Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) interacts with cell surface heparan sulfate (HS) and α3β1 integrin during the early stages of infection of human dermal microvascular endothelial cells (HMVEC-d) and human foreskin fibroblasts (HFF), and these interactions are followed by virus entry overlapping with the induction of preexisting host cell signal pathways. KSHV also utilizes the amino acid transporter protein xCT for infection of adherent cells, and the xCT molecule is part of the cell surface heterodimeric membrane glycoprotein CD98 (4F2 antigen) complex known to interact with α3β1 and αVβ3 integrins. KSHV gB mediates adhesion of HMVEC-d, CV-1, and HT-1080 cells and HFF via its RGD sequence. Anti-αV and -β1 integrin antibodies inhibited the cell adhesion mediated by KSHV-gB. Variable levels of neutralization of HMVEC-d and HFF infection were observed with antibodies against αVβ3 and αVβ5 integrins. Similarly, variable levels of inhibition of virus entry into adherent HMVEC-d, 293 and Vero cells, and HFF was observed by preincubating virus with soluble α3β1, αVβ3, and αVβ5 integrins, and cumulative inhibition was observed with a combination of integrins. We were unable to infect HT1080 cells. Virus binding and DNA internalization studies suggest that αVβ3 and αVβ5 integrins also play roles in KSHV entry. We observed time-dependent temporal KSHV interactions with HMVEC-d integrins and CD98/xCT with three different patterns of association and dissociation. Integrin αVβ5 interaction with CD98/xCT predominantly occurred by 1 min postinfection (p.i.) and dissociated at 10 min p.i., whereas α3β1-CD98/xCT interaction was maximal at 10 min p.i. and dissociated at 30 min p.i., and αVβ3-CD98/xCT interaction was maximal at 10 min p.i. and remained at the observed 30 min p.i. Fluorescence microscopy also showed a similar time-dependent interaction of αVβ5-CD98. Confocal-microscopy studies confirmed the association of CD98/xCT with α3β1 and KSHV. Preincubation of KSHV with soluble heparin and α3β1 significantly inhibited this association, suggesting that the first contact with HS and integrin is an essential element in subsequent CD98-xCT interactions. Anti-CD98 and xCT antibodies did not block virus binding and entry and nuclear delivery of viral DNA; however, viral-gene expression was significantly inhibited, suggesting that CD98-xCT play roles in the post-entry stage of infection, possibly in mediating signal cascades essential for viral-gene expression. Together, these studies suggest that KSHV interacts with functionally related integrins (αVβ3, α3β1, and αVβ5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression.
The γ-2 herpesvirus Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), or human herpesvirus 8, is etiologically associated with KS; primary effusion lymphoma, or body cavity-based B-cell lymphoma (BCBL); and multicentric Castleman's disease. The in vivo host cell range of KSHV is not yet fully characterized but appears to be broad, as viral DNA and transcripts have been detected in B cells from peripheral blood, B cells in BCBL and multicentric Castleman's disease, KS spindle cells, and KS lesion-associated CD45+/CD68+ monocytes, keratinocytes, and epithelial cells (13, 23, 47). Similar to in vivo tropism, KSHV has broad in vitro tropism. KSHV from BCBL cells can infect human B cells, lymphocytes, endothelial and epithelial cells, fibroblasts, and CD34+ stem cell precursors of dendritic cells. KSHV also infects a variety of animal cells, such as owl monkey kidney cells, BHK-21 cells, Chinese hamster ovary (CHO) cells, and mouse fibroblasts (3, 22, 50, 61, 66). The tropism and properties of wild-type KSHV from the saliva of infected individuals and KSHV isolates from Africa and other areas where KS is endemic are not known at present.
For any virus, the first key step in the infection of target cells is the interaction with cell surface molecules. Compared to the advances in other areas of KSHV research, knowledge regarding KSHV entry and infection is limited for several reasons, such as the complexity of the process, the rapidity of the events, involvement of multiple KSHV envelope glycoproteins, the wide range of target cells, and the inherent difficulties in studying virus-receptor interactions. The available studies demonstrate that KSHV enters adherent human foreskin fibroblasts (HFF), human dermal microvascular endothelial cells (HMVEC-d), and human embryonic kidney epithelial cells (293), as well as nonadherent BJAB (KSHV- and Epstein-Barr virus [EBV]-negative B-lymphoma) cells by endocytosis (1, 4, 34). KSHV's ability to bind human B, endothelial, and epithelial cells; monocytes (but not T and NK cells); and a variety of animal cells is probably due in part to KSHV's interaction with the ubiquitous cell surface heparan sulfate (HS) proteoglycan (2, 4, 12). KSHV binds to HSs of several in vitro target cells, such as BJAB, BCBL-1, and 293 cells, HMVEC-d, and HFF (2, 4, 79). Treatment of virus with heparin blocked the binding of radiolabeled virus to both adherent and nonadherent target cells. KSHV infection can be inhibited in a dose-dependent manner by soluble heparin, but not by chondroitin sulfates A and C (4). Removal of HFF surface HS with heparinase reduced KSHV infectivity. Soluble heparin blocked or displaced KSHV binding to target cells, and this binding was drastically reduced in mutant CHO cells deficient in HS (4). KSHV gB and gpK8.1A bind to cell surface HS molecules (2, 7, 79), and the binding of soluble forms of gB and gpK8.1A proteins generated in baculovirus is saturable and can be blocked by soluble heparin (79, 80). Virion envelope-associated full-length gB and gpK8.1A specifically bind heparin-agarose, which can be eluted by high concentrations of soluble heparin (2, 79). All these studies suggest that HS probably serves as an initial contact receptor for several in vitro target cells.
KSHV also interacts with α3β1 integrin of HMVEC-d and HFF (3). Integrins are a large family of heterodimeric receptors containing noncovalently associated transmembrane α and β glycoprotein subunits (27, 56). There are 17 α and 9 β subunits, generating more than 24 known combinations of αβ cell surface receptors. Each cell expresses several combinations of αβ integrins, and each αβ combination has its own binding specificity and signaling properties (27, 56). Anti-α3 or -β1 antibodies or soluble α3β1 integrin blocked KSHV infection of HMVEC-d and HFF with about 50% reduction in infection as measured by green fluorescent protein (GFP) expression (3). Integrin involvement in KSHV infection of B cells and other cells has not been demonstrated. Thus, integrin could be one of the KSHV receptors in some adherent target cells, but not in all cells. Studies by us and others suggest that KSHV also utilizes αVβ3 and αVβ5 integrins for infection of adherent target cells (24). KSHV also utilizes the dendritic-cell (DC)-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN; CD209) as a receptor for infection of human primary myeloid DCs, macrophages, and B cells (60). DC-SIGN was required for virus attachment to these cells and DC-SIGN-expressing cell lines. KSHV binding and infection were blocked by anti-DC-SIGN monoclonal antibody (MAb), by mannan (a natural ligand for DC-SIGN), and by soluble DC-SIGN (60). Pretreatment of cells with anti-DC-SIGN antibodies could not completely block KSHV binding and infection, which can be attributed to additional receptors, such as HS and other molecules, for KSHV on these cells.
A recent report demonstrated that the 12-transmembrane xCT protein is a possible fusion-entry receptor for KSHV in adherent cells (39). Surprisingly, xCT mRNA was not detected in human CD19 primary B cells isolated from fresh peripheral blood mononuclear cells (39), which are known target cells for KSHV. These studies further suggest that, like EBV (89), KSHV may interact with one set of receptors in adherent cells and may use an alternative receptors(s) in nonadherent cells, while other molecules besides xCT may be involved in infection of B cells. The xCT molecule is part of the cell surface 125-kDa disulfide-linked heterodimeric membrane glycoprotein complex containing a common glycosylated heavy-chain CD98 molecule (4F2 antigen; 80 kDa) and a group of six 45-kDa light chains (11, 58, 78). The xCT-associated CD98 is one part of the CD98 heterodimer (CD98/xCT) (64, 78). CD98 is a multifunctional molecule involved in amino acid transport, regulation of cell adhesion, cell fusion, cell proliferation, and integrin activation. It is interesting that CD98 was initially identified as a molecule associated with integrin α3, while CD98 and integrin α3 were originally named fusion regulation protein 1 (FRP-1) and FRP-2, respectively (35, 53, 54). CD98 has also been reported to be involved in the signal transduction cascade of αVβ3 integrin (38). CD98 association with integrin α3 plays an important role in cell-cell fusion and virus-induced cell fusion (53, 54). For example, the Newcastle disease virus and parainfluenza virus type 2-induced cell fusion is regulated by α3 integrin and CD98 (35, 55). CD98 has also been shown to be associated with β1 integrins and involved in membrane clustering and β1 integrin-mediated signaling events (15, 16, 41, 62, 84). CD98 stimulates integrin α3β1-dependent adhesion in small-cell lung cancer cells and in certain breast cancer cell lines (10). Recent studies have shown that the cell fusion induced by the human immunodeficiency virus (HIV) envelope glycoprotein gp160 is also regulated by CD98, integrin, and the activation of tyrosine kinases (54, 71).
The use of multiple integrins, interaction between receptors, and sequential interaction of receptors for gaining entry into the target cells has been reported in many viral systems (30, 32, 73, 86). KSHV interactions with HS and integrins in HMVEC-d and HFF is followed by virus entry, overlapping with the induction of preexisting integrin-associated host cell signal pathways, such as focal adhesion kinase (FAK), Src, phosphatidylinositol 3-kinase (PI3-K), protein kinase C-ζ, Rho-GTPases, mitogen-activated protein kinase kinase (MEK), extracellular signal-regulated kinase (ERK1/2), and nuclear factor kappa B (NF-κB), which are essential for the internalization of viral DNA, modulation of actin and microtubules, transport of capsid via the dynein motor along the microtubules, nuclear delivery of viral DNA, and initiation of viral-gene expression (3, 50, 51, 59, 63, 67, 74). The role of CD98/xCT in the infectious process of KSHV is not known. In the present study, we carried out a comprehensive analysis of KSHV interactions with integrins, CD98, and xCT molecules. Our studies demonstrate that, in addition to α3β1, αVβ3 and αVβ5 integrins also play roles in KSHV infection of adherent target cells and that infection of HMVEC-d leads to the formation of a multimolecular complex containing α3β1, αVβ3, and αVβ5 integrins and CD98/xCT. Our studies also demonstrate that CD98 and xCT play roles in the post-entry stage of KSHV infection, possibly in mediating signal cascades essential for viral-gene expression, and suggest that KSHV interacts with functionally related αVβ3, α3β1, and αVβ5 integrins and CD98/xCT molecules during the early stages of endothelial cell infection.
MATERIALS AND METHODS
Cells.
HMVEC-d (CC-2543; Clonetics, Walkersville, MD), HFF (Clonetics), 293 cells (human embryonic kidney cells), Vero cells (ATCC CCL-81), BCBL-1 cells (KSHV-carrying human B cells), recombinant GFP-KSHV (GFP-rKSHV.152)-harboring BCBL-1 cells (GFP-BCBL-1) (a gift from Jeffrey Vieira, Fred Hutchinson Cancer Research Center, Seattle, WA), and BJAB cells used in this study were propagated and maintained according to procedures described previously (1-4, 80). The human HT1080 fibrosarcoma cell line from connective tissue described as adherent cells with epithelial cell morphology containing an activated N-ras oncogene was obtained from the ATCC (ATCC CCL-121).
Virus.
Induction of the KSHV lytic cycle in BCBL-1 cells, supernatant collection, and virus purification procedures were described previously (49, 50, 51, 59, 63, 68, 74), and virus purity was assessed by general guidelines established in our laboratory. KSHV DNA was extracted from the virus, and the copies were quantitated by real-time DNA PCR using primers amplifying the KSHV open reading frame 73 (ORF73) gene as described previously (3, 42, 50).
Recombinant KSHV proteins and anti-KSHV gB antibodies.
Cloning of the BCBL-1 KSHV gB ORF, the gBΔTM gene region encoding amino acids 1 to 702 lacking the transmembrane and cytoplasmic domains, the gBΔTM-RGA mutant (RGD to RGA), and the generation of recombinant baculovirus containing gBΔTM and gBΔTM-RGA have been described previously (80). New Zealand White male rabbits were immunized with purified gBΔTM (80), and immunoglobulin G (IgG) fractions were purified by protein-A Sepharose 4B columns (Amersham Pharmacia Biotech, Piscataway, NJ). Nonspecific antibodies were removed by columns of cyanogen bromide-activated Sepharose 4B covalently coupled with purified glutathione S-transferase protein and BJAB cell lysate.
Antibodies and reagents.
The hybridoma cell line 4F2 (C13; anti-CD98) was obtained from the ATCC. The CD98 MAb secreted in the culture medium of hybridoma cells was purified by protein-A Sepharose affinity chromatography. Function-blocking mouse MAbs against integrins B3B11 (anti-β1; IgG1), P1E6 (anti-α2; IgG1), ASC-6 (anti-α3; IgG1), P1D6 (anti-α5; IgG3), LM609 (anti-αVβ3; IgG1), PiF6 (anti-αVβ5; IgG1), 10D6 (anti-αVβ6; IgG2a), and M-KID2 (ant-α3β1; IgG1) and rabbit polyclonal antibodies against integrins α1 (AB1934), α3 (AB1920), αV (AB1030), β3 (AB1932), and β5 (AB1926) were obtained from Chemicon International, Temecula, CA. Function-blocking MAb L230 (anti-αV; IgG1; ATCC HB-8448) was purchased from the ATCC. Rabbit polyclonal antibodies against β1 integrin (SC-8978), β6 integrin (SC-15329), and goat polyclonal CD98 (C-20) and anti-CD71 MAb were from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. Tetradecanoyl phorbol acetate was obtained from Sigma, St. Louis, MO. Rabbit anti-LAT1 polyclonal antibody was from Novus Biologicals, Littleton, CO. Anti-goat, anti-rabbit, and anti-mouse antibodies linked to horseradish peroxidase, alkaline phosphatase, fluorescein isothiocyanate, Alexa 488, Alexa 594, and Alexa 647 were purchased from KPL Inc., Gaithersburg, MD., or Molecular Probes, Eugene, OR. Protein A- and G-Sepharose CL-4B beads were from Amersham Pharmacia Biotech, Piscataway, NJ.
The rabbit anti-xCT peptide antibodies utilized in this work were raised against the following peptides of the xCT protein: P25-40 (CPSLGNKEPPGQEKVQL; intracellular), P66-77 (CPSLGNKEPPGQEKVQL; extracellular), P97-109 (CYAELGTTIKKSGG; intracelluar), P218-238 (TQNFKDAFSGRDSSIC; extracelluar), and P255-270 (VTEEVENPEKTIPLAIC; intracellular) (39). IgGs from the rabbit antisera were purified using protein-A Sepharose affinity chromatography. Human cellular fibronectin (120 kDa) was purchased from Upstate Biotechnology Inc. (Lake Placid, NY), and galectin 3 was from Sigma Chemical Co. (St. Louis, MO). Purified soluble α3β1, α5β1, αVβ3, and αVβ5 (n-octyl pyranoside preparation) and vitronectin were obtained from Chemicon International, Temecula, CA.
Radiolabeled-KSHV binding assay.
HMVEC-d were preincubated with CD98 antibody (10 μg), xCT peptide antibodies (10 μg), and control CD71 antibody (10 μg) at 4°C for 1 h. The cells were then washed three times with ice-cold phosphate-buffered saline (PBS) before the addition of [3H]thymidine-labeled, density gradient-purified KSHV (5,000 cpm). As a control, labeled KSHV was incubated with 100 μg of heparin/ml for 1 h at 37°C, added to HMVEC-d, and incubated for 90 min at 4°C. After incubation, the cells were washed five times and lysed with 1% sodium dodecyl sulfate (SDS) and 1% Triton X-100, and the radioactivity was precipitated with trichloroacetic acid and counted in a scintillation counter.
Measurement of KSHV internalization by real-time DNA PCR.
Target cells treated with antibody or untreated cells were infected with KSHV at 10 DNA copies (multiplicity of infection [MOI]) per cell. After 2 h of incubation, the cells were washed twice with PBS to remove the unbound virus, treated with 0.25% trypsin-EDTA for 5 min at 37°C to remove the bound but noninternalized virus, and washed. Total DNA was isolated from infected or uninfected cells using a DNeasy kit (Qiagen, Inc., Valencia, CA) as described previously (42). In each reaction, a total of 100 ng of DNA sample, KSHV ORF73 gene TaqMan probe, and QuantiTect PCR mixture were used. The KSHV ORF73 gene cloned in the pGEM-T vector (Promega) was used for the external standard. Known amounts of ORF73 plasmid were used in the amplification reactions, along with the test samples. The cycle threshold values were used to plot the standard graph and to calculate the relative copy numbers of viral DNA in the samples.
Nucleus isolation and KSHV DNA nuclear delivery assay.
HMVEC-d were preincubated with anti-CD98 antibody (10 μg), anti-xCT peptide antibodies (10 μg), and control CD71 antibody (10 μg) at 4°C for 1 h. Cells treated with antibody and untreated cells were infected with KSHV at 10 DNA copies per cell. To monitor the delivery of KSHV DNA to HMVEC-d nuclei, nucleus isolation was performed by using a Nuclei EZ isolation kit (Sigma) according to the manufacturer's recommendations. Briefly, cells infected with KSHV were collected after 2 h, washed, treated with trypsin-EDTA (0.25% trypsin and 5 mM EDTA) to remove noninternalized virus, and lysed on ice for 5 min with a mild lysis buffer (Sigma), and the nuclei were concentrated by centrifugation at 500 × g for 5 min. DNA was isolated from the nuclei using a DNeasy kit (Qiagen) as described previously (42). Internalized KSHV DNA was quantitated by amplification of the ORF73 gene by real-time DNA PCR (42).
Real-time RT-PCR.
Total RNA was isolated from infected or uninfected cells using an RNeasy kit (Qiagen) as described previously (42). ORF73 and ORF50 RNA expression was detected by real-time reverse transcription (RT)-PCR using gene specific real-time primers and specific TaqMan probes as described previously (42). The samples were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression.
GFP-KSHV infection.
Unless otherwise stated, cells were infected with five DNA copies per cell. The effects of anti-integrin antibodies and soluble integrins on KSHV infection were measured by using GFP-KSHV (75) according to procedures described previously (3, 49). Briefly, monolayers in eight-well chamber slides (Nalge Nunc International, Naperville, IL) were preincubated with anti-integrin MAbs at 4°C before incubation with GFP-KSHV at 37°C. A predetermined amount of GFP-KSHV was incubated with soluble human integrins, and the integrin-GFP-KSHV mixture was added to the cells. After 3 days, green fluorescent cells were examined under a fluorescence microscope and were counted using the Nikon Magna Firewire digital imaging system (3, 49).
Flow cytometric analysis.
Suspension cells (BJAB) and single-cell suspensions of adherent HMVEC-d, 293 cells, and HFF were washed and incubated with anti-α3β1, anti-αVβ3, anti-αVβ5, or anti-xCT antibodies at room temperature for 30 min. The cells were washed twice and incubated with Alexa 488-labeled anti-mouse or anti-rabbit secondary antibodies at room temperature for 30 min, washed again, and analyzed in a FACScan cytometer (Becton Dickinson, Bedford, MA) with appropriate gating parameters. Unstained cells, an isotype control, and cells incubated with secondary antibody alone were used for controls. To quantify the expression of integrins and xCT on the cells, the mean fluorescence intensity for each staining was measured.
Immunoprecipitation and Western blotting.
Target cells grown to confluence were serum starved by incubation with EBM2 for 8 h and infected with KSHV at an MOI of 10 for different times at 37°C, washed with PBS, and lysed in NP-40 buffer containing 15 mM NaCl, 1 mM MgCl2, 1 mM MnCl2, 2 mM CaCl2, 2 mM phenylmethylsulfonyl fluoride, and protease inhibitor mixture (Sigma). The lysates were clarified by centrifugation for 10 min at 4°C and normalized to equal amounts of total proteins. The lysate was incubated for 2 h with immunoprecipitating antibody at 4°C, and immune complexes were captured using 15 μl of protein G-Sepharose. They were washed three times with lysis buffer, boiled with SDS-polyacrylamide gel electrophoresis sample buffer, and run on a 10% gel. The proteins were transferred to nitrocellulose membranes, blocked, and then incubated with primary antibody overnight at 4°C. Species-specific horseradish peroxidase- and alkaline phosphatase-conjugated antibodies were used for secondary labeling. Immunoreactive bands were identified by enhanced chemiluminescence (68) according to the manufacturer's instructions. The bands were scanned and quantitated with the ImageQuanta software program (Molecular Dynamics).
Immunofluorescence assay.
The colocalization of CD98 with αVβ5 or CD98 with α3β1 integrin and its subunits α3 and β1 or xCT with α3β1 was detected using an immunofluorescence assay. HMVEC-d were serum starved and infected with KSHV at an MOI of 10 for 10 min or for different times at 37°C, followed by fixation with 4% paraformaldehyde for 15 min and permeabilized with 0.2% Triton X-100 in 1× PBS for 5 min at room temperature After being blocked for 45 min, the cells were processed for double immunostaining with antibodies against integrins, CD98, and xCT. This was followed by 1 h of incubation at room temperature with donkey anti-goat, goat anti-rabbit, or goat anti-mouse antibodies labeled with Alexa 594 or Alexa 488. Following washing with PBS, slides were mounted with mounting medium and examined under a Nikon fluorescence microscope equipped with a Metamorph digital imaging system.
Laser-scanning confocal immunofluorescence.
HMVEC-d infected with KSHV at an MOI of 10 were fixed with 4% paraformaldehyde. The cells were then permeabilized with 0.2% Triton X-100 for 5 min, washed, and blocked with bovine serum albumin (BSA) for 30 min. For triple-color confocal microscopy, the infected cells were stained with monoclonal anti-gpK8.1A antibody (91), followed by Alexa Fluor 488-labeled goat anti-mouse secondary antibody. For CD98 staining, the cells were incubated with anti-CD98 antibody, followed by a donkey anti-goat Alexa Fluor 594-labeled secondary antibody. For integrin staining, the cells were incubated with a polyclonal rabbit antibody, followed by goat anti-rabbit Alexa Fluor 647-labeled antibody. The Olympus Fluoview 300 fluorescence confocal microscope was used for imaging, and analysis was performed using Fluoview software (Olympus, Melville, NY).
Cell adhesion assay.
Target cell adhesion to KSHV gBΔTM or gBΔTM-RGA was performed as described previously (80). Briefly, Maxisorp enzyme-linked immunosorbent assay plates (Nunc, Roskilde, Denmark) were coated with 100 μl of fibronectin, vitronectin, gBΔTM, or gBΔTM-RGA overnight at 4°C in sterilized PBS (2 μg/ml). The plates were washed, blocked with 1% BSA, washed, collected with 0.05% trypsin-0.2% EDTA, washed, and resuspended in 0.1% BSA-serum-free Dulbecco's modified Eagle's medium (DMEM) (supplemented only with glutamine), and plated at 2 × 104 cells/well in 100 μl. The cells were then incubated at 37°C in a 5% CO2 atmosphere with 100% humidity for 45 min. The plates were washed, and the adherent cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. The stained cells were extracted with 0.1 M sodium citrate, and absorbance at 595 nm was measured with an enzyme-linked immunosorbent assay reader (Bio Kinetics Reader EL340; Bio-Tek Instruments, Winooski, VT). To test the effects of anti-integrin antibodies, the cells were incubated with a predetermined concentration of anti-integrin antibodies (10 μg/ml) in 0.1% BSA-serum-free DMEM for 45 min on ice before being seeded in KSHV gBΔTM-coated plates.
RESULTS
Cell adhesion induced by KSHV gBΔTM requires β1 and αV integrins.
The RGD sequence found in most extracellular matrix (ECM) proteins, such as fibronectin, collagen, and vitronectin, interacts with several different integrins, which forms the initial step of the outside-in signal cascade controlling various cell functions, such as gene expression, activation of FAK, activation of cytoskeleton elements, endocytosis, cell attachment, motility, cell cycle progression, cell growth, differentiation, and apoptosis (27, 56). We have shown previously that KSHV gB mediated the adhesion of HFF, HMVEC-d, and CV-1 cells via its RGD sequence (80), and here we examined the identity of the integrin(s) involved in this process. So far, nine integrins have been identified as mediating RGD-dependent interactions with integrins (in addition to non-RGD interactions); they are integrins α3β1, α5β1, α8β1, αVβ1, αVβ3, αVβ5, αVβ6, αVβ8, and αIIbβ3 (48, 56). Integrins α8β1, αVβ8, and αIIbβ3 were not analyzed in this study, since their expression is limited to certain cell types and there are no good commercially available antibodies against them (40, 48, 65).
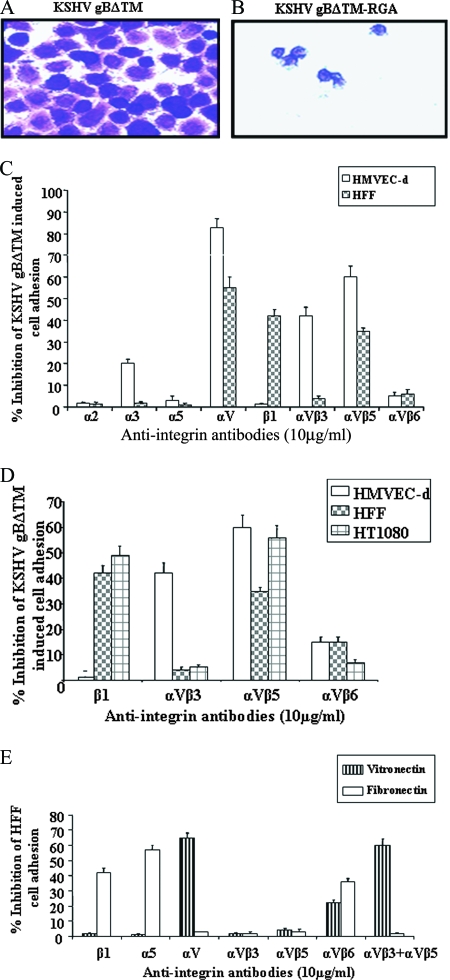
In wells coated with gBΔTM, we observed monolayers of spread out cells with clear cellular projections (Fig. 1A), and in contrast, only a few rounded cells were seen in gBΔTM-RGA-coated wells (Fig. 1B). When cells were mixed first with 10 μg of anti-integrin antibodies, varying levels of cell-type- and antibody-dependent inhibition of adhesion were observed (Fig. 1C and D). Anti-α3 antibody blocked about 20% of HMVEC-d adhesion to gBΔTM, and no inhibition was seen with HFF (Fig. 1C). No inhibition was observed with antibodies against RGD-dependent α5 or RGD-independent α2 integrins (Fig. 1C). In contrast, the anti-αV (L230) antibodies blocked 82% ± 4% of HMVEC-d and 56% ± 5% of HFF adhesion to gBΔTM (Fig. 1C). The function-blocking anti-β1 antibody blocked about 42% ± 3% and 50% ± 2% of HFF and HT1080 cell adhesion, respectively, and no effect was seen with HMVEC-d (Fig. 1D). The αVβ3 antibody did not block HFF binding but blocked HMVEC-d adhesion. This was not due to nonavailability or something unique about the αVβ3 in HFF, since anti-αV, a potent MAb, binds both αVβ3 and αVβ5 integrins and showed efficient inhibition of both in HMVEC-d and HFF adhesion. The variations could be due to the affinities and avidities of and sites recognized by the αVβ3 anti-integrin antibodies used.
FIG. 1.
Role of integrins in KSHV gB-mediated cell adhesion. (A and B) Maxisorp plates were coated with different concentrations of purified gBΔTM and gBΔTM-RGA in PBS (2 μg/ml; 100 μl/well) overnight at 4°C. The plates were washed and blocked with 1% BSA-PBS, and adhesion assays were performed (80). The adhering cells were fixed with 4% paraformaldehyde, washed, and stained with crystal violet. Shown is a photomicrograph of HFF adhering to gBΔTM and gBΔTM-RGA proteins. (C and D) Adhesion of HMVEC-d, HFF, and HT1080 cells to gBΔTM in the presence of anti-integrin antibodies. The cells were incubated with anti-integrin antibodies in 0.1% BSA-serum-free DMEM for 45 min on ice before being seeded onto gBΔTM-coated wells. The crystal violet-stained adhering cells were extracted with 0.1 M sodium citrate and quantified by measurement of the absorbance at 595 nm. Each experiment was done in duplicate, and each bar represents the average plus the standard deviation (SD) of three experiments. (E) Specificities of anti-integrin antibodies inhibiting cell adhesion. HMVEC-d, HFF, and HT1080 cells were incubated with anti-integrin antibodies (10 μg/ml) for 45 min on ice before being seeded in fibronectin- or vitronectin-coated wells. The results with HFF are shown. Each experiment was done in duplicate, and each bar represents the average plus SD of three experiments.
To identify the involved αV integrin, three function-blocking antibodies against integrins αVβ3, αVβ5, and αVβ6 were used. Anti-αVβ3 antibodies inhibited about 42% ± 3% of HMVEC-d adhesion, and no effect was seen on the adhesion of HFF and HT1080 cells (Fig. 1D). In contrast, anti-αVβ5 antibody blocked HMVEC-d (60% ± 5%), HFF (37% ± 1%), and HT1080 cell (56% ± 5%) adhesion (Fig. 1D). Only about 5 to 10% inhibition was seen with anti-αVβ6 antibodies (Fig. 1C and D). The combination of anti-αVβ3 and anti-αVβ5 antibodies inhibited about 80% of HFF and HMVEC-d adhesion to gBΔTM, while combinations of anti-αVβ6 and anti-αVβ5 had no additive effect (data not shown).
To ascertain the specificities of the anti-integrin antibodies used, we tested their abilities to block HFF adhesion to fibronectin and vitronectin. Human fibroblasts adhere to the 40-kDa fragment of fibronectin via integrin α4β1 independently of the RGD motif and to the 120-kDa fibronectin fragment via integrin α5β1 in an RGD-dependent way (20, 21). Similarly, anti-β1 and anti-α5 antibodies blocked HFF adhesion to fibronectin. To adhere to vitronectin, human fibroblasts use both integrins αVβ3 and αVβ5 and can be blocked only by a combination of anti-αVβ3 and anti-αVβ5 antibodies (19). Individually, antibodies against αVβ3 and αVβ5 did not block HFF adhesion to fibronectin (Fig. 1E), and in contrast, the combination of anti-αVβ3 and anti-αVβ5 antibodies blocked about 60% of HFF adhesion to vitronectin, a level similar to that of the anti-αV antibody L230 (Fig. 1E). Anti-αVβ6 blocked cell adhesion to both fibronectin and vitronectin (Fig. 1E), confirming that integrin αVβ6 acts as a receptor for both fibronectin and vitronectin (56, 85). These data show that the antibodies used in this study are function blocking and that our data are reliable.
KSHV infection of endothelial cells and fibroblasts is blocked by antibodies against αV, β1, and α3 integrins.
In our previous studies, we used several available antibodies against a range of RGD and non-RGD binding integrin molecules and reported the abilities of anti-α3, -β1, and -α3β1 integrin antibodies to block the KSHV infection of HMVEC-d and HFF (3). However, anti-α3 and -β1 antibodies, as well as soluble α3β1 integrin, did not completely block viral infection. Moreover, in experiments with CHO-B2 cells and CHO-B2-B3 cells expressing human α3 integrins, even though CHO-B2-B3 cells were four times more permissive to KSHV infection than CHO-B2 cells, infection was still threefold less than with HMVEC-d and HFF (3). These results suggested that there are other receptors besides α3β1 integrin for KSHV infection.
Since several viruses utilize multiple integrins for target cell infection, in addition to the previously tested anti-integrin antibodies, we tested the abilities of a new panel of commercially available function-blocking antibodies against several RGD and non-RGD binding integrin molecules. Among the antibodies tested, anti-αV antibody (L230) inhibited KSHV infectivity by about 40 to 48% in HMVEC-d and HFF (Fig. 2A), a level similar to the inhibition observed with anti-α3 or anti-β1 antibodies (Fig. 2A) (3). Similar isotype-specific antibodies against α5 and αVβ6 (Fig. 2A), as well as α1, α6, and β4, did not show (data not shown) any significant inhibition of KSHV infectivity. KSHV infection of HMVEC-d was significantly (P < 0.01) blocked by anti-αVβ3 antibodies (38%) and by anti-αVβ5 (28%) antibodies (Fig. 2A). Although anti-αVβ5 antibodies blocked HFF infection by about 18%, no significant reduction in KSHV infectivity in HFF was observed with anti-αVβ3 antibodies (Fig. 2A). However, when we used a combination of equal concentrations of anti-αVβ5 and anti-αVβ3 antibodies, about 50% reduction in KSHV infection of HFF was observed (Fig. 2A). This is similar to the effect observed with these antibodies in adenovirus 2 infection (86). When used individually, these antibodies affected adenovirus 2 infectivity minimally; however, when used in combination, virus infectivity was reduced by more than 50% (86). These results clearly show that, in addition to α3β1 integrins, αVβ3 and αVβ5 also play roles in the infectious process of KSHV.
FIG. 2.
Inhibition of KSHV infection by anti-integrin antibodies and soluble integrins. (A) Inhibition of GFP-KSHV infection by anti-integrin antibodies. Monolayers of HMVEC-d and HFF were incubated with 10 μg/ml of MAbs against integrins or integrin subunits for 1 h at 4°C, washed, and infected with GFP-KSHV at an MOI of 5 DNA copies/cell. After incubation for 2 h at 37°C, the cells were washed and incubated for 72 h, and the number of GFP-positive cells was estimated under a fluorescence microscope. Each experiment was done in duplicate, and each bar represents the average plus the standard deviation (SD) of three experiments. (B) Effects of anti-integrin antibodies on KSHV binding. HFF were incubated with anti-integrin antibodies (10 μg/ml) at 4°C for 90 min and incubated with a predetermined concentration of [3H]thymidine-labeled purified KSHV. The labeled KSHV was also mixed with 10 μg/ml of heparin or soluble integrins for 90 min at 4°C and then added to the cells. After incubation for 90 min at 4°C with the virus, the cells were washed, lysed, precipitated with trichloroacetic acid, and counted. The bound virus cpm in the absence of any treatment was considered to be 100% and was used to calculate the percentage inhibition of virus binding. Each reaction was done in triplicate, and each bar represents the average plus the SD of three independent experiments. The results with HFF are shown. (C) Inhibition of KSHV infection by soluble integrins. KSHV (5 DNA copies/cell) was mixed with 10 μg/ml of heparin or 10 μg/ml soluble integrins for 2 h at 37°C and added to the cells. After 2 h, the cells were washed to remove the unbound virus, treated with 0.25% trypsin-EDTA for 5 min at 37°C to remove the bound but noninternalized viruses, and washed, and total DNA was isolated. The number of KSHV ORF73 copies was estimated by real-time DNA PCR. The cycle threshold values were used to plot the standard graph and to calculate the relative copy numbers of viral DNA in the samples. The data are presented as percentages of inhibition of KSHV DNA internalization obtained when the cells were incubated with the virus alone. Each reaction was done in duplicate, and each bar represents the average ± SD of three experiments. (D) KSHV was mixed with 10 μg/ml soluble integrin combinations for 2 h at 37°C and added to HFF. After 2 h, the cells were washed to remove the unbound virus and treated with 0.25% trypsin-EDTA for 5 min at 37°C to remove the bound but noninternalized viruses, and the internalized viral DNA was estimated by real-time DNA PCR. Each reaction was done in duplicate, and each bar represents the average ± the SD of three experiments.
KSHV interactions with αVβ3, αVβ5, and α3β1 integrins do not affect virus binding to the target cells.
To determine the roles of αVβ3 and αVβ5 integrins in KSHV infection, [3H]thymidine-labeled KSHV binding to target cells was examined. Similar to our previous observation, heparin inhibited 95% of KSHV binding to HFF, while none of the anti-integrin antibodies or soluble integrins inhibited KSHV binding to HFF (Fig. 2B). Similar observations were also made with HMVEC-d (data not shown). These data clearly show that αVβ3 and αVβ5 integrins play very limited roles in the initial binding of KSHV to target cells, indicating a role in the post-cell attachment stage of infection.
Soluble α3β1, αVβ3, and αVβ5 block KSHV infection.
We next tested the ability of soluble integrin to block KSHV entry in four adherent target cells (HMVEC-d, HFF, 293, and Vero). We used the commercially available human soluble α3β1, αVβ3, αVβ5, and α5β1 integrins, which are nontoxic up to 20-μg/ml concentrations in 10 mM n-octyl-β-d-glucopyranoside formulation. KSHV preincubated with soluble integrins was added to the target cells and incubated at 37°C for 2 h, unbound virus was removed, and the cells were treated with trypsin-EDTA to remove the bound but noninternalized viruses. The internalized KSHV genome copies were quantified by a real-time PCR method (42). Preincubation of virus with heparin blocked >85% of KSHV entry in all target cells (Fig. 2C), thus demonstrating that HS must be the initial contact receptor for these cells, concentrating virus on the cell surfaces. As seen in experiments with anti-integrin antibodies, variable levels of KSHV entry, ranging from 35 to 60%, were neutralized by preincubating virus with 10 μg of soluble α3β1, αVβ3, and αVβ5 integrins (Fig. 2C). These inhibitions were statistically significant, and in contrast, soluble integrin α5β1 did not show any significant effect on KSHV entry (Fig. 2C). About 35%, 40%, and 49% inhibitions of viral DNA entry were observed in HMVEC-d when virus was pretreated with α3β1, αVβ3, and αVβ5 integrins, respectively. When the virus was pretreated with a combination of integrins (10 μg), an increase of α3β1 inhibition from about 40% to 53%, in combination with αVβ3, and to 51% with αVβ5 integrins was observed (Fig. 2D). A maximum cumulative inhibition of about 63% was observed with an αVβ3 and αVβ5 combination (Fig. 2D). The increase in neutralization when integrins were used in combination was small and not cumulative. Though this was surprising, since both integrin complexes are involved in the binding, it could be due to the integrin concentrations used and competition among the integrins to bind RGD and non-RGD domains of KSHV glycoproteins, as well as to the use of other, alternative receptors by KSHV. Nevertheless, these results further verified that, along with α3β1, αVβ5 and αVβ3 integrins also play roles in adherent target cell infection by KSHV.
CD98hc associates with αVβ5, αVβ3, β1, and α3β1 integrins in KSHV-infected HMVEC-d in a time-dependent manner.
The amino acid transporter xCT is one of six light-chain molecules that are covalently linked to the membrane glycoprotein 4F2hc/CD98, which has been shown to be associated with β1 and α3β1 integrins, forming functional complexes on the cell surface and modulating integrin-dependent functions (10, 15, 16, 41). CD98 has also been reported to be involved in cross-linking and the signal transduction cascade of αVβ3 integrin (38). Here, we designed experiments to determine (i) whether KSHV interacts with CD98 during infection of endothelial cells (HMVEC-d), (ii) whether integrins play a role in this interaction, (iii) the identities of the interacting integrins, and (iv) the kinetics of these interactions.
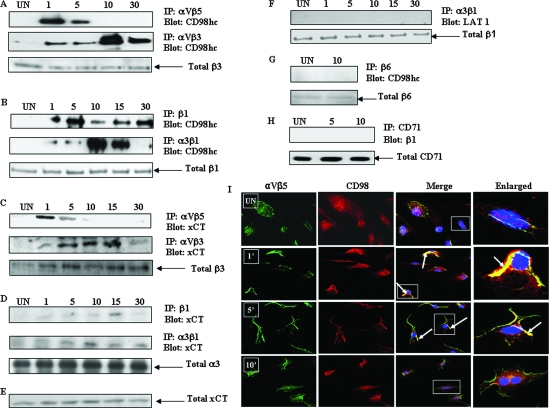
To determine whether CD98/xCT associates with integrins, we infected HMVEC-d with KSHV for various times and lysed them under mild conditions with NP-40-containing buffer, and the cell lysates were immunoprecipitated with anti-αVβ5, -αVβ3, -β1, and -α3β1 antibodies, followed by blotting with anti-CD98hc antibodies. We observed a maximum association of CD98 with αVβ5 integrin as early as 1 min postinfection (p.i.), which was reduced considerably at 5 min p.i., and no association was seen at 10 min and 30 min p.i. (Fig. 3A, top). Immunofluorescence analysis also showed a strong association of CD98 with αVβ5 at 1 min p.i., which was reduced at 5 min p.i. and completely dissociated at 10 min p.i. (Fig. 3I). In contrast, the association between αVβ3 and CD98 was observed as early as 1 min p.i. and increased to a maximum at 10 min p.i., and the strong association was detectable even at 30 min p.i. (Fig. 3A, middle). Equal loading of samples in the immunoprecipitates was confirmed by the detection of an equal quantity of total β3 integrin (Fig. 3A, bottom). The CD98hc-β1 integrin association was first evident at 1 min p.i., had increased by 5 min p.i., and was observed until 30 min p.i. (Fig. 3B, top). The interaction of CD98hc with α3β1 integrin was observed as early as 1 min p.i., and the extent of coimmunoprecipitation increased in a time-dependent manner, with a maximum at 10 min p.i.; continued to be detected at 15 min p.i.; and then returned to basal level at 30 min p.i. (Fig. 3B, middle). Equal loading of samples in the immunoprecipitates was confirmed by the detection of an equal quantity of total β1 integrin (Fig. 3B, bottom).
FIG. 3.
KSHV interactions with integrins and CD98/xCT molecules. (A, B, C, and D) Serum- starved HMVEC-d were uninfected (UN) or infected with KSHV at an MOI of 10 DNA copies/cell for different time periods (minutes). Infected and uninfected cells were lysed with NP-40-containing buffer, and the protein complexes were immunoprecipitated. (A) CD98hc coimmunoprecipitates with αVβ5 and αVβ3 integrins in KSHV-infected HMVEC-d. Shown is immunoprecipitation (IP) with anti-αVβ5 integrin antibody (top) or anti-αVβ3 integrin antibodies (middle). The immunoprecipitated proteins were Western blotted and probed with anti-CD98hc antibodies. The immunoprecipitated blot was stripped and reprobed with anti-β3 integrin antibody (bottom). (B) CD98hc coimmunoprecipitates with β1 and α3β1 integrins in KSHV-infected HMVEC-d. Shown is IP with anti-β1 integrin (top) or anti-α3β1 integrin antibody (middle). The immunoprecipitates were Western blotted and probed for CD98hc. Equal quantities of total cell lysates were probed with anti-β1 antibody (bottom). (C) xCT coimmunoprecipitates with αVβ5 and αVβ3 integrins in KSHV-infected HMVEC-d. Infected and uninfected cell lysates were immunoprecipitated with anti-αVβ5 integrin antibody (top) or anti-αVβ3 integrin antibody (middle). The immunoprecipitated proteins were Western blotted and probed with xCT antibodies. The blot was stripped and reprobed with anti-β3 integrin antibody (bottom). (D) xCT coimmunoprecipitates with β1 and α3β1 integrins in KSHV-infected cells. Shown is IP with β1 (top) or α3β1 (middle) integrin antibody. The immunoprecipitates were blotted and probed with anti-xCT antibody. The immunoprecipitated blot was stripped and reprobed with anti-α3 antibody (bottom). (E) Western blot analysis of xCT expression in HMVEC-d. Equal quantities of total cell lysates of the samples in Fig. 1D were subjected to SDS- polyacrylamide gel electrophoresis followed by blotting with anti xCT antibody. (F) There is no association between α3β1 integrin and LAT1 in KSHV-infected cells. Infected and uninfected HMVEC-d were lysed, and the lysates were immunoprecipitated using specific anti-α3β1 integrin antibodies. The immunoprecipitated proteins were blotted and probed with anti-LAT1 antibodies (top). Equal quantities of total cell lysates were probed with anti-β1 antibody (bottom). (G) CD98 does not coimmunoprecipitate with β6 integrin. Serum-starved HMVEC-d infected with KSHV for 5 min and 10 min were immunoprecipitated with anti-β6 integrin antibody and blotted for CD98hc (top). Equal quantities of total cell lysates were probed with anti-β6 integrin antibody (bottom). (H) β1 integrin does not coimmunoprecipitate with CD71. Serum-starved HMVEC-d were infected with KSHV for 10 min. Uninfected and infected cell lysates were immunoprecipitated using anti-CD71 antibody and blotted for β1 integrin (top). Equal quantities of total cell lysates were probed with anti-CD71 antibodies (bottom). (I) Immunofluorescence analysis of CD98 association with αVβ5 integrin. HMVEC-d were infected with KSHV at an MOI of 10 for 1 min, 5 min, and 10 min. The cells were fixed in 2% paraformaldehyde for 15 min, permeabilized with 0.2% Triton X-100 for 5 min, and blocked. Infected and uninfected cells were stained with CD98 and αVβ5 antibodies. CD98 was visualized by incubation with Alexa 594-labeled secondary antibody (red), and αVβ5 integrin was visualized by Alexa 488-conjugated secondary antibody (green). In the merged column, yellow shows the colocalization of integrins with CD98. Areas with colocalizing proteins are indicated by arrows. The boxed areas are enlarged in the rightmost column.
These results (i) suggested that αVβ5, αVβ3, α3β1, and CD98 are components of a multimeric receptor complex formed during the early stages of KSHV infection in HMVEC-d, (ii) indicated temporal interactions between KSHV and these molecules, (iii) suggested that the association of each of the integrins might take place in a temporally coordinated manner, and (iv) suggested that in the absence of one set of integrins, KSHV can still associate with another integrin molecule. The roles of αVβ3 and αVβ5 in KSHV infection and their association with viral glycoproteins and signal molecules are currently under investigation.
The xCT molecule associates with αVβ5, αVβ3, β1, and α3β1 integrins during KSHV infection of HMVEC-d.
Since xCT is the light-chain component of the CD98 molecule and has been identified as a receptor mediating KSHV cell entry in the adherent target cells, we examined the possible association of αVβ5, αVβ3, β1, and α3β1 integrins with the xCT molecule. When lysates from infected HMVEC-d were immunoprecipitated with αVβ5 or αVβ3 MAbs and probed, the xCT molecule coimmunoprecipitated with the integrins in a time-dependent manner (Fig. 3C, top and middle). The time course of association was very similar to that seen for the association of CD98hc with the integrins αVβ5 and αVβ3. Equal amounts of protein in the samples were confirmed by stripping and reprobing of the membrane with an antibody against β3 (Fig. 3C, bottom). Immunoprecipitation with β1 or α3β1 and blotting for xCT also showed an immunoprecipitation profile similar to that seen for the association of β1 and α3β1 with CD98hc (Fig. 3D, top and middle). There was no change in the total α3 levels (Fig. 3D bottom). When the expression of xCT in these samples was tested by Western blotting of the lysates with xCT-specific antibody, no change in xCT expression was observed in any of the samples (Fig. 3E). No detectable association of α3β1 integrin with LAT1, another light-chain subunit of CD98, was observed (Fig. 3F, top). This suggested that KSHV interacts during infection of HMVEC-d with the CD98-xCT light-chain complex that is also associated with the αVβ5, αVβ3, and α3β1 integrins.
CD98/xCT-α3β1 integrin association in HMVEC-d during KSHV infection is specific.
To assess the specificity of CD98/xCT binding to β1 integrins and to determine whether β1 integrin associates with other membrane proteins, two sets of experiments were carried out. Since we observed the association of all three integrins at 5 min p.i. and maximum association of two integrins at 10 min p.i., we infected the cells for 5 min and 10 min. In one experiment, we immunoprecipitated the infected and uninfected cell lysates using anti-β6 integrin antibody and blotted for CD98hc. In another set of experiments, we performed immunoprecipitation with an antibody against CD71, a plasma membrane transferrin receptor protein, and probed for β1 integrin. To ensure equal amounts of protein loading, the membranes were stripped and reprobed with antibodies against β6 integrin (Fig. 3G, bottom) and CD71 (Fig. 3H, bottom) in the respective blots. In these studies, we did not observe any association between CD98 and β6 integrin (Fig. 3G, top) or between CD71 and β1 integrin (Fig. 3H, top) during KSHV infection, suggesting that the observed interactions between CD98 and β1 integrins during KSHV infection are specific.
Varying distributions of integrins and xCT in different cell types.
We next utilized flow cytometry to analyze the cell surface expression and distribution densities of xCT and integrin α3β1, αVβ3, and αVβ5 molecules in HFF, HMVEC-d, and HEK 293, HT1080, and BJAB cells. As shown in Table 1, both α3β1 and xCT molecules were expressed in >90% of human adherent HFF and HMVEC-d and 293 cells. Integrin αVβ3 was expressed in more than 90% of HFF, while its expression was lower in HMVEC-d and 293 cells. About 60% of 293 cells expressed moderate levels of αVβ5 integrin, and lower levels were expressed in HMVEC-d and HFF. In contrast, in the nonadherent human B-cell line (BJAB), we observed only 30% of cells expressing α3β1 and xCT molecules, while αVβ3 and αVβ5 integrins were barely detectable (Table 1). More than 90% of HT1080 cells expressed α3 integrin, but the expression of αVβ3 and αVβ5 integrins was very low. These results suggest that the presence and distribution of KSHV receptors may vary between different human adherent and nonadherent cells. Since both α3β1 integrins and CD98/xCT serve as receptors for KSHV, we evaluated further the association between these receptors in KSHV-infected cells.
TABLE 1.
Flow cytometric analysis of integrins and xCT molecule expression in different cell types
| Integrin or xCT | Distributions (%) and levels (MFI) on target cellsa
|
||||
|---|---|---|---|---|---|
| HMVEC-d | HFF | 293 | BJAB | HT1080 | |
| α3 | 94 (41) | 98 (118) | 92 20) | 31 (47) | 99 (1070) |
| α3β1 | 93 (53) | 98 (161) | 93 (28) | 30 (44) | ND |
| αVβ3 | 32 (28) | 95 (46) | 15 (16) | 1.17 (3) | 5 (261) |
| αVβ5 | 6 (40) | 33 (26) | 61 (11) | <1 | 12 (260) |
| xCT | 93 (139) | 96 (451) | 97 (191) | 28 (539) | ND |
Single cell suspensions of BJAB, HMVEC-d, HFF, and 293 cells were first incubated with antibodies against various integrins and xCT molecules, followed by Alexa 488-conjugated secondary antibodies, and examined by fluorescence-activated cell sorting. The table shows the percentages of fluorescent cells positive for integrins and xCT, while the Mean Fluorescence Intensity (MFI) values as an indication of expression levels are given in parenthesis. ND: not done.
CD98 and xCT molecules colocalize with α3β1, α3, and β1 during KSHV infection of HMVEC-d.
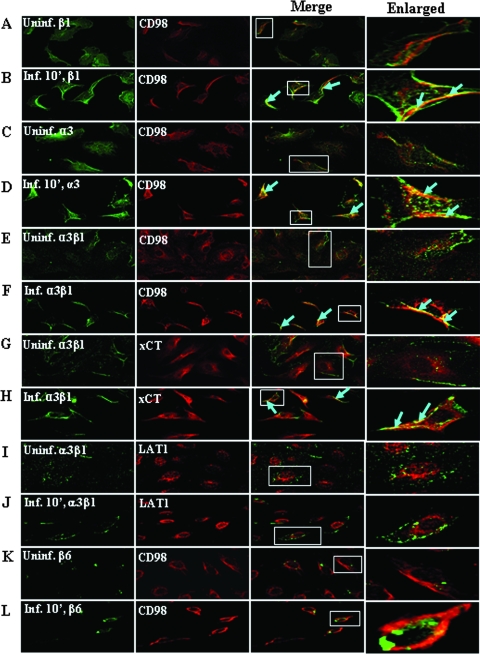
To further demonstrate the physical association between α3β1 integrin and CD98/xCT, HMVEC-d infected for 10 min were examined for the colocalization of CD98 with α3β1, α3, and β1 or xCT with α3β1 using anti-α3, -β1, -α3β1, -CD98, or -xCT antibodies. As can be seen in Fig. 4, compared to the uninfected cells, CD98 colocalized with β1 (Fig. 4A and B), α3 (Fig. 4C and D), and α3β1 (Fig. 4E and F) integrins in the infected cells. We also detected the colocalization of xCT and α3β1 integrin in the infected cells (Fig. 4G and H). Since β1 integrin heterodimers lie in close proximity to CD98 family members, we have observed a very moderate colocalization in the uninfected control cells. However, compared to the uninfected control, we always observed a strong colocalization of these molecules in the infected cells due to KSHV interactions with these molecules, forming the multimolecular complex.
FIG. 4.
Colocalization of CD98/xCT with α3, β1, and α3β1 integrins in KSHV-infected HMVEC-d. Serum-starved HMVEC-d were infected with KSHV at an MOI of 10 at 37°C for 10 min. Uninfected and infected cells were fixed with 4% paraformaldehyde in PBS for 30 min. The cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min, blocked, and then incubated with primary antibodies (anti-β1, anti-α3, anti-α3β1, anti-CD98, anti-β6, anti-xCT, or anti-LAT1) for 1 h at room temperature. The staining was visualized by incubation with Alexa 594-labeled anti-goat antibody (red) for CD98, Alexa 594-labeled anti-rabbit antibody (red) for xCT and LAT1, and anti-mouse Alexa 488-conjugated secondary antibody (green) for integrins. (A and B, C and D, E and F) Colocalization of β1, α3, and α3β1 integrins (green) with CD98 (red) in uninfected and infected cells, respectively. (G and H) Colocalization of α3β1 (green) with xCT (red) in uninfected and infected cells. (I and J) Colocalization of α3β1 (green) with LAT1 (red) in uninfected and infected cells. (K and L) Colocalization of β6 (green) with CD98 (red) in uninfected and infected cells. In the merged panel, yellow shows the colocalization of integrins with CD98/xCT. Areas with colocalizing proteins are indicated by arrows. Magnification, ×40. The boxed areas are enlarged in the rightmost column.
Together with the immunoprecipitation studies, these results clearly demonstrated the occurrence of a physical association between α3β1 integrin and CD98/xCT during KSHV infection. No colocalization was observed when the uninfected and infected cells were stained for α3β1 and LAT1 (another light-chain subunit of CD98) (Fig. 4I and J) and for CD98 and β6 integrin antibodies (Fig. 4K and L). These control experiments confirmed the observation that a specific association exists between CD98/xCT and α3β1 integrins during the early stages of KSHV infection of HMVEC-d.
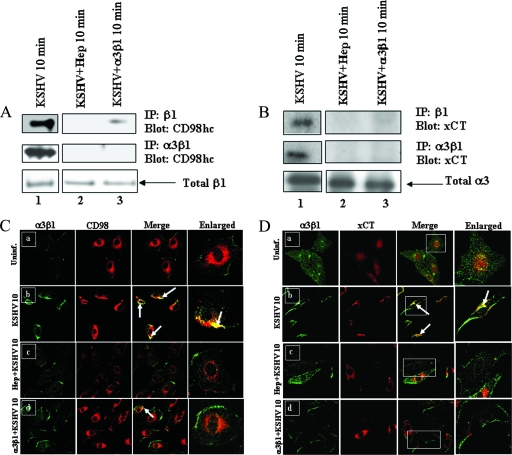
Preincubation of KSHV with soluble heparin and α3β1 blocks virus-induced association of α3β1 and CD98/xCT molecules.
KSHV binds initially to HS molecules in HFF and HMVEC-d and then to the α3β1 integrin (3). To determine whether interactions with HS and α3β1 integrin are prerequisites for KSHV interactions with CD98/xCT, KSHV at an MOI of 10 was preincubated with soluble heparin or α3β1 at 37°C for 1 h before infection of HMVEC-d for 10 min, a time point at which maximum interaction was observed (Fig. 3), and the cell lysates were utilized for immunoprecipitation with β1 or α3β1 antibodies. As shown in Fig. 5A, lane 2 (top and middle), heparin strongly blocked the interaction of CD98hc with both β1 and α3β1 integrins. When cells were infected with soluble-α3β1-treated virus, although more than 90% of the association between β1 integrin and CD98hc was blocked, we could still detect a trace amount of CD98hc (Fig. 5A, lane 3, top). This shows that, in addition to α3β1, other β1 integrin heterodimers may also be involved in the CD98 association. However, the association between α3β1 and CD98hc was completely blocked by α3β1-treated virus (Fig. 5A, lane 3, middle), suggesting that the interaction of KSHV with α3β1 is essential for the association between α3β1 and CD98. Similar patterns of inhibition were also observed for the interaction between β1 integrin and xCT (Fig. 5B, top), as well as α3β1 and xCT (Fig. 5B, middle), in both heparin-treated and α3β1-treated virus.
FIG. 5.
Soluble α3β1 and heparin inhibit CD98/xCT association with integrins in KSHV-infected HMVEC-d. Serum-starved HMVEC-d were infected for 10 min with untreated KSHV, KSHV preincubated (37°C for 1 h) with 100 μg heparin sulfate, or KSHV preincubated (37°C for 1 h) with 10 μg of soluble α3β1 at an MOI of 10. (A and B) Cell lysates were immunoprecipitated with anti-β1 integrin antibody (top) or anti-α3β1 integrin antibody (middle). The immunoprecipitated proteins were Western blotted and probed for CD98hc (A) or for xCT (B). The membranes were reprobed with anti-β1 integrin antibodies (A, bottom), and equal quantities of total cell lysates were probed with anti-α3 integrin antibodies (B, bottom). (C and D) Confocal analysis of α3β1 colocalization with CD98/xCT in KSHV-infected cells. Serum-starved HMVEC-d were infected with untreated KSHV, KSHV preincubated (37°C for 1 h) with 10 μg of soluble α3β1, or KSHV preincubated (37°C for 1 h) with 100 μg heparin for 10 min at an MOI of 10. Uninfected and infected cells were fixed with paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked, and incubated with anti-CD98, anti-xCT, or anti-α3β1 primary antibodies. The cells were then stained with Alexa 488-labeled secondary antibodies for α3β1 (green) or Alexa 594-labeled secondary antibodies for CD98 (red) or xCT (red). (C) Colocalization of α3β1 (green) with CD98 (red) in uninfected (Uninf.) (a), untreated KSHV-infected (b), heparin-treated KSHV-infected (c), and soluble-α3β1-treated KSHV-infected (d) cells. (D) Colocalization of α3β1 (green) with xCT (red) in uninfected (a), untreated KSHV-infected (b), heparin-treated KSHV-infected (d), and soluble-α3β1-treated KSHV-infected (c) cells. The arrows indicate colocalization of proteins. Magnification, ×60. The boxed areas are enlarged in the rightmost column.
To confirm the effects of soluble-HS- and α3β1-treated virus, colocalization of CD98 with α3β1 was examined by confocal immunofluorescence microscopy. HMVEC-d were infected for 10 min using untreated KSHV or virus pretreated with soluble HS and α3β1. When colocalizations of CD98, xCT, and α3β1 integrin were compared, we observed a drastic reduction in the specific colocalization of CD98 and xCT with α3β1 integrin in cells infected with HS- or α3β1-treated virus compared to the cells infected with untreated virus (Fig. 5C, a to d, and D, a to d). These results further supported the findings in Fig. 5A and B and suggested that the initial attachment of the virus to HS and the subsequent interaction with α3β1 are essential for efficient association of α3β1 and CD98/xCT in KSHV-infected cells.
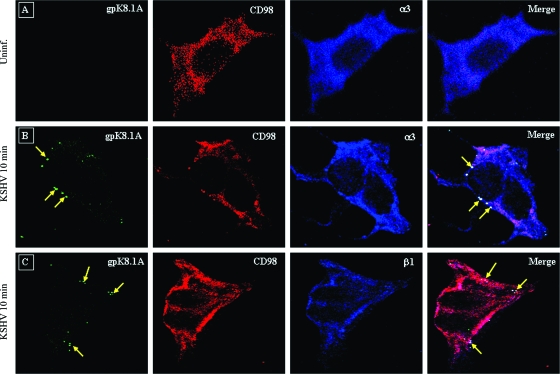
CD98 and α3β1 colocalize with KSHV during the early stages of infection of HMVEC-d.
We performed triple-color immunofluorescence confocal microscopy to study the colocalization of receptors with KSHV during infection. HMVEC-d infected with KSHV at an MOI of 20 for 10 min were immunostained with antibodies against KSHV envelope glycoprotein gpK8.1A, along with anti-CD98 and α3/β1 integrin antibodies, so that the colocalization of KSHV with both receptors could be visualized in the same samples. When the individual color channels were merged, the areas of triple-color colocalization stained white. These studies showed a very clear colocalization of CD98 and α3 integrin with most of the KSHV virions (Fig. 6B). A similar pattern of colocalization was also observed with anti-β1 integrin antibodies (Fig. 6C). In the absence of virus infection, no gpK8.1A staining and colocalization with receptors was observed (Fig. 6A). Taken together, our results confirmed the specific interaction of KSHV with α3β1 and CD98/xCT receptors during the early stages of infection of HMVEC-d.
FIG. 6.
Confocal microscopic analysis of the interaction of KSHV with CD98 and α3/β1 integrin in infected cells. HMVEC-d were infected with KSHV at an MOI of 10 at 37°C for 10 min. The cells were fixed in 4% paraformaldehyde, permeabilized, and blocked. Triple-color confocal microscopy was performed on the uninfected (Uninf.) (A) and infected cells using anti-gpK 8.1A antibody for the detection of virus and anti-CD98 and anti-α3 (B) or anti-β1 (C) antibody for the detection of receptors. gpK8.1A was visualized with secondary antibodies conjugated to Alexa 488 (green), CD98 was visualized with Alexa 594 (red), and α3 or β1 integrin was visualized with Alexa 647 (blue). The overlays show the association of KSHV with CD98 and α3 or β1 integrin. The arrows indicate virus and the site of association of virus with CD98 and α3 or β1 integrin. Magnification, ×80.
Natural ligand of α3β1 induces the association between CD98 and α3β1 molecules.
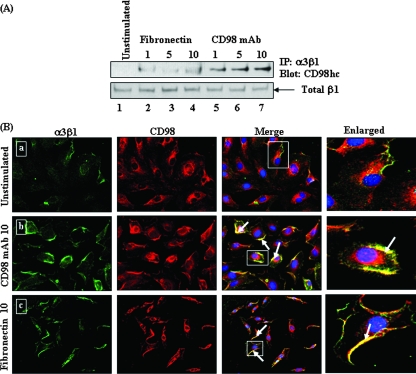
ECM proteins, such as laminin, fibronectin, and collagen, bind to α3β1 and induce integrin clustering and downstream signaling events (14, 46). To determine whether the natural ligands binding to α3β1 lead to specific interactions between CD98/xCT and α3β1, as seen in KSHV infection, we incubated HMVEC-d with 10 μg/ml of fibronectin for different times, and the cell lysates were immunoprecipitated with α3β1 antibodies, followed by immunoblotting with CD98 antibody. Our results showed a time-dependent increase in the association between CD98 and α3β1 at 1 min, 5 min, and 10 min post-fibronectin treatment (Fig. 7A, top, lanes 2 to 4), which was similar to the association induced by KSHV in the infected cells.
FIG. 7.
Effects of fibronectin and CD98 MAb on α3β1-CD98 association in HMVEC-d. (A) HMVEC-d were left unstimulated (top, lane 1), stimulated with fibronectin (10 μg/ml) for different time periods (minutes) (top, lanes 2 to 4), or stimulated with anti-CD98 MAbs (10 μg/ml) for different time periods (top, lanes 5 to 7). The cells were lysed, and the lysates were immunoprecipitated by anti-α3β1 integrin antibody and then blotted with CD98hc antibody. Loading was verified by blotting with total β1 integrin (bottom). (B) Effects of fibronectin and CD98 MAb on an α3β1-CD98 colocalization immunofluorescence assay in HMVEC-d. Cells unstimulated (a) or stimulated with CD98 MAb (10 μg/ml) (b) or with fibronectin (10 μg/ml) (c) were incubated with antibodies against α3β1 and CD98, and the staining was observed by incubation with Alexa 488 (green) for α3β1 and Alexa 594 (red) for CD98. The arrows indicate colocalization of activated α3β1 with CD98. Magnification, ×40. The boxed areas are enlarged in the rightmost column.
Incubation of HMVEC-d with anti-CD98 antibody induces association between CD98 and α3β1 molecules.
Since cross-linking of CD98 molecules by anti-CD98 antibodies has been shown to induce clustering of α3β1 integrins (15, 41, 62), we next evaluated the association of CD98 with α3β1 in HMVEC-d stimulated with anti-CD98 MAbs. Cell lysates from unstimulated cells and cells stimulated with CD98 MAb for different times were immunoprecipitated with α3β1 antibody and probed for the presence of CD98 by immunoblotting. The CD98 MAb stimulated the association between CD98 and α3β1, and a time-dependent increase in the association between CD98 and α3β1 was observed at 1 min, 5 min, and 10 min after exposure to the MAb (Fig. 7A, top, lanes 5 to 7). There was no change in total β1 levels (Fig. 7A, bottom).
To confirm this result, HMVEC-d incubated in the presence of CD98 MAb or fibronectin were stained with CD98 and anti-α3β1 antibody. As shown in Fig. 7B, compared to the untreated cells (Fig. 7B, a), CD98 and α3β1 were colocalized at the cell periphery in both anti-CD98 antibody (Fig. 7B, b)- and fibronectin (Fig. 7B, c)-treated cells. These results suggested that cross-linking of CD98 by antibody or α3β1 ligand triggers an association between CD98 and α3β1 in HMVEC-d, and KSHV infection probably mimics this induction, recruiting CD98 to the protein complex containing α3β1 and other integrins, thereby leading to the physical association of the various receptors. The presence of CD98 in the complex may be critical for enhancement of the association between the receptors, as evidenced by the antibody-treated cells, and for the formation of a multimolecular complex.
Anti-CD98 and anti-xCT antibodies do not block KSHV binding and entry.
Using GFP-KSHV, Kaleeba and Berger (39) have shown that GFP expression from the KSHV genome is reduced by pretreating cells with anti-xCT antibodies. However, the mechanism of this inhibition is not known. Although our results presented above demonstrated the association of CD98/xCT with α3β1 integrin during the early stages of KSHV infection, it remained unclear whether this interaction plays a role in regulating virus infection. Therefore, we next conducted experiments to determine whether CD98 and xCT play roles in the binding, entry, and gene expression stages of KSHV infection. In addition to the anti-CD98 antibodies, we used anti-xCT antibodies raised against the different extracellular and intracellular regions of xCT protein at positions 25 to 40 (intracellular), 66 to 77 (extracellular), 97 to 109 (intracellular), 218 to 232 (extracellular), and 255 to 270 (intracellular), which were identical to the anti-peptide antibodies used by Kaleeba and Berger (39).
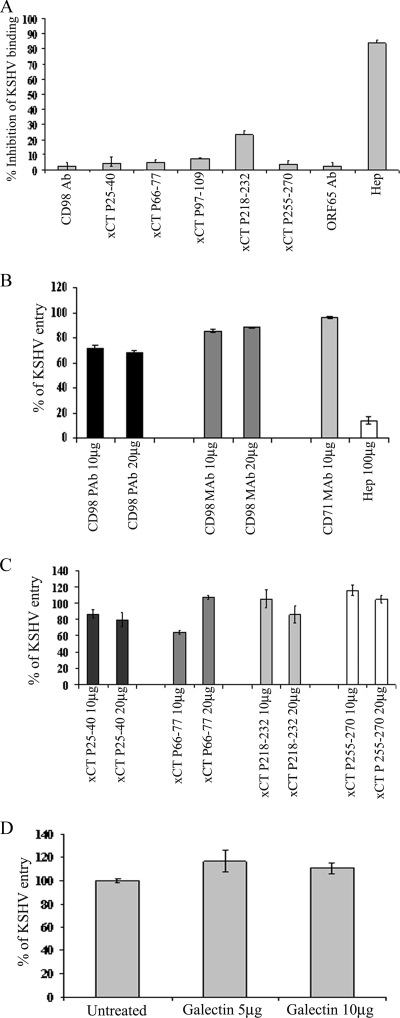
To determine the roles of CD98 and xCT in KSHV binding, we analyzed the ability of [3H]thymidine-KSHV to bind HMVEC-d pretreated with anti-CD98 and anti-xCT peptide antibodies (P25-40, P66-77, P97-109, P218-232, and P255-270) or control anti-ORF65 antibodies. No significant inhibition of KSHV binding was observed in cells incubated with anti-CD98, anti-xCT, or control antibodies. In contrast, as we have observed before (2), incubation of virus with heparin (100 μg/ml) inhibited >80% of radiolabeled KSHV binding to HMVEC-d (Fig. 8A). These studies demonstrated that CD98 and xCT do not play roles in KSHV binding to HMVEC-d.
FIG. 8.
(A) Effects of anti-CD98 and anti-xCT peptide antibodies on KSHV binding. HMVEC-d, untreated or treated with 10 μg/ml of various antibodies at 4°C for 1 h, were incubated with a fixed concentration of [3H]thymidine-labeled virus in RPMI 1640 for 1 h at 4°C with gentle rotation. For control, [3H]thymidine-labeled KSHV was preincubated with 100 μg of heparin/ml for 1 h at 37°C before addition to the cells. After incubation at 4°C, the cells were washed, lysed, and precipitated with trichloroacetic acid, and cell-associated virus radioactivity (in cpm) was measured. The cpm of bound virus in the absence of any treatment was considered to be 100% and was used to calculate the percentage inhibition of virus binding. Each reaction was done in triplicate, and each bar represents the average ± standard deviation (SD) of three independent experiments. (B and C) Effects of anti-CD98 and anti-xCT peptide antibodies on KSHV DNA internalization. HMVEC-d were untreated or treated with various concentrations of anti-CD98 MAb (CD98 MAb) and polyclonal antibody (CD98 PAb) or anti-CD71 antibodies (B) or with anti-xCT peptide antibodies (C) at 4°C for 1 h and then infected with KSHV at an MOI of 10 for 2 h. Unbound virus was removed by washing the cells, and the cells were treated with 0.25% trypsin-EDTA for 5 min at 37°C to remove the bound but noninternalized virus. The cells were washed, DNA was extracted, and the internalized KSHV DNA was quantitated by amplification of the ORF73 gene by real-time DNA PCR. Copy numbers were calculated from the standard graph generated by real-time DNA PCR using known concentrations of a cloned ORF73 gene. Each reaction was done in duplicate, and each bar represents the average ± SD of three experiments. (D) Effect of CD98 ligand, galectin 3, on KSHV DNA internalization. HMVEC-d were first incubated with the CD98 ligand galectin at 5- and 10-μg/ml concentrations for 1 h at 4°C. The cells were washed and then infected with KSHV at an MOI of 10 for 2 h. Internalized KSHV DNA was quantitated by amplification of the ORF73 gene by real-time DNA PCR. Each reaction was done in duplicate, and each bar represents the average ± the SD of three experiments. KSHV DNA internalization in the absence of any treatment was considered to be 100%.
Since the Kaleeba and Berger (39) study suggested that KSHV requires xCT for fusion with and entry into many cell types, including HFF and HMVEC-d, we analyzed the roles of CD98 and xCT in KSHV entry. HMVEC-d were preincubated with different concentrations of CD98 MAb and polyclonal antibody, different xCT peptide antibodies, and control anti-CD71 antibodies at 4°C for 60 min to allow antibody binding. These cells were washed and incubated with KSHV at 4°C for 60 min to allow virus attachment and then shifted to 37°C for 2 h. Internalization of viral DNA was determined by real-time DNA PCR analysis of viral ORF73 copy numbers. Under our experimental conditions, the anti-CD98 MAbs did not significantly affect the entry of KSHV (Fig. 8B). We did observe about 30% inhibition of virus entry with the polyclonal anti-CD98 antibodies. The control antibody against CD71 had no effect on virus entry. Similarly, we did not observe any significant inhibition of virus entry with the various anti-xCT antibodies (Fig. 8C), and only about 15 to 20% inhibition was seen with anti-xCT antibodies directed against the intracellular 25 to 40 amino acids of xCT (Fig. 8C). These studies demonstrated that CD98 and xCT do not play major roles in KSHV binding to and entry into HMVEC-d.
CD98 ligand does not block entry of KSHV.
Since galectin 3 has been shown to be a natural ligand for CD98, HMVEC-d were treated with 5- and 10-μg/ml concentrations of galectin 3 for 1 h at 4°C, washed, and infected with KSHV for 2 h at 37°C, and entry was measured. No significant inhibition of KSHV entry was observed with CD98 ligand (Fig. 8D). In fact, we observed a moderate enhancement of viral-DNA internalization. These studies further confirmed that CD98 does not play a major role in KSHV entry into HMVEC-d.
Anti-CD98 and anti-xCT antibodies block KSHV viral-gene expression.
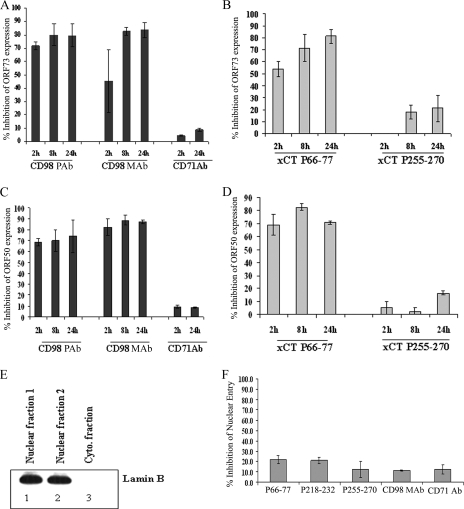
The above-mentioned results demonstrated that CD98 and xCT do not play major roles in the binding and entry of KSHV under anti-CD98 or anti-xCT peptide antibody treatment conditions. To investigate the effects of CD98 and xCT antibodies on viral-gene expression, we incubated HMVEC-d with CD98 and different xCT peptide antibodies for 1 h and then infected them with KSHV for 2 h, 8 h, and 24 h. Viral-gene expression determined by real-time RT-PCR analysis showed about 70 to 80% inhibition of KSHV latency-associated ORF73 (Fig. 9A) and lytic-cycle ORF50 (Fig. 9C) gene expression in CD98 MAb- and polyclonal-antibody-treated infected cells. The xCT extracellular peptide antibody against P66-77 also showed similar levels of inhibition of ORF73 (Fig. 9B) and ORF50 (Fig. 9D) gene expression, and no significant inhibition was observed with intracellular-peptide antibody P255-270 (Fig. 9B and D). Our results show that CD98 and xCT are involved in mediating viral-gene expression rather than viral internalization. These findings suggest the possibility that CD98 and xCT may be involved in the post-entry stage of KSHV infection.
FIG. 9.
Effects of anti-CD98 and anti-xCT antibodies on KSHV gene expression and nuclear delivery. HMVEC-d were untreated or treated with 10 μg/ml of anti-CD98 MAb (CD98 MAb) and polyclonal antibody (CD98 PAb), with anti-CD71 antibodies (A and C), or with anti-xCT peptide antibodies (B and D) at 4°C for 1 h. The cells were washed with ice-cold PBS and then infected with KSHV at an MOI of 10 DNA copies/cell at 37°C for different times. Total RNA was isolated at 2 h, 8 h, and 24 h p.i., and 250 ng of DNase-treated RNA was subjected to real-time RT-PCR with ORF73 (A and B) and ORF50 (C and D) gene-specific primers and TaqMan probes. Known concentrations of DNase-treated in vitro-transcribed ORF50 and ORF73 transcripts were used in a real-time RT-PCR to construct a standard graph, from which the relative copy numbers of viral transcripts were calculated and normalized with GAPDH. Each reaction was done in duplicate, and each bar represents the average ± standard deviation (SD) of three experiments. The histograms depict the percent inhibition in RNA copy numbers for KSHV ORF73 and ORF50 genes in the presence of antibodies, which were calculated by comparison to viral-gene expression in the absence of any treatment. (E) Nuclear fractions from HMVEC-d infected at 10 DNA copies/cell for 2 h were isolated, and the purity of the nuclear fraction was confirmed by lamin B Western blots. (F) The total nuclear DNA was isolated, normalized to contain 100 ng/5 μl, and analyzed by real-time DNA PCR with KSHV ORF73 primers and probe. Each reaction was done in duplicate, and each bar represents the average ± SD of three experiments. KSHV DNA associated with infected-cell nuclei in the absence of any treatment was considered to be 100%.
Anti-CD98 and anti-xCT antibodies do not block viral-DNA delivery to the nucleus.
We next determined whether the inhibition of viral-gene expression by xCT and CD98 antibodies is due to interference at postentry steps, such as transport of the virus capsid and delivery of the viral DNA to the nucleus. To investigate the effects of CD98 and xCT antibodies on nuclear delivery, we incubated HMVEC-d with CD98 and xCT peptide antibodies for 1 h and then infected them with KSHV for 2 h. Nuclear fractions were isolated from these cells, and the purity was confirmed by probing for the nuclear marker lamin B. Nuclear fractions were highly positive for the nuclear marker lamin B (Fig. 9E), but it was not detected in the cytoplasmic fractions. DNA isolated from the nuclei was subjected to real-time DNA PCR to investigate the nucleus-associated KSHV DNA. We did not observe any significant inhibition of nucleus-associated KSHV DNA in anti-xCT and -CD98 antibody-treated cells, which showed only about 10 to 20% inhibition (Fig. 9F), which was similar to the inhibition observed with the control anti-CD71 antibody (Fig. 9F). These data suggest CD98 and xCT molecules do not have any significant effect on nuclear delivery of viral DNA into HMVEC-d and that they have roles in KSHV gene expression.
DISCUSSION
Identification of the cellular receptor(s) utilized by KSHV to bind and gain entry into the different target cells is an evolving area of study. Detailed knowledge about host cell surface molecules recognized by KSHV is crucial, not only to understand the tropism and pathogenesis of KSHV, but also for the development of strategies to block infection. The interaction of herpesviruses with cell surface molecules is a very complex process involving multiple receptors and multiple viral proteins, and receptors may vary according to the cell types (70). For example, γ1-EBV initiates the infection of human primary B cells via its envelope glycoprotein gp350/220 interacting with the C3d complement receptor CR2. Subsequently, EBV binds to the HLA class II molecule via its gp42 in the gH/gL complex (18, 45, 83). In contrast, infection of epithelial cells is believed to occur via the gH/gL complex and BMRF2 glycoprotein interactions with integrin molecules (82). Based on the studies conducted with herpes simplex virus type 1 and human cytomegalovirus, the current working model is that herpesvirus glycoproteins interact with multiple receptors in a temporally coordinated manner, leading to the internalization of viral DNA (69, 81). This is probably very similar to HIV type 1 gp120 interaction with the CD4 molecule, which leads to subsequent conformation changes in gp120, leading to interaction with chemokine receptors, which then leads to the conformation change in HIV gp41 and fusion of the viral envelope with host cell membranes (88).
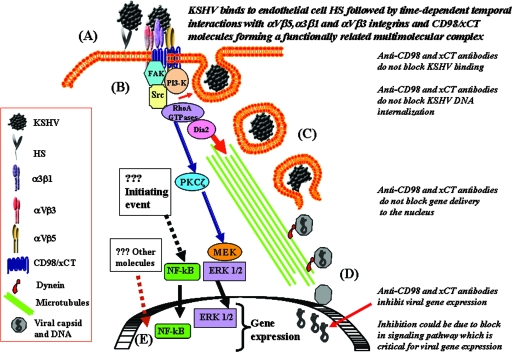
Here, we present convincing evidence (Fig. 10) to suggest that KSHV interactions with HMVEC-d leads to the formation of a multimolecular complex composed of integrins (α3β1, αVβ3, and αVβ5) and CD98/xCT molecules and that these interactions occur in a temporally coordinated manner. Whether HS is also a part of this complex is not examined, and all of these interactions may be critical for modulating the downstream events, such as the various host cell signal cascades, that we have demonstrated previously during the early stages of KSHV infection (50, 51, 63, 67, 74), which are critical for the completion of entry and infection by KSHV. Previous studies have demonstrated the interaction of CD98 with both α3β1 and αVβ3 integrins, leading to a multimolecular complex and signaling network (10, 38). KSHV probably induces this multicomponent complex to serve as the potential site of virus entry and to coordinate endocytosis, fusion, and induction of host cell signaling. The roles of individual molecules in different steps of KSHV entry and infection need to be examined further. Similarly, additional studies are required to determine whether complex formation is due to the simultaneous interaction of multiple glycoproteins with cell surface receptors and whether the variations between receptor molecule interactions in turn affect the kinetics of subsequent interactions. The interaction of different glycoproteins of human cytomegalovirus with different receptors, gB with epidermal growth factor receptor (EGFR) and gH with αVβ3, forming a complex composed of EGFR and integrins, has been reported (81). Detailed studies of individual receptors silenced by short interfering RNA are in progress, which will provide substantial information on the roles of receptors and their importance in KSHV entry.
FIG. 10.
Model diagram summarizing the interaction of KSHV with receptors, the different stages involved in KSHV infection, and the various signal pathways induced by KSHV. The process of infection is divided into several distinct phases. (A) Binding and interaction with receptors. The initial attachment of the virus with the binding receptor HS molecules via its envelope glycoproteins gpK8.1A and gB is followed by temporally coordinated interactions with integrins (α3ß1, αVß3, and αVß5) and CD98-xCT molecules, leading to the formation of a multimolecular complex. (B) Induction of signal pathways and entry. Interactions with cell surface receptors trigger the cascades of host cell preexisting signal pathways. Integrin activation of FAK and Src leads to the activation of PI3-K and Rho-GTPases. These signal cascades lead to the formation of endocytic vesicles, and the internalization of KSHV occurs by endocytosis. (C) Transport of the virus. The endosome moves in the cytoplasm, and the release of the viral nucleocapsid into the cytoplasm occurs. RhoA facilitates the transport of capsid toward the nucleus by inducing microtubule stabilization and regulating microtubule dynamics via Dia-2. (D) Nuclear delivery. The viral DNA is delivered to the nucleus. (E) Gene expression. The initiation of viral-gene expression occurs with the help of KSHV binding- and entry-induced cellular signaling molecules and transcription factors, such as NF-κB and ERK1/2. The stage at which anti-CD98/xCT antibodies could be exerting their effect on blocking of viral-gene expression during KSHV infection is shown on the right.
Integrins play a major role in the infectious processes of many microbes, including several viruses (8, 72, 76). For numerous viruses, cell attachment and internalization are distinct steps, and several viruses utilize multiple integrins during their interactions with host cells. Adenovirus types 2 and 5 use αVβ3, αVβ5, and αVβ1 for their entry (44, 52). Foot-and-mouth disease virus type A utilizes αVβ3, αVβ6, and αVβ1 for its binding and entry, while foot-and-mouth disease virus type 0 utilizes αVβ6, αVβ1, and αVβ3 for its binding and entry (6, 36, 37). Rotavirus utilizes α2β1 and sialic acid R for attachment and αVβ3 and hsc70 protein for entry (29, 32). The pathogenic strain of hantavirus utilizes αVβ3 and αIIβ3 for entry, and the nonpathogenic strain utilizes β1 integrin (25, 26). Our studies presented here clearly demonstrate that α3β1, αVβ3, and αVβ5 integrins play important roles in KSHV infection.
In our earlier studies, we observed about 50% reduction in KSHV infection of primary human fibroblasts and endothelial cells by RGD peptides, antibodies against KSHV-gB RGD peptide (RGDTFQTSSSPTPPGSSS), and fibronectin, which was the starting point to suggest a role for integrins in KSHV infection (3). The suggestion that α3β1 integrin plays a role in KSHV infection of HMVEC-d and HFF came from observations that about 30 to 50% of KSHV infection was inhibited by pretreating cells with function-blocking antibodies against the α3 and β1 subunits and by mixing virus with soluble α3β1 integrin molecules before infection (3). Immunoprecipitation of virus-α3 and -β1 complexes from HFF by anti-KSHV-gB antibodies demonstrated KSHV interaction with α3β1 integrin. Infection of CHO cells expressing human α3 integrin increased virus entry and infection (3). Furthermore, mixing virus or KSHV-gB with soluble α3β1 integrin molecules decreased the ability of KSHV to induce FAK in Du17 (FAK+/+) cells and HFF, Pyk-2 in Du3 cells (FAK−/−), and RhoA and ERK1/2 in HFF (43, 50, 67). In addition, the ability of KSHV-gBΔTM to induce FAK in HFF was inhibited by preincubating gB with α3β1 integrin. Incubation of HMVEC-d with KSHV-gBΔTM, but not KSHV-gBΔTM-RGA, induced the phosphorylation of VEGFR-3 in HMVEC-d, and α3 was detected with immunoprecipitation by anti-VEGFR-3 antibodies in gB-treated endothelial cells (90). In addition, the results shown here demonstrate the specific association of α3β1 integrin with CD98/xCT molecules in the infected cells. All this evidence suggests that KSHV interacts with α3β1 integrin during infection. Whether KSHV recognizes α3β1 integrin by “RGD” or other motifs was not determined.
Neutralization of KSHV infection of fibroblasts and endothelial cells by anti-integrin antibodies (αV, αVβ3, αVβ5, and α3β1) and by soluble integrins (αVβ3, αVβ5, and α3β1) and virus binding and DNA internalization studies suggest that, similar to α3β1 integrin, αVβ3 and αVβ5 integrins also play roles in KSHV entry. The ability of anti-αV antibodies to neutralize KSHV better than anti-αVβ3 and -αVβ5 integrins clearly suggests that results may vary depending upon the antibody and therefore a need for caution in interpreting results with antibodies. Our results show that integrins β1, αVβ3, and αVβ5 play roles in KSHV gBΔTM-mediated cell adhesion. The differences in the inhibition of gB-mediated adhesion and neutralization of KSHV infection by the anti-αVβ3, -αVβ5, and -α3 integrin antibodies is not unexpected. For example, endothelial cells from AIDS-KS lesions adhere to HIV Tat via integrins α5β1 and αVβ3 (5), while adhesions of human leiomyosarcoma cells to Tat occur via αVβ5 integrin (77). Moreover, it is common for different cells to express different combinations of integrins, and one integrin ligand generally binds several different integrins (27, 56). For example, fibronectin and vitronectin are two common ECM proteins widely used in cell adhesion studies. Vitronectin binds to integrins αVβ1, αVβ3, αVβ5, and αIIbβ1 through the RGD sequence, while fibronectin binds to integrins α3β1, α5β1, α8β1, αVβ1, αVβ3, αVβ5, αVβ6, αVβ8, and αIIbβ3 through the RGD sequence and to integrins α2β1, α4β1, and α4β7 in an RGD-independent manner (56).
A very recent study used a 15-mer AHSRGDTFQTSSGCG peptide of KSHV gB (24). The GCG amino acids in this peptide are not present in the KSHV gB sequence and may potentially give rise to dimers and multimers due to the cystine residue (24). Using this peptide, the authors showed that it mediated HT1080 cell adhesion, which was blocked by αVβ3 antibodies. This is in contrast to our studies, where we observed the inhibition of purified KSHV gB (702 amino acids long)-mediated HT1080 cell adhesion by αVβ5 antibody, but not by αVβ3 antibodies. When the 15-mer AHSRGDTFQTSSGCG peptide-bound beads were incubated with αVβ3 and α3β1 integrins, or with CHO cell lysates, only αVβ3 binding was detected, but not αVβ1 integrin binding (24). These data suggested that the RGD domain of KSHV gB may potentially interact with αVβ3 integrin. In this study, HT1080 cell infection was also inhibited by preincubating cells with anti-αVβ3 antibodies; however, anti-α3β1 and -αVβ5 antibodies were not tested (24). So far, we have been unable to infect HT1080 cells (data not shown).
Mouse keratinocytes lacking α3β1 were infected with KSHV, and expression of human α3 resulted in only 55% infection (24). Even though the level of αVβ3 and the ability of anti-αVβ3 to block KSHV infection were not tested in these cells, the authors concluded that α3β1 expression must have a dominant-negative effect on αVβ3 integrins. Since the dominant effect of α3β1 has been demonstrated only after specific ligand binding to α3β1 (9, 33), it is not clear whether virus binding to α3β1 generates such an effect, which itself is an interesting area to study. In our ongoing studies with CHO cells, we did not observe any dominant-negative effect in CHO cells expressing α3β1 and αVβ3 integrins, and in contrast, we observed an additive effect of increased KSHV infectivity in these cells (data not shown). The discrepancies between these studies could be due to cell type and procedural differences and suggest that further work is essential to fully understand the contributions of different integrins to KSHV infection. Moreover, since herpesvirus-cell receptor interactions are temporally coordinated events mediated by interactions of viral glycoproteins with one receptor, leading to conformational changes of viral glycoproteins, leading to the interaction with the next receptor(s), our detection of α3β1 in immunoprecipitation reactions of HFF or HMVEC-d that were first incubated with the virus (3) or purified gB (68) could represent events occurring during physiological virus-host cell interactions. Hence, there are many important gaps remaining to be filled, and the determinants of KSHV tropism remain incompletely defined.
Integrin functions and signaling are modulated by several proteins and protein complexes (31, 57, 87). CD98 is a protein that may act at various levels to modulate integrin function, as well as facilitating signaling downstream of integrin ligation (15-17, 62, 84). As demonstrated by immunoprecipitation, KSHV interactions with β1 integrin lead to association with CD98hC, which probably allows the specific virus interactions with the xCT molecule and not with other light chains. Our results indicate that the entry mechanism of KSHV is a three-step process that involves the initial binding to the cell surface HS, which in turn leads to the interaction with integrins and CD98/xCT, which may lead to the internalization of KSHV. Through the engagement of integrin, KSHV can modulate a variety of signaling molecules and subsequent cellular activities, including endocytosis, transport, and gene expression of the virus (1, 50, 51, 63, 67). It is possible that the antibody can block the interaction of the virus with the receptor at some other stages of infection, rather than attachment and entry, such as intracellular trafficking of the virus, fusion of the virus envelope with the endosome, viral-gene delivery to the nucleus, and viral-gene expression. A functionally significant role for the integrin-CD98 interaction in virus-induced cell fusion has been previously proposed (53, 54). Inhibition of endosomal fusion with antibodies used to block infection by the flavivirus West Nile virus has been shown to block pH-dependent endosomal fusion with the viral envelope after internalization of the antibody-virion complex without affecting binding and entry (28). Interaction of integrins with CD98/xCT, resulting in modulation of signaling molecules, might be another mechanism that is involved in the regulation of viral-gene expression.
Previous study by Kaleeba et al. (39) showed that antibodies against xCT blocked KSHV fusion and entry with naturally permissive target cells. Their study did not actually identify the stage of KSHV infection at which xCT played a role in infection and whether the antibodies blocked binding or entry into the target cell, movement into the target cell, nuclear delivery, or viral-gene expression. What was measured was the expression of GFP from the KSHV genome 3 days p.i., which was considered the measure of virus entry. Our studies differentiated the different stages of KSHV infection systematically and methodically. Our studies showed that xCT antibodies did not block viral binding. We measured viral-DNA entry by a more sensitive technique of real-time DNA PCR, and our studies showed that xCT antibodies do not block viral-DNA entry. Furthermore, our studies also showed that gene delivery to the nucleus was not blocked by anti-xCT antibodies, but they blocked the gene expression of KSHV. This suggests that the decreased expression of GFP observed by Kaleeba et al. after 3 days post-GFP-KSHV infection and the inhibition of viral-gene expression observed by us are probably due to a block in the signaling molecules associated with xCT molecules that are critical for viral-gene expression.
The inhibition of viral-gene expression observed in anti-CD98 and xCT antibody-treated cells could be due to the roles of CD98 and xCT in gene expression subsequent to nuclear delivery, perhaps due to a role in the signal transduction needed for viral-gene expression (Fig. 10). Our ongoing experiments show that xCT antibodies inhibit several key signaling molecules in KSHV-infected cells, which suggests that the antibodies block a major signaling pathway that is necessary for viral-gene expression. Further studies are in progress to explore the mechanism by which anti-CD98/xCT antibodies block viral-gene expression, viral glycoproteins involved in the interactions with integrins/CD98/xCT, and the role of CD98-xCT in KSHV-induced signal pathways, all of which will lead to a better understanding of KSHV biology.
Acknowledgments
This study was supported in part by Public Health Service grant CA 075911 and by the Rosalind Franklin University of Medicine and Science-H. M. Bligh Cancer Research Fund to B.C.
We thank Keith Philibert for critically reading the manuscript. We acknowledge Rita Levine for her assistance in flow cytometry performed at the Rosalind Franklin University Flow Cytometry/Cell Sorting Research Core Facility.
Footnotes
Published ahead of print on 1 October 2008.
REFERENCES
- 1.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 777978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284235-249. [DOI] [PubMed] [Google Scholar]
- 3.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108407-419. [DOI] [PubMed] [Google Scholar]
- 4.Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282245-255. [DOI] [PubMed] [Google Scholar]
- 5.Barillari, G., R. Gendelman, R. C. Gallo, and B. Ensoli. 1993. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc. Natl. Acad. Sci. USA 907941-7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin alpha V beta 3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 692664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkmann, A., K. Mahr, A. Ensser, S. Yaguboglu, F. Titgemeyer, B. Fleckenstein, and F. Neipel. 2001. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J. Virol. 7511583-11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliska, J. B., J. E. Galan, and S. Falkow. 1993. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell 73903-920. [DOI] [PubMed] [Google Scholar]
- 9.Borza, C. M., A. Pozzi, D. B. Borza, V. Pedchenko, T. Hellmark, B. G. Hudson, and R. Zent. 2006. Integrin α3β1, a novel receptor for α3(IV) noncollagenous domain and a trans-dominant Inhibitor for integrin αvβ3. J. Biol. Chem. 28120932-20939. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekaran, S., N. H. Guo, R. G. Rodrigues, J. Kaiser, and D. D. Roberts. 1999. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by α3β1 integrin and regulated by insulin-like growth factor-1 and CD98. J. Biol. Chem. 27411408-11416. [DOI] [PubMed] [Google Scholar]
- 11.Deves, R., and C. A. Boyd. 2000. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J. Membr. Biol. 173165-177. [DOI] [PubMed] [Google Scholar]
- 12.Dezube, B. J., M. Zambela, D. R. Sage, J. F. Wang, and J. D. Fingeroth. 2002. Characterization of Kaposi sarcoma-associated herpesvirus/human herpesvirus-8 infection of human vascular endothelial cells: early events. Blood 100888-896. [DOI] [PubMed] [Google Scholar]
- 13.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elices, M. J., L. A. Urry, and M. E. Hemler. 1991. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J. Cell Biol. 112169-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenczik, C. A., T. Sethi, J. W. Ramos, P. E. Hughes, and M. H. Ginsberg. 1997. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 39081-85. [DOI] [PubMed] [Google Scholar]
- 16.Feral, C. C., N. Nishiya, C. A. Fenczik, H. Stuhlmann, M. Slepak, and M. H. Ginsberg. 2005. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. USA 102355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feral, C. C., A. Zijlstra, E. Tkachenko, G. Prager, M. L. Gardel, M. Slepak, and M. H. Ginsberg. 2007. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J. Cell Biol. 178701-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingeroth, J. D., J. J. Weis, T. F. Tedder, J. L. Strominger, P. A. Biro, and D. T. Fearon. 1984. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc. Natl. Acad. Sci. USA 814510-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gailit, J., and R. A. Clark. 1996. Studies in vitro on the role of alpha v and beta 1 integrins in the adhesion of human dermal fibroblasts to provisional matrix proteins fibronectin, vitronectin, and fibrinogen. J. Investig. Dermatol. 106102-108. [DOI] [PubMed] [Google Scholar]
- 20.Gailit, J., M. Pierschbacher, and R. A. Clark. 1993. Expression of functional alpha 4 beta 1 integrin by human dermal fibroblasts. J. Investig. Dermatol. 100323-328. [DOI] [PubMed] [Google Scholar]
- 21.Gailit, J., J. Xu, H. Bueller, and R. A. Clark. 1996. Platelet-derived growth factor and inflammatory cytokines have differential effects on the expression of integrins alpha 1 beta 1 and alpha 5 beta 1 by human dermal fibroblasts in vitro. J. Cell Physiol. 169281-289. [DOI] [PubMed] [Google Scholar]
- 22.Ganem, D. 1998. Human herpesvirus 8 and its role in the genesis of Kaposi's sarcoma. Curr. Clin. Top. Infect. Dis. 18237-251. [PubMed] [Google Scholar]
- 23.Ganem, D. 1997. KSHV and Kaposi's sarcoma: the end of the beginning? Cell 91157-160. [DOI] [PubMed] [Google Scholar]
- 24.Garrigues, H. J., Y. E. Rubinchikova, C. M. Dipersio, and T. M. Rose. 2008. Integrin αVβ3 Binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 821570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavrilovskaya, I. N., E. J. Brown, M. H. Ginsberg, and E. R. Mackow. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by β3 integrins. J. Virol. 733951-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavrilovskaya, I. N., M. Shepley, R. Shaw, M. H. Ginsberg, and E. R. Mackow. 1998. β3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 957074-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 2851028-1032. [DOI] [PubMed] [Google Scholar]
- 28.Gollins, S. W., and J. S. Porterfield. 1986. A new mechanism for the neutralization of enveloped viruses by antiviral antibody. Nature 321244-246. [DOI] [PubMed] [Google Scholar]
- 29.Guerrero, C. A., E. Mendez, S. Zarate, P. Isa, S. Lopez, and C. F. Arias. 2000. Integrin alpha(v) beta(3) mediates rotavirus cell entry. Proc. Natl. Acad. Sci. USA 9714644-14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haywood, A. M. 1994. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J. Virol. 681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemler, M. E. 2001. Specific tetraspanin functions. J. Cell Biol. 1551103-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewish, M. J., Y. Takada, and B. S. Coulson. 2000. Integrins α2β1 and α4β1 can mediate SA11 rotavirus attachment and entry into cells. J. Virol. 74228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodivala-Dilke, K. M., C. M. DiPersio, J. A. Kreidberg, and R. O. Hynes. 1998. Novel roles for α3β1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J. Cell Biol. 1421357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue, N., J. Winter, R. B. Lal, M. K. Offermann, and S. Koyano. 2003. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J. Virol. 778147-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito, Y., H. Komada, S. Kusagawa, M. Tsurudome, H. Matsumura, M. Kawano, H. Ohta, and M. Nishio. 1992. Fusion regulation proteins on the cell surface: isolation and characterization of monoclonal antibodies which enhance giant polykaryocyte formation in Newcastle disease virus-infected cell lines of human origin. J. Virol. 665999-6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson, T., A. P. Mould, D. Sheppard, and A. M. King. 2002. Integrin αvβ1 is a receptor for foot-and-mouth disease virus. J. Virol. 76935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. King. 2000. The epithelial integrin αvβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 744949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabir-Salmani, M., M. N. Fukuda, M. Kanai-Azuma, N. Ahmed, S. Shiokawa, Y. Akimoto, K. Sakai, S. Nagamori, Y. Kanai, K. Sugihara, and M. Iwashita. 2008. The membrane-spanning domain of CD98 heavy chain promotes αvβ3 integrin signals in human extravillous trophoblasts. Mol. Endocrinol. 22707-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaleeba, J. A., and E. A. Berger. 2006. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 3111921-1924. [DOI] [PubMed] [Google Scholar]
- 40.Kamata, T., and Y. Takada. 2001. Platelet integrin αIIbβ3-ligand interactions: what can we learn from the structure? Int. J. Hematol. 74382-389. [DOI] [PubMed] [Google Scholar]
- 41.Kolesnikova, T. V., B. A. Mannion, F. Berditchevski, and M. E. Hemler. 2001. β1 integrins show specific association with CD98 protein in low density membranes. BMC Biochem. 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 783601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan, H. H., N. Sharma-Walia, D. N. Streblow, P. P. Naranatt, and B. Chandran. 2006. Focal adhesion kinase is critical for entry of Kaposi's sarcoma-associated herpesvirus into target cells. J. Virol. 801167-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 755405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, Q., M. K. Spriggs, S. Kovats, S. M. Turk, M. R. Comeau, B. Nepom, and L. M. Hutt-Fletcher. 1997. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 714657-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyamoto, S., H. Teramoto, J. S. Gutkind, and K. M. Yamada. 1996. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 1351633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mocarski, E. S., Jr. 1997. Propagating Kaposi's sarcoma-associated herpesvirus. N. Engl. J. Med. 336214-215. [DOI] [PubMed] [Google Scholar]
- 48.Mu, D., S. Cambier, L. Fjellbirkeland, J. L. Baron, J. S. Munger, H. Kawakatsu, D. Sheppard, V. C. Broaddus, and S. L. Nishimura. 2002. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J. Cell Biol. 157493-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naranatt, P. P., S. M. Akula, and B. Chandran. 2002. Characterization of γ2-human herpesvirus-8 glycoproteins gH and gL. Arch. Virol. 1471349-1370. [DOI] [PubMed] [Google Scholar]
- 50.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 771524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naranatt, P. P., H. H. Krishnan, M. S. Smith, and B. Chandran. 2005. Kaposi's sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J. Virol. 791191-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nemerow, G. R. 2000. Cell receptors involved in adenovirus entry. Virology 2741-4. [DOI] [PubMed] [Google Scholar]
- 53.Ohgimoto, S., N. Tabata, S. Suga, M. Nishio, H. Ohta, M. Tsurudome, H. Komada, M. Kawano, N. Watanabe, and Y. Ito. 1995. Molecular characterization of fusion regulatory protein-1 (FRP-1) that induces multinucleated giant cell formation of monocytes and HIV gp160-mediated cell fusion. FRP-1 and 4F2/CD98 are identical molecules. J. Immunol. 1553585-3592. [PubMed] [Google Scholar]
- 54.Ohta, H., M. Tsurudome, H. Matsumura, Y. Koga, S. Morikawa, M. Kawano, S. Kusugawa, H. Komada, M. Nishio, and Y. Ito. 1994. Molecular and biological characterization of fusion regulatory proteins (FRPs): anti-FRP MAbs induced HIV-mediated cell fusion via an integrin system. EMBO J. 132044-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okamoto, K., S. Ohgimoto, M. Nishio, M. Tsurudome, M. Kawano, H. Komada, M. Ito, Y. Sakakura, and Y. Ito. 1997. Paramyxovirus-induced syncytium cell formation is suppressed by a dominant negative fusion regulatory protein-1 (FRP-1)/CD98 mutated construct: an important role of FRP-1 in virus-induced cell fusion. J. Gen. Virol. 78775-783. [DOI] [PubMed] [Google Scholar]
- 56.Plow, E. F., T. A. Haas, L. Zhang, J. Loftus, and J. W. Smith. 2000. Ligand binding to integrins. J. Biol. Chem. 27521785-21788. [DOI] [PubMed] [Google Scholar]
- 57.Porter, J. C., and N. Hogg. 1998. Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol. 8390-396. [DOI] [PubMed] [Google Scholar]
- 58.Quackenbush, E., M. Clabby, K. M. Gottesdiener, J. Barbosa, N. H. Jones, J. L. Strominger, S. Speck, and J. M. Leiden. 1987. Molecular cloning of complementary DNAs encoding the heavy chain of the human 4F2 cell-surface antigen: a type II membrane glycoprotein involved in normal and neoplastic cell growth. Proc. Natl. Acad. Sci. USA 846526-6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raghu, H., N. Sharma-Walia, M. V. Veettil, S. Sadagopan, A. Caballero, R. Sivakumar, L. Varga, V. Bottero, and B. Chandran. 2007. Lipid rafts of primary endothelial cells are essential for Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J. Virol. 817941-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rappocciolo, G., F. J. Jenkins, H. R. Hensler, P. Piazza, M. Jais, L. Borowski, S. C. Watkins, and C. R. Rinaldo, Jr. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J. Immunol. 1761741-1749. [DOI] [PubMed] [Google Scholar]
- 61.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 725182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rintoul, R. C., R. C. Buttery, A. C. Mackinnon, W. S. Wong, D. Mosher, C. Haslett, and T. Sethi. 2002. Cross-linking CD98 promotes integrin-like signaling and anchorage-independent growth. Mol. Biol. Cell 132841-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sadagopan, S., N. Sharma-Walia, M. V. Veettil, H. Raghu, R. Sivakumar, V. Bottero, and B. Chandran. 2007. Kaposi's sarcoma-associated herpesvirus induces sustained NF-κB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J. Virol. 813949-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato, H., M. Tamba, T. Ishii, and S. Bannai. 1999. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 27411455-11458. [DOI] [PubMed] [Google Scholar]
- 65.Schnapp, L. M., N. Hatch, D. M. Ramos, I. V. Klimanskaya, D. Sheppard, and R. Pytela. 1995. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J. Biol. Chem. 27023196-23202. [DOI] [PubMed] [Google Scholar]
- 66.Schulz, T. F., J. Sheldon, and J. Greensill. 2002. Kaposi's sarcoma associated herpesvirus (KSHV) or human herpesvirus 8 (HHV8). Virus Res. 82115-126. [DOI] [PubMed] [Google Scholar]
- 67.Sharma-Walia, N., H. H. Krishnan, P. P. Naranatt, L. Zeng, M. S. Smith, and B. Chandran. 2005. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 7910308-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma-Walia, N., P. P. Naranatt, H. H. Krishnan, L. Zeng, and B. Chandran. 2004. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J. Virol. 784207-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 2751-8. [DOI] [PubMed] [Google Scholar]
- 70.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 7710179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabata, N., M. Ido, S. Suga, S. Ohgimoto, M. Tsurudome, M. Kawano, M. Nishio, N. Watanabe, K. Okamoto, H. Komada, M. Sakurai, and Y. Ito. 1997. Protein tyrosine kinase activation provides an early and obligatory signal in anti-FRP-1/CD98/4F2 monoclonal antibody induced cell fusion mediated by HIV gp160. Med. Microbiol. Immunol. 186115-123. [DOI] [PubMed] [Google Scholar]
- 72.Triantafilou, K., Y. Takada, and M. Triantafilou. 2001. Mechanisms of integrin-mediated virus attachment and internalization process. Crit. Rev. Immunol. 21311-322. [PubMed] [Google Scholar]
- 73.Tufaro, F. 1997. Virus entry: two receptors are better than one. Trends Microbiol. 5257-259. [DOI] [PubMed] [Google Scholar]
- 74.Veettil, M. V., N. Sharma-Walia, S. Sadagopan, H. Raghu, R. Sivakumar, P. P. Naranatt, and B. Chandran. 2006. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J. Virol. 8011432-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vieira, J., P. O'Hearn, L. Kimball, B. Chandran, and L. Corey. 2001. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J. Virol. 751378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Virji, M. 1996. Microbial utilization of human signalling molecules. Microbiology 1423319-3336. [DOI] [PubMed] [Google Scholar]
- 77.Vogel, B. E., S. J. Lee, A. Hildebrand, W. Craig, M. D. Pierschbacher, F. Wong-Staal, and E. Ruoslahti. 1993. A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. J. Cell Biol. 121461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner, C. A., F. Lang, and S. Broer. 2001. Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. Cell Physiol. 281C1077-C1093. [DOI] [PubMed] [Google Scholar]
- 79.Wang, F. Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J. Virol. 757517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, F. Z., S. M. Akula, N. Sharma-Walia, L. Zeng, and B. Chandran. 2003. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J. Virol. 773131-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang, X., D. Y. Huang, S. M. Huong, and E. S. Huang. 2005. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nat. Med. 11515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang, X., and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J. Virol. 72158-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 725552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Warren, A. P., K. Patel, Y. Miyamoto, J. N. Wygant, D. G. Woodside, and B. W. McIntyre. 2000. Convergence between CD98 and integrin-mediated T-lymphocyte co-stimulation. Immunology 9962-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weinacker, A., A. Chen, M. Agrez, R. I. Cone, S. Nishimura, E. Wayner, R. Pytela, and D. Sheppard. 1994. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J. Biol. Chem. 2696940-6948. [PubMed] [Google Scholar]
- 86.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73309-319. [DOI] [PubMed] [Google Scholar]
- 87.Woods, A., and J. R. Couchman. 2000. Integrin modulation by lateral association. J. Biol. Chem. 27524233-24236. [DOI] [PubMed] [Google Scholar]
- 88.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384179-183. [DOI] [PubMed] [Google Scholar]
- 89.Yoshiyama, H., S. Imai, N. Shimizu, and K. Takada. 1997. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J. Virol. 715688-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang, X., J. F. Wang, B. Chandran, K. Persaud, B. Pytowski, J. Fingeroth, and J. E. Groopman. 2005. Kaposi's sarcoma-associated herpesvirus activation of vascular endothelial growth factor receptor 3 alters endothelial function and enhances infection. J. Biol. Chem. 28026216-26224. [DOI] [PubMed] [Google Scholar]
- 91.Zhu, L., V. Puri, and B. Chandran. 1999. Characterization of human herpesvirus-8 K8.1A/B glycoproteins by monoclonal antibodies. Virology 262237-249. [DOI] [PubMed] [Google Scholar]