Abstract
The envelopment of the nucleocapsid is an important step in white spot syndrome virus (WSSV) assembly. Previous studies showed that VP26, a major envelope protein of WSSV, can interact with viral nucleocapsid. In this study, using the biotin label transfer technique, we found that the biotin label was transferred from Bio-rVP26 to the viral capsid protein VP51 or from Bio-MBP-VP51 to VP26. Far-Western analyses provided further evidence for direct interaction between VP26 and VP51. Therefore, we conclude that VP26 functions as a matrix-like linker protein between the viral envelope and nucleocapsid, which suggests that VP26 is a key factor in the envelopment of WSSV virion.
White spot syndrome virus (WSSV) is among the largest bacilliform viruses and has a very complex structure, in which the nucleocapsid containing a double-stranded circular DNA genome is surrounded by a lipid-containing envelope (24). WSSV is a very virulent pathogen which is responsible for high mortality in cultured shrimp populations (3, 22) and can also infect most species of crustaceans (1, 4, 9, 10). So far, three geographic WSSV isolates have been sequenced, and the complete genome sequence contains approximately 180 putative open reading frames (2, 14, 21). On the basis of phylogenetic analysis, WSSV has been classified as the sole member in a novel virus genus, Whispovirus, of the family Nimaviridae (16).
It is known that the structural proteins play very important roles in virus infection and the morphogenesis process. In recent years, there has been considerable progress in determining the protein composition of WSSV virions. First, 18 structural proteins of WSSV were discovered by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS) or electrospray ionization-quadrupole TOF-MS (5). After that, 33 viral structural proteins were identified by liquid chromatography-nano-electrospray ionization-tandem MS (13). Later, by separating WSSV virions into envelope and nucleocapsid fractions, we identified 22 proteins in the envelope fraction by MALDI-TOF MS, with 7 in the nucleocapsid fraction and 1 in both fractions (20). Recently, about 50 viral structural proteins were identified by using shotgun proteomics (8). However, due to the absence of appropriate cell lines, little is known about the organization of viral envelope and nucleocapsid components and how they functionally connect. In this study, we proposed to elucidate the assembly mechanism of WSSV by studying the interaction between structural proteins.
VP26 was first believed to be a nucleocapsid protein (15), but later it was identified as a viral envelope protein by immunoelectron microscopy (23). Recently, VP26 was regarded as a tegument protein because it could be solubilized by increasing salt concentration in Triton X-100 buffer, and it lies between the virus envelope and nucleocapsid, as determined by immunogold labeling electron microscopy (12). In our laboratory, when purified virions were treated with low-salt buffer containing 1% Triton X-100, VP26 was found present mainly in the envelope fraction and less in the nucleocapsid fraction (18). In addition, VP26 was found to interact with viral nucleocapsid in vitro (18), as well as with VP28 (the most abundant WSSV envelope protein) (20). Therefore, we consider VP26 to be the best candidate to act as a molecular bridge between the envelope and nucleocapsid of WSSV.
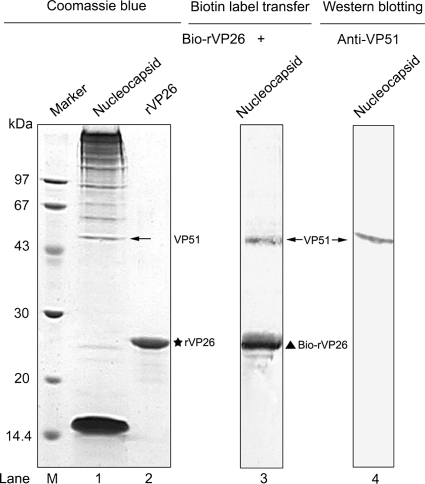
To determine which viral capsid protein(s) interacts directly with VP26, the biotin label transfer assay was performed using a ProFound Sulfo-SBED biotin label transfer kit (Pierce) according to the manufacturer's instructions. The advantage of this approach is that the target protein can be identified within a large protein complex. In brief, the recombinant VP26 (rVP26) protein with His tag was expressed and purified as previously described (18) and conjugated with Sulfo-SBED in the dark for 30 min at room temperature (RT). The excess cross-linking reagent was removed by dialysis overnight at 4°C in 1× label transfer buffer (supplied with the kit). Highly purified WSSV virions, as well as viral envelope and nucleocapsid fractions, were prepared according to previously described procedures (19, 20). After Sulfo-SBED-conjugated rVP26 (Bio-rVP26) was incubated with the viral nucleocapsid fraction, the reaction mixture was then cross-linked by exposure to a 365-nm 6-W hand-held UV lamp for 15 min at a distance of 5 cm. Protein samples were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (7) and either stained with Coomassie blue or blotted onto a polyvinylidene fluoride (PVDF; GE Healthcare) membrane. Biotin-labeled protein was detected with streptavidin-alkaline phosphatase (AP) (Promega). After incubation with NBT/BCIP substrate (Roche), a distinct band (Fig. 1, lane 3, upper band) was visible, which corresponds to VP51 (Fig. 1, lane 4), as detected by Western blotting with anti-VP51 antibody, besides Bio-rVP26 itself (Fig. 1, lane 3, lower band). The result suggests that the biotin label was transferred exclusively from Bio-rVP26 to its interacting protein, VP51.
FIG. 1.
The biotin label is transferred from Bio-rVP26 to VP51, as determined by biotin transfer assay. The left panel shows the Coomassie blue-stained SDS-PAGE gel of the WSSV nucleocapsid fraction (lane 1) and rVP26 (lane 2). The middle panel shows that rVP26 conjugated with Sulfo-SBED is mixed with the nucleocapsid fraction and was subjected to UV cross-linking. The protein mixtures were separated by SDS-PAGE, transferred onto PVDF membranes, and detected with streptavidin-AP (lane 3). The right panel shows that the VP51 protein present in the nucleocapsid fraction is visualized by Western blotting using anti-VP51 antibody (lane 4). The star indicates the position of rVP26. The triangle indicates the position of Bio-rVP26. The VP51 bands are indicated by arrows. Lane M, molecular mass reference markers (kDa).
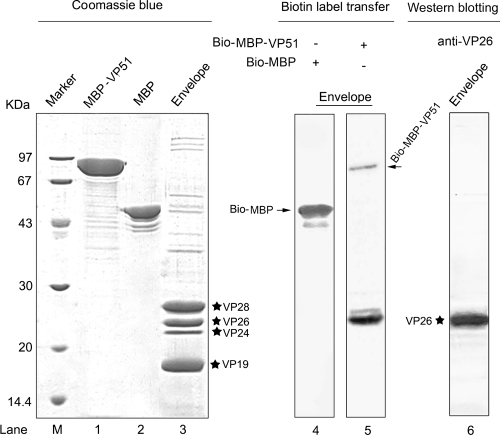
VP51, also termed VP466 or VP51C, is encoded by the wsv308 gene (21) and was initially thought to be a viral envelope protein (5). However, Tsai et al. (12) and Li et al. (8) demonstrated by immunoblotting that VP51 was present in the viral nucleocapsid fraction. Likewise, Wu et al. (17) found that VP51 was located exclusively in the viral capsid by Western blotting and immunoelectron microscopy. Moreover, MALDI-TOF MS results also showed that VP51 was one of eight viral nucleocapsid components (20). To verify the above-described interaction, VP51 was cloned by PCR amplification (with primers 5′-GCATGGATCCTCTGCATCTTTAATATTGGAC-3′ and 5′-GCGCAAGCTTTTATGACACAAACCTATTCC-3′ [BamHI and HindIII sites, respectively, are underlined]) and expressed in Escherichia coli strain BL21(DE3), using the pMAL-c2x vector (New England Biolabs) to generate N-terminal maltose-binding protein (MBP)-tagged fusion protein. The MBP-VP51 or MBP purified by amylose resin (New England Biolabs) was also labeled using Sulfo-SBED. The results showed that a strong biotin-labeled band (Fig. 2, lane 5) that corresponds to VP26 (Fig. 2, lane 6), as determined by Western blotting with anti-VP26 antibody, was detected when the viral envelope fraction was incubated with Bio-MBP-VP51 but not with Bio-MBP (Fig. 2, lane 4). Moreover, the biotin moiety was not found to transfer to other viral envelope proteins, suggesting that the interaction between VP26 and VP51 is specific.
FIG. 2.
The biotin label was transferred from Bio-MBP-VP51 to VP26 as determined by biotin transfer assay. The left panel shows the Coomassie blue-stained SDS-PAGE gel of MBP-VP51 (lane 1), MBP (lane 2), and the WSSV envelope fraction (lane 3). The middle panel shows that biotin-labeled MBP and MBP-VP51 react with the envelope fraction and were detected with streptavidin-AP (lanes 4 and 5). The right panel shows that the VP26 protein contained in the envelope fraction was visualized by Western blotting using anti-VP26 antibody (lane 6). The four major envelope proteins, VP28, VP26, VP24 and VP19, are marked with stars. Biotin-labeled MBP and MBP-VP51 bands are indicated by arrows. Lane M, molecular mass reference markers (kDa).
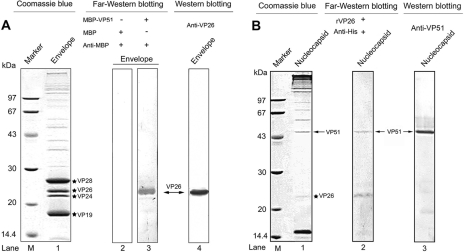
To further confirm the interaction between VP26 and VP51, far-Western blotting was performed as described previously (6), with some modifications. The protein sample (viral envelope or nucleocapsid fraction) was separated by SDS-PAGE, transferred onto PVDF membranes, and renatured by incubation with renaturation buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 2% nonfat milk, 0.1% Tween 20, 10% glycerol [pH 7.5]) containing 6 M, 3 M, 1.5 M, 0.75 M, 0.375 M, and 0.15 M guanidine-HCl at RT for successive 30-min periods, respectively. Finally, the membrane was immersed in renaturation buffer overnight at 4°C. Subsequently, the membrane containing viral envelope proteins was incubated with MBP-VP51 or MBP alone as the negative control in renaturation buffer for 2 h at RT, followed by incubation with anti-MBP monoclonal antibody (1:2,000; BioLabs) for 30 min at RT. After three washes with phosphate-buffered solution, the membrane was incubated with AP-conjugated goat anti-mouse immunoglobulin G (diluted 1:7,500) for 30 min at 4°C. The immunoblot signals were visualized by the AP reaction. As shown in Fig. 3A, MBP-VP51 did specifically bind to VP26 (Fig. 3, lane 3) in the viral envelope fraction, as visualized by Coomassie blue staining or Western blotting (Fig. 3, lane 1 or 4, respectively), while MBP alone did not (Fig. 3, lane 2). Similarly, when the membrane containing viral nucleocapsid proteins was incubated with the rVP26 protein and immunoblotted with anti-His monoclonal antibody (1:3,000; GE Healthcare), one immunoreactive band (Fig. 3B, lane 2, upper band) corresponding to VP51 was observed. This result further indicates that the interaction that occurs between VP26 and VP51 is direct and does not require other virus proteins. Interestingly, a band (Fig. 3B, lane 2, lower band) corresponding to VP26 was detected. We suppose this was due to rVP26 binding to the residual VP26 present in the nucleocapsid fraction, suggesting that VP26 can self-interact and form homomultimers. This is consistent with a recent report that VP26 crystallizes as trimers (11). VP26 homomultimers might be important for the interaction with other viral structural proteins, such as VP28.
FIG. 3.
The interaction between VP26 and VP51 is confirmed by far-Western analysis. (A) The WSSV envelope fraction was separated by SDS-PAGE and transferred onto PVDF membranes. The MBP-VP51 or MBP (negative control) is used as an overlay protein and detected with anti-MBP monoclonal antibody (lanes 2 and 3). The position of VP26 was visualized by Coomassie blue staining (lane 1) or Western blotting (lane 4). (B) The WSSV nucleocapsid fraction was separated by SDS-PAGE and transferred onto PVDF membranes. The rVP26 was used as the overlay protein and detected with anti-His monoclonal antibody (lane 2). The position of VP51 is visualized by Coomassie blue staining (lane 1) or Western blotting (lane 3). The four major envelope proteins, VP28, VP26, VP24 and VP19, are marked with stars. Biotin-labeled MBP and MBP-VP51 bands are indicated by arrows. Lane M, molecular mass reference markers (kDa).
Previous studies have shown that the VP28, VP26, and VP24 proteins can form a complex (20). To understand the physical association of VP28 or VP24 with the viral capsid, we also performed biotin label transfer assays and far-Western blotting as described above using rVP28 or rVP24 as a probe. All test results were negative (data not shown), indicating that there were no interactions occurring between the capsid proteins and VP28 or VP24.
In this study, using biotin label transfer and far-Western blotting techniques, we demonstrated for the first time the interaction between the viral envelope protein VP26 and the capsid protein VP51. Therefore, we conclude that VP26 functions as a linker protein interacting directly with VP51 to bridge the envelope to the nucleocapsid of WSSV, which suggests that VP26 is a key factor in the envelopment of the WSSV virion. Taken together, our finding not only lays the foundation for understanding the mechanisms of morphogenesis and assembly of WSSV but also provides potential targets for antiviral drug design. However, many interesting questions still require further research, such as determining the exact domains of VP26 and VP51 involved in the interaction, which capsid protein(s) can bind with VP51, and whether the process of envelopment was blocked or was interfered with when either VP26 or VP51 was silenced by RNA interference.
Acknowledgments
This investigation was supported by the Natural Science Foundation of China (3077164035), the National 863 Program of China (2006AA100312 and 2006AA09Z445), and the National 973 Program of China (2006CB101801).
Footnotes
Published ahead of print on 8 October 2008.
REFERENCES
- 1.Chang, P. S., H. C. Chen, and Y. C. Wang. 1998. Detection of white spot syndrome associated baculovirus in experimentally infected wild shrimp, crab and lobsters by in situ hybridization. Aquaculture 164233-242. [Google Scholar]
- 2.Chen, L. L., J. H. Leu, C. J. Huang, C. M. Chou, S. M. Chen, C. H. Wang, C. F. Lo, and G. H. Kou. 2002. Identification of a nucleocapsid protein (VP35) gene of shrimp white spot syndrome virus and characterization of the motif important for targeting VP35 to the nuclei of transfected insect cells. Virology 29344-53. [DOI] [PubMed] [Google Scholar]
- 3.Chou, H. Y., C. Y. Huang, C. H. Wang, and C. F. Lo. 1995. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis. Aquat. Org. 23165-173. [Google Scholar]
- 4.Hameed, A. S., G. Balasubramanian, S. S. Musthaq, and K. Yoganandhan. 2003. Experimental infection of twenty species of Indian marine crabs with white spot syndrome virus (WSSV). Dis. Aquat. Organ. 57157-161. [DOI] [PubMed] [Google Scholar]
- 5.Huang, C., X. Zhang, Q. Lin, X. Xu, Z. Hu, and C. L. Hew. 2002. Proteomic analysis of shrimp white spot syndrome viral proteins and characterization of a novel envelope protein VP466. Mol. Cell. Proteomics 1223-231. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita, T., and K. Shimazaki. 2001. Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant Cell. Physiol. 42424-432. [DOI] [PubMed] [Google Scholar]
- 7.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 8.Li, Z., Q. Lin, J. Chen, J. L. Wu, T. K. Lim, S. S. Loh, X. Tang, and C. L. Hew. 2007. Shotgun identification of the structural proteome of shrimp white spot syndrome virus and iTRAQ differentiation of envelope and nucleocapsid subproteomes. Mol. Cell. Proteomics 61609-1620. [DOI] [PubMed] [Google Scholar]
- 9.Lo, C. F., C. H. Ho, S. E. Peng, C. H. Chen, H. C. Hsu, Y. L. Chiu, C. F. Chang, K. F. Liu, M. S. Su, C. H. Wang, and A. G. H. Kou. 1996. White spot syndrome baculovirus (WSBV) detected in culture and captured shrimp, crabs and other arthropods. Dis. Aquat. Org. 27215-225. [Google Scholar]
- 10.Sanchez-Martinez, J. G., G. Aguirre-Guzman, and H. Mejia-Ruiz. 2007. White spot syndrome virus in cultured shrimp: a review. Aquac. Res. 381339-1354. [Google Scholar]
- 11.Tang, X., J. Wu, J. Sivaraman, and C. L. Hew. 2007. Crystal structures of major envelope proteins VP26 and VP28 from white spot syndrome virus shed light on their evolutionary relationship. J. Virol. 816709-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai, J. M., H. C. Wang, J. H. Leu, A. H. Wang, Y. Zhuang, P. J. Walker, G. H. Kou, and C. F. Lo. 2006. Identification of the nucleocapsid, tegument, and envelope proteins of the shrimp white spot syndrome virus virion. J. Virol. 803021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai, J. M., H. C. Wang, J. H. Leu, H. H. Hsiao, A. H. Wang, G. H. Kou, and C. F. Lo. 2004. Genomic and proteomic analysis of thirty-nine structural proteins of shrimp white spot syndrome virus. J. Virol. 7811360-11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Hulten, M. C., J. Witteveldt, S. Peters, N. Kloosterboer, R. Tarchini, M. Fiers, H. Sandbrink, R. K. Lankhorst, and J. M. Vlak. 2001. The white spot syndrome virus DNA genome sequence. Virology 2867-22. [DOI] [PubMed] [Google Scholar]
- 15.van Hulten, M. C., M. Westenberg, S. D. Goodall, and J. M. Vlak. 2000. Identification of two major virion protein genes of white spot syndrome virus of shrimp. Virology 266227-236. [DOI] [PubMed] [Google Scholar]
- 16.Vetten, H. J., P. W. G. Chu, J. L. Dale, R. Harding, J. Hu, L. Katul, M. Kojima, J. W. Randles, Y. Sano, and J. E. Thomas. 2005. Nanoviridae, p. 343-352. In C. M. Faquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy, 8th report of the ICTV. Elsevier/Academic Press, London, United Kingdom.
- 17.Wu, C., and F. Yang. 2006. Localization studies of two white spot syndrome virus structural proteins VP51 and VP76. Virol. J. 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie, X., and F. Yang. 2005. Interaction of white spot syndrome virus VP26 protein with actin. Virology 33693-99. [DOI] [PubMed] [Google Scholar]
- 19.Xie, X., H. Li, L. Xu, and F. Yang. 2005. A simple and efficient method for purification of intact white spot syndrome virus (WSSV) viral particles. Virus Res. 10863-67. [DOI] [PubMed] [Google Scholar]
- 20.Xie, X., L. Xu, and F. Yang. 2006. Proteomic analysis of the major envelope and nucleocapsid proteins of white spot syndrome virus. J. Virol. 8010615-10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang, F., J. He, X. Lin, Q. Li, D. Pan, X. Zhang, and X. Xu. 2001. Complete genome sequence of the shrimp white spot bacilliform virus. J. Virol. 7511811-11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan, W. B., and Y. H. Wang. 1998. White spot syndrome virus infection of cultured shrimp in china. J. Aquat. Anim. Health 10405-410. [Google Scholar]
- 23.Zhang, X., C. Huang, X. Xu, and C. L. Hew. 2002. Transcription and identification of an envelope protein gene (p22) from shrimp white spot syndrome virus. J. Gen. Virol. 83471-477. [DOI] [PubMed] [Google Scholar]
- 24.Zhou, Q., H. Li, Y. P. Qi, and F. Yang. 2008. Lipid of white spot syndrome virus originating from host-cell nuclei. J. Gen. Virol. 892909-2914. [DOI] [PubMed] [Google Scholar]