween 20, pH 10.2), and the protein solution was passed through a MicroSpin empty column unit (GE Healthcare UK Ltd., United Kingdom). The filtrate was mixed with 50 μl of chitin beads (New England Biolabs, Ipswich, MA) at room temperature for 18 h. The mixture was filtrated in another empty column unit by centrifugation at 10,000 × g for 5 min. The beads in the column unit were washed five times with 500 μl of HEPES-T buffer (0.5 M HEPES buffer containing 500 mM sodium chloride, 0.1 M EDTA, and 0.1% Triton X-100, pH 8.0) and resuspended in 150 μl of the dissolving solution. The resuspended chitin beads were mixed with identical volumes of SDS sample buffer and analyzed by Western blotting using an antibody to the AncuEPV spindles (26). BSA was used as a negative control in the binding assay, and its detection was carried out using a monoclonal antibody to BSA (clone BSA-33; Sigma).
Stabilities of fusolin and its mutants in digestive juice harvested from B. mori.
Digestive juice was collected from fasting fifth-instar larvae of B. mori (hybrid strain, C146 × N137) with electric shock (100 V for a few seconds), and undigested contents in the fluid were removed by low-speed centrifugation. An aliquot (2.5 μg) of each protein was treated with 10 μl of undiluted B. mori digestive juice for 5 min, and the stability of each sample was assessed by Western blot analysis using an antibody to the AncuEPV spindles (26).
Identification of the N-terminal amino acid sequence of fusolin after treatment with digestive juice.
The B. mori digestive juice was diluted 150-fold with dissolving solution (50 mM sodium carbonate containing 10 mM dithiothreitol and 0.05% [vol/vol] Tween 20, pH 10.2), and 5 μg of recombinant fusolin was solubilized in 50 μl of the diluted digestive juice. After incubation at room temperature for 30 min, the digestive juice-treated and untreated proteins were subjected to SDS-PAGE and then blotted onto a PVDF membrane. Each protein band was excised from the membrane and sequenced directly by sequential Edoman degradation using a protein sequencer (Hewlett Packard model 241 N/C).
Inhibition assay of C-terminal degradation of fusolin.
Five micrograms of recombinant fusolin was dissolved in 40 μl of the 150-fold-diluted digestive juice containing one of the following protease inhibitors: 1.25 μg/μl Pefabloc SC Plus (serine protease inhibitor; Roche Diagnostics), 125 ng/μl E64 (cysteine protease inhibitor; Calbiochem), 6.25 ng/μl pepstatin A (aspartic protease inhibitor; Alexis), or 25 mM EDTA (metalloprotease inhibitor). After incubation at room temperature for 30 min, fusolin degradation was analyzed by SDS-PAGE.
RESULTS
Expression and purification of recombinant fusolin.
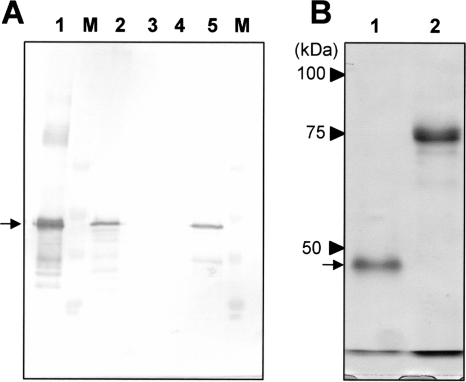
To express the fusolin gene, a recombinant AcMNPV-based baculovirus expression vector harboring His-tagged AncuEPV fusolin gene was constructed and inoculated into Sf21 cells. As shown in Fig. 2A, protein expression was detected in the vector-infected cell culture by Western blot analysis (lane 2). The infected cells were lysed with Nonidet P-40 detergent, and the lysate obtained was fractionated into insoluble (precipitated) and soluble (supernatant) fractions by centrifugation. Expressed recombinant fusolin was detected in the insoluble protein fraction (lane 5), while no recombinant fusolins could be detected in the soluble and secreted (conditioned medium) fractions (lanes 3 and 4, respectively). The insoluble fraction was solubilized in 5 M guanidine hydrochloride, and recombinant fusolin was purified using Talon metal affinity resin. The SDS-PAGE analysis of the purified recombinant fusolin exhibited a single 49-kDa band, indicating that the recombinant fusolin was highly purified (Fig. 2B, lane 1).
FIG. 2.
Expression of AncuEPV fusolin gene using a baculovirus expression system. (A) Western blot analysis of Sf21 cells infected with a baculovirus-based vector harboring a His-tagged AncuEPV fusolin gene. Lane 1, native fusolin derived from AncuEPV spindles (positive control); lane 2, whole-cell culture; lane 3, conditioned medium (secreted fraction); lane 4, soluble protein fraction; lane 5, insoluble protein fraction; lane M, molecular mass standard. The position of the fusolin visualized by alkaline phosphatase-BCIP-NBT staining is indicated by an arrow. (B) SDS-PAGE analysis of the recombinant fusolin purified from vector-infected cells. Lane 1, recombinant AncuEPV fusolin; lane 2, His-tagged β-glucuronidase. The position of the fusolin stained with Coomassie brilliant blue is indicated by an arrow.
Glycosylation of recombinant fusolin.
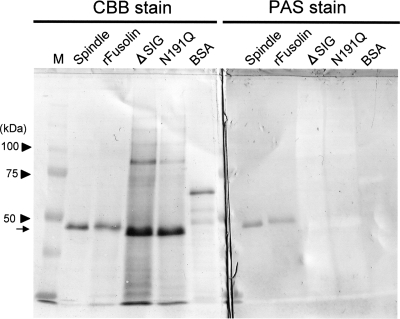
AncuEPV fusolin has a signal peptide (amino acids [aa] 1 to 16) which could be involved in the targeting of the endoplasmic reticulum and one putative N-glycosylation site (Asn191). Indeed, it has been already demonstrated that the fusolin derived from AncuEPV spindles is glycosylated (28). PAS stain analysis was performed to examine the glycosylation of the recombinant fusolin. As shown in Fig. 3, the carbohydrate chain was detected in the recombinant fusolin. The ΔSIG mutant, whose signal peptide is deleted, and N191Q, whose Asn191 is replaced with Gln, were expressed using the baculovirus vector (Fig. 1), and each protein was subjected to PAS staining. No band was detected in these mutants (Fig. 3), indicating that the signal peptide is actually necessary for glycosylation and a carbohydrate chain is linked at Asn191.
FIG. 3.
Glycosylation of the recombinant fusolin. Recombinant proteins were subjected to SDS-PAGE and electroblotted onto a PVDF membrane. Blotted membranes were stained with Coomassie brilliant blue (CBB) (left) or PAS (right). Spindle, native fusolin derived from AncuEPV spindles; rFusolin, recombinant fusolin; M, molecular mass standard. BSA was used as a negative control. The position of the fusolin is indicated by an arrow.
Lectin-binding experiments were carried out to compare the profiles of the carbohydrate chains between the spindle-derived (native) and baculovirus-expressed (recombinant) fusolins. As indicated in Table 2, both proteins bound to only mannose-binding lectins (GNA, concanavalin A [ConA], and LCA), and there was no difference in the binding specificities between native and recombinant fusolins.
TABLE 2.
Lectin binding profiles for native and recombinant fusolins
| Lectin | Binding
|
Binding specificity of each lectin | |
|---|---|---|---|
| Native | Recombinant | ||
| GNA | + | + | Mannose, terminal linked α1-3, α1-4, α1-2 to mannose |
| SNA | − | − | α2-6-Linked sialic acid |
| MAA | − | − | Terminal α2-3-linked sialic acid |
| PNA | − | − | Galactose β-(1-3)-N-acetylgalactosamine, usually forms the core unit of O-glycans |
| DSA | − | − | Galactose β-(1-4)-N-acetylglucosamine |
| ConA | + | + | α-Linked mannose or glucose |
| DBA | − | − | N-Acetylgalactosamine α-(1-3)-N-acetylgalactosamine in O-glycan |
| RCA120 | − | − | β-Linked galactose |
| LCA | + | + | β-Linked mannose |
It has been shown that, in native fusolin, the N-terminal signal peptide (aa 1 to 16) is cleaved immediately prior to an HGY motif which is conserved in all known sequences of EPV fusolins (28). The N-terminal sequence of the recombinant fusolin was determined to be HGYVTFP, indicating that the signal sequence of the recombinant fusolin is cleaved prior to the HGY motif as well as that of native fusolin.
Enhancement activity of recombinant fusolin in peroral virus infection.
To evaluate the enhancement activity of the recombinant fusolin in peroral infectivity, we compared the 50% lethal dose (LD50) of the BmNPV dilution series combined with the recombinant fusolin to that of the same dilution series combined with sterile water. The LD50 was reduced about 320-fold when the recombinant fusolin was added to the virus inocula, while there was no significant difference in the ratio of infected to uninfected larvae between virus inocula with sterile water and with His-tagged rGUS (Table 3). The results indicated that the recombinant fusolin is active in enhancing peroral infection with BmNPV. In addition, the ratio of infected to uninfected larvae in the inoculation of AncuEPV spheroids combined with the recombinant fusolin was significantly higher than that with inoculation of the spheroids alone (Table 4), indicating that the recombinant fusolin enhances also the infectivity of AncuEPV. Previously, we demonstrated that AncuEPV spindles purified from insects facilitate the peroral infection with AncuEPV (23, 26, 30). This is the first demonstration of the enhancement of EPV infection by fusolin produced using a foreign gene expression system. The C-terminal His tag itself seems not to influence the enhancing activity, because His-tagged rGUS did not enhance the infectivity of either BmNPV or AncuEPV.
TABLE 3.
Enhancing activity of recombinant fusolin in peroral infectivity of BmNPV
| Additive | No. of dead larvae/no. of larvae tested for indicated no. of BmNPV OBs administered/larvaa
|
LD50 | 95% fiducial limit of LD50b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 101 | 102 | 103 | 104 | 105 | 106 | 107 | |||
| Sterile water | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 2/20 | 12/20 | 18/20 | 8.24 × 105 | 4.10 × 105 to 1.68 × 106 |
| rFusolin | 0/20 | 8/20* | 13/20* | 16/20* | 2.55 × 103 | 2.54 × 101 to 1.04 × 104 | ||||
| rGUS | 0/20 | 0/20 | 0/20 | 5/20 | ||||||
| Spindles | 0/20 | 15/20* | 20/20* | <1.00 × 101 | ||||||
| dSpindles | 0/20 | 20/20* | 20/20* | 20/20* | 20/20* | 20/20* | 20/20* | <1.00 × 101 | ||
Third-instar first-day larvae of B. mori were used in this test. Nine micrograms of each additive (equivalent to ca. 1×106 of spindles) was administrated per larva. *, significant difference (P < 0.01, chi-square test) in the ratio of infected to uninfected larvae compared to inoculation of OBs alone.
The LD50 and its 95% fiducial limit were calculated by probit analysis using the computer program PriProbit (version 1.63; Masayuki Sakuma, Kyoto University).
TABLE 4.
Enhancing activity of recombinant fusolin in peroral infectivity of AncuEPVa
| Inoculum | No. of infected larvae/no. of tested larvaeb | Infection rate (%) |
|---|---|---|
| AncuEPV spheroids alone | 5/32 | 15.6 |
| AncuEPV spheroids combined with rFusolin | 23/33* | 69.7 |
| AncuEPV spheroids combined with rGUS | 0/18 | 0 |
| rFusolin | 0/30 | 0 |
| Sterile water | 0/29 | 0 |
Second-instar first-day larvae of A. cuprea were used in this test. A total of 1.5×103 of AncuEPV spheroids was inoculated per larva, and 9 μg of rFusolin or rGUS was administrated per larva as an additive.
*, significant difference (P < 0.01, chi-square test) in the ratio of infected to uninfected larvae compared to inoculation of the spheroids alone.
Although the recombinant fusolin enhanced the peroral infectivity of BmNPV by about 320-fold, AncuEPV spindles showed much higher (>8.24 × 104-fold) enhancing activity (Table 3). It has been reported that AncuEPV spindles increase the infectivity of BmNPV by up to 106-fold (24). One of the major differences between native and recombinant fusolins is that the recombinant fusolin is denatured with 5 M guanidinium hydrochloride during the His tag purification. Therefore, we prepared denatured AncuEPV spindles (dSpindles) following the procedure for purification of the recombinant fusolin. The dSpindles that were obtained no longer formed a bipyramidal structure, but their enhancing activity was not at all affected (Table 3). This result indicates that the bipyramidal shape itself is not essential for high-level enhancing.
Mapping of the essential regions for peroral infectivity enhancement.
To investigate the essential regions for the enhancing activity of fusolin in peroral infection, N- and C-terminal deletion mutants were prepared (Fig. 1), and their activities were analyzed with the bioassay using BmNPV OBs. As indicated in Table 5 (experiments 1 and 2), no N-terminal deletion mutants had enhancing activity, and the C-terminal deletion up to amino acid 254 (DC4) did not have a significant influence on the activity. These results indicate that the N-terminal region (aa 1 to 253) is essential for the enhancing activity. This region contains highly conserved sequence elements as well as one N-glycosylation site and is predicted to be a chitin-binding domain, chitin-binding domain 3 (24, 28). To examine the requirement of the carbohydrate chain for infectivity enhancement, glycosylation-defective mutants N191Q and ΔSIG were subjected to the bioassay. The N191Q mutant harboring the single amino acid substitution at the N-glycosylation site (Asn191 to Gln) enhanced infectivity only when administered in combination with a high concentration (104 or 105) of BmNPV OBs (Table 5, experiments 3-1 and 3-2). The ΔSIG mutant was no longer able to facilitate the peroral infectivity of BmNPV (Table 5, experiments 3-1 and 3-2). These results indicate that the carbohydrate chain is important for the enhancement of peroral infection.
TABLE 5.
Enhancing activity of the mutants in peroral infectivity of BmNPV
| Expt no.a | Additiveb | No. of dead larvae/no. of tested larvae for indicated no. of BmNPV OBs administered/larvac
|
||||
|---|---|---|---|---|---|---|
| 0 | 101 | 103 | 104 | 105 | ||
| 1 | Sterile water | 0/24 | 0/24 | 8/24 | ||
| rFusolin | 0/24 | 24/24* | 24/24* | |||
| DN1 | 0/24 | 0/24 | 5/24 | |||
| DN2 | 0/24 | 2/24 | 7/24 | |||
| DN3 | 0/24 | 0/24 | 8/24 | |||
| DN4 | 0/24 | 0/24 | 7/24 | |||
| DN5 | 0/24 | 0/24 | 7/24 | |||
| 2 | Sterile water | 0/24 | 0/24 | 0/24 | 9/24 | |
| rFusolin | 0/24 | 24/24* | 24/24* | 24/24* | ||
| DC1 | 0/24 | 20/24* | ||||
| DC2 | 0/24 | 19/24* | ||||
| DC3 | 0/24 | 24/24* | 24/24* | |||
| DC4 | 0/24 | 16/24* | ||||
| DC5 | 0/24 | 0/24 | 0/24 | 11/24 | ||
| DC6 | 0/24 | 0/24 | 0/24 | 11/24 | ||
| DC7 | 0/24 | 0/24 | 0/24 | 10/24 | ||
| 3-1 | Sterile water | 0/24 | 0/24 | 0/24 | 7/24 | 13/24 |
| rFusolin | 0/24 | 24/24* | 24/24* | 24/24* | ||
| ΔSig | 0/24 | 0/24 | 0/24 | 12/24 | ||
| N191Q | 0/24 | 0/24 | 2/24 | 23/24* | 23/24* | |
| 3-2 | Sterile water | 0/24 | 0/24 | 0/24 | ||
| rFusolin | 0/24 | 24/24* | 24/24* | |||
| ΔSig | 0/24 | 0/24 | ||||
| N191Q | 0/24 | 0/24 | 21/24* | |||
| 4 | Sterile water | 0/24 | 0/24 | 3/24 | 5/24 | |
| rFusolin | 0/24 | 24/24* | 24/24* | 24/24* | ||
| YMF | 0/24 | 1/24 | 2/24 | 4/24 | ||
| IHE | 0/24 | 1/24 | 0/24 | 6/24 | ||
Each experiment (numbers 1 to 4) was carried out independently, but the same batch of BmNPV OBs dilution series was used in all experiments.
Second-instar first-day larvae of B. mori were used in all tests. Six micrograms of each additive was administrated per larva.
Evaluation of enhancing activity was carried out with at least two different concentrations of BmNPV OBs. *, significant difference (P < 0.01, chi-square test) in the ratio of infected to uninfected larvae compared to inoculation of OBs alone.
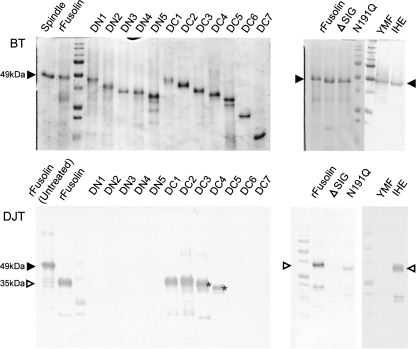
Stability in insect digestive juice.
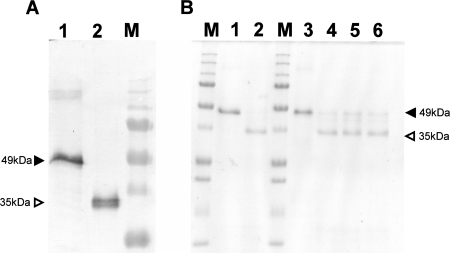
To examine stability in digestive juice, recombinant fusolin was treated with the digestive juice harvested from B. mori larvae, and its stability was assessed by Western blot analysis. Interestingly, the treatment of fusolin with the digestive juice resulted in a loss of apparent molecular mass (ca. 35 kDa) compared to untreated fusolin (ca. 49 kDa) (Fig. 4A). The same result was obtained when native fusolin derived from AncuEPV spindles was treated with the digestive juice (data not shown). To investigate the digested region, the N-terminal amino acid sequence of fusolin was analyzed after treatment with digestive juice. The sequence determined was HGYVTFP, which is identical to that of untreated recombinant fusolin, indicating that the C-terminal region is degraded in digestive juice.
FIG. 4.
Proteolytic processing of fusolin in insect digestive juice. (A) Western blot analysis of the recombinant fusolin treated with digestive juice harvested from B. mori larvae. The recombinant fusolins were analyzed before (lane 1) and after (lane 2) treatment with the digestive juice. Closed and open arrowheads indicate positions of full-length (49-kDa) and processed (35-kDa) fusolins, respectively. Lane M, molecular mass standard. (B) Inhibition assay of the C-terminal degradation of fusolin. Lane 1, recombinant fusolin (untreated); lane 2, recombinant fusolin treated with digestive juice; lanes 3 to 6, recombinant fusolin treated with digestive juice containing protease inhibitors (Pefabloc SC Plus [serine protease inhibitor]) (lane 3), E64 (cysteine protease inhibitor) (lane 4), pepstatin A (aspartic protease inhibitor) (lane 5), and EDTA (metalloprotease inhibitor) (lane 6); lane M, molecular mass standard. Closed and open arrowheads indicate the same as described for panel A.
The characterization of the digestive enzyme participating in the C-terminal degradation was carried out using several protease inhibitors. As shown in Fig. 4B, a serine protease inhibitor (Pefabloc SC Plus) blocked the C-terminal degradation (lane 3), while a cysteine protease inhibitor (E64), aspartic protease inhibitor (pepstatin A), and metalloprotease inhibitor (EDTA) had no apparent effects (lanes 4 to 6). These results suggest that the C-terminal region of fusolin is degraded by a serine protease(s) in digestive juice.
To identify the essential region for stability in digestive juice, deletion mutants (Fig. 1) were treated with digestive juice and then assessed by Western blotting. As shown in Fig. 5, no N-terminal deletion mutants were detected, and deletion from the C terminus up to amino acid 254 (DC4) exhibited tolerance to the digestive juice. To examine the contribution of the carbohydrate chain to stability, the N191Q and ΔSIG mutants (Fig. 1) were subjected to the same assay. The N191Q mutant showed some tolerance to digestive enzymes but the tolerance was considerably decreased in comparison to that of wild-type recombinant fusolin (Fig. 5). The ΔSIG mutant was highly unstable in the digestive juice, as no band was detected by Western blot analysis (Fig. 5). These results indicate that the N-terminal conserved region (aa 1 to 253) is necessary for stability in digestive juice and the carbohydrate chain is also important. These requisites for stability are consistent with those for enhancing activity in peroral infection (summarized below in Fig. 7). The molecular masses of the DC1 and DC2 mutants were decreased by treatment with digestive juice, whereas those of the DC3 and DC4 mutants were not affected (Fig. 5). This suggests that the C-terminal degradation of fusolin by treatment with digestive juice ends between amino acids 267 and 306 (Fig. 1; see also Fig. 7, below).
FIG. 5.
Stabilities of rFusolin and its derivates in digestive juice. BT, before treatment. Each protein was run on a 17% SDS-polyacrylamide gel before treatment with the digestive juice. DJT, digestive juice treatment. Each protein was treated with the B. mori digestive juice, and their stabilities were assessed by Western blotting using antibody to AncuEPV spindles. Closed and open arrowheads indicate the positions of full-length and processed fusolins, respectively. The molecular masses of DC3 and DC4 were not influenced by treatment with digestive juice (asterisks).
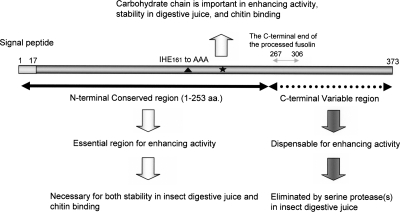
FIG. 7.
Summary of essential and nonessential regions of AncuEPV fusolin. The N-terminal conserved region (aa 1 to 253; horizontal black arrow) is essential for its enhancing activity in peroral infection and is necessary for both stability in insect digestive juice and chitin binding. The carbohydrate chain (black star) is important in the enhancing activity, stability in digestive juice, and chitin binding. On the other hand, the C-terminal variable region (horizontal dotted arrow) is dispensable and is digested by an intestinal serine protease(s). The C-terminal end of the processed fusolin, whose C-terminal dispensable region is eliminated by a serine protease(s), is expected to be located between amino acids 267 and 306 (horizontal gray arrow). The closed triangle indicates a triple amino acid replacement mutant, IHE161 to AAA, which causes no changes in chitin binding and stability in digestive juice but causes loss of enhancing activity.
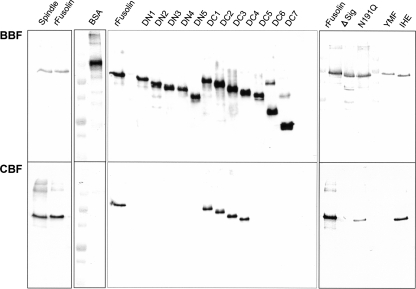
Chitin-binding ability.
EPV fusolins possess a putative chitin-binding domain named chitin-binding domain 3 (20, 24) and show 30 to 40% identity to GP37s of NPVs (1, 6, 10, 11, 19-21, 28, 34, 40). The chitin-binding ability of GP37 has been demonstrated in Spodoptera litura multiple nucleopolyhedrovirus (20), whereas that of fusolin has not yet been reported. Therefore, we examined the chitin-binding ability of AncuEPV fusolin in vitro using chitin beads. As shown in Fig. 6, both native and recombinant fusolins were detected in the chitin-binding fraction, whereas BSA used as a negative control could not bind to chitin beads. To map the essential region for chitin binding, the in vitro binding assay was carried out with the deletion mutants (Fig. 1). No N-terminal deletion mutants bound to chitin beads, and the C-terminal deletion mutants up to amino acid 254 (DC4) exhibited binding ability (Fig. 6). To examine the involvement of the carbohydrate chain in chitin binding, the N191Q and ΔSIG mutants were analyzed in the binding assay. The N191Q mutant exhibited reduced affinity compared to the wild-type recombinant fusolin, and the ΔSIG mutant showed no positive binding signal (Fig. 6). These results indicate that the N-terminal conserved region (aa 1 to 253) is required for chitin binding and that the carbohydrate chain is also important for effective binding. As with stability in digestive juice, these requisites for chitin binding were consistent with those for enhancing activity in peroral infection (summarized in Fig. 7).
FIG. 6.
In vitro chitin-binding assay. BBF, before-binding fraction. Each protein was solubilized in the dissolving solution and assessed by Western blotting before the chitin-binding step. CBF, chitin-binding fraction. Each protein solubilized in the dissolving solution was mixed with chitin beads at room temperature for 18 h. Binding proteins on the chitin beads were detected by Western blot analysis. BSA was used as a negative control, and its detection was carried out using a monoclonal antibody to BSA.
The enhancing activity of fusolin may involve more than stability in digestive juice and chitin-binding ability.
Serratia marcescens CBP21 is a chitin-binding protein which harbors chitin-binding domain 3. Recently, several amino acids of the CBP21 involved in chitin binding were identified (39). To provide chitin-binding-defective mutants, triple amino acid mutants YMF (Tyr-Met-Phe79 to Ala-Ala-Ala) and IHE (Ile-His-Glu161 to Ala-Ala-Ala) (Fig. 1) were constructed by reference to the above-mentioned report, and their enhancing activities, stabilities in digestive juice, and chitin-binding abilities were analyzed. These mutants showed no enhancing activity in peroral infection with BmNPV (Table 5, experiment 4). In the YMF mutant, no chitin binding was detected and it was not stable in digestive juice (Fig. 5 and 6). On the other hand, the IHE mutant exhibited both chitin-binding ability and tolerance to digestive enzymes, similar to the wild-type recombinant fusolin (Fig. 5 and 6). Thus, the results suggest that tolerance to digestive juice and chitin-binding ability alone are not sufficient for the enhancing activity of fusolin, and therefore other unknown factors appear to be required for the enhancement of peroral infection.
DISCUSSION
The recombinant fusolin enhanced the peroral infectivity of BmNPV approximately 320-fold in third-instar larvae of B. mori. On the other hand, AncuEPV spindles enhanced the infectivity of BmNPV up to approximately 106-fold (24). One of the major differences between the spindle-derived (native) and baculovirus-expressed (recombinant) fusolins is that the recombinant fusolin is denatured with 5 M guanidinium hydrochloride during His tag purification. However, the dSpindles, whose bipyramidal structures are completely disrupted, still exhibited high-level enhancing activity similar to the spindles before denaturing treatment. This suggests that the spindle shape per se is not required for high-level enhancing ability. It is unknown at present why the enhancing activity of the recombinant fusolin is reduced in comparison to that of native fusolin. High-resolution structural analysis, such as nuclear magnetic resonance spectrometry, may find a structural difference between native and recombinant fusolins.
Although the recombinant fusolin was detected in the insoluble fraction of vector-infected cells, no spindle-shaped inclusions were observed (data not shown), as in the case of Melolontha melolontha entomopoxvirus (10). It was predicted that the C-terminal His tag of recombinant fusolin may disturb the formation of the bipyramidal structure. Therefore, expression of untagged fusolin was also tried, but no spindles were found in vector-infected cells (data not shown). These results suggest that the formation of the bipyramidal structure probably requires another AncuEPV protein(s) and/or a proper host factor(s) derived from A. cuprea.
The mutagenesis of the fusolin revealed that the N-terminal region (aa 1 to 253) is essential for the enhancement of peroral virus infection, while the C-terminal region is dispensable. Furthermore, the N-terminal region was necessary for stability in digestive juice and chitin-binding ability, suggesting that these abilities are necessary for the enhancing activity. However, the IHE mutant was stable in digestive juice and showed chitin-binding ability but had no enhancing activity. Therefore, other unknown properties of EPV fusolins appear to be necessary for the enhancement of peroral infection.
The C-terminal regions of EPV fusolins are not conserved and are variable in length (5, 20, 34). Intriguingly, in this study, the C-terminal region was eliminated by a serine protease(s) in digestive juice. It is well-known that the C-terminal regions of Bacillus thuringiensis Cry toxins are digested by insect midgut proteases to create the active form (36). The jasmonic acid-inducible threonine deaminase (TD), which is a plant defense protein against insect herbivores, is known to act in insect gut to deplete isoleucine, which is required for insect growth (3, 4). Recently, it was reported that the C-terminal portion of the TD is removed by midgut enzymes of Manduca sexta and that the enzymatic activity of the processed TD is increased compared to that of the full-length TD (3, 4). Therefore, it would be interesting to study the functional meaning of elimination of the C-terminal region in EPV fusolins.
The glycosylation-defective mutants N191Q and ΔSIG showed reduced and abolished enhancing activities, respectively. The results indicate that the carbohydrate chain of fusolin is important for enhancing activity. However, in the case of PsEPV, bacterium-expressed EF (fusolin), which has no carbohydrate chain, has been shown to have enhancing activity in peroral infection (16). As the N191Q mutant did not completely lose the enhancing activity, the carbohydrate chain does not seem to act directly to enhance peroral virus infection, but rather it may participate in proper protein folding.
There are reports regarding various proteins which cause PM disintegration. For instance, in granuloviruses, a viral metalloprotease called enhancin is known to disrupt the PM of the host insect by degrading intestinal insect mucin, which is the major PM protein (7, 18, 33, 37, 41). Wheat germ agglutinin, which is a chitin-binding lectin, is speculated to interfere with the assembly of normal PM structure, leading to voids in the network (12, 14). Recently, maize insect resistance cysteine protease (Mir1-CP), a chitin-binding cysteine protease, was shown to be capable of directly permeabilizing the PM by its cysteine protease activity (31, 32). EPV fusolins harbor a chitin-binding domain at the N-terminal region, but no catalytic domain has been found thus far. There are at least two possible mechanisms which may explain the ability of fusolins to disintegrate the PM. First, fusolin may act as a chitin-binding competitor that prevents assembly of the PM in a manner similar to the wheat germ agglutinin lectin (12, 14) and to the chitin-binding reagent calcofluor (42, 43), although this theory is premised on frequent regeneration of the PM. Second, fusolin may cause structural changes in the chitin network, leading to PM disruption. Recently, it was reported that S. marcescens CBP21 binds to crystalline chitin and gives rise to conformational changes that facilitate hydrolysis by exogenous chitinases (38). Conformational changes in the chitin network may facilitate the attack on the PM by intestinal enzymes. Further experiments to elucidate the molecular mechanism of the PM disintegration by AncuEPV fusolin are in progress at the in vivo level.
Acknowledgments
We thank Shogo Atsumi, Naoya Wasano, Shoji Asano, Toru Arakawa, Hiroaki Noda, and Satoshi Takeda of NIAS for all of their fruitful discussions, and we appreciate François J. Kraus of Administration des services techniques de l'agriculture, Luxembourg, for critical reading of the manuscript and helpful suggestions.
Footnotes
Published ahead of print on 1 October 2008.
REFERENCES
- 1.Arif, B. M. 1995. Recent advances in the molecular biology of entomopoxviruses. J. Gen. Virol. 761-13. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty, M., K. Narayanan, and M. K. Sivaprakash. 2004. In vivo enhancement of nucleopolyhedrovirus of oriental armyworm, Mythimna separata using spindles from Helicoverpa armigera. Indian J. Exp. Biol. 42121-123. [PubMed] [Google Scholar]
- 3.Chen, H., C. G. Wilkerson, J. A. Kuchar, B. S. Phinney, and G. A. Howe. 2005. Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. USA 5219237-19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, H., E. Gonzales-Vigil, C. G. Wilkerson, and G. A. Howe. 2007. Stability of plant defense proteins in the gut of insect herbivores. Plant Physiol. 1431954-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dall, D., T. Luque, and D. O'Reilly. 2001. Insect-virus relationships: sifting by informatics. Bioessays 23184-193. [DOI] [PubMed] [Google Scholar]
- 6.Dall, D., A. Sriskantha, A. Vera, J. Lai-Fook, and T. Symonds. 1993. A gene encoding a highly expressed spindle body protein of Heliothis armigera entomopoxvirus. J. Gen. Virol. 741811-1818. [DOI] [PubMed] [Google Scholar]
- 7.Derksen, A. C., and R. R. Granados. 1988. Alteration of a lepidopteran peritrophic membrane by baculoviruses and enhancement of viral infectivity. Virology 167242-250. [DOI] [PubMed] [Google Scholar]
- 8.Devine, P. L., and J. A. Warren. 1990. Glycoprotein detection on Immobilon PVDF transfer membrane using the periodic acid/Schiff reagent. BioTechniques 8492-495. [PubMed] [Google Scholar]
- 9.Furuta, Y., W. Mitsuhashi, J. Kobayashi, S. Hayasaka, S. Imanishi, Y. Chinzei, and M. Sato. 2001. Peroral infectivity of non-occluded viruses of Bombyx mori nucleopolyhedrovirus and polyhedrin-negative recombinant baculoviruses to silkworm larvae is drastically enhanced when administered with Anomala cuprea entomopoxvirus spindles. J. Gen. Virol. 82307-312. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier, L., F. Cousserans, J. C. Veyrunes, and M. Bergoin. 1995. The Melolontha melolontha entomopoxvirus (MmEPV) fusolin is related to the fusolins of lepidopteran EPVs and to the 37K baculovirus glycoprotein. Virology 208427-436. [DOI] [PubMed] [Google Scholar]
- 11.Gross, C. H., G. M. Wolgamot, R. L. Q. Russell, M. N. Pearson, and G. F. Rohrmann. 1993. A 37-kilodalton glycoprotein from a baculovirus of Orgyia pseudotsugata is localized to cytoplasmic inclusion bodies. J. Virol. 67469-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harper, M. S., T. L. Hopkins, and T. H. Czapla. 1998. Effect of wheat germ agglutinin on formation and structure of the peritrophic membrane in European corn borer (Ostrinia nubilalis) larvae. Tissue Cell 30166-176. [DOI] [PubMed] [Google Scholar]
- 13.Hirano, H. 1989. Microsequence analysis of winged bean seed proteins electroblotted from two-dimensional gel. J. Protein Chem. 8115-130. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins, T. L., and M. S. Harper. 2001. Lepidopteran peritrophic membranes and effects of dietary wheat germ agglutinin on their formation and structure. Arch. Insect Biochem. Physiol. 47100-109. [DOI] [PubMed] [Google Scholar]
- 15.Hukuhara, T., T. Hayakawa, and A. Wijonarko. 1999. Increased baculovirus susceptibility of armyworm larvae feeding on transgenic rice plants expressing an entomopoxvirus gene. Nat. Biotechnol. 171122-1124. [DOI] [PubMed] [Google Scholar]
- 16.Hukuhara, T., T. Hayakawa, and A. Wijonarko. 2001. A bacterially produced virus enhancing factor from an entomopoxvirus enhances nucleopolyhedrovirus infection in armyworm larvae. J. Invertebr. Pathol. 7825-30. [DOI] [PubMed] [Google Scholar]
- 17.Lai-Fook, J., and D. J. Dall. 2000. Spindle bodies of Heliothis armigera entomopoxvirus develop in structures associated with host cell endoplasmic reticulum. J. Invertebr. Pathol. 75183-192. [DOI] [PubMed] [Google Scholar]
- 18.Lepore, L. S., P. R. Roelvink, and R. R. Granados. 1996. Enhancin, the granulosis virus protein that facilitates nucleopolyhedrovirus (NPV) infections, is a metalloprotease. J. Invertebr. Pathol. 68131-140. [DOI] [PubMed] [Google Scholar]
- 19.Li, X., J. Barrett, A. Pang, R. J. Klose, P. J. Krell, and B. M. Arif. 2000. Characterization of an overexpressed spindle protein during a baculovirus infection Virology 26856-67. [DOI] [PubMed] [Google Scholar]
- 20.Li, Z., C. Li, K. Yang, L. Wang, C. Yin, Y. Gong, and Y. Pang. 2003. Characterization of chitin-binding protein GP37 of Spodoptera litura multicapsid nucleopolyhedrovirus. Virus Res. 96113-122. [DOI] [PubMed] [Google Scholar]
- 21.Liu, J. J., and E. B. Carstens. 1996. Identification, molecular cloning, and transcription analysis of the Choristoneura fumiferana nuclear polyhedrosis virus spindle-like protein gene. Virology 223396-400. [DOI] [PubMed] [Google Scholar]
- 22.Liu, S., H. Li, S. Sivakumar, and B. C. Bonning. 2006. Virus-derived genes for insect-resistance plants, p. 427-457. In B. C. Bonning, K. Maramorosh, and A. J. Shatkin (ed.), Advances in virus research, vol. 68. Academic Press, San Diego, CA. [DOI] [PubMed] [Google Scholar]
- 23.Mitsuhashi, W. 2002. Further evidence that spindles of an entomopoxvirus enhance its infectivity in a host insect. J. Invertebr. Pathol. 7959-61. [DOI] [PubMed] [Google Scholar]
- 24.Mitsuhashi, W. 2006. Enhancement of the infectivity of insect viruses: a protein produced by entomopoxviruses is a potential synergist of viral pesticides, p. 147-159. In S. G. Pandalai (ed.), Recent research developments in entomology, vol. 5. Research Signpost, Kerala, India. [Google Scholar]
- 25.Mitsuhashi, W., Y. Furuta, and M. Sato. 1998. The spindles of an entomopoxvirus of Coleoptela (Anomala cuprea) strongly enhance the infectivity of a nucleopolyhedrovirus in Lepidoptera (Bombyx mori). J. Invertebr. Pathol. 71186-188. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuhashi, W., H. Kawakita, R. Murakami, Y. Takemoto, T. Saiki, K. Miyamoto, and S. Wada. 2007. Spindles of an entomopoxvirus facilitate its infection of the host insect by disrupting the peritrophic membrane. J. Virol. 814235-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsuhashi, W., and K. Miyamoto. 2003. Disintegration of the peritrophic membrane of silkworm larvae due to spindles of an entomopoxvirus. J. Invertebr. Pathol. 8234-40. [DOI] [PubMed] [Google Scholar]
- 28.Mitsuhashi, W., H. Saito, and M. Sato. 1997. Complete nucleotide sequence of the fusolin gene of an entomopoxvirus in the cupreous chafer, Anomala cuprea Hope (Coleoptera: Scarabaeidae). Insect Biochem. Mol. Biol. 27869-876. [DOI] [PubMed] [Google Scholar]
- 29.Mitsuhashi, W., and M. Sato. 2000. Enhanced infection of a nucleopolyhedrovirus in a lepidopteran pest (Spilosoma imparilis) by spindles of a coleopteran entomopoxvirus (EPV) (Anomala cuprea EPV). J. Forest Res. 5285-287. [Google Scholar]
- 30.Mitsuhashi, W., M. Sato, and Y. Hirai. 2000. Involvement of spindles of an entomopoxvirus (EPV) in infectivity of the EPVs to their host insect. Arch. Virol. 1451465-1471. [DOI] [PubMed] [Google Scholar]
- 31.Mohan, S., P. W. K. Ma, T. Pechan, E. R. Bassford, W. P. Williams, and D. S. Luthe. 2006. Degradation of the S. frugiperda peritrophic matrix by an inducible maize cysteine protease. J. Insect Physiol. 5221-28. [DOI] [PubMed] [Google Scholar]
- 32.Pechan, T., A. Cohen, W. P. Williams, and D. S. Luthe. 2002. Insect feeding mobilizes a unique plant defense protease that disrupts the peritrophic matrix of caterpillars. Proc. Natl. Acad. Sci. USA 9913319-13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng, J., J. Zhong, and R. R. Granados. 1999. A baculovirus enhancing alters the permeability of a mucosal midgut peritrophic matrix from lepidopteran larvae. J. Insect Physiol. 45159-166. [DOI] [PubMed] [Google Scholar]
- 34.Phanis, C. G., D. P. Miller, S. C. Cassar, M. Tristem, S. M. Thiem, and D. R. O'Reilly. 1999. Identification and expression of two baculovirus gp37 genes. J. Gen. Virol. 801823-1831. [DOI] [PubMed] [Google Scholar]
- 35.Sawano, A., and A. Miyawaki. 2000. Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic Acids Res. 28e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slavicek, J. M., and H. J. R. Popham. 2005. The Lymantria dispar nucleopolyhedrovirus enhancins are components of occlusion-derived virus. J. Virol. 7910578-10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaaje-Kolstad, G., S. J. Horn, D. M. F. Van Aalten, B. Synstad, and V. G. H. Eijsink. 2005. The non-catalytic chitin-binding protein CBP21 from Serratia marcescens is essential for chitin degradation. J. Biol. Chem. 28028492-28497. [DOI] [PubMed] [Google Scholar]
- 39.Vaaje-Kolstad, G., D. R. Houston, A. H. K. Riemen, V. G. H. Eijsink, and D. M. F. Van Aalten. 2005. Crystal structure and binding properties of the Serratia marcescens chitin-binding protein CBP21. J. Biol. Chem. 28011313-11319. [DOI] [PubMed] [Google Scholar]
- 40.Vialard, J. E., L. Yuen, and C. D. Richardson. 1990. Identification and characterization of a baculovirus occlusion body glycoprotein which resembles spheroidin, an entomopoxvirus protein. J. Virol. 645804-5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, P., and R. R. Granados. 1997. An intestinal mucin is the target substrate for a baculovirus enhancin. Proc. Natl. Acad. Sci. USA 946977-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, P., and R. R. Granados. 2000. Calcofluor disrupts the midgut defense system in insects. Insect Biochem. Mol. Biol. 30135-143. [DOI] [PubMed] [Google Scholar]
- 43.Wang, P., and R. R. Granados. 2001. Molecular structure of the peritrophic membrane (PM): identification of potential PM target sites for insect control. Arch. Insect Biochem. Physiol. 47110-118. [DOI] [PubMed] [Google Scholar]
- 44.Wijonarko, A., and T. Hukuhara. 1998. Detection of a virus enhancing factor in the spheroid, spindle, and virion of an entomopoxvirus. J. Invertebr. Pathol. 7282-86. [DOI] [PubMed] [Google Scholar]
- 45.Yuen, L., J. Dionne, B. M. Arif, and C. Richardson. 1990. Identification and sequencing of the spheroidin gene of Choristoneura biennis entomopoxvirus. Virology 175427-433. [DOI] [PubMed] [Google Scholar]