Abstract
Superinfection by a second human immunodeficiency virus type 1 (HIV-1) strain indicates that gaps in protective immunity occur during natural infection. To define the role of HIV-1-specific neutralizing antibodies (NAbs) in this setting, we examined NAb responses in 6 women who became superinfected between ∼1 to 5 years following initial infection compared to 18 women with similar risk factors who did not. Although superinfected individuals had less NAb breadth than matched controls at ∼1 year postinfection, no significant differences in the breadth or potency of NAb responses were observed just prior to the second infection. In fact, four of the six subjects had relatively broad and potent NAb responses prior to infection by the second strain. To more specifically examine the specificity of the NAbs against the superinfecting virus, these variants were cloned from five of the six individuals. The superinfecting variants did not appear to be inherently neutralization resistant, as measured against a pool of plasma from unrelated HIV-infected individuals. Moreover, the superinfected individuals were able to mount autologous NAb responses to these variants following reinfection. In addition, most superinfected individuals had NAbs that could neutralize their second viral strains prior to their reinfection, suggesting that the level of NAbs elicited during natural infection was not sufficient to block infection. These data indicate that preventing infection by vaccination will likely require broader and more potent NAb responses than those found in HIV-1-infected individuals.
Human immunodeficiency virus type 1 (HIV-1) superinfection occurs when an individual chronically infected with one strain of HIV-1 becomes infected with a second strain, indicating that natural immune responses to HIV-1 are not always protective. Since superinfection occurs despite ongoing immune responses to the first HIV-1 strain, it provides an avenue to explore how specific immune deficits allow HIV-1 infection to become established. To date, approximately 30 well-characterized cases of HIV-1 superinfection have been described based on longitudinal follow-up (1, 7, 10, 13, 14, 28, 37, 38, 41, 43, 45, 51, 56); many other presumed cases have been defined in cross-sectional studies, where there is evidence of dual infection at the time when viral sequences were examined (reviewed in reference 43). Many of the cases of superinfection identified in longitudinal studies occurred within the first year following initial infection, when immune responses to HIV-1 are often not fully mature. However, HIV-1 superinfections have also been found frequently during chronic infection (38), when the immune response to HIV-1 should be fully developed.
The frequency of superinfection likely depends on a variety of factors, including the nature of the superinfecting strains, the use of antiretroviral medications, and the immune status of the individual. Several studies, which screened more than 3,000 individuals, found no evidence of HIV-1 superinfection, though many of these individuals were receiving antiretroviral therapy (6, 9, 50). In contrast, a study of Thai intravenous drug users found two cases of HIV-1 superinfection among 130 chronically infected individuals (41). More recently, three population-based studies found that HIV-1 superinfection occurred at a rate close to that of initial infection. In a study of high-risk women in Kenya, the incidence of superinfection was approximately 4% per year (7, 38), approximately half the incidence of primary infection in the same cohort of 8% per year (15). Among a cohort of men in southern California, the incidence of superinfection was 5% (45), which was equal to the initial infection rate of 5% per year in a similar cohort (12). The frequent detection of superinfection in these more recent studies calls into question what role, if any, immunity to the first strain has in protection from the second strain.
The relatively small number of well-characterized cases of superinfection has limited analysis of the role of the immune response in superinfection. Thus, it remains unclear whether only a subset of individuals with particularly poor immune responses succumb to superinfection or whether immune responses during HIV-1 infection are in general inadequate to prevent infection. All six superinfected subjects in whom cellular immune responses have been assessed had cytotoxic T lymphocytes (CTL) directed toward their initial strain, as measured by gamma interferon enzyme-linked immunospot assay (1, 13, 41, 47, 55). While there were differences between the studies in the number of potential epitopes evaluated, the breadth of the immune responses to the initial HIV-1 strain varied in these superinfected individuals, with four individuals having very broad responses to multiple epitopes (1, 41, 55) and two individuals having relatively narrow responses predominantly directed to a single epitope (13, 47). In four of these cases, at least some of the CTL present were cross-reactive with the superinfecting strain prior to reinfection (1, 41, 47, 55). However, in all six cases at least some of the targeted CTL epitopes were altered in the superinfecting strains, which could have contributed to the ability of these strains to establish infection (1, 13, 41, 47, 55). Furthermore, as CTL play a critical role in controlling an established infection but are ineffective in preventing initial infection (reviewed in references 5 and 35), it is perhaps not surprising that these cellular responses were insufficient to prevent reinfection.
Unlike CTL, neutralizing antibodies (NAbs) to HIV-1 can prevent infection in animal models (reviewed in reference 46); NAbs, therefore, might be able to prevent superinfection in humans if sufficiently broad and potent. Among two cases of superinfection described by Ramos et al., binding antibodies to the V3 region were generated to the initial strain though these did not appear to cross-react with the superinfection strain (41). Moreover, in this study, neutralization was not assessed (41). In a third case, only weak NAbs were present to the initial virus, and these antibodies did not neutralize the superinfecting strain (1). In the only study to compare superinfected individuals to those with similar risk factors who did not become superinfected, three individuals who became superinfected had weak NAb responses to their initial infection (44). These three superinfections all occurred relatively early, within 6 months of initial infection. In addition, this study examined the ability of plasma from the superinfected individuals to neutralize just three viruses, and responses to the superinfecting virus itself were not examined. In order to more rigorously evaluate the potential role of NAb responses in protection from superinfection, we assessed the NAb responses in six cases of superinfection that occurred throughout the course of chronic HIV-1 infection using a larger panel of viruses, including the superinfecting strains.
MATERIALS AND METHODS
Study population.
The individuals in this study were part of a prospective cohort study of high-risk women from Mombasa, Kenya, in which timing of the first infection is defined by both HIV-1 serology and HIV RNA testing (23-25). All of the women were HIV-1 infected through heterosexual contact, and none reported antiretroviral therapy during follow-up for this study although some have since started therapy due to CD4 counts of <200/μl. From this cohort, a total of 56 individuals were screened for superinfection by analysis of env sequences (7) or both env and gag sequences (38) in peripheral blood mononuclear cells (PBMCs) within the first year of infection and again ∼5 years later. Presumed cases of superinfection were confirmed using phylogenetic analyses, and the time of superinfection was determined by examining the HIV sequences in intervening PBMC samples, typically collected at 3-month intervals, using both phylogenetic analysis and subtype-specific PCR (7, 38). The superinfection cases and controls for the present study were identified from among these 56 women. Verbal or written informed consent was obtained from all patients. The ethical review committees of the University of Nairobi, the University of Washington, and the Fred Hutchinson Cancer Research Center approved this study.
Cloning of full-length, functional env genes.
In most cases, full-length env genes for the virus panel and from superinfection cases were cloned directly from PBMC DNA by limiting dilution nested PCR as described previously (21, 54). Because insufficient PBMC DNA was available, env clones from subjects QD022 and QB008 were cloned from DNA from cultured PBMCs. The virus culture was maintained for a maximum of 21 days, during which time there is limited impact on the representation of the major viral species (53). Because there is variability in the sequence targets for amplification, conditions were optimized for each subject; primers and PCR conditions for each case are listed in Table S1 in the supplemental material. The full-length sequences obtained were highly representative of the sequences obtained previously by amplification of the V1-V5 region (7, 38). The PCR products were digested with MluI and NotI restriction enzymes (Invitrogen) and cloned into the pCIneo vector (Promega). In the one case where the restriction sites were not compatible (the env genes from subject QA013), PCR products were cloned into pcDNA3.1/V5-His-TOPO vector (Invitrogen). All env sequences were evaluated to determine if recombination had occurred between the initial and the superinfecting strains, as defined previously (7, 38). Only one sequence, from subject QB008, was a recombinant sequence. As it could not be conclusively established whether this recombination event occurred in the subject or during the PCR amplification, this variant was not studied further. To generate pseudotyped viral particles, plasmid DNA was transfected into 293T cells along with an envelope-deficient HIV-1 subtype A proviral plasmid, Q23Δenv, as described previously (21). Pseudotyped viruses were screened for infectivity by a single-round infection of TZM-bl cells (29) (AIDS Research and Reference Reagent Program, National Institutes of Health). Approximately 50% of cloned env genes were capable of mediating infection; the complete sequences of these functional clones, each from an independent PCR, were determined using a BigDye Terminator, version 3.1, cycle sequencing kit (Applied Biosystems) according to the manufacturer's instructions.
Phylogenetic analyses.
Full-length env sequences were examined using the BLAST search tool from the National Center for Biotechnology Information (NCBI; at http://www.ncbi.nlm.nih.gov/blast/Blast.cgi) to rule out contamination from other laboratory strains or sample mix-up. Full-length env sequences from the novel clones as well as sequences from the original V1-V5 sequences used to define the superinfection cases (7, 38) were assembled using Sequencher software (Gene Codes). The V2-V5 regions were then aligned using Clustal X (49), and manually edited using MacClade, version 4.01 (22), to remove regions that could not be unambiguously aligned, as described previously (7, 38). Phylogenetic trees were then constructed by neighbor-joining using an HKY85 model in PAUP* (version 4.01b10) (48). Viral subtype was defined using the NCBI genotyping database (http://www.ncbi.nlm.nih.gov/) and by phylogenetic analyses with reference sequences from the Los Alamos National Laboratory HIV database (http://www.hiv.lanl.gov/).
Neutralization assays.
Neutralization was assessed in triplicate with at least two independent preparations of pseudoviral stock using the TZM-bl neutralization assay as described previously (3, 29, 54). Briefly, 500 infectious particles of pseudovirus, as determined by infection of TZM-bl cells, were incubated with serial dilutions of plasma for 1 h; then TZM-bl indicator cells were added, and infection levels were determined by assessing β-galactosidase activity after 48 h. Median inhibitory concentrations (IC50s) were defined as the reciprocal dilution of plasma that resulted in 50% inhibition, calculated as described previously using the linear portion of the neutralization curve (3, 54). The standard plasma pool collected from 30 HIV-1-infected individuals in Kenya between 1998 and 2000 has been previously described (3).
Calculation of breadth and potency scores.
Breadth and potency scores were calculated for each plasma sample to compare neutralization between cases and controls. A median IC50 value was assigned to each panel virus, based on the median of all the IC50 values from every plasma sample tested against that virus. To define a breadth score for an individual plasma sample, the plasma/virus combinations in which the IC50 was above the median IC50 defined for that virus with all plasma samples were given a score of 1, and those below were scored as 0. The overall breadth score was determined by summing these numbers for all 16 viruses tested. The potency score was derived by dividing the IC50 value of a given plasma/virus combination by the median virus IC50 value. As with the breadth score, the potency scores against all 16 panel viruses were added to obtain an overall potency score. Thus, if a plasma sample had an IC50 of 250 for a virus whose median score was 50, this plasma would receive 1 breadth point but 5 potency points toward its total score.
Statistical analyses.
All statistical analyses were performed using Intercooled Stata, version 10.0 (College Station, TX). In order to compare median IC50, mean IC50, breadth score, and potency score between superinfection cases and controls, the values for the three controls for each case were averaged. The matched values for superinfection cases and controls were compared by a Wilcoxon signed ranks test. In order to determine whether the timing of the superinfection sample influenced the neutralization scores, the number of years postinfection (ypi) was compared with the mean, median, breadth, or potency scores using a Spearman rank correlation. A two-sample Wilcoxon rank sum test was used to compare the plasma pool neutralization sensitivity of the superinfecting variants to that of the initial variants. The same test was used to compare neutralization sensitivity of superinfecting and early variants.
Nucleotide sequence accession numbers.
The novel sequences from the superinfected subjects have been deposited in the GenBank database under accession numbers FJ396012 through FJ396031.
RESULTS
Breadth and potency of NAb responses in superinfection cases and controls.
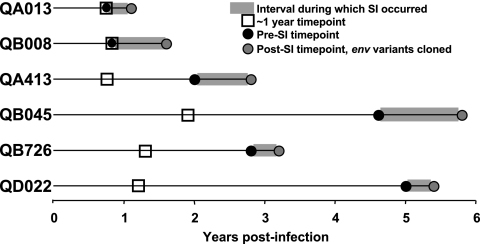
Six cases of superinfection that occurred between ∼1 to 5 years following initial infection were previously identified within a female sex worker cohort study in Kenya (Fig. 1 and Table 1) (7, 38). Viral loads were approximately 40,000 copies/ml in most individuals near the time of superinfection (7, 38). Absolute CD4 counts were available prior to superinfection in only two subjects, and both had CD4 counts exceeding 500 cells/μl (38). The remaining four subjects had CD4 counts exceeding 200 cells/μl at the first available time point following superinfection, which ranged from 1 to 4 years following superinfection (7, 38). Thus, none of these individuals appeared to be in an advanced state of immunosuppression at the time of reinfection. In order to determine whether deficits in the breadth and potency of their NAb responses were a correlate of superinfection, we examined three controls in which we could not detect superinfection for each case. Controls were matched to cases according to the initial HIV subtype (A, C, or D), the timing of plasma samples in relation to initial infection, and the viral load (Table 2). NAb responses in cases and controls were examined at approximately 1 year following initial infection in all subjects to allow us to compare all individuals at the same time point (Fig. 1 and Table 2), as well as just prior to documented superinfection (Fig. 1 and Table 2). In order to assess the breadth and potency of the plasma from the superinfection cases and controls, we tested a panel of 16 HIV-1 pseudoviruses representing transmitted variants from various HIV-1 subtypes including A, A/D recombinant, C, D, and B. The majority of variants were cloned from individuals from Kenya (3, 54; also unpublished data), except for 6535.3 (B5), THRO4156.18 (B15), and Du156.12 (C1), which are part of the previously described standard subtype B and C virus panels (17, 18). These variants were selected for the panel on the basis of their neutralization sensitivity to pooled plasma from HIV-1-infected individuals in Kenya. Selecting variants that showed detectable neutralization with pooled plasma increased the chances of observing, and thus differentiating, neutralizing activity between individual plasma samples.
FIG. 1.
Timing of analyzed samples for the six superinfection cases. Each of the six superinfection cases is identified on the left, with a horizontal line indicating the time since the initial infection, according to the scale at the bottom. The interval during which superinfection occurred is indicated by the gray bar. The ∼1-year time point from which plasma samples were evaluated is indicated by the open squares, and the presuperinfection time point is indicated by the black circles. For subjects QA013 and QB008, the ∼1-year time point and the presuperinfection time point were the same since superinfection occurred at approximately 1 year. The gray circles indicate the first time point at which the superinfecting variants were identified; all env variants were cloned from this time point. SI, superinfection.
TABLE 1.
Characteristics of superinfection casesa
| Case | Estimated timing of superinfection (ypi) | Initial infecting subtype | Superinfecting subtype |
|---|---|---|---|
| QA013 | 0.72-1.1 | D | A |
| QB008 | 0.83-1.6 | C | C/A |
| QA413 | 2.0-2.8 | A | A |
| QB045 | 4.6-5.8 | A2 | A |
| QB726 | 2.8-3.2 | A | A |
| QD022 | 5.0-5.4 | A | C |
TABLE 2.
Selection of cases and controls for assessment of breadth and potency of NAb responses
| Group and case | Case time point (ypi) | Case VL (log10 copies/ml) | Control time point mean (ypi)a | Control VL mean (log10 copies/ml) |
|---|---|---|---|---|
| Cases at ∼1 year | ||||
| QA013 | 0.72 | 5.12 | 0.76 | 4.79 |
| QB008 | 0.83 | 4.51 | 0.86 | 4.50 |
| QA413 | 0.76 | 5.60 | 0.97 | 4.70 |
| QB045 | 1.9 | 4.49 | 1.9 | 4.24 |
| QB726 | 1.3 | 3.90 | 1.1 | 3.88 |
| QD022 | 1.2 | NDb | 1.2 | 4.47 |
| Presuperinfection cases | ||||
| QA013 | 0.72 | 5.12 | 0.76 | 4.79 |
| QB008 | 0.83 | 4.51 | 0.86 | 4.50 |
| QA413 | 2.0 | 4.94 | 2.0 | 4.91 |
| QB045 | 4.6 | 3.78 | 4.7 | 4.93 |
| QB726 | 2.8 | 1.70 | 2.8 | 4.08 |
| QD022 | 5.0 | 3.81 | 5.0 | 4.67 |
For each case, 3 controls per case were matched according to HIV-1 subtype, timing of plasma samples tested, and VLs. Insufficient data were available to compare CD4 counts between cases and controls.
ND, not done.
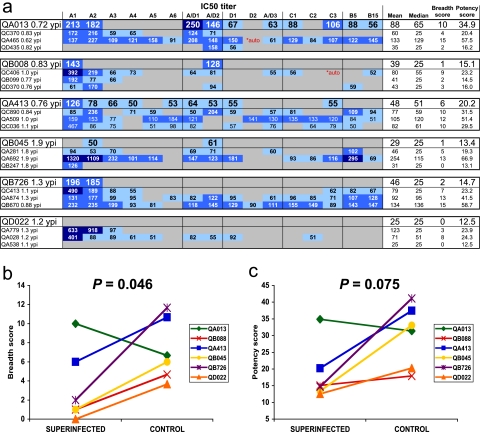
We assessed the ability of plasma to neutralize the panel viruses by determining the median IC50 (defined as the reciprocal dilution of plasma that resulted in 50% inhibition) for each plasma/virus pair at ∼1 ypi (Fig. 1 and Fig. 2a). Plasma from both cases and controls exhibited a broad range of neutralizing potency against these transmitted variants (Fig. 2a). To quantitate potential differences between superinfection cases and matched controls, each case was compared to the average of the three controls per case. The mean IC50 value was lower in the superinfecting plasma than in the controls (P = 0.05) while there was a trend for a lower median IC50 value in superinfection cases than in controls (P = 0.07). Because each panel virus had a different overall neutralization sensitivity, we normalized the IC50 value of each virus/plasma pair to the median IC50 for each virus with all plasma to calculate breadth and potency scores. At ∼1 year after initial infection, the superinfection cases had significantly lower breadth scores than the matched controls (Wilcoxon signed ranks test, P = 0.046) (Fig. 2b). In addition, there was a trend toward less potency in the NAb responses at ∼1 year in superinfection cases than in controls (P = 0.075) (Fig. 2c). To ensure that variability in the sample timing was not altering results, we compared the breadth or potency scores with the number of years postinfection by Spearman rank correlation. There was no significant relationship between the timing of the plasma samples and the breadth (P = 0.36) or the potency (P = 0.51) scores. As much of the neutralizing activity observed was relatively weak, we also calculated 70 and 80% inhibitory concentration (IC70 and IC80, respectively) values (see Fig. S1 and S2 in the supplemental material). On the basis of IC70 values, we observed less potency and a trend for less breadth among superinfection cases than in controls (see Fig. S1 in the supplemental material). IC80 values were generally below the limit of detection, and no significant differences in neutralizing activity were observed between superinfection cases and controls (see Fig. S2 in the supplemental material).
FIG. 2.
NAb responses at approximately 1 ypi. (a) The plasma samples tested are displayed along the left, with a subject identification code followed by the number of years postinfection at which the plasma sample was obtained. Each superinfection case is displayed in larger font at the top of a group, with the three matched controls in the box beneath. The 16 viruses tested are shown at the top, followed by a column for the mean, median, breadth score, and potency scores for each plasma sample (four rightmost columns). The 16 panel viruses are abbreviated by subtype along the top. The virus variants are: A1, Q461d1; A2, Q168b23; A3, Q842d16; A4, BJ613.E1; A5, BS208.B1; A6,Q769b9; A/D1, BF535.A1; A/D2, QA790.204I.ENV.C1; D1, QD435.100 M.ENV.A4; D2, QA465.59 M.ENV.D1; A/D3, QZ100.ENV.D83; C1, Du156.12; C2,QB099.391 M.ENV.C8; C3, QC406.70 M.ENV.F3; B5, 6535.3; B15, THRO4156.18. IC50 values are shown as numerical values in the table. The data are color coded, with darker blue boxes denoting more potent neutralization. A gray bar indicates that <50% neutralization was observed at a plasma dilution of 1:50, which was the highest dilution tested. For the purposes of statistical analyses, these IC50 values were assigned a level of 25. The two values marked “*auto” in red indicate that the plasma sample was autologous to the panel virus and was therefore not included in calculation of the breadth and potency scores. (b) Comparison of breadth scores between superinfection cases and the average value from the three matched controls for each case. Breadth scores are shown along the y axis, and the superinfecting and control groups are compared along the x axis. The lines between the data points denote comparison between the superinfection cases and the matched controls. P values for the superinfection cases compared to controls were obtained by comparing scores with the Wilcoxon signed ranks test. (c) Comparison of potency scores, displayed as per breadth scores in panel b.
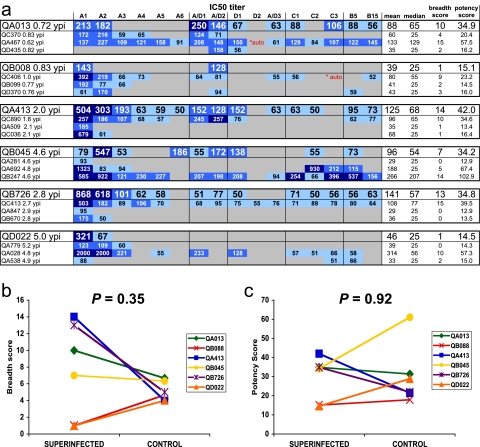
Plasma samples from the superinfection cases and controls were then compared at the time point immediately prior to superinfection, as the breadth and the potency of the NAbs at this time should reflect those faced by the entering superinfecting virus (Fig. 1 and 3a and Table 2). NAbs present in four of the six superinfection cases (QA013, QA413, QB045, and QB726) could neutralize the majority of the heterologous panel viruses at this time (Fig. 3a). There was no detectable difference between the superinfection cases and controls in the breadth (P = 0.35) (Fig. 3b) or the potency (P = 0.92) (Fig. 3c) of their NAb responses just prior to superinfection. Similarly, there were no significant differences in either mean (P = 0.92) or median (P = 0.92) IC50 values between superinfection cases and controls. Furthermore, as with the ∼1-year time point, there was no significant correlation between the timing of the samples and the breadth (P = 0.31) or potency (P = 0.65) scores. Finally, comparison of IC70 and IC80 values also did not reveal any significant differences between cases and controls (see Fig. S3 and S4 in the supplemental material).
FIG. 3.
NAb responses immediately prior to superinfection. (a) IC50 values for plasma/virus combinations are presented as described in the legend to Fig. 2a. Here, the viruses tested are the same as in the experiment shown in Fig. 2, but the plasma tested was from a different time point, i.e., the time point immediately prior to documented superinfection. (b) Comparison of breadth scores between superinfection cases and matched controls prior to superinfection as described in the legend of Fig. 2b. (c) Comparison of potency scores between superinfection cases and matched controls prior to superinfection as described in the legend of Fig. 2c.
Characterization of superinfecting envelope variants.
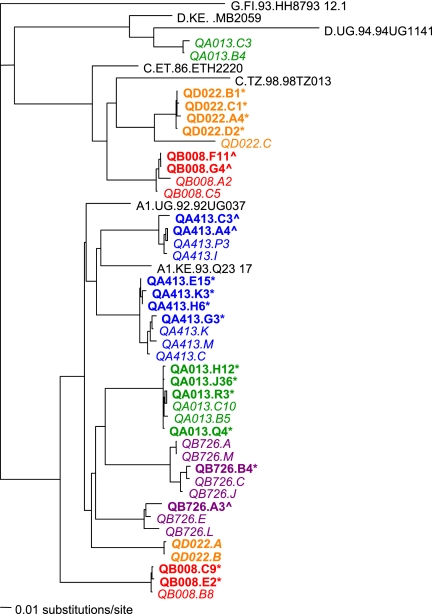
In order to evaluate the neutralization profile of the superinfecting variants, we cloned full-length, functional HIV-1 envelope variants from the first time point in which superinfecting variants were detected. Clones representing the envelope from the superinfecting variants were obtained for five of the six cases; in subject QB045 the superinfecting variant was always a minority variant (38), and despite cloning >10 functional variants, we were unable to obtain a superinfecting variant and could not evaluate this case further (data not shown). In three cases (QB008, QA413, and QB726), we cloned variants representative of both the initial and superinfecting variants, as defined by sequence analyses in previous studies (Fig. 4) (7, 38). In two cases, only clones representing the superinfecting variant were obtained (Fig. 4), presumably because the superinfecting strain became dominant (QA013) or replaced the initial strain (QD022). The V1-V5 sequences within the full-length env variants were representative of the previously described sequences (Fig. 4). The diversity observed at this early time point could reflect the transmission of multiple variants, as has been previously observed among women infected heterosexually (20). The diversity and evolution of the superinfecting variants following reinfection have been discussed in more detail previously (7, 38).
FIG. 4.
Neighbor-joining phylogenetic tree of V2-V5 sequences from superinfection cases. Reference sequences for subtype G, D, C, and A from the Los Alamos HIV database (http://www.hiv.lanl.gov/content/index) are displayed in black. Sequences from each subject are denoted in a separate color. For reference, sequences that were originally obtained from these cases using primers that amplify a subgenomic portion of envelope (V1-V5) (7, 38) are shown in italics. The case reference sequences were obtained from the first time point at which the superinfecting variants were detected and contain representatives of both the initial and superinfecting strains, except for case QD022, in which the superinfecting variants completely replaced the initial variants. Thus, the QD022.A and QD022.B initial sequences in bold italics were from a presuperinfection time point (0.14 ypi). Sequences from the full-length env clones from these same individuals isolated as part of this study are shown in bold print, with an asterisk denoting a superinfecting sequence and a caret denoting a sequence from the initial virus population, as defined in the previous study. Full-length envelope sequences were cloned either directly from PBMC DNA (subjects QA013, QB726, and QA413) or from PBMC DNA following short-term coculture with uninfected PBMCs (subjects QB008 and QD022) from the first time point at which superinfecting sequences were detected.
In order to determine whether the superinfecting variants were particularly neutralization resistant, the sensitivity of these variants to a plasma pool derived from 30 HIV-positive individuals in Kenya (3) was examined. No significant differences in the neutralization sensitivity of the superinfecting variants was observed compared to either the initial viruses from the superinfection cases or a collection of viruses pseudotyped with envelope variants of HIV-1 derived from other infected individuals early in their infections (Table 3). Thus, the superinfecting variants did not appear to be inherently neutralization resistant.
TABLE 3.
Neutralization sensitivity of superinfecting, initial, and early variants of HIV-1 to a pool of plasma from HIV-1 infected individuals
| HIV-1 variant typea | No. of subjects | Median IC50b | P valuec |
|---|---|---|---|
| Superinfecting | 5 | 58.9 | |
| Initial | 3 | 114 | 0.18 |
| Early | 13 | 60.0 | 0.80 |
Initial variants from the superinfection cases were cloned at the time point when the superinfecting variants were first detected. Early variants represent full-length functional HIV-1 variants from within 1 year of infection that were cloned from individuals infected with subtypes A, A/D recombinant, C, and D in Kenya. The plasma pool was collected from 30 HIV-1-infected individuals in Kenya between 1998 and 2000 and has been previously described (13).
Since more than one variant was examined from each subject and these variants might be more similar to each other than those from other subjects, a median IC50 value of all the variants per person was determined. These per person IC50 values were then used to compare between superinfecting, initial, and early variants.
P values were determined by two-sample Wilcoxon rank sum testing for superinfecting variants compared to either initial or early variants.
Neutralization of superinfecting variants by plasma from the superinfected individuals.
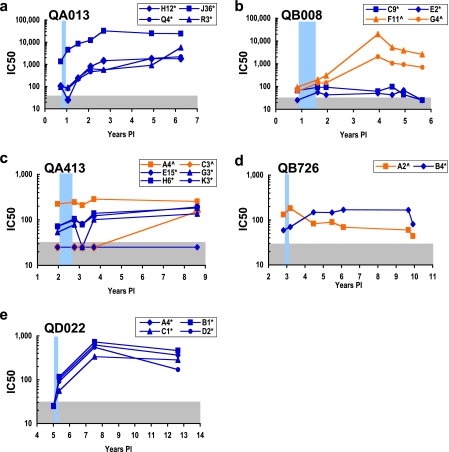
To more specifically examine the NAb sensitivity of the envelope of variants from superinfected individuals near the predicted time of exposure, each variant was tested against plasma from the time point just prior to documented superinfection (Fig. 5). In addition, variants were tested against plasma samples from later in infection in order to determine whether these individuals were able to mount an autologous response to these variants (Fig. 5). In subject QA013, superinfection occurred between 0.72 and 1.1 ypi (7). Three of the four superinfecting variants (denoted by an asterisk) from 1.1 ypi were neutralized by plasma from 0.72 ypi, with IC50 values of 106 for QA013.H12*, 113 for QA013.Q4*, and 94 for QA013.R3* (Fig. 5a). Variant QA013.J36* was remarkably neutralization sensitive, with an IC50 value of 1,343 with the presuperinfection plasma and IC50 values exceeding 25,000 with later plasma samples (Fig. 5a). This subject mounted a potent autologous NAb response, with IC50 values of >1,800 to all of the superinfecting variants at 6.3 ypi (Fig. 5a).
FIG. 5.
Neutralization of superinfecting and initial variants by plasma from the superinfected individuals. Each panel represents the evolving NAb response in the superinfected subject indicated in the upper left. The autologous plasma IC50 value against the various initial and superinfecting env variants is plotted over time. Superinfecting variants are denoted with blue symbols, and initial variants are indicated with orange symbols. All the env variants were cloned from the time point immediately after superinfection, and the light-blue bars denote the interval in which superinfection occurred. The gray bar indicates the limit of detection of our assay. When a virus was neutralized <50% at a plasma dilution of 1:50, the highest dilution tested, the IC50 value was assigned a level of 25 and is within the gray area. PI, postinfection.
In subject QB008, superinfection occurred between 0.83 and 1.6 ypi (7), and two initial and two superinfecting variants were cloned at 1.6 ypi (Fig. 4). One of the two superinfecting variants (QB008.C9*) was weakly neutralized by the plasma from 0.83 ypi, with an IC50 of 66 (Fig. 5b). The other superinfecting variant, QB008.E2*, failed to reach 50% neutralization at a 1:50 dilution of plasma, which was the highest concentration tested, making it resistant in this assay (Fig. 5b). All of the variants were neutralized by autologous plasma at the contemporaneous time point with IC50 values of 56 to 202, and this subject went on to develop a preferential NAb response to the initial virus variants (Fig. 5b).
We first detected superinfection in subject QA413 at 2.8 ypi (38) and cloned six viral variants from that time point: two representing the initial strain and four representing the superinfecting virus. Plasma from 2.0 ypi was able to weakly neutralize three of the four superinfecting variants, with IC50 values of 53 for QA413.G3*, 72 for QA413.H6*, and 72 for QA413.K3* (Fig. 5c). The fourth superinfecting variant, QA413.E15*, was resistant to neutralization by plasma from 2.0 ypi. One of the initial viral variants (denoted by the caret), QA413.C3^, was resistant to neutralization at all but the latest time point evaluated, while the other initial variant, QA413.A4^, was moderately sensitive to neutralization, with IC50 values from 210 to 285 throughout the course of infection. This subject developed only moderate autologous NAb responses, with IC50 values from 77 to 193 to all the variants tested (Fig. 5c), despite good breadth in the heterologous NAb responses (Fig. 3a).
Subject QB726 was superinfected between 2.8 and 3.2 ypi (38). The superinfecting variant cloned at 3.2 ypi was neutralized by the plasma from 2.8 ypi with a IC50 of 59, while the initial variant was slightly more susceptible to this plasma sample with a IC50 value of 132 (Fig. 5d). This subject, despite a broad and potent heterologous NAb response prior to superinfection (Fig. 3a), developed only moderate NAb responses to the variants cloned at 3.2 ypi, with IC50 values of 50 to 170 to these autologous variants (Fig. 5d).
Subject QD022 was superinfected between 5.0 and 5.4 ypi (38). In this subject, all of the superinfecting variants cloned from 5.4 ypi were resistant to the 5.0 ypi plasma sample (Fig. 5e). These variants were weakly neutralized by the contemporaneous plasma sample, with IC50 values of 56 to 104, but subsequent plasma samples neutralized these variants more potently, with IC50 values of 170 to 726 (Fig. 5e).
Overall, one of the two subjects with relatively narrow NAb responses prior to superinfection (Fig. 3a, QB008) was still able to neutralize one of her superinfecting strains, while the other subject with relatively narrow responses (QD022) was reinfected with viral strains that were resistant to host NAbs. Three subjects with relatively broad NAb responses prior to superinfection (QA013, QA413, and QB726) (Fig. 3a) were able to neutralize the majority of their superinfecting viruses prior to their reinfection with these strains. All individuals were able to mount autologous NAb responses to their superinfecting variants following reinfection (Fig. 5), indicating that these variants were not inherently neutralization resistant, consistent with the findings using pooled plasma (Table 3).
DISCUSSION
We comprehensively evaluated the role of the breadth and the potency of the HIV-1 NAb response in protection from infection using six cases of superinfection that were identified among a cohort of female sex workers with extensive long-term follow-up (7, 38). At the time of superinfection, no significant deficits in NAb responses were observed in the superinfected individuals compared to matched controls. Thus, even NAb levels typically found during chronic infection can fail to protect from reinfection with circulating strains of HIV-1. Furthermore, in four of five cases evaluated, superinfection occurred despite preexisting plasma NAbs capable of neutralizing the strains that established the second infection.
The breadth and potency of the NAb responses were heterogeneous among these individuals at the time of exposure to the superinfecting virus. As the variants within the virus panel were chosen on the basis of their neutralization sensitivity to pooled plasma, most individuals were able to neutralize at least one of these heterologous variants. Overall, the breadth and potency of the NAb responses were similar to those found in the matched controls and other chronically infected individuals. In particular, four subjects had relatively robust NAb responses, while two others had comparatively narrow responses. In fact, the four superinfected subjects with the broadest NAb responses could neutralize the ∼70% of the viruses within the panel with average IC50s of ∼110, while the matched controls neutralized ∼40% of the panel viruses with average IC50s of ∼90. Admittedly, limitations in the numbers of cases and controls limited the robustness of the statistical analyses. We therefore compared the NAb responses of the superinfected individuals to those of 72 individuals from the same cohort whose NAb responses were assessed at 5 ypi (K. Bosch, D. Panteleeff, and J. Overbaugh, unpublished data). Three superinfected subjects (QA413, QB045, and QB726) had breadth and potency scores within the upper quartile of these 5-year responses. Subject QA013 had breadth and potency scores at the median of the 5-year NAb responses despite having been superinfected relatively early after the first infection (∼1 year), at a time when the breadth and potency of the NAb response has not yet peaked (42). These 72 women were not selected based on any clinical or immunological findings; thus, they represent typical NAb responses in high-risk African women during chronic infection. Four of the six superinfections therefore occurred in individuals with relatively broad and potent NAb responses at the time of exposure. It is difficult to draw precise comparisons with our data and NAb responses observed in other cohorts because of differences in assays and test strains used. In addition, most of the published studies have focused on selected individuals, particularly long-term nonprogressors, which is not an ideal comparison group (2, 8, 19, 30, 32, 39, 57). While rare individuals in these populations exhibited apparently broader and more potent NAb responses (e.g., neutralization of ∼90% of viruses tested at average IC50s of ∼230) (8) than the women in our study, many other individuals exhibited less breadth and potency in NAb responses against the test strains used. On the basis of all these comparisons—including to matched controls, to chronically infected women in the same population, and to published studies—we conclude that the women who became superinfected did not have deficits in the breadth or potency of their NAb responses relative to other HIV-infected groups when they became superinfected. Overall, NAb responses in superinfected individuals were typical of HIV-1 infection, ranging from narrow to relatively broad.
At ∼1 ypi, we observed relatively narrow NAb responses in superinfected individuals compared to controls. This early lack of breadth in the superinfected women mirrors the findings in three superinfected men who acquired HIV-1 through sex with men (44). Interestingly, this same association was observed despite the fact that the study of Smith et al. used a small number of primarily laboratory-adapted viruses whereas ours employed a large collection of variants cloned directly from infected individuals near the time of transmission. This early association between breadth and risk of superinfection in both studies suggests that some association between HIV-1-specific antibodies and risk of superinfection may exist early in infection. However, we did not observe a correlation between NAb breadth at the time of superinfection and whether a woman became superinfected. At these later times, which seem to be a more relevant measure of the role of NAbs in protection from infection, there was a broadening of the NAb responses in most individuals prior to their documented superinfection.
Perhaps a more important measure of the role of NAbs in protection from superinfection is the study of the antigenicity of the specific viruses that established the second infection. In this study, we examined such viruses derived from the first time point following documented superinfection. We found that these viruses were not unusually neutralization resistant as there were no differences in the neutralization sensitivities of these variants and other circulating variants to pooled plasma from HIV-1-infected individuals. Moreover, the infected individuals were able to mount autologous NAb responses to the superinfecting variants, suggesting that the strains could themselves elicit NAbs. Importantly, the superinfecting variants were often susceptible to neutralization by plasma from the person who became infected by these strains: in four of five subjects, at least one superinfecting variant was susceptible to the host plasma from the time prior to superinfection. Even if a “sieve” effect weeded out the majority of the neutralization-sensitive viral variants from the donor virus, at least some of these variants apparently established infection despite encountering NAbs capable of neutralizing them. While we cannot rule out that these variants evolved within a narrow window after transmission, it is not clear what would drive them to become more susceptible to neutralization. Overall, these data suggest that the levels of NAbs found in these individuals were insufficient to prevent infection even by variants that showed some susceptibility.
Several caveats to these data need to be considered. First, as with all cases of superinfection, it remains possible that the superinfecting viruses were present at low levels and/or compartmentalized prior to their first detection. We have reasonable confidence that the superinfecting strains were detected soon after they became established in these cases because we used a sensitive subtype-specific PCR assay that gives >92% probability of detecting a strain present with a prevalence of 5% to define the time of superinfection (38). Moreover, the women in this cohort have relatively few partners (on average 1 to 2 per week) (16), making superinfection in a short time frame less likely than reported in women who have many more partners (11). Secondly, we cannot conclude that control subjects were protected from superinfection because superinfections could be missed if the second virus did not persist (56) or recombined with the initial virus within the regions of the genome analyzed (38). If such cases were missed within the control group, this would decrease our ability to detect differences between cases and controls. Third, new infections are generally established at mucosal sites, where NAb levels could be lower than those assessed in the plasma, possibly allowing local establishment and spread of infection before immune control could be attained. It is therefore possible that differences could be observed in NAb levels at the mucosal sites, in particular, against the superinfecting strains near the time of infection.
While this study suggests that the presence of any detectable NAbs of the proper specificity may not protect against HIV-1 infection, it does not rule out the possibility that such antibodies would be effective if present at higher levels. These data are consistent with the nonhuman primate (NHP) model, where sterile protection from infection generally required very high NAb levels that produced >99% in vitro neutralization of the challenge simian-human immunodeficiency virus with between 1:8 and 1:200 dilutions of plasma (26, 27, 31, 34, 36). While differences in the assays used to assess neutralization between the NHP studies and our human study make direct comparisons difficult, the NAb levels in the superinfected individuals were probably not at this potency. Even the unusually neutralization-sensitive variant QA013.J36* was neutralized at 90% by host plasma at a 1:78 dilution but did not achieve 99% neutralization at the lowest plasma dilution tested (1:50). The remaining superinfecting variants were neutralized at levels of <90% with a 1:50 dilution of plasma and were therefore not at the levels required for sterilizing immunity in the NHP model. Thus, it remains unclear whether the higher levels of NAbs achieved by passive transfer within the NHP model would be protective in humans.
These findings in exposed humans, where there is extensive diversity in potential infecting strains, unfortunately suggest a high bar for the levels of antibodies required for eliciting protective immunity. While weak, narrow NAb responses could have contributed to superinfection in a subset of individuals, others became reinfected despite relatively robust NAb responses to their first strain. Since NAbs can clear virus without dependence on the cellular immune system, the levels of antibodies required for protection in these previously infected individuals are likely similar to those required in uninfected, vaccinated individuals. Furthermore, most effective vaccines are thought to provide protection primarily by stimulating neutralizing antibodies to clear cell-free virus (35, 40); thus, the assays used here should provide a valid measure of viral protection by this mechanism. An effective HIV-1 vaccine will therefore need to elicit more robust NAb responses than found during natural infection. Indeed, this is the case for some other viral vaccines, such as those for hepatitis B and human papillomavirus, which elicit equivalent or higher levels of NAbs than natural infection (4, 33, 52). HIV-1 presents additional challenges because of the extreme genetic diversity of the virus. Our results suggesting that reinfection occurs even in individuals who have antibodies capable of neutralizing diverse strains further underscore this challenge.
Supplementary Material
Acknowledgments
We thank like the Mombasa research team for their support and efforts, Stephanie Rainwater for assistance with phylogenetic analyses, and Anne Piantadosi for helpful comments on the manuscript. We also gratefully acknowledge the women who participated in the study.
This study was supported by NIH grant AI38518 to J.O. and grant K08 AI068424-01 to C.A.B. C.A.B. was also supported in part by NIH training grant T32AI07140.
Footnotes
Published ahead of print on 8 October 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420434-439. [DOI] [PubMed] [Google Scholar]
- 2.Beirnaert, E., P. Nyambi, B. Willems, L. Heyndrickx, R. Colebunders, W. Janssens, and G. van der Groen. 2000. Identification and characterization of sera from HIV-infected individuals with broad cross-neutralizing activity against group M (Env clade A-H) and group O primary HIV-1 isolates. J. Med. Virol. 6214-24. [PubMed] [Google Scholar]
- 3.Blish, C., R. Nedellec, K. Mandaliya, D. Mosier, and J. Overbaugh. 2007. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS 6693-702. [DOI] [PubMed] [Google Scholar]
- 4.Bocher, W. O., S. Herzog-Hauff, W. Herr, K. Heermann, G. Gerken, K. H. Meyer Zum Buschenfelde, and H. F. Lohr. 1996. Regulation of the neutralizing anti-hepatitis B surface (HBs) antibody response in vitro in HBs vaccine recipients and patients with acute or chronic hepatitis B virus (HBV) infection. Clin. Exp. Immunol. 10552-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, S. A., J. L. Hurwitz, X. Zhan, P. C. Doherty, and K. S. Slobod. 2005. CD8+ T-cells: are they sufficient to prevent, contain or eradicate HIV-1 infection? Curr. Drug Targets Infect. Disord. 5113-119. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty, B., L. Valer, C. De Mendoza, V. Soriano, and M. E. Quinones-Mateu. 2004. Failure to detect human immunodeficiency virus type 1 superinfection in 28 HIV-seroconcordant individuals with high risk of reexposure to the virus. AIDS Res. Hum. Retrovir. 201026-1031. [DOI] [PubMed] [Google Scholar]
- 7.Chohan, B., L. Lavreys, S. M. J. Rainwater, and J. Overbaugh. 2005. Evidence for frequent reinfection with human immunodeficiency virus type 1 of a different subtype. J. Virol. 7910701-10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhillon, A. K., H. Donners, R. Pantophlet, W. E. Johnson, J. M. Decker, G. M. Shaw, F. H. Lee, D. D. Richman, R. W. Doms, G. Vanham, and D. R. Burton. 2007. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J. Virol. 816548-6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzales, M. J., E. Delwart, S. Y. Rhee, R. Tsui, A. R. Zolopa, J. Taylor, and R. W. Shafer. 2003. Lack of detectable human immunodeficiency virus type 1 superinfection during 1072 person-years of observation. J. Infect. Dis. 188397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb, G. S., D. C. Nickle, M. A. Jensen, K. G. Wong, J. Grobler, F. Li, S. L. Liu, C. Rademeyer, G. H. Learn, S. S. Karim, C. Williamson, L. Corey, J. B. Margolick, and J. I. Mullins. 2004. Dual HIV-1 infection associated with rapid disease progression. Lancet 363619-622. [DOI] [PubMed] [Google Scholar]
- 11.Grobler, J., C. M. Gray, C. Rademeyer, C. Seoighe, G. Ramjee, S. A. Karim, L. Morris, and C. Williamson. 2004. Incidence of HIV-1 dual infection and its association with increased viral load set point in a cohort of HIV-1 subtype C-infected female sex workers. J. Infect. Dis. 1901355-1359. [DOI] [PubMed] [Google Scholar]
- 12.Harro, C. D., F. N. Judson, G. J. Gorse, K. H. Mayer, J. R. Kostman, S. J. Brown, B. Koblin, M. Marmor, B. N. Bartholow, and V. Popovic. 2004. Recruitment and baseline epidemiologic profile of participants in the first phase 3 HIV vaccine efficacy trial. J. Acquir. Immune Defic. Syndr. 371385-1392. [DOI] [PubMed] [Google Scholar]
- 13.Jost, S., M. C. Bernard, L. Kaiser, S. Yerly, B. Hirschel, A. Samri, B. Autran, L. E. Goh, and L. Perrin. 2002. A patient with HIV-1 superinfection. N. Engl. J. Med. 347731-736. [DOI] [PubMed] [Google Scholar]
- 14.Koelsch, K. K., D. M. Smith, S. J. Little, C. C. Ignacio, T. R. Macaranas, A. J. Brown, C. J. Petropoulos, D. D. Richman, and J. K. Wong. 2003. Clade B HIV-1 superinfection with wild-type virus after primary infection with drug-resistant clade B virus. AIDS 17F11-F16. [DOI] [PubMed] [Google Scholar]
- 15.Lavreys, L., J. M. Baeten, V. Chohan, R. S. McClelland, W. M. Hassan, B. A. Richardson, K. Mandaliya, J. O. Ndinya-Achola, and J. Overbaugh. 2006. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin. Infect. Dis. 421333-1339. [DOI] [PubMed] [Google Scholar]
- 16.Lavreys, L., J. M. Baeten, J. Overbaugh, D. D. Panteleeff, B. H. Chohan, B. A. Richardson, K. Mandaliya, J. O. Ndinya-Achola, and J. K. Kreiss. 2002. Virus load during primary human immunodeficiency virus (HIV) type 1 infection is related to the severity of acute HIV illness in Kenyan women. Clin. Infect. Dis. 3577-81. [DOI] [PubMed] [Google Scholar]
- 17.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 7910108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, M., J. F. Salazar-Gonzalez, C. A. Derdeyn, L. Morris, C. Williamson, J. E. Robinson, J. M. Decker, Y. Li, M. G. Salazar, V. R. Polonis, K. Mlisana, S. A. Karim, K. Hong, K. M. Greene, M. Bilska, J. Zhou, S. Allen, E. Chomba, J. Mulenga, C. Vwalika, F. Gao, M. Zhang, B. T. Korber, E. Hunter, B. H. Hahn, and D. C. Montefiori. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 8011776-11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y., S. A. Migueles, B. Welcher, K. Svehla, A. Phogat, M. K. Louder, X. Wu, G. M. Shaw, M. Connors, R. T. Wyatt, and J. R. Mascola. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 131032-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long, E. M., H. L. Martin, Jr., J. K. Kreiss, S. M. Rainwater, L. Lavreys, D. J. Jackson, J. Rakwar, K. Mandaliya, and J. Overbaugh. 2000. Gender differences in HIV-1 diversity at time of infection. Nat. Med. 671-75. [DOI] [PubMed] [Google Scholar]
- 21.Long, E. M., S. M. J. Rainwater, L. Lavreys, K. Mandaliya, and J. Overbaugh. 2002. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res. Hum. Retrovir. 18567-576. [DOI] [PubMed] [Google Scholar]
- 22.Maddison, D., and W. Maddison. 2005. MacClade 4: analysis of phylogeny and character evolution. Sinauer, Sunderland, MA. [DOI] [PubMed]
- 23.Martin, H. L., D. J. Jackson, K. Mandaliya, J. Bwayo, J. P. Rakwar, P. Nyange, S. Moses, J. O. Ndinya-Achola, K. Holmes, F. Plummer, E. Ngugi, and J. Kreiss. 1994. Preparation for AIDS vaccine evaluation in Mombasa, Kenya: establishment of seronegative cohorts of commercial sex workers and trucking company employees. AIDS Res. Hum. Retrovir. 10S235-S237. [PubMed] [Google Scholar]
- 24.Martin, H. L., P. M. Nyange, B. A. Richardson, L. Lavreys, K. Mandaliya, D. J. Jackson, J. O. Ndinya-Achola, and J. Kreiss. 1998. Hormonal contraception, sexually transmitted diseases, and the risk of heterosexual transmission of HIV-1. J. Infect. Dis. 1781053-1059. [DOI] [PubMed] [Google Scholar]
- 25.Martin, H. L., B. A. Richardson, P. M. Nyange, L. Lavreys, S. L. Hillier, B. Chohan, K. Mandaliya, J. O. Ndinya-Achola, J. Bwayo, and J. Kreiss. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 1801863-1868. [DOI] [PubMed] [Google Scholar]
- 26.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 734009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6207-210. [DOI] [PubMed] [Google Scholar]
- 28.McCutchan, F. E., M. Hoelscher, S. Tovanabutra, S. Piyasirisilp, E. Sanders-Buell, G. Ramos, L. Jagodzinski, V. Polonis, L. Maboko, D. Mmbando, O. Hoffmann, G. Riedner, F. von Sonnenburg, M. Robb, and D. L. Birx. 2005. In-depth analysis of a heterosexually acquired human immunodeficiency virus type 1 superinfection: evolution, temporal fluctuation, and intercompartment dynamics from the seronegative window period through 30 months postinfection. J. Virol. 7911693-11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montefiori, D. 2004. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays, p. 1-15. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, W. Strober, and R. Coico (ed.), Current protocols in immunology, vol. 12. John Wiley and Sons, New York, NY. [DOI] [PubMed] [Google Scholar]
- 30.Montefiori, D. C., G. Pantaleo, L. M. Fink, J. T. Zhou, J. Y. Zhou, M. Bilska, G. D. Miralles, and A. S. Fauci. 1996. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J. Infect. Dis. 17360-67. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura, Y., T. Igarashi, N. Haigwood, R. Sadjadpour, R. J. Plishka, A. Buckler-White, R. Shibata, and M. A. Martin. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 762123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyambi, P. N., J. Nkengasong, P. Lewi, K. Andries, W. Janssens, K. Fransen, L. Heyndrickx, P. Piot, and G. van-der-Groen. 1996. Multivariate analysis of human immunodeficiency virus type 1 neutralization data. J. Virol. 706235-6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsson, S. E., L. L. Villa, R. L. Costa, C. A. Petta, R. P. Andrade, C. Malm, O. E. Iversen, J. Hoye, M. Steinwall, G. Riis-Johannessen, A. Andersson-Ellstrom, K. Elfgren, G. von Krogh, M. Lehtinen, J. Paavonen, G. M. Tamms, K. Giacoletti, L. Lupinacci, M. T. Esser, S. C. Vuocolo, A. J. Saah, and E. Barr. 2007. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 254931-4939. [DOI] [PubMed] [Google Scholar]
- 34.Pal, R., V. S. Kalyanaraman, B. C. Nair, S. Whitney, T. Keen, L. Hocker, L. Hudacik, N. Rose, I. Mboudjeka, S. Shen, T. H. Wu-Chou, D. Montefiori, J. Mascola, P. Markham, and S. Lu. 2006. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology 348341-353. [DOI] [PubMed] [Google Scholar]
- 35.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10806-810. [DOI] [PubMed] [Google Scholar]
- 36.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 758340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pernas, M., C. Casado, R. Fuentes, M. J. Perez-Elias, and C. Lopez-Galindez. 2006. A dual superinfection and recombination within HIV-1 subtype B 12 years after primoinfection. J. Acquir. Immune Defic. Syndr. 4212-18. [DOI] [PubMed] [Google Scholar]
- 38.Piantadosi, A., B. Chohan, V. Chohan, R. S. McClelland, and J. Overbaugh. 2007. Chronic HIV-1 infection frequently fails to protect against superinfection. PLoS Pathog. 3e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilgrim, A. K., G. Pantaleo, O. J. Cohen, L. M. Fink, J. Y. Zhou, J. T. Zhou, D. P. Bolognesi, A. S. Fauci, and D. C. Montefiori. 1997. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J. Infect. Dis. 176924-932. [DOI] [PubMed] [Google Scholar]
- 40.Plotkin, S. A. 2001. Immunologic correlates of protection induced by vaccination. Pediatr. Infect. Dis. J. 2063-75. [DOI] [PubMed] [Google Scholar]
- 41.Ramos, A., D. J. Hu, L. Nguyen, K. O. Phan, S. Vanichseni, N. Promadej, K. Choopanya, M. Callahan, N. L. Young, J. McNicholl, T. D. Mastro, T. M. Folks, and S. Subbarao. 2002. Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J. Virol. 767444-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, D. M., D. D. Richman, and S. J. Little. 2005. HIV superinfection. J. Infect. Dis. 192438-444. [DOI] [PubMed] [Google Scholar]
- 44.Smith, D. M., M. C. Strain, S. D. W. Frost, S. K. Pillai, J. K. Wong, T. Wrin, C. J. Petropolous, E. S. Daar, S. J. Little, and D. D. Richman. 2006. Lack of neutralizing antibody response to HIV-1 predisposes to superinfection. Virology 3551-5. [DOI] [PubMed] [Google Scholar]
- 45.Smith, D. M., J. K. Wong, G. K. Hightower, C. C. Ignacio, K. K. Koelsch, E. S. Daar, D. D. Richman, and S. J. Little. 2004. Incidence of HIV superinfection following primary infection. JAMA 2921177-1178. [DOI] [PubMed] [Google Scholar]
- 46.Srivastava, I. K., J. B. Ulmer, and S. W. Barnett. 2005. Role of neutralizing antibodies in protective immunity against HIV. Hum. Vaccine 145-60. [DOI] [PubMed] [Google Scholar]
- 47.Streeck, H., B. Li, A. F. Poon, A. Schneidewind, A. D. Gladden, K. A. Power, D. Daskalakis, S. Bazner, R. Zuniga, C. Brander, E. S. Rosenberg, S. D. Frost, M. Altfeld, and T. M. Allen. 2008. Immune-driven recombination and loss of control after HIV superinfection. J. Exp. Med. 2051789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swofford, D. 1991. PAUP: phylogenetic analysis using parsimony, 3rd ed. Illinois Natural History Survey, University of Illinois, Champaign.
- 49.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 254876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsui, R., B. L. Herring, J. D. Barbour, R. M. Grant, P. Bacchetti, A. Kral, B. R. Edlin, and E. L. Delwart. 2004. Human immunodeficiency virus type 1 superinfection was not detected following 215 years of injection drug user exposure. J. Virol. 7894-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Kuyl, A. C., K. Kozaczynska, R. van den Burg, F. Zorgdrager, N. Back, S. Jurriaans, B. Berkhout, P. Reiss, and M. Cornelissen. 2005. Triple HIV-1 infection. N. Engl. J. Med. 3522557-2559. [DOI] [PubMed] [Google Scholar]
- 52.Villa, L. L., K. A. Ault, A. R. Giuliano, R. L. Costa, C. A. Petta, R. P. Andrade, D. R. Brown, A. Ferenczy, D. M. Harper, L. A. Koutsky, R. J. Kurman, M. Lehtinen, C. Malm, S. E. Olsson, B. M. Ronnett, F. E. Skjeldestad, M. Steinwall, M. H. Stoler, C. M. Wheeler, F. J. Taddeo, J. Yu, L. Lupinacci, R. Railkar, R. Marchese, M. T. Esser, J. Bryan, K. U. Jansen, H. L. Sings, G. M. Tamms, A. J. Saah, and E. Barr. 2006. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 245571-5583. [DOI] [PubMed] [Google Scholar]
- 53.Voronin, Y., B. Chohan, M. Emerman, and J. Overbaugh. 2007. Primary isolates of human immunodeficiency virus type 1 are usually dominated by the major variants found in blood. J. Virol. 8110232-10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu, X., A. B. Parast, B. A. Richardson, R. Nduati, G. John-Stewart, D. Mbori-Ngacha, S. M. Rainwater, and J. Overbaugh. 2006. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J. Virol. 80835-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang, O. O., E. S. Daar, B. D. Jamieson, A. Balamurugan, D. M. Smith, J. A. Pitt, C. J. Petropoulos, D. D. Richman, S. J. Little, and A. J. Brown. 2005. Human immunodeficiency virus type 1 clade B superinfection: evidence for differential immune containment of distinct clade B strains. J. Virol. 79860-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yerly, S., S. Jost, M. Monnat, A. Telenti, M. Cavassini, J. P. Chave, L. Kaiser, P. Burgisser, and L. Perrin. 2004. HIV-1 co/super-infection in intravenous drug users. AIDS 181413-1421. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, Y. J., C. Fracasso, J. R. Fiore, A. Bjorndal, G. Angarano, A. Gringeri, and E. M. Fenyo. 1997. Augmented serum neutralizing activity against primary human immunodeficiency virus type 1 (HIV-1) isolates in two groups of HIV-1-infected long-term nonprogressors. J. Infect. Dis. 1761180-1187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.