Abstract
Gammaherpesviruses establish life-long persistency inside the host and cause various diseases during their persistent infection. However, the systemic interaction between the virus and host in vivo has not been studied in individual hosts continuously, although such information can be crucial to control the persistent infection of the gammaherpesviruses. For the noninvasive and continuous monitoring of the interaction between gammaherpesvirus and the host, a recombinant murine gammaherpesvirus 68 (MHV-68, a gammaherpesvirus 68) was constructed to express a firefly luciferase gene driven by the viral M3 promoter (M3FL). Real-time monitoring of M3FL infection revealed novel sites of viral replication, such as salivary glands, as well as acute replication in the nose and the lung and progression to the spleen. Continuous monitoring of M3FL infection in individual mice demonstrated the various kinetics of transition to different organs and local clearance, rather than systemically synchronized clearance. Moreover, in vivo spontaneous reactivation of M3FL from latency was detected after the initial clearance of acute infection and can be induced upon treatment with either a proteasome inhibitor Velcade or an immunosuppressant cyclosporine A. Taken together, our results demonstrate that the in vivo replication and reactivation of gammaherpesvirus are dynamically controlled by the locally defined interaction between the virus and the host immune system and that bioluminescence imaging can be successfully used for the real-time monitoring of this dynamic interaction of MHV-68 with its host in vivo.
Herpesviruses are large DNA genome viruses, which persist inside a host through the combination of a productively replicating lytic cycle and a dormantly latent cycle. The persistent infection of herpesvirus causes most herpesviral diseases, and thus understanding the mechanism of herpesviral persistence in vivo is critical for the control of herpesviral diseases. Among the subfamilies of herpesvirus, the gammaherpesviruses are important human pathogens because they cause a variety of diseases in humans, such as infectious mononucleosis, nasopharyngeal carcinoma, Burkitt's lymphoma, and Kaposi's sarcoma (30, 34). However, studies of two known human gammaherpesviruses, Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), have been limited mostly to in vitro investigation as a result of their restricted host range (30, 34).
On the basis of its genomic and biological similarities to human gammaherpesviruses, murine gammaherpesvirus 68 (MHV-68, a gammaherpesvirus 68) has been used as a small animal model to study the in vivo host interaction of human gammaherpesviruses and to develop therapeutic or preventive strategies against gammaherpesvirus-associated diseases (12, 37). During intranasal infection of inbred mice with MHV-68, MHV-68 establishes acute productive infection in the lung and subsequent latent infection in the spleen (37). In response to poorly defined stimuli, MHV-68 may reactivate from latency and replenish the latent pool (50). Like other gammaherpesviruses, latent infection of MHV-68 leads to splenomegaly and lymphoproliferative diseases in the host. Furthermore, it promotes the development of lymphomas in immune-deficient mice (44, 47).
Studies of MHV-68 in mouse models typically rely on sacrifice of infected mice to determine the distribution and extent of viral replication (37). Although these postmortem experimental methods have been useful for defining virus-host interactions that regulate the replication and pathogenesis of MHV-68 in vivo, these assay techniques preclude the continuous real-time monitoring of MHV-68 infection in the same animal. In addition, MHV-68 infection of unexpected anatomic sites, which might give significant insights into viral pathogenesis, may not be identified simply because the infected tissue is not sampled (16, 45). Thus, the real-time whole-body monitoring of MHV-68 infection in living mice can provide new insights into virus-host interaction and viral pathogenesis.
Recently, a bioluminescence imaging technique has been developed to measure the activity of luciferase reporters in living mice noninvasively and repetitively (1, 7, 8, 53). This technique has several benefits. First, it has little background and thus is a very sensitive method for detecting reporter activities (53). Second, d-luciferin, the substrate for the firefly luciferase (FL) reporter, can easily cross cell membranes, including the intact blood-brain barrier after intraperitoneal administration into mice (4, 5). Thus, the FL reporter can be detected in most anatomic sites, although the light coming from within is attenuated by overlying tissues of the mice (36). Third, the concentration of d-luciferin used for bioluminescence imaging is too low to be toxic or immunogenic and thus allows serial imaging examinations in the same mouse (18, 28). Finally, bioluminescence measured in vivo highly correlates with FL activity measured in vitro in the lysate of extracted tissues (53). On the basis of these advantages, bioluminescence imaging in living mice has been successfully used to localize and measure FL reporter activities from tumor xenografts to microbial infection (6, 7, 24-26).
In this study, we constructed a recombinant MHV-68 expressing FL by a viral M3 promoter (MHV-68/M3FL, henceforth referred to as M3FL) and used the bioluminescence imaging system to monitor the systemic infection of mice with MHV-68. Our data demonstrated that in vivo replication and reactivation of gammaherpesvirus are dynamically controlled by the locally defined interaction between the virus and the host immune system and that M3FL is an effective model for studying the in vivo interaction of gammaherpesvirus with its host.
MATERIALS AND METHODS
Cells and viruses.
All cells were propagated in Dulbecco's modified Eagle medium containing 10% fetal bovine serum and antibiotics. NIH 3T12 cells were mainly used to propagate the wild-type MHV-68 (WT) and its mutant derivatives for in vitro and in vivo study. Vero cells were used to determine the titers of infectious viruses.
To make specific mutations in MHV-68, a two-step allelic exchange method was performed using a bacterial artificial chromosome clone of MHV-68 (BAC MHV-68) as the target (39, 40). The consequent mutation was confirmed by DNA sequencing, and the genomic integrity of mutated BAC MHV-68 was investigated by restriction enzyme digestion and Southern blot analysis as previously described (40). Subsequently, mutant viruses were produced by transfecting the mutant BAC plasmid with Cre recombinase-expressing plasmid, which removes the BAC vector sequence, into NIH 3T12 cells using Lipofectamine Plus (Invitrogen).
For multistep growth curve analysis of viruses, the cells were infected at a multiplicity of infection (MOI) of 0.05 and harvested at 1, 24, 48, 72, and 96 h postinfection. The infected cells were frozen and thawed three times, and the titers of the infectious viruses in the whole-cell lysate were determined by plaque assay.
Mouse experiments.
All animal handling was performed in accordance with the Animal Research Committee guidelines of the University of California, Los Angeles. Mice were purchased from Charles River Laboratories, Inc. (Wilmington, MA). All mice were infected after anesthesia, and the infected mice were sacrificed at 5 days postinfection (dpi) (5 × 105 PFU/mouse) or 7 dpi (500 PFU/mouse) to measure the acute viral infection in the lung or at 14 dpi to measure the viral latent load in the spleen. Statistical analysis of the data was performed by Student's t test. The infectious viral titer in the lung and the reactivatable viral latency was measured by plaque assay and infectious center assay, respectively, as previously described (45). T cells were purified from the thymus with the Pan T-cell isolation kit, which negatively selects T cells in a magnetic field, as instructed by the manufacturer (Miltenyi Biotec).
For in vivo reactivation of MHV-68, 50 mg of cyclosporine A (CspA)/kg of body weight or 0.3 mg of Velcade/kg of body weight were injected intraperitoneally into the infected mice on the days indicated on figures. For ex vivo reactivation and amplification of the latent MHV-68, single-cell suspensions were prepared from the infected organs and cocultivated with a monolayer of Vero cells in the presence of antibiotic cocktail (50 μg/ml kanamycin, 34 μg/ml chloramphenicol, and 100 μg/ml ampicillin). When the cocultivated cells showed 80 to 90% cytopathic effect, the infected cells were harvested. Total DNA was harvested by lysing the infected cells with proteinase K and precipitating the DNA with ethanol as previously described (54). Total DNA was subjected to restriction enzyme digestion overnight, electrophoresed on 0.8% agarose gels, and visualized after staining with ethidium bromide.
Quantification of viral genome and transcript.
The total genomic DNA from the infected lungs or spleen tissue was prepared using the DNeasy kit (Qiagen) and then subjected to quantitative real-time PCR as previously described (40). The total RNA from the infected spleen tissue was prepared using the RNeasy kit (Qiagen) and transcripts of ORF50 (immediate-early), ORF57 (early), ORF29 (late), and ORF73 (latent) genes were detected via PCR using the following sets of gene-specific primers, respectively: SH-50F (5′-GATTCCCCTTCAGCCGATAAG-3′) and SH-50R (5′-CAGACATTGTAGAAGTTCACCT-3′), ORF57SP-F1 (5′-ACCAAATGATGGAAGGACTAC-3′) and ORF57SP-R (5′-GCAGAGGAGAGTTGTGGAC-3′), ORF29R1 (5′-TTCTCATTGGCATCTTTGAGG-3′) and ORF29L4 (5′-GGAAAATGGGGTGATCCTGT-3′), and SH-73-5F (5′-GATGAGGGAAGTGTTGGTGATG-3′) and SH-73-3R (5′-CTCGTGAGTAGCGCCGACTAG-3′).
Bioluminescence optical imaging.
Bioluminescence imaging of M3FL replication in mice was performed using the in vivo imaging system (IVIS; Xenogen Corp., Alameda, CA), which consists of a cooled charge-coupled-device (CCD) camera mounted on a light-tight specimen chamber, camera controller, and a computer system for data acquisition and analysis. Every other day postinfection with 5 × 105 PFU of M3FL, mice were anesthetized with a mixture of xylazine and ketamine, and imaging was performed immediately after administration of d-luciferin by intraperitoneal injection (3 mg/mouse) from a 15-mg/ml stock solution in phosphate-buffered saline. For ex vivo imaging, mice were anesthetized and given d-luciferin by intraperitoneal injection. At ca. 10 min after injection of substrate, the mice were sacrificed and dissected to identify the signaling organ.
A grayscale surface image of each mouse was obtained first at 1.5-cm subject height focus, 20-cm field of view, 0.2-s exposure time, and 8 f-stop (aperture). Overlapping bioluminescence images were acquired at the same field of view, 60 s, 1 f-stop, and an open emission filter. Bioluminescence imaging was performed after rotating each mouse toward the CCD camera to detect signal on four sides (dorsal, ventral, and two lateral sides) until the luminescent signal reached its peak and waned. The pseudocolor scale bar of each image shows the relative photon flux for each image with different minimum and maximum values to visualize signals with different intensities in each image. All imaging experiments were performed at least two times, and the representative data are shown here.
Quantitation of luciferase activity.
Relative intensities of in vivo bioluminescence were represented as an overlapping pseudocolor images from violet (least intense) to red (most intense). Grayscale photographs and corresponding color images were superimposed with LivingImage (Xenogen) and Igor (Wavemetrics, Lake Oswego, OR). A region of interest (ROI) was manually selected, and the intensity was expressed as photon flux (photons/s/cm2/steradian). For the reliable comparison of signal intensities, the maximum photon flux values were measured from each ROI and analyzed.
For in vitro correlation of FL activity with viral replication, NIH 3T12 cells were infected in duplicate with M3FL at multiple MOIs (1 to 0.000001). At 3 dpi, the infected cells were frozen and thawed three times, and the titers of the infectious viruses in the whole-cell lysate were determined by plaque assay. For the measurement of FL activity, the infected cells were lysed in 1× passive lysis buffer (Promega), and the relative number of luciferase unit (RLU) and protein amount of each lysate were determined. For in vivo correlation of FL activity with viral replication, mice (n = 12) were infected intranasally with 5 × 105 PFU of M3FL, and the lungs of the infected mice were harvested at the peak time of acute replication. The titer of infectious virus was determined by plaque assay using the homogenized lung lysate in complete medium. For the measurement of luciferase activity, the infected lung tissues were homogenized in 1× passive lysis buffer, and the number of RLU and protein amount of each lysate were determined.
RESULTS
Construction and growth kinetics of recombinant MHV-68 expressing firefly luciferase.
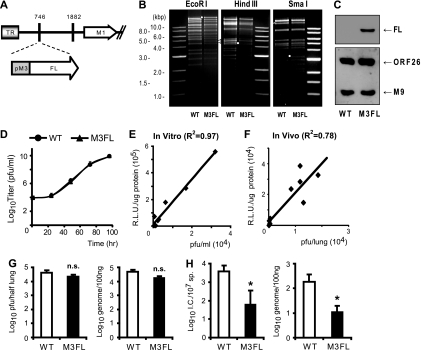
Between the two distinct life cycles of herpesviruses, a productively replicating lytic cycle and a dormantly latent cycle, the herpesvirus is highly active during the lytic phase. In contrast, during the latent phase, there is little or limited gene expression of the herpesvirus. In this study, we focused on the virus-host interaction during the lytic cycle of herpesvirus. Therefore, to monitor the in vivo replication of MHV-68 noninvasively and repetitively in the same mouse over time by using bioluminescence imaging, we constructed a recombinant MHV-68 by inserting a viral M3 promoter-driven firefly luciferase cassette between viral tRNA-1 and -2 (between genomic coordinates 746 and 747 of MHV-68 WUMS [GenBank no. U97553]) (M3FL) (Fig. 1A) (27). The M3 promoter is highly responsive to the MHV-68 lytic cycle regulator, regulator of transcription activation (RTA), and the robust expression of the M3 gene has been detected during lytic replication of MHV-68 both in vitro and in vivo (27). We hypothesized that the luminescent signal from the M3 promoter- driven luciferase activity can represent the active in vivo lytic replication of MHV-68. The genomic integrity of the constructed M3FL was confirmed by restriction enzyme digestions in comparison to the WT (Fig. 1B), and the expression of the firefly luciferase from M3FL was confirmed (Fig. 1C).
FIG. 1.
Construction of recombinant MHV-68 for bioluminescent imaging. (A) Schematic diagram of M3FL. Viral M3 promoter-driven firefly luciferase (FL) gene cassette was inserted between genomic coordinates 746 and 747 of MHV-68 WUMS (GenBank no. U97553). Nucleotide 1882 is the insertion site of the bacterial artificial chromosome vector sequence. TR, terminal repeat. (B) Genomic integrity of M3FL. BAC DNA from wild-type MHV-68 (WT) and M3FL was digested with EcoRI, HindIII, and SmaI and analyzed by agarose gel electrophoresis. Small white circles indicate the shift of digested fragment due to the insertion of the M3 promoter-driven FL gene cassette. The white triangle indicates the heterogenous fragment in the EcoRI-digested pattern, which includes the 40-bp repeat region. (C) Expression of FL from M3FL virus. The lysate of the cells infected with either WT or M3FL was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the expression of FL was analyzed by Western blotting using anti-FL (sc-57603; Santa Cruz). A Western blot using antibodies against viral structural proteins (ORF26 and M9) was included as a control for viral replication. (D) Multistep growth curve of wild-type MHV-68 and M3FL in NIH 3T12 cells. The data were compiled from three independent experiments, and standard deviations are shown as error bars. (E) In vitro correlation of FL activity with viral replication. NIH 3T12 cells were infected with M3FL at multiple MOIs, and the titer of infectious virus and the relative luciferase units (R.L.U.)/μg of protein in the whole-cell lysate were determined as described in Materials and Methods. (F) In vivo correlation of FL activity with viral replication. Mice were infected intranasally with 5 × 105 PFU of M3FL, and the titer of infectious virus and the number of RLU in the lungs of the infected mice were determined as described in Materials and Methods. (G) Acute replication of WT and M3FL in the lungs of the infected mice at 7 dpi. The titer of infectious virus and number of viral genome copies were determined by plaque assay (left panel) and quantitative real-time PCR (q-PCR) (right panel), respectively. (H) Latency establishment of WT and M3FL in the spleen at 14 dpi. The reactivatable latency and viral genome load were determined by infectious center (I.C.) assay (left panel) and quantitative PCR (right panel), respectively. (Left) The reactivatable latency as determined by an infectious center (I.C.) assay for 107 splenocytes (107 sp) is shown (four to six mice per group and per time point). Data are represented as means plus standard deviations (error bars). M3FL values that were not significantly different (n.s.) or were significantly different (P < 0.05 [ast]) from the value for the WT are indicated.
The multistep growth curve assay was performed to investigate the growth of M3FL and the parental wild-type MHV-68 during multiple rounds of replication in mouse fibroblast NIH 3T12 cell line at an MOI of 0.05. As shown in Fig. 1D, there was no significant difference between the in vitro growth of M3FL and that of WT. In addition, the viral titers of M3FL measured by a standard plaque assay are highly correlated with the activity of the expressed FL, demonstrating that the luminescent signal driven by the M3 promoter can reliably indicate the replication of M3FL (Fig. 1E and F).
Next, the in vivo growth of M3FL and that of WT were compared after intranasal infection of BALB/c mice. At the peak time of acute infection in the lung (7 dpi), there was no significant difference in infectious viral titers and genome replication between M3FL and WT (Fig. 1G). After virus in lytic replication was cleared from the lung, MHV-68 establishes latency in the spleen with a peak time at day 14 (15). The viral latency and genome load in the spleen at 14 dpi was measured by an infectious center assay and real-time monitoring, respectively. In an infectious center assay, single-cell splenocytes were cocultivated with Vero cells that support viral lytic replication. During cocultivation, the latent virus in splenocytes reactivates to produce infectious viruses that subsequently infect Vero cells and generate plaques. The numbers of plaques (infectious centers) were counted to quantitate the reactivating viruses. For a control to show that there was no preformed infectious virus in the splenocytes, cells were killed prior to cocultivation by freeze-thawing, which does not affect the infectivity of preformed virions, and we found no plaques in the control (data not shown). The results of infectious center assays were presented in Fig. 1H, and there were ca. 10-fold-less reactivating viruses detected in M3FL-infected mice than in those infected with WT. A similar change in reduction was observed in the viral genome load. Taken together, the in vivo data indicate that the insertion of the M3FL expression cassette does not significantly affect viral productive infection in the lung but may reduce the establishment of viral latency (Fig. 1H).
Distinct spatial and temporal progression of MHV-68 infection following different routes of inoculation.
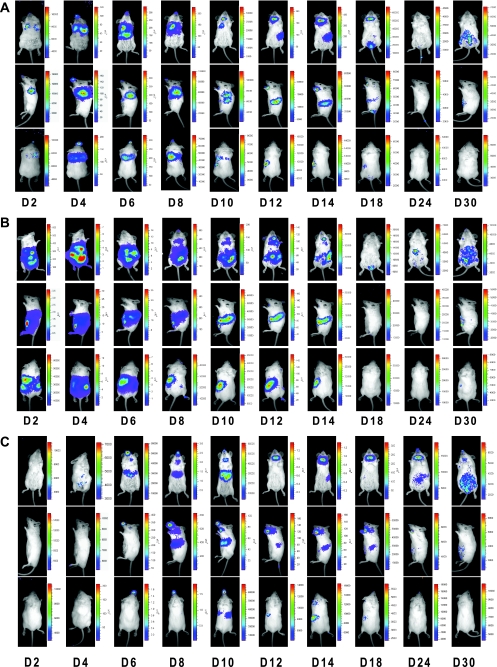
In vivo characterization of MHV-68 replication has been focused on performing standard virological assays for the lung and spleen, which are the two main sites of infection following intranasal or intraperitoneal inoculation of mice. Furthermore, the time to harvest tissues for assays depends upon routes and doses of inoculation, and very often tissues need to be taken at multiple time points to examine the kinetics of viral replication. The recombinant bioluminescent M3FL virus provides an alternative approach to study in vivo viral replication by tracking the light signals generated by the cells infected with M3FL within a living animal noninvasively and continuously. As shown in Fig. 2A, the luminescent signals were captured with a CCD camera and provided an indication of M3FL activity within BALB/c mice over a month following intranasal infection. The signals in the lung increased over a week and gradually cleared by D12 (12 dpi). In the spleen, the lights were first detected at D10 and lasted for a week with a peak time at D14. The kinetics of signals in the lung and spleen were consistent with what has been reported in the literature using standard virological assays (15). However, there are two novel findings from examining these in vivo bioluminescence images. First, the imaging has revealed other sites of infection, in areas of the salivary gland and thymus. The infection in the salivary gland area started to be detected at D10 and continued until D18, while the signals in the thymus area could be seen only at D10. Additional sites, such as urogenital tract (D18), tail (D18), and peritoneal cavity (D30), were found to emit signals at later times after infection, indicating M3FL infection of these tissues. Second, the kinetics of viral replication varies among organs and tissues within a mouse. In other words, the luminescent signals peaked and cleared at different times depending upon which site of replication was examined. Furthermore, after initial clearance of all signals (Fig. 2A, D24), M3FL activity reappeared in the peritoneum of the infected mice (Fig. 2A, D30).
FIG. 2.
Spatial and temporal progression of MHV-68 infection via different routes of inoculation. Mice were infected intranasally (A), intraperitoneally (B), or orally (C) with 5 × 105 PFU of M3FL. Images at different time points are shown to represent acute infection in the primary site of infection, the spatiotemporal progression of viral replication, and the clearance and reappearance of viral replication. The day postinfection (e.g., D2 is day 2 postinfection) is shown at the bottom of each series of images.
After intraperitoneal inoculation of M3FL, the luminescent signals displayed a different pattern of distribution and progression (Fig. 2B) compared to that seen after intranasal infection. For the first week, the signals were primarily detected at the site of inoculation, the peritoneal cavity, including the liver and spleen as the target organs for viral replication. M3FL started to be cleared from the liver at D8 but remained active in the spleen until D14. Interestingly, similar to intranasal infection, the signals could also be found in the salivary gland and thymus areas (Fig. 2B, D8 to D14), as well as in the urogenital tract (Fig. 2B, D18). Consistent with Fig. 2A, the luminescent signals could be detected again in the peritoneum at later times (D24 and D30) after being cleared at D18.
Although intranasal and intraperitoneal routes of inoculations are most commonly used for in vivo studies of MHV-68, oral administration has been previously reported (32). Furthermore, one major transmission mode for herpesviruses is through an oral route. We therefore also examined the whole-body imaging of M3FL activity following the oral route of inoculation. The bioluminescence pattern after oral infection (Fig. 2C) is very similar to that of intranasal infection (Fig. 2A). However, infection by an oral route led to an earlier appearance of signals in the salivary gland area (Fig. 2C, D6), which lasted for a much longer period of time up to D30.
Taken together, in all three routes of infection examined, M3FL replicated robustly within mice and ample luminescent signals were generated, allowing us to monitor the spatial and temporal progression of MHV-68 infection. The bioluminescence imaging clearly showed initial replication in the primary sites of infection, such as the nose and lung (intranasal), peritoneum (intraperitoneal), and salivary gland (oral), which depends upon the infection routes. Eventually, all the signals progressed to the spleen, a major reservoir of viral latency.
Novel sites of MHV-68 replication.
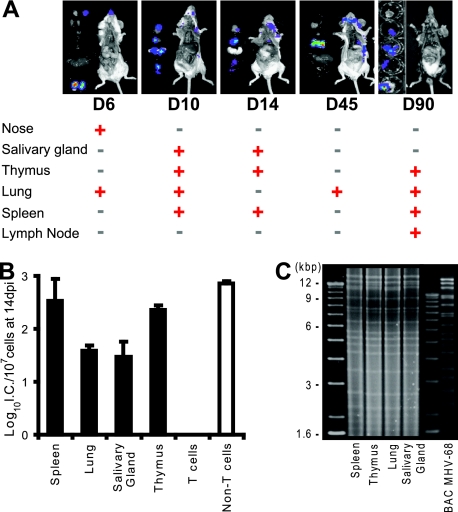
One important finding from whole-body imaging of M3FL-infected live mice is the revelation of novel sites of viral replication other than lung and spleen. To confirm the sites of infection and to further identify other tissues or organs which were infected but may be difficult to detect by noninvasive imaging due to its limit of sensitivity, ex vivo imaging of the individual organs/tissues was performed after euthanizing the M3FL-infected mice. Consistent with the whole-body noninvasive imaging results, luminescent signals were detected in areas of the nose, salivary glands, lung, and spleen at different times postinfection (Fig. 3A). In addition, the signal was consistently detected in area of the thymus and mesenteric lymph nodes (Fig. 3A) and occasionally in adrenal glands and the liver (unpublished data). Furthermore, even after the initial clearance of acute lytic replication (Fig. 3A, D14), M3FL showed signals again later, suggesting spontaneous reactivation of MHV-68 from latency (Fig. 3A, D45 and D90).
FIG. 3.
Identification of novel sites of MHV-68 replication. (A) Ex vivo bioluminescent imaging showing the in situ localization of luciferase signal after intranasal infection. At the indicated day (D) postinfection in the figure, the infected mice were sacrificed and dissected to identify the signaling organs. Whether a particular organ showed signal (red plus signs) or not (black minus signs) at the given time is shown below the images. (B) Latency establishment of M3FL in the spleen, lung, salivary glands, thymus, and the subpopulations of cells in the thymus. Mice were infected with 5 × 105 PFU of M3FL, and the organs of the infected mice were harvested at 14 dpi. Reactivatable latency was measured by infectious center (I.C.) assay. (C) Restriction enzyme (EcoRI) digestion pattern of the reactivated viruses in different organs. The rightmost lane shows the EcoRI digestion pattern of BAC MHV-68.
To further investigate MHV-68 replication in the newly identified sites, the tissues around the salivary glands and thymus were examined for viral replication in comparison with the lung and spleen. Among those organs, preformed infectious virus was detected only in the lung before viruses in the initial acute replication stage are cleared, suggesting that the detectable level of productive replication might be restricted to the lung. However, all four organs were found to harbor reactivatable latent viruses by an infectious center assay (Fig. 3B). After the single-cell suspension from the infected organs was cocultivated with monolayer cells, the virus reactivated and underwent lytic replication. The total DNA harvested from the cocultures of newly identified organs was analyzed by restriction enzyme digestion, which displayed a similar pattern to the digestion of harvested DNA from the lung and spleen and parental MHV-68 DNA (Fig. 3C), indicating that MHV-68 was the virus reactivating from those organs. Collectively, these data suggest that the luminescent signals from M3FL reliably indicate the site of MHV-68 infection and replication.
The development of T lymphocytes (T cells), which are the key components of the cell-mediated immune system, takes place in the thymus. T cells have been shown to control both acute infection in the lung and latent infection in the spleens of MHV-68-infected mice (19). Interestingly, we found that the frequency of latent MHV-68 in the thymus was similar to that in the spleen (Fig. 3B), although the total amount of latent virus in the thymus area is less than that in the spleen, taking into consideration the fact that there are fewer cells in the thymus. The major cell type in the thymus is developing immature thymocytes. However, there are other cell types that play crucial roles in T-cell maturation by positively and negatively selecting functional T cells. Thus, to further delineate the biological consequence of MHV-68 infection of the thymus, the cell type harboring latent MHV-68 was investigated. Single cells were prepared from the infected thymus and associated lymph nodes at 14 dpi and were sorted using a Pan T-cell isolation kit, which isolates T cells by depleting non-T-cells with antibodies. After cell isolation, latent MHV-68 and viral genome were detected only in the non-T-cell fraction (Fig. 3B and unpublished data) (19). These data suggest that MHV-68 infects non-T-cells in the thymus area. One limitation of our experiments is that we did not distinguish the lymph nodes that are attached to salivary glands and thymus. In subsequent experiments, we separated lymph nodes and found that the major signals are from the lymph nodes.
Persistent infection and spontaneous reactivation of MHV-68.
After collection of the noninvasive bioluminescence imaging data and time of infection, the spatial and temporal progression of M3FL replication was quantitatively analyzed by comparing the maximum photon signals among the different regions of interest at multiple time points. Although the order of progression of M3FL replication was consistent among different mice after intranasal infection with 5 × 105 PFU of M3FL (Fig. 4A), averaging data from multiple mice would eliminate the distinctive kinetics of each organ (Fig. 4B). Therefore, analysis was conducted separately on individual mice, and the representative data are shown in Fig. 4C, which indicates how MHV-68 replication progresses spatially and temporally in a single mouse after intranasal infection.
FIG. 4.
Investigation of M3FL infection in different sites at multiple time points. (A) Comparable replication of M3FL among mice after intranasal infection with 5 × 105 PFU of M3FL. Images depicted represent acute infection in the nose and the lung (day 6 postinfection [D6]), the transition of replication from the lung to the spleen (D14), and the reappearance of viral replication (D45). (B and C) A region of interest was manually selected over the signals, and the intensity was analyzed using LivingImage software and expressed as photon flux (photons/s/cm2/steradian). As representative data, the comparison of bioluminescence signals among the four mice (B) and the spatiotemporal progression of the signals in a single mouse (C) are shown. For the reliable comparison of signal intensities, the maximum photon flux values were measured from each ROI and shown here.
As demonstrated in Fig. 4C, M3FL infection progressed to different organs with different kinetics. While viruses that were replicating were cleared in one organ, the virus started replicating in another organ. This spatiotemporal progression and local clearance of M3FL infection vividly illustrates that virus-host interaction is defined locally, not systemically. Moreover, several months after the initial clearance of acute infection, M3FL activity could be detected again in some organs (Fig. 3A and 4C), suggesting that latent MHV-68 reactivates spontaneously and sporadically. Taken together, these data demonstrated the dynamics of MHV-68 infection among organs and tissues within mice, a consequence of interactions between the virus and host.
In vivo reactivation of MHV-68 from latency.
During their life-long latency in the host, herpesviruses can reactivate to the productive lytic cycle. However, the reactivation process of herpesvirus in vivo is not clearly understood. Currently, the assays to investigate reactivations, such as infectious center assay, are heavily dependent on ex vivo reactivation of latent viruses from the infected cells. The frequency of reactivation in vitro is strongly influenced by culture conditions, and it is questionable whether the stimuli that induce ex vivo reactivation are the same as those for in vivo reactivation. As previously described, we were able to detect what appeared to be reactivation of M3FL after initial clearance (Fig. 3A and 4B). Therefore, we examined whether we can utilize M3FL and the bioluminescence imaging to study induction of in vivo reactivation after the initial clearance of signals.
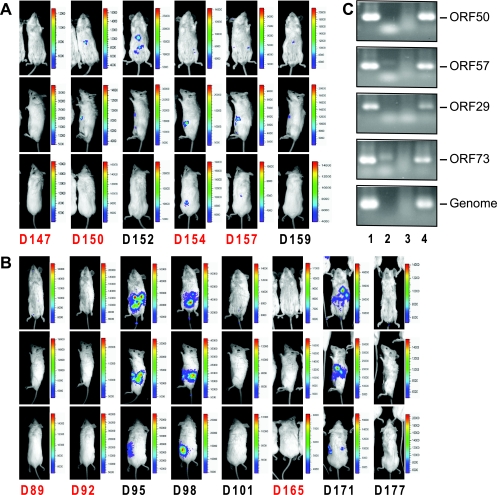
To reactivate latent M3FL in vivo, two approaches were taken. One was inducing the lytic replication using a drug which is known to induce the reactivation of human gammaherpesviruses in vitro (2, 3), and the other was suppressing immune surveillance. Velcade (PS-341, bortezomib) is clinically approved by the FDA for the treatment of multiple myelomas and has been previously shown to reactivate KSHV and EBV from latently infected B-cell lines through inhibiting the NF-κB activity (2, 3). However, the in vivo inductive effect of Velcade on viral reactivation has not been examined, although clinical trials are ongoing at several institutions. Thus, we administered Velcade into the infected mice when there was no significant signal detected in the whole body to test whether it could induce in vivo reactivation of MHV-68. As shown in Fig. 5A, the luminescent signal appeared in the peritoneum upon treatment with Velcade, indicating M3FL reactivation to express the luciferase. Because latency and reactivation of MHV-68 are mainly controlled by the T-cell-mediated immune response, we reasoned that the inhibition of T-cell function might relieve host immune restrictions on the reactivation of MHV-68. Therefore, we also examined in vivo reactivation of M3FL by the treatment with an immunosuppressant, cyclosporine A, which inhibits T-cell activation (44, 52). As shown in Fig. 5B, the signals appeared in the peritoneum 3 days after the second administration of CspA and waned 6 days after. Moreover, after the signal was resolved, the repeated CspA treatment made the luminescent signal appear again (Fig. 5B), mimicking recurrent reactivation of herpesvirus in humans. To confirm that the signals were due to viral reactivation, we examined the expression of viral latent and lytic transcripts as well as the viral genomes in the spleen. The reverse transcription-PCR results clearly showed an increase of viral gene expression and viral DNA after CspA treatment (Fig. 5C, lane 4), consistent with induced viral replication in the spleen. Collectively, by using the bioluminescence imaging with M3FL, we demonstrated that Velcade and CspA were able to induce in vivo reactivation. Moreover, it emphasizes the dynamic and fine balance between the host immune system and viral reactivation and replication.
FIG. 5.
In vivo reactivation of MHV-68 from latency. When there was no significant bioluminescent signal in the whole body of infected mice, the mice were intraperitoneally treated with 0.3 mg of Velcade/kg (at ca. 150 dpi) (A) or 50 mg/kg of cyclosporine A (at ca. 90 dpi) (B) on the days indicated in red (D147, 147 dpi) and continuously monitored. (C) Total RNAs were extracted from the infected NIH 3T12 cells (3 dpi; MOI of 0.05) (lane 1), uninfected NIH 3T12 cells (lane 2), the spleen of the infected mouse (ca. 100 dpi) (lane 3), or the spleen of the infected mouse (ca. 100 dpi) after CspA treatment (50 mg/kg, twice) (lane 4). cDNAs were prepared, and the transcript levels of ORF50 (immediate-early), ORF57 (early), ORF29 (late), and ORF73 (latent) were detected by PCR. D, days postinfection.
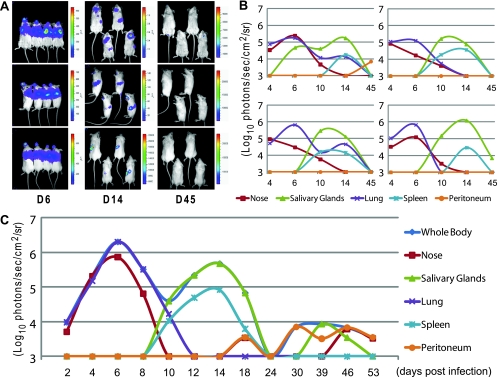
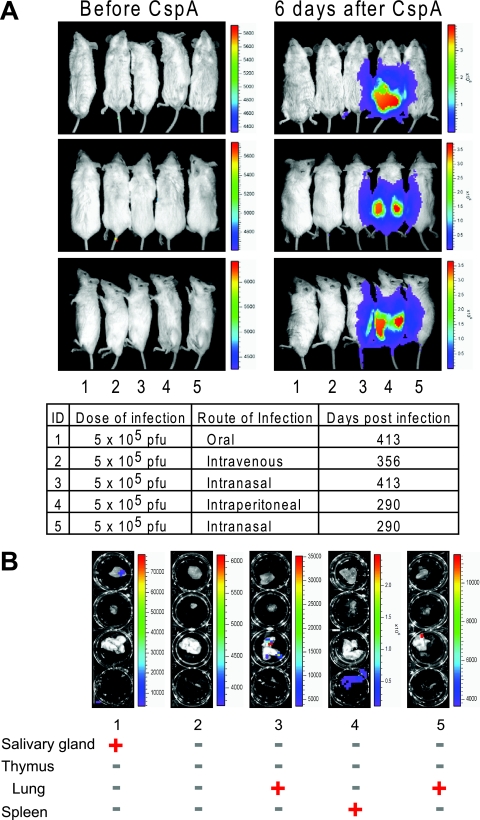
Notably, when we treated mice infected by different routes with CspA to reactivate M3FL, we found that the extent of the reactivation is strongest and the period of reactivation is the longest in mice infected intraperitoneally (Fig. 6A). This result suggests that the latent reservoir established by intraperitoneal infection is greater and survives longer than that of the other routes of infection. To further investigate the sites of reactivation, we conducted ex vivo imaging on those CspA-treated mice. M3FL reactivation could be found in all mice infected by different routes, suggesting that a combination of in vivo and ex vivo data becomes more informative. Interestingly, the major site of reactivation depends upon the routes of infection and corresponds to the primary site of infection. In other words, after CspA treatment of mice infected for a long time (ca. 10 months), the strongest signals were detected in the lungs of intranasally infected mice, in the salivary glands of orally infected mice, and in the spleens of intraperitoneally infected mice (Fig. 6B). Taken together, these results suggest that the initial phase of viral infection may determine either the preferential reservoir of long-term viral latency or the local host immune system controlling the reactivation of latent virus (23, 42, 50).
FIG. 6.
Different reactivation of M3FL via different routes of infection. (A) In vivo reactivation of M3FL a long time after infection via different routes. All the long-term infected mice were intraperitoneally treated with 50 mg of CspA/kg twice (every third day). Before and 6 days after initial administration of CspA, the mice were imaged. ID, identification number. (B) Ex vivo bioluminescent imaging showing the in situ localization of luciferase signal from M3FL a long time after infection via different routes. Whether a particular organ showed signal (red plus sign) or not (green minus sign) at the given time is shown below the photographs.
DISCUSSION
MHV-68 has been used as an experimental model for studying the in vivo virus-host interaction of gammaherpesviruses, which exemplify persistent viral infection. In this study, a bioluminescence imaging system was introduced to monitor MHV-68 infection in the whole mouse. Imaging of a recombinant MHV-68 expressing firefly luciferase (M3FL) has enabled us not only to analyze the spatial and temporal progression of MHV-68 infection in the whole body of the host but also to investigate the fine balance between the virus and the host immune system, which controls the replication and reactivation of the virus.
M3 expression as the representation of lytic replication.
In this study, we studied the virus-host interaction during the lytic cycle of gammaherpesvirus by using the promoter of M3 to control the expression of firefly luciferase. M3 is one of the most highly expressed lytic genes during the natural course of MHV-68 infection both in vitro and in vivo (20, 38, 41, 42, 51). The kinetic class of M3 expression is early-late, which requires the products of immediate-early transcripts, but its maximal expression further necessitates viral DNA synthesis (27, 49). Previously, the expression of M3 during latency was found at least 1 month postinfection (38). This M3 expression is most likely to represent the lytic gene expressed in a small subset of reactivating cells, considering the high level of M3 expression during the lytic phase (27, 41), which is consistent with our imaging data. The promoter, containing 600 bp of upstream sequence of the M3 TATA box, was previously shown to yield the highest level of luciferase activity in the presence of RTA (27). This promoter region was used to construct M3FL.
Our data indicated that M3FL is attenuated in establishing latency in the spleen (Fig. 1H). However, the luminescent signals from M3FL could be detected in multiple organs after a long period of time, supporting the idea that M3FL is able to establish long-term latency and to reactivate from latent reservoirs (Fig. 3A, 5, and 6). Although the locus where M3FL is inserted does not interrupt any coding region, we could not exclude the possibility that it may interfere with the expression of other viral genes, which results in the reduction of latency. Another possibility is that the reduction may be immune mediated. Firefly luciferase alone has been known to be nonimmunogenic (18), but expression of the luciferase in the context of MHV-68 infection may elicit antiluciferase immunity, which targets and limits the amplification of latently infected cells at the peak time. It has been previously shown that failure of viral immune evasion has a significant impact on viral latency but not lytic replication.
Interestingly, although we consistently detected the luciferase activity driven by the M3 promoter from multiple organs and the viral lytic transcripts in those signaling organs, preformed lytic virus was rarely detected, except in the lung at the initial peak of acute infection. One possible explanation of this phenomenon is that, during in vivo lytic replication of MHV-68, there may be limited production of extracellular viruses, which is below the detection limit for our current plaque assays. This hypothesis is supported by the gp150-deficient MHV-68 mutant study, which emphasizes the importance of cell-to-cell virus spread as the major route of virus propagation in an infected host (10, 43), and our plaque assays are not able to quantitate this mode of viral replication. Alternatively, host immune responses might prevent the viral replication from being completed and producing infectious viruses. Furthermore, there might be host immune restrictions on the ability of the extracellular viruses to produce plaques. For example, the infected host may generate neutralizing antibodies in the later stages of MHV-68 infection, which can impede the extracellular viruses in establishing de novo infection and thus producing plaques (17).
Spatial and temporal progression of MHV-68 infection.
Two inoculation routes are frequently used to study MHV-68 infection of mice. One is intranasal infection, in which viral inoculum in the form of small droplets is administered into the nostrils of a mouse. The other is intraperitoneal infection, in which viral inoculum is injected into the peritoneal cavity of a mouse. We also examined another possible route of inoculation, oral infection, by delivering viral inoculum through the oral cavity. Consistent with the previous studies, we observed that M3FL infection by all routes of inoculation reached and established infection in the spleen, a major reservoir of MHV-68 latency, after acute replication in the primary site of infection. All routes of infection showed persistent viral replication, local clearance, and sporadic reappearance of the bioluminescent signal. Moreover, using this new bioluminescence imaging system, we monitored the progression of MHV-68 infection within individual mice longitudinally. Our data clearly demonstrate the dynamic history of MHV-68 infection in a single mouse after each different route of infection.
In this study, the intranasal infection was most intensively examined because it presents the viral interactions with mucosal immune responses. To further investigate the previously uncharacterized anatomic sites, both noninvasive whole-body imaging and ex vivo organ imaging were used after intranasal infection. In contrast to the traditional postmortem examination, which focused on the lung and spleen, bioluminescence imaging identified the replication and reactivation of MHV-68 in other locations, such as the nose, salivary glands, and thymus (Fig. 3A). Infection of these organs with MHV-68 was verified by isolating the virus from the tissues and/or by detecting the viral genome in the tissues using PCR (Fig. 3 and unpublished data).
Among the newly identified organs where MHV-68 infection was consistently detected, the salivary glands are interesting. For other herpesviruses, including cytomegalovirus, human herpesvirus 6 and 7, EBV, and KSHV, a tropism for salivary gland tissue has been shown, and shedding of those viruses into saliva is common during their reactivation, which facilitates transmission (9, 11, 21, 22, 33). Thus, the discovery of MHV-68 in salivary glands suggests that MHV-68 may be also transmitted through saliva, similar to the other ubiquitous human herpesviruses. Furthermore, this poses an intriguing possibility of using MHV-68-infected mice as an animal model for the study of salivary infection of human herpesviruses.
Dynamic interaction of MHV-68 and the host immune system.
It was traditionally thought that the acute lytic replication of MHV-68 is systemically cleared from the infected host within 2 weeks postinfection. However, using our bioluminescence imaging system, we demonstrated that the lytic replication of MHV-68 peaked and cleared with different kinetics among organs and tissues. Moreover, the viral lytic replication was cleared at one point and reappeared in some other organs, suggesting the spontaneous and sporadic reactivation of MHV-68.
The progression of viral infection among organs and tissues greatly depends upon the routes of inoculation and mode of transmission among tissues. In other words, the signals appear first at the sites close to where the virus is inoculated. And when the virus replicates, the host immune responses are elicited and recruited to the sites of infection to clear the virus. After infection at the primary sites, the virus spreads to secondary sites. We do not know exactly how the virus spreads. Based upon no detectable viremia during infection, migration of infected cells is believed to be the way for the virus to disseminate. Therefore, the virus is continuously on the move in association with specific types of cells. The infection at any given site is controlled by permissiveness of the infected cells and the host immunity induced at the site. The abilities of herpesvirus to establish latency and to evade the immune responses further complicate the dynamics of this competition. Consequently, viral infection within mice is a dynamic process, varying among organs and tissues as well as times after infection. Our data did not directly reveal the underlying mechanism but suggest that a different approach is required to study this complex issue.
We further demonstrated that the bioluminescence imaging of M3FL can be utilized to study the induction of viral reactivation in vivo. Previously we have shown that NF-κB inhibits the initiation of MHV-68 lytic replication and blocking the NF-κB activity by Velcade reactivates KSHV and EBV (2, 3). Indeed, M3FL reactivation could be induced by Velcade to a level beyond the control of the host immune system and detected by imaging. While using CspA, we were able to suppress the host immune surveillance and allowed the reactivating virus to replicate and generate sufficient signals to be detected. Although we cannot completely exclude the possible effect of Velcade on the immune system and that of CspA on the lytic replication of gammaherpesvirus (29, 31, 48), the results clearly indicate that the reactivation of the latent virus is controlled by a delicate balance between the ability of the virus to replicate and the ability of the host to restrict reactivation.
Importantly, we also found that the preferential site of reactivation depends upon the route of infection. The biggest latent reservoirs for reactivation depend upon the route of infection. This result has significant implications for in vivo study, since very often, the spleen is the only organ harvested from long-term infected mice for analysis.
In conclusion, our data highlight the use of bioluminescence imaging for real-time monitoring of the interaction between persistent gammaherpesvirus and the host immune system. By enabling serial studies of the gammaherpesvirus infection in the same host over time, this noninvasive imaging illustrates the dynamic virus-host interaction which controls the replication and reactivation of gammaherpesvirus in vivo. This imaging system can facilitate the development of drugs for controlling gammaherpesviruses in vivo by enabling real-time monitoring of the systemic infection after drug treatment. Furthermore, the roles of host and virus genes in in vivo gammaherpesvirus replication can be efficiently investigated either by infecting the virus in mice with different genetic backgrounds or by investigating a mutant virus in a healthy host.
Acknowledgments
We thank Christine A. Holten for critically reading and editing the manuscript, the staff of the Crump Institute for Molecular Imaging at UCLA for help with the bioluminescence imaging experiments, and all members in the Sun lab for helpful discussions. We also appreciate Philip Stevenson's communication and suggestions.
This work was supported by U.S. National Institutes of Health (NIH) grants R01-DE15752, R21-CA120761, RO1-AI52002 and P50-CA86306. S.H. was supported by UCLA AIDS Institute and UCLA Center for AIDS Research (AI28697) and Universitywide AIDS Research Program Dissertation Award (D06-LA-4). T.-T.W. was supported by NIH grant 1R21DE018337-01.
Footnotes
Published ahead of print on 8 October 2008.
REFERENCES
- 1.Benaron, D. A., P. R. Contag, and C. H. Contag. 1997. Imaging brain structure and function, infection and gene expression in the body using light. Philos. Trans. R. Soc. Lond. B 352755-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, H. J., W. H. McBride, J. A. Zack, and R. Sun. 2005. Prostratin and bortezomib are novel inducers of latent Kaposi's sarcoma-associated herpesvirus. Antivir. Ther. 10745-751. [PubMed] [Google Scholar]
- 3.Brown, H. J., M. J. Song, H. Deng, T. T. Wu, G. Cheng, and R. Sun. 2003. NF-κB inhibits gammaherpesvirus lytic replication. J. Virol. 778532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choy, G., P. Choyke, and S. K. Libutti. 2003. Current advances in molecular imaging: noninvasive in vivo bioluminescent and fluorescent optical imaging in cancer research. Mol. Imaging 2303-312. [DOI] [PubMed] [Google Scholar]
- 5.Choy, G., S. O'Connor, F. E. Diehn, N. Costouros, H. R. Alexander, P. Choyke, and S. K. Libutti. 2003. Comparison of noninvasive fluorescent and bioluminescent small animal optical imaging. BioTechniques 351022-1026, 1028-1030. [DOI] [PubMed] [Google Scholar]
- 6.Contag, C. H., P. R. Contag, J. I. Mullins, S. D. Spilman, D. K. Stevenson, and D. A. Benaron. 1995. Photonic detection of bacterial pathogens in living hosts. Mol. Microbiol. 18593-603. [DOI] [PubMed] [Google Scholar]
- 7.Contag, C. H., S. D. Spilman, P. R. Contag, M. Oshiro, B. Eames, P. Dennery, D. K. Stevenson, and D. A. Benaron. 1997. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem. Photobiol. 66523-531. [DOI] [PubMed] [Google Scholar]
- 8.Contag, P. R., I. N. Olomu, D. K. Stevenson, and C. H. Contag. 1998. Bioluminescent indicators in living mammals. Nat. Med. 4245-247. [DOI] [PubMed] [Google Scholar]
- 9.Correia-Silva, J., J. M. N. Victoria, A. L. S. Guimaraes, U. E. Salomao, M. de Abreu, H. Bittencourt, and R. S. Gomez. 2007. Cytomegalovirus shedding in the oral cavity of allogeneic haematopoietic stem cell transplant patients. Oral Dis. 13163-169. [DOI] [PubMed] [Google Scholar]
- 10.de Lima, B. D., J. S. May, and P. G. Stevenson. 2004. Murine gammaherpesvirus 68 lacking gp150 shows defective virion release but establishes normal latency in vivo. J. Virol. 785103-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Luca, D., P. Mirandola, T. Ravaioli, R. Dolcetti, A. Frigatti, P. Bovenzi, L. Sighinolfi, P. Monini, and E. Cassai. 1995. Human herpesviruses 6 and 7 in salivary glands and shedding in saliva of healthy and human immunodeficiency virus positive individuals. J. Med. Virol. 45462-468. [DOI] [PubMed] [Google Scholar]
- 12.Doherty, P. C., J. P. Christensen, G. T. Belz, P. G. Stevenson, and M. Y. Sangster. 2001. Dissecting the host response to a gamma-herpesvirus. Philos. Trans. R. Soc. Lond. B 356581-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donskoy, E., and I. Goldschneider. 2003. Two developmentally distinct populations of dendritic cells inhabit the adult mouse thymus: demonstration by differential importation of hematogenous precursors under steady state conditions. J. Immunol. 1703514-3521. [DOI] [PubMed] [Google Scholar]
- 14.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 1651074-1081. [DOI] [PubMed] [Google Scholar]
- 15.Flano, E., Q. Jia, J. Moore, D. L. Woodland, R. Sun, and M. A. Blackman. 2005. Early establishment of gamma-herpesvirus latency: implications for immune control. J. Immunol. 1744972-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flano, E., I. J. Kim, J. Moore, D. L. Woodland, and M. A. Blackman. 2003. Differential gamma-herpesvirus distribution in distinct anatomical locations and cell subsets during persistent infection in mice. J. Immunol. 1703828-3834. [DOI] [PubMed] [Google Scholar]
- 17.Gill, M. B., L. Gillet, S. Colaco, J. S. May, B. D. de Lima, and P. G. Stevenson. 2006. Murine gammaherpesvirus-68 glycoprotein H-glycoprotein L complex is a major target for neutralizing monoclonal antibodies. J. Gen. Virol. 871465-1475. [DOI] [PubMed] [Google Scholar]
- 18.Hakamata, Y., T. Murakami, and E. Kobayashi. 2006. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation 811179-1184. [DOI] [PubMed] [Google Scholar]
- 19.Hogquist, K. A., T. A. Baldwin, and S. C. Jameson. 2005. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 5772-782. [DOI] [PubMed] [Google Scholar]
- 20.Husain, S. M., E. J. Usherwood, H. Dyson, C. Coleclough, M. A. Coppola, D. L. Woodland, M. A. Blackman, J. P. Stewart, and J. T. Sample. 1999. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8+ T lymphocytes. Proc. Natl. Acad. Sci. USA 967508-7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Idesawa, M., N. Sugano, K. Ikeda, M. Oshikawa, M. Takane, K. Seki, and K. Ito. 2004. Detection of Epstein-Barr virus in saliva by real-time PCR. Oral Microbiol. Immunol. 19230-232. [DOI] [PubMed] [Google Scholar]
- 22.Klussmann, J. P., A. Muller, M. Wagner, O. Guntinas-Lichius, M. Jungehuelsing, T. Sloots, D. V. Ablashi, and G. R. Krueger. 2000. Human herpesvirus type 8 in salivary gland tumors. J. Clin. Virol. 16239-246. [DOI] [PubMed] [Google Scholar]
- 23.Krug, L. T., J. M. Moser, S. M. Dickerson, and S. H. Speck. 2007. Inhibition of NF-kappaB activation in vivo impairs establishment of gammaherpesvirus latency. PLoS Pathog. 3e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luker, G. D., J. P. Bardill, J. L. Prior, C. M. Pica, D. Piwnica-Worms, and D. A. Leib. 2002. Noninvasive bioluminescence imaging of herpes simplex virus type 1 infection and therapy in living mice. J. Virol. 7612149-12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luker, G. D., and D. A. Leib. 2005. Luciferase real-time bioluminescence imaging for the study of viral pathogenesis. Methods Mol. Biol. 292285-296. [DOI] [PubMed] [Google Scholar]
- 26.Luker, G. D., J. L. Prior, J. Song, C. M. Pica, and D. A. Leib. 2003. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J. Virol. 7711082-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Guzman, D., T. Rickabaugh, T. T. Wu, H. Brown, S. Cole, M. J. Song, L. Tong, and R. Sun. 2003. Transcription program of murine gammaherpesvirus 68. J. Virol. 7710488-104503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massoud, T. F., and S. S. Gambhir. 2007. Integrating noninvasive molecular imaging into molecular medicine: an evolving paradigm. Trends Mol. Med. 13183-191. [DOI] [PubMed] [Google Scholar]
- 29.Mattingly, L. H., R. A. Gault, and W. J. Murphy. 2007. Use of systemic proteasome inhibition as an immune-modulating agent in disease. Endocr. Metab. Immune Disord. Drug Targets 729-34. [DOI] [PubMed] [Google Scholar]
- 30.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 31.Nencioni, A., F. Grunebach, F. Patrone, A. Ballestrero, and P. Brossart. 2006. The proteasome and its inhibitors in immune regulation and immune disorders. Crit. Rev. Immunol. 26487-498. [DOI] [PubMed] [Google Scholar]
- 32.Peacock, J. W., and K. L. Bost. 2000. Infection of intestinal epithelial cells and development of systemic disease following gastric instillation of murine gammaherpesvirus-68. J. Gen. Virol. 81421-429. [DOI] [PubMed] [Google Scholar]
- 33.Pica, F., and A. Volpi. 2007. Transmission of human herpesvirus 8: an update. Curr. Opin. Infect. Dis. 20152-156. [DOI] [PubMed] [Google Scholar]
- 34.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2628. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 35.Savino, W. 2006. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah, K., and R. Weissleder. 2005. Molecular optical imaging: applications leading to the development of present day therapeutics. NeuroRx 2215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simas, J. P., and S. Efstathiou. 1998. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 6276-282. [DOI] [PubMed] [Google Scholar]
- 38.Simas, J. P., D. Swann, R. Bowden, and S. Efstathiou. 1999. Analysis of murine gammaherpesvirus-68 transcription during lytic and latent infection. J. Gen. Virol. 8075-82. [DOI] [PubMed] [Google Scholar]
- 39.Smith, G. A., and L. W. Enquist. 1999. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J. Virol. 736405-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song, M. J., S. Hwang, W. H. Wong, T. T. Wu, S. Lee, H. I. Liao, and R. Sun. 2005. Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis. Proc. Natl. Acad. Sci. USA 1023805-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevenson, P. G. 2004. Immune evasion by gamma-herpesviruses. Curr. Opin. Immunol. 16456-462. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson, P. G., and S. Efstathiou. 2005. Immune mechanisms in murine gammaherpesvirus-68 infection. Viral Immunol. 18445-456. [DOI] [PubMed] [Google Scholar]
- 43.Stewart, J. P., O. J. Silvia, I. M. Atkin, D. J. Hughes, B. Ebrahimi, and H. Adler. 2004. In vivo function of a gammaherpesvirus virion glycoprotein: influence on B-cell infection and mononucleosis. J. Virol. 7810449-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145818-826. [PMC free article] [PubMed] [Google Scholar]
- 45.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 732347-2356. [DOI] [PubMed] [Google Scholar]
- 46.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1993. Interactions of murine gammaherpesvirus 68 with B and T cell lines. Virology 193825-833. [DOI] [PubMed] [Google Scholar]
- 47.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 733275-3279. [DOI] [PubMed] [Google Scholar]
- 48.Tanner, J. E., and J. Menezes. 1994. Interleukin-6 and Epstein-Barr virus induction by cyclosporine A: potential role in lymphoproliferative disease. Blood 843956-3964. [PubMed] [Google Scholar]
- 49.van Berkel, V., K. Preiter, H. W. Virgin IV, and S. H. Speck. 1999. Identification and initial characterization of the murine gammaherpesvirus 68 gene M3, encoding an abundantly secreted protein. J. Virol. 734524-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2003. Maintenance of gammaherpesvirus latency requires viral cyclin in the absence of B lymphocytes. J. Virol. 775118-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virgin, H. W., IV, R. M. Presti, X. Y. Li, C. Liu, and S. H. Speck. 1999. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J. Virol. 732321-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, P., and J. Heitman. 2005. The cyclophilins. Genome Biol. 6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, J. C., G. Sundaresan, M. Iyer, and S. S. Gambhir. 2001. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol. Ther. 4297-306. [DOI] [PubMed] [Google Scholar]
- 54.Wu, T.-T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 743659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]