Abstract
Respiratory syncytial virus (RSV) readily infects and reinfects during infancy and throughout life, despite maternal antibodies and immunity from prior infection and without the need for significant antigenic change. RSV has two neutralization antigens, the F and G virion glycoproteins. G is expressed in both membrane-bound (mG) and secreted (sG) forms. We investigated whether sG might act as a decoy for neutralizing antibodies by comparing the in vitro neutralization of wild-type (wt) RSV versus recombinant mG RSV expressing only mG. wt RSV indeed was less susceptible than mG RSV to monovalent G-specific and polyvalent RSV-specific antibodies, whereas susceptibility to F-specific antibodies was equivalent. This difference disappeared when the virus preparations were purified to remove sG. Thus, sG appears to function as a neutralization decoy. We evaluated this effect in vivo in mice by comparing the effects of passively transferred antibodies on the pulmonary replication of wt RSV versus mG RSV. Again, wt RSV was less sensitive than mG RSV to G-specific and RSV-specific antibodies; however, a similar difference was also observed with F-specific antibodies. This confirmed that sG helps wt RSV evade the antibody-dependent restriction of replication but indicated that in mice, it is not acting primarily as a decoy for G-specific antibodies, perhaps because sG is produced in insufficient quantities in this poorly permissive animal. Rather, we found that the greater sensitivity of mG versus wt RSV to the antiviral effect of passively transferred RSV antibodies required the presence of inflammatory cells in the lung and was Fcγ receptor dependent. Thus, sG helps RSV escape the antibody-dependent restriction of replication via effects as an antigen decoy and as a modulator of leukocytes bearing Fcγ receptors.
Human respiratory syncytial virus (RSV) is the leading viral agent of serious pediatric respiratory tract disease worldwide (10). Yearly infections and deaths due to RSV worldwide are estimated to be 64 million and 160,000, respectively (53). A striking feature of RSV is its ability to infect neonates and infants very early in life despite the presence of maternally derived virus-neutralizing serum antibodies. Indeed, the peak of serious RSV disease occurs at 2 months of age, a time in life when maternal antibodies protect infants against most other pathogens. Another striking characteristic of RSV is its ability to reinfect and cause disease throughout life, sometimes even during the same epidemic season, despite having only a single serotype (17, 19, 20, 22; reviewed in reference 10). The ability of RSV to infect very early in life despite maternal antibodies and to reinfect throughout life despite immunity from prior infection accounts for much of its impact on human health.
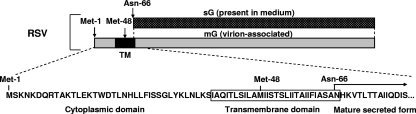
RSV has two major virion envelope proteins, the fusion F and major attachment G glycoproteins, which are the two viral neutralization antigens. The full-length RSV membrane-bound G protein (mG), which is anchored by a transmembrane domain near the N terminus, also is expressed in a secreted version (sG) that lacks the transmembrane domain due to an alternative initiation of translation at the second Met (amino acid 48) in the open reading frame, followed by proteolytic trimming to make a new N terminus at amino acid position 66 (Fig. 1). In the medium of RSV-infected cells, approximately 80% of the total released G protein is present as sG, while the remaining 20% is present as mG incorporated into virion particles (24, 39). Although the RSV G protein is characterized by extensive sequence diversity among different viral isolates (8, 16, 26, 46, 49), all of the many available G protein sequences contain the second Met at position 48, suggesting that the expression of the secreted form is highly conserved and confers some selective advantage. A number of other enveloped viruses express both membrane-bound and secreted forms of a major surface glycoprotein and neutralization antigen, indicating that the expression of two forms of a neutralization antigen, one anchored and one secreted, is a common theme in animal virology (see Discussion). We were interested in investigating whether the RSV sG glycoprotein—and, by extrapolation, the secreted forms of these other viral glycoproteins—might help the virus evade host immunity. One possible mechanism would be to function as a decoy molecule to bind virus-neutralizing antibodies, thereby reducing the efficiency of antibody-mediated virus neutralization. This question was addressed in vitro in the present study by evaluating the relative sensitivity of recombinant wild-type (wt) RSV, which expresses both sG and mG, or an RSV mutant that expresses only mG (designated mG RSV) to neutralization by RSV antibodies in the presence or absence of sG. This was also studied in vivo in a mouse model in which the replication of wt RSV and mG RSV in the lungs was compared in animals that were passively administered RSV G or F antibodies. There, we identified a second effect of sG that involves modulation of the inflammatory leukocyte response.
FIG. 1.
The secreted and membrane-bound forms of the RSV G glycoprotein. Vertically aligned rectangles represent the same amino acid sequence but differ with regard to being secreted (upper bar diagram, dotted) or membrane-bound (lower bar diagram, shaded). The transmembrane domain (TM) is depicted in black. The sequence of the first 100 amino acids of the protein is shown at the bottom to illustrate the two alternative translational start sites at Met-1 and Met-48, the transmembrane domain, and the new N terminus that is generated at asparagine-66 by proteolysis (24, 39).
Previous studies provided conflicting results regarding the level of replication, the level of inflammatory cell infiltration, and the levels of cytokines that were induced by wt RSV versus mG RSV. In one study, sG was found to decrease both virus replication and pulmonary cell infiltration in mice (44), whereas in the second study, sG promoted both replication and pulmonary cell infiltration (29). In the present study, we addressed these disparate findings using wt RSV and mG RSV preparations that had been sequenced completely, had minimal adventitious changes, and were found to replicate comparably in vitro and in vivo. The results showed that sG reduces the sensitivity of wt RSV to passively transferred antibodies through an effect that depends on the presence of pulmonary Fc receptor-bearing leukocytes.
The evasion and manipulation of the host immune system by virally expressed proteins is well known and has been described with regard to the effects on antigen presentation, cellular responses, inflammation, and other cytokine-mediated responses (2, 30, 38). The present report describes a new facet involving the evasion of virus-neutralizing antibodies by two distinct mechanisms.
MATERIALS AND METHODS
Viruses, cells, and analysis of viral proteins.
Recombinant RSV was previously engineered to contain the Met-48-Ile and Ile-49-Val amino acid substitutions that ablated the expression of sG, resulting in the virus mG RSV (48). We recovered a fresh preparation of mG RSV from cDNA constructed as previously described (48). Recombinant wt RSV and mG RSV were propagated in HEp-2 human epithelial cells (American Type Culture Collection, Manassas, VA) in 2% fetal bovine serum (Invitrogen, Carlsbad, CA). The virus preparations used in this study were sequenced in their entirety except for nucleotides 1 to 29 and 15192 to 15223 at the 3′ and 5′ ends, respectively, that were bound by reverse transcriptase PCR (RT-PCR) primers and were not directly confirmed. Sequence analysis was performed directly on uncloned RT-PCR products. For the experiment for which the results are shown in Fig. 2, the medium overlying the virus-infected cells was collected and clarified by low-speed centrifugation (300 × g at 4°C for 10 min) and used without further treatment. For the experiment for which the results are shown in Fig. 3, virus in the clarified medium was purified by ultracentrifugation in a discontinuous 30% to 60% (wt/wt) sucrose gradient (8,000 × g at 4°C for 2 h). The virus was collected from the interface between the two sucrose layers and aliquoted. For the experiments for which the results are shown in Fig. 4 to 9, the viruses in the clarified medium were concentrated 20-fold (by volume) using Vivaspin 20 centrifugal concentrators with a pore size of 300 kDa (Sartorius AG, Goettingen, Germany) according to the manufacturer's recommendations. Since sG should readily pass through the concentrator pores, its concentration should have remained essentially unchanged. Titration of the viruses was performed by plaque assay on monolayers of HEp-2 cells with immunostaining of the plaques by monoclonal antibodies specific to RSV F protein (32).
FIG. 2.
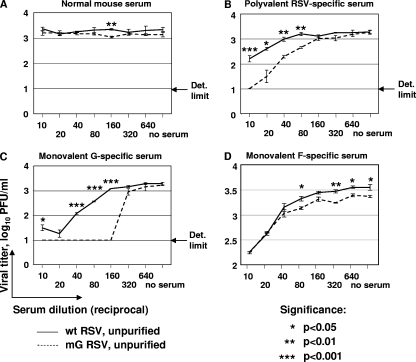
Neutralization of 103.0 to 103.5 PFU/ml of unpurified wt RSV or mG RSV with various dilutions of normal (nonimmune) mouse serum (A), polyvalent RSV-specific mouse antiserum (B), or monovalent mouse antiserum specific to the G (C) or F (D) protein. The viral titers shown are the means of three replicate samples per each serum dilution, with the SE indicated. The limit of detection (Det. limit) in the assay is indicated except for the F-specific serum, which was also 1.0 log10 PFU/ml. Statistical significances of the differences between the titers of the two viruses are shown.
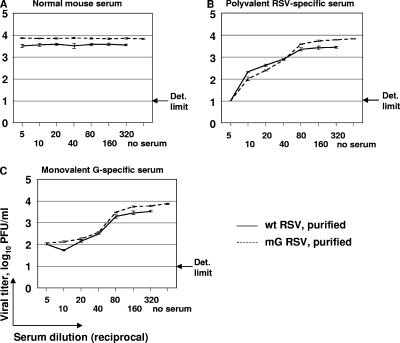
FIG. 3.
Neutralization of wt RSV or mG RSV as described in the legend to Fig. 2, except that the virus preparations were purified to remove sG that is otherwise present in wt RSV preparations. (A) Normal (nonimmune) serum; (B) polyvalent RSV-specific antiserum; (C) monovalent G-specific antiserum.
FIG. 4.
Replication of wt RSV or mG RSV in the lungs of mice in the presence of RSV-specific antibodies of various specificities. (A) Map of the experiment. (B to D) Pulmonary titers of wt RSV or mG RSV in mice that had previously received passive intraperitoneal transfer of the indicated dilutions of polyvalent RSV-specific serum (B), monovalent RSV G-specific serum (C), or monovalent RSV F-specific serum (D). The passive transfer was on day −1, infection was on day 0, and the tissue harvest was on day 4. The limit of detection (Det. limit; 1.7 log10 PFU/g of lung tissue) is indicated by an arrow. Mean viral titers and SE are shown. Groups A to F in each panel involved the following respective numbers of mice per group (and the numbers in parentheses indicate the number of mice in that group that lacked detectable virus): panel B, 6, 6, 6, 7, 6, and 6 (2); panel C, 5, 6, 6, 6, 6 (3), and 6 (5); panel D, 6, 5, 6, 6, 6, and 6 (2). For the animals in which virus was not detected, an arbitrary value of 1.4 log10 PFU/g (twofold below the detection limit) was assigned for the purpose of calculating the means; in the case of group F in panel C, this resulted in a mean titer of 1.45 log10 PFU/g that was below the detection level and is indicated with a plus. The n-fold reduction in the viral titers in the antibody-administered groups compared to that in the PBS-administered groups receiving the same virus are shown above the bars of the former groups. For each antibody-administered group, the statistical significance of the reduction versus the PBS-administered group infected with the same virus is shown. The differences between the viral titers of PBS-treated mice in different experiments depicted in panels B, C, and D are related to slightly variable titers of viral inoculums, as determined by their back titration.
FIG. 9.
Production of cytokines and chemokines in the lungs of mice treated or mock-treated with triamcinolone and infected with wt RSV or mG RSV, following the experimental scheme shown in Fig. 5A. Groups of mice treated with triamcinolone are indicated with a “t.” (A) Q-RT-PCR analysis of pulmonary mRNA. The difference in mRNA abundance in the infected-cell sample (normalized to an internal housekeeping mRNA control) compared to that of the mock-treated sample (normalized to an internal housekeeping control) was expressed in log2 units. The values of the mock group were equal to 1. (B) Analysis of cytokine and chemokine expression at the protein level by ELISA, expressed as pg per g of lung tissue. All of the cytokines and chemokines shown in panel A also were analyzed in panel B, but the results are shown only for those where there was a clear change compared to the mock. Mean values ± SE are calculated based on four mice per group infected with wt RSV or mG RSV and treated with triamcinolone; all other groups contained three mice per group. Statistically significant differences between the values for the wt RSV-infected groups and the mG RSV-infected groups are indicated with asterisks.
For viral protein analysis, sucrose gradient-purified virions were lysed using NuPage LDS sample buffer (Invitrogen, Mountain View, CA), and the proteins were electrophoretically separated under denaturing and reducing conditions on 4 to 12% gradient bis-Tris acrylamide gels (NuPage system; Invitrogen). Following separation, the proteins were analyzed by silver staining using a SilverQuest kit (Invitrogen), according to the manufacturer's recommendations, or by Western blotting. Magic XP marker proteins (Invitrogen) were used as molecular weight markers. Quantitative analysis of the protein bands in scanned silver-stained gels was performed with IP lab gel software (Signal Analytics Corp., Vienna, VA). For the Western blotting analysis, the separated proteins were transferred to a Hybond-P membrane (GE Healthcare, Piscataway, NJ) and incubated with the polyvalent mouse RSV-specific antibodies (see below), followed by the goat anti-mouse immunoglobulin G (IgG) plus IgM labeled with fluorescein (Invitrogen) (both at a 1:1,000 dilution). The fluorescent bands were scanned with a Typhoon 8600 phosphorimager (Molecular Dynamics, Sunnyvale, CA) and analyzed as described above.
Generation of polyvalent and monovalent RSV-specific antisera.
To generate the polyvalent RSV-specific serum, 26 10- to 12-week-old BALB/c mice or C57BL/6 mice (Charles River Laboratories) under methoxyflurane anesthesia were inoculated by the intranasal route with 5 × 105 PFU (in a volume of 100 μl) of wt RSV. Four weeks later, the animals were euthanized and sera were collected and pooled. To generate monovalent serum specific to RSV G or F protein, recombinant vaccinia viruses expressing the G (14) or F (33) gene of RSV strain A2 (the same strain as was used for the construction of recombinant wt RSV and mG RSV in the present study) were propagated in Vero cells (American Type Culture Collection) containing 2% fetal bovine serum (Invitrogen). To harvest the virus, the cells were scraped into the medium, collected by low-speed centrifugation as described above, subjected to three consecutive cycles of freezing and thawing to disrupt the cells and release the virus particles, and sonicated. The viruses were titrated on monolayers of Vero African green monkey cells, and plaques were identified by crystal violet staining. The viruses were used to inoculate 7- to 18-week-old BALB/c mice (Charles River Laboratories) (35 and 55 animals for the vaccinia viruses expressing RSV F or G, respectively) under methoxyflurane anesthesia by intranasal and intradermal inoculations in the footpad (50 μl into each site), with a combined dose of 3 × 106 or 3 × 107 PFU of vaccinia virus expressing RSV G or F, respectively, per animal. For generation of the G-specific serum, the inoculations were repeated 4 weeks later. Four weeks after the last dose, the animals were euthanized and serum samples were collected and pooled.
Determination of antibody titers.
The titers of antibodies specific to the RSV G and F protein in the polyvalent and monovalent mouse sera were determined by enzyme-linked immunosorbent assay (ELISA) as follows. Immulon IB plates (Thermo Scientific, Waltham, MA) were coated with 100 ng/well of purified G or F protein diluted in carbonate buffer. The plates were then incubated overnight at 4°C and washed three times with phosphate-buffered saline containing 0.05% Tween 20 (PBST). Dilutions of mouse sera in PBST containing 1% fetal bovine serum were applied to antigen-coated and noncoated wells; the plates were incubated overnight at room temperature and washed as described above. A 1:2,000 dilution of goat anti-human IgG conjugated to alkaline phosphatase (Jackson ImmunoResearch, West Grove, PA) in PBST was added, and the plates were incubated at room temperature overnight followed by washing as described above. To develop the plates, 100 μl per well of alkaline phosphatase yellow (pNPP) liquid substrate system for ELISA (Sigma-Aldrich, St. Louis, MO) was added, the plates were incubated for 20 min at room temperature, and the reaction was stopped by adding 50 μl per well of 1 N sodium hydroxide. The optical density was determined at 405 nm, and the titer was defined as the inverse dilution resulting in an optical density that was at least double that of the background and was greater than 0.1. In the polyvalent serum raised by RSV infection and used in the experiments for which the results are shown in Fig. 2 and 3, the reciprocal titers of the G- and F-specific antibodies were 16.3 and 18.3 log2 units, respectively. In the polyvalent RSV-specific serum used in the experiments for which the results are shown in Fig. 4, the respective titers were 18.3 and 20.3, respectively. In the monovalent RSV G-specific or F-specific sera, the reciprocal titer of the G-specific or F-specific antibodies was 17.3 log2 units in each case. Following the passive antibody transfer (see below), serum neutralizing-antibody titers were determined by a 60% RSV plaque reduction assay as previously described (9).
In vitro neutralization of wt RSV or mG RSV by sera of various specificities.
A total of 75 μl of Opti-MEM medium (Invitrogen) containing 1 × 104 PFU of recombinant wt RSV or mG RSV and 10% guinea pig complement (Cambrex Corporation, East Rutherford, NJ) were combined with 75 μl of various dilutions of polyvalent RSV-specific, monovalent G- or F-specific, or control serum in Opti-MEM medium and were incubated at 37°C for 1 h. The residual infectivity was then determined by titration in HEp-2 monolayers as described above.
Infection of mice and replication of wt RSV or mG RSV in lungs following the passive transfer of RSV-specific antibodies.
Eight- to 10-week-old BALB/c mice (Charles River Laboratories, Wilmington, MA) (Fig. 4, 5, 6, 8, and 9) or 9-week-old Fcγ receptor knockout (KO) mice on a BALB/c background (Taconic, Germantown, NY) (Fig. 7) were used in the study. For the passive-transfer experiments (Fig. 4, 7, and 8), the animals were injected by the intraperitoneal route with 0.5 ml of the indicated dilution of polyvalent RSV-specific or monovalent G-specific or F-specific serum in PBS or with PBS alone. The next day, the serum samples were collected in some experiments, and the animals were inoculated intranasally under methoxyflurane anesthesia with 6 × 106 PFU of wt RSV or mG RSV, which had been concentrated with Vivaspin concentrators as described above, in a volume of 100 μl. On day 4 after infection, the mice were euthanized; the lungs were isolated, homogenized in Leibowitz L-15 medium (Invitrogen) containing antibiotics, and clarified by low-speed centrifugation as described above; and the supernatants containing the virus were divided into aliquots and frozen. The viral concentrations were determined by plaque titration as described above. For some passive-transfer experiments, as well as for histopathological and immunohistochemical studies and for the quantitation of cytokines and chemokines (see below), mice were given daily intramuscular injections of triamcinolone acetonide suspension (Kenalog-40) (Bristol Myers Squibb Company, Princeton, NJ) at a dose of 30 mg/kg and were infected as described above.
FIG. 5.
Weights of mice infected with wt RSV or mG RSV and treated or mock-treated with triamcinolone. (A) Map of the experiment. (B) Body weight expressed as a percentage of the original weight on day −1. The groups included the following numbers of mice that were mock-treated or treated with triamcinolone, respectively: wt RSV, 5 and 4; mG RSV, 5 and 4; and mock-infected, 3 and 3. In the wt RSV/triamcinolone group, one mouse was found dead on day 4, and the mean titer was calculated based on three mice. In the no-triamcinolone treatment group, significant differences for the wt RSV-infected mice compared to the mG RSV-infected mice were observed on days 1, 2, and 3; in the triamcinolone treatment group, a significant difference was observed on day 3 only (indicated with asterisks). Similar data were obtained in a separate independent experiment (not shown).
FIG. 6.
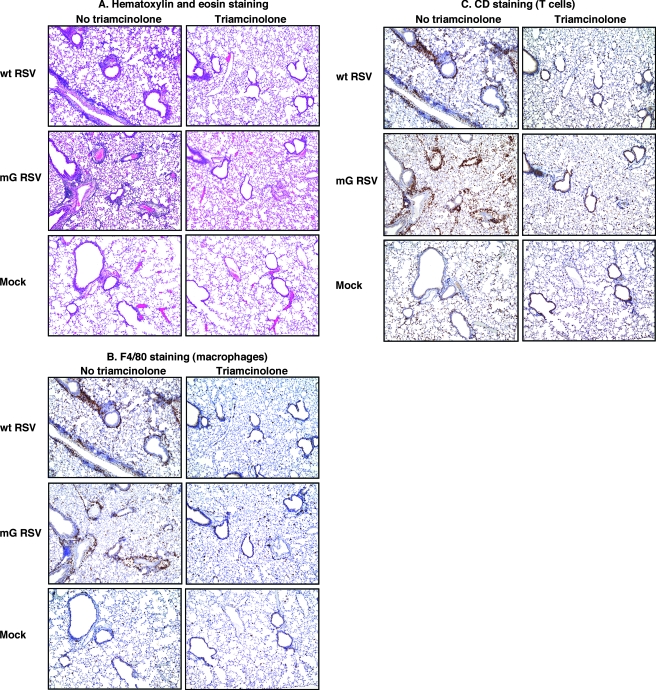
Histopathology and influx of macrophages and T cells in the lungs of mice infected with wt RSV or mG RSV and treated or mock-treated with triamcinolone from the same experiment as shown in Fig. 5. Lungs were harvested 4 days postinfection and were fixed and embedded in paraffin. Thin-layer sections were stained with hematoxylin and eosin (A), antibody specific to the F4/80 antigen (B), which is a marker for macrophages and other myeloid cells, or antibody specific to CD3, a marker for T cells (C). Scores for all of the mice in the experiment are shown in Table 1. For each virus and treatment combination, the sections shown in A, B, and C represent adjacent tissue slices.
FIG. 8.
Replication of wt RSV or mG RSV in the lungs of Fc receptor γ KO mice that had received a 1:20 dilution of monovalent F-specific antiserum or PBS as a control. (A) Map of the experiment. (B) Pulmonary titers of wt RSV and mG RSV. The n-fold reductions of the viral titers in the antibody-administered groups compared to that of the PBS-administered groups with the statistical significances of the differences are shown above the bars for the latter groups. Each group contained four mice, with the exception of the wt RSV group injected with PBS, which contained five mice. Det. limit, limit of detection.
FIG. 7.
Replication of wt RSV or mG RSV in the lungs of mice that had received RSV G-specific and F-specific antibodies and were treated with triamcinolone. (A) Map of the experiment. (B, C) Pulmonary titers of the viruses in mice that received a 1:20 dilution of monovalent G-specific antiserum (B) or a 1:30 dilution of monovalent F-specific antiserum (C) with control groups that received PBS instead of antiserum. Groups A, B, C, and D included 5, 6, 6, and 3 mice in panel B and 3, 4, 5, and 5 mice in panel C, respectively. Panels B and C depict the results of two independent experiments. For each of the four treatments (B and C), no statistical difference was observed between the log10 titers of wt RSV and mG RSV.
Histopathological and immunohistochemical analyses of lung tissues.
Lung tissues were fixed in 10% neutral buffered formalin and embedded in paraffin, and 4-μm sections were processed for staining with hematoxylin and eosin. Additional lung sections were stained for F4/80 (macrophage marker) or CD3 (T-cell marker) using rat anti-mouse F4/80 (Serotec, Inc., Raleigh, NC) or rabbit anti-human CD3 (Dako, Glostrup, Denmark), respectively, as the primary antibodies. For F4/80, a secondary biotinylated goat anti-rat IgG antibody (Biocare Medical, Concord, CA) was applied after incubation with the primary antibody, followed by a horseradish peroxidase-streptavidin complex (Biocare Medical). For CD3, a biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories, Burlingame, CA) was applied, followed by R.T.U. Vectastain Elite ABC reagent (Vector). The staining of both F4/80 and CD3 was completed with DAB chromogen.
Analysis of pulmonary cytokines and chemokines.
On day 4 after infection, the mice were euthanized, the lungs were isolated, and RNA from one lung of each mouse was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's recommendations, and additionally purified by two consecutive phenol-chloroform extractions, followed by ethanol precipitation. Quantitative analyses of the pulmonary mRNA for the cytokines and chemokines were performed using Taqman gene expression assays (Applied Biosystems, Foster City, CA), according to the manufacturer's recommendations. Quantitation was done by the comparative threshold cycle (CT) method, where ΔΔCT = ΔCT(target) − ΔCT(mock). Here, ΔΔCT is the change in the expression of the target RNA of interest in the infected-cell sample compared to that of the mock-treated control, expressed as log2 units. ΔCT(target) is the CT for the target RNA in the infected-cell sample normalized to the CT for an internal housekeeping gene, and ΔCT(mock) is the CT for the target RNA in the mock-treated control normalized to the CT for an internal housekeeping gene. CT is the number of cycles needed to reach a threshold value.
A second lung from each mouse was homogenized in cold Pierce radioimmunoprecipitation assay buffer with Halt protease inhibitor, Halt phosphatase inhibitor, and EDTA (Thermo Scientific, Rockford, IL), and the lysates were prepared according to the radioimmunoprecipitation assay buffer manufacturer's recommendations. Quantitative analyses of the cytokines and chemokines in the lysates were performed with Quantikine immunoassays (R&D Systems, Minneapolis, MN) according to the manufacturer's recommendations.
Statistical analysis of the data.
Differences in the viral titers in mouse lungs were evaluated by the Student's t test and considered significant when the P value was <0.05. Data are shown as means ± standard errors of the means (SE).
RESULTS
In vitro, sG protects RSV from neutralization by G-specific but not F-specific antibodies.
Recombinant RSV was previously engineered to contain two codon substitutions, Met-48-Ile and Ile-49-Val, in the G open reading frame (Fig. 1). These mutations were previously shown to completely abrogate the expression of the sG protein and did not affect the translation of the full-length G protein or change the hydrophobicity of the transmembrane domain (39, 48). The resulting mutant (mG RSV) lacking the sG protein was demonstrated to replicate with wt-like efficiency in cell culture and in the lungs of seronegative mice (48). The virus preparations used in this study were sequenced in their entirety (except for short segments at each genome end representing sites of primer binding [see Materials and Methods]) by direct analysis of the uncloned RT-PCR products. This showed that the sequence of the wt RSV preparation was identical to its cDNA clone (which had previously been shown to encode wt virus on the basis of causing respiratory tract disease in chimpanzees) except for a single nucleotide substitution at position 12205 (L gene) that resulted in an amino acid change (Thr-1236-Ile) and a single nucleotide substitution at position 12329 (L gene) that did not change the amino acid coding and was considered to be silent. This specific preparation of virus replicated in cell culture and in mice with an efficiency consistent with being a wt virus, suggesting that these changes were phenotypically silent. The mG RSV, whose cDNA was isogenic to wt RSV apart from the engineered change in G, contained a single nucleotide substitution at position 9849 (L gene) that did not affect the amino acid coding and thus was considered to be silent.
To determine whether sG might serve as a decoy molecule for RSV-specific antibodies, we compared the sensitivity of wt and mG RSV to neutralization in vitro by polyvalent mouse antiserum made in response to infection with RSV (and shown by ELISA to contain high levels of G-specific and F-specific antibodies [see Materials and Methods]) or by monovalent F-specific or G-specific mouse antiserum made in response to infection with recombinant vaccinia virus expressing the RSV F or G glycoprotein. The virus-antibody mixtures were incubated for 1 h at 37°C in the presence of 5% guinea pig complement (to enhance neutralization of the viruses by the antibodies and to better mimic the in vivo situation), and residual infectivity was determined by plaque titration.
Initially, we evaluated preparations of both wt RSV and mG RSV that were produced in cell culture but which had not been further purified and thus contained plentiful sG protein in the case of wt RSV but not mG RSV. We found that the polyvalent RSV-specific serum neutralized mG more efficiently than wt RSV (Fig. 2B). This difference was evident at low serum dilutions (dilutions of 1:10 to 1:80), whereas at high dilutions the neutralizing activity diminished and the difference between the two viruses disappeared, as would be expected. An even greater difference in neutralization between the two viruses was observed with the G-specific monovalent serum (Fig. 2C). At a serum dilution of 1:160, the residual infectivity of wt RSV was >1,200 PFU/ml, while that of mG was below the detection limit of 10 PFU/ml (>120-fold difference). In contrast, incubation with the F-specific serum resulted in equivalent neutralization of the two viruses (Fig. 2D), confirming that the difference in susceptibility was specific to G.
The reduced susceptibility of wt RSV to G-specific antibodies in vitro depends on sG.
We then purified wt RSV and mG RSV by ultracentrifugation in sucrose gradients to remove sG from the preparation of wt RSV. The ultracentrifugation of RSV in sucrose gradient was previously demonstrated to completely separate the virus from the sG protein (23). With the removal of sG, wt RSV and mG RSV became equally susceptible to neutralization by the polyvalent RSV-specific (Fig. 3B) and monovalent G-specific (Fig. 3C) antisera. Since the level of incorporation of full-length G protein into the viral particles may affect their susceptibility to neutralization by G-specific antibodies both in vitro and in vivo (below), we compared the levels of G protein in purified particles of wt RSV and mG RSV. We analyzed the protein content by denaturing gel electrophoresis and silver staining, followed by densitometric analysis of the protein bands, as well as by Western blotting with primary antibodies specific to the total RSV proteins and fluorescently labeled secondary antibodies, followed by phosphorimager analysis of the protein bands. We found no consistent difference between the two viruses in the ratios of G versus P protein or G versus M protein in either the silver stain or Western blot analysis (data not shown), suggesting that the abrogation of sG translation by introducing the two conserved mutations does not affect the level of incorporation of the full-length G protein into the viral particles. This is consistent with the expectation that ablation of the expression of sG would not affect the expression of mG, since the Met-48 translational start site of sG is downstream from the Met-1 start site of mG. These results indicated that the presence of sG in the preparation of wt RSV helped the virus evade neutralization by G-specific antibodies, thus showing that sG can act as an antigen decoy.
In vivo, sG protects RSV not only from G-specific antibodies but also from F-specific antibodies.
We next sought to determine if sG could function as a decoy for virus-neutralizing antibodies in vivo. For practical purposes, this was evaluated in the mouse model, but it is important to note that rodents are only semipermissible for RSV replication, with only scattered infected cells. Thus, the amount of sG elaborated by RSV replication in the respiratory tract of the mouse would be considerably less than that in the natural human host, such that its effect might be reduced in the animal model. BALB/c mice were injected intraperitoneally with polyvalent RSV-specific serum at dilutions of 1:20 and 1:40 or with PBS. The next day (day 0), the mice were infected intranasally with unpurified wt RSV or mG RSV. On day 4, corresponding to the peak of RSV replication in mice (18), the animals were sacrificed (Fig. 4A), and the viral titers in the lungs were determined by plaque titration (Fig. 4B). In animals infected with wt RSV, the prior administration of the 1:40 dilution of polyvalent serum did not reduce the pulmonary viral titers. In contrast, in mG-infected animals, viral titers were reduced by 15-fold. Moreover, the administration of the 1:20 dilution of serum resulted in a marginal (2.6-fold), statistically insignificant reduction in wt RSV titers, while the titers of mG RSV were reduced 257-fold. This indicated that mG RSV was much more sensitive than wt RSV to the antiviral effect of polyvalent antibodies in vivo, although it did not address whether G or F antibodies or both were mediating the effect.
We next tested the effect of monovalent G-specific antibodies on RSV replication in vivo (Fig. 4C). The administration of a 1:20 dilution of serum resulted in only a marginal (2.4-fold), statistically insignificant reduction in the pulmonary wt RSV titers, while titers of mG RSV were reduced 8.2-fold. The administration of a 1:5 dilution of serum resulted in a 17-fold reduction of wt RSV titers and, at minimum, a 31-fold reduction of mG RSV titers. In this case, the reduction of mG RSV titers is likely to be underestimated, since in three of the six animals infected with wt RSV and in five of the six animals infected with mG RSV, viral titers were below the limit of detection. These data suggest that the sG protein produced by wt RSV decreased the ability of passively transferred polyvalent RSV-specific and monovalent G-specific antibodies to restrict the replication of RSV in mouse lungs. We next tested whether we could observe a similar effect in animals injected with monovalent F-specific antibodies, which were expected to provide a negative control as it did in vitro (Fig. 4D). To our surprise, the administration of F-specific serum also resulted in a greater restriction of replication of mG RSV than wt RSV. In animals injected with a 1:40 dilution of F-specific serum, titers of wt RSV were not reduced, while titers of mG RSV were reduced 7.6-fold. The administration of a 1:20 dilution of F-specific serum resulted in 12-fold and 53-fold reductions of wt RSV and mG RSV, respectively.
Thus, in the mouse model, sG decreased the ability of either G- or F-specific antibodies to restrict the replication of RSV. This indicated that the mechanism of action of sG in this system cannot simply involve acting as a decoy to sequester G-specific neutralizing antibodies and instead must employ some other mechanism. One possibility is that the antibody-dependent restriction of virus replication effected by sG might involve its immunomodulatory activities, activities that remain to be fully defined (see the introduction). Of particular interest, a number of immune cell types bear Fc receptors that can bind antibody and contribute to viral clearance by the opsonization of complexes of antibody bound with free virus. In addition, virus replication can be restricted by antibody-dependent cellular cytotoxicity (ADCC) in which Fc-bearing inflammatory cells kill RSV-infected epithelial cells that have been bound by antibodies. Interestingly, the different susceptibilities of wt RSV and mG RSV to passively transferred antibodies were most pronounced when the polyvalent RSV-specific antibodies were used (Fig. 4B), compared to the G-specific (Fig. 4C) or F-specific (Fig. 4D) antibodies, suggesting that the effects of the G- and F-specific antibodies were additive.
The greater sensitivity of mG RSV to RSV antibodies is reversed by anti-inflammatory treatment.
If ADCC- and/or antibody-mediated opsonization was mediating the decrease in the replication of RSV in the lungs, then the elimination of the influx of inflammatory cells into the lung should abrogate the difference in replication of wt RSV and mG RSV in passively immunized mice. To address this, we first compared the extent of the gross pulmonary inflammation and histopathology induced by infection with wt RSV versus mG RSV. We also evaluated whether inflammation caused by the two viruses could be reduced or eliminated by the administration of a glucocorticoid anti-inflammatory drug. Glucocorticoid treatment is known to reduce inflammation in response to pulmonary viral infections; this has been shown to result in increased viral replication, indicating that the inflammatory response contributes to restricting virus replication (12, 36).
Groups of mice were infected with wt RSV or mG RSV or were mock-infected, with or without the daily administration of anti-inflammatory glucocorticoid triamcinolone acetonide beginning 1 day prior to infection (Fig. 5A). To evaluate the overall disease, the mice were weighed daily. In mice that did not receive triamcinolone, infection with wt RSV caused a substantial weight loss, with a maximum reduction of 9% observed on day 2 postinfection (Fig. 5B, left). In contrast, infection with mG RSV did not cause any significant weight loss. Thus, the ability to express sG was associated with increased disease. In mice treated with triamcinolone, all of the groups including the mock-treated control sustained substantial losses of weight on days 2 to 3 (Fig. 5B, right), consistent with the previously published data and presumably related to the catabolic effect of glucocorticoid hormones (41). However, no significant difference was observed between the groups except on day 3, when there was a slightly greater loss of weight for the wt RSV-infected group compared to that of the mG RSV-infected group.
On day 4 postinfection, the lungs were harvested and analyzed by histopathological examination of fixed thin-layer sections. The examination of lung sections stained with hematoxylin and eosin demonstrated that infection with wt RSV or mG RSV resulted in a substantial lymphocyte infiltration and formation of perivascular and peribronchial cuffs; however, no difference was observed between the two viruses (Fig. 6A and Table 1). Similarly, immunostaining of the thin-layer sections for the F4/80 antigen, which is a marker of myeloid cells, mostly macrophages, or for CD3, a T-cell marker, revealed a strong influx of both types of cells following infection with either virus, but no difference was observed between the two viruses (Fig. 6B and C; Table 1). Treatment with triamcinolone eliminated the histopathological changes and the influx of myeloid cells and T lymphocytes associated with infection by either virus (Fig. 6A to C; Table 1). These data suggest that the reduced sensitivity of wt RSV to the antiviral activity of RSV-specific antibodies, mediated by sG, is not a consequence of an altered level of gross pulmonary pathology or inflammation, although the possibility remained that it might be associated with modulation of the numbers of certain cell populations not identified in this gross analysis or, alternatively, with cell function.
TABLE 1.
Histopathological changes and influx of immune cells caused by infection with wt RSV or mG RSV in mice treated or mock-treated with triamcinolone
| Virus | Triamcinolone | Histopathological changes in lungsa
|
|||
|---|---|---|---|---|---|
| Lung lymphocytes and perivascular/peribronchiolar cuffs | Lung interstitial lesions | Macrophages and other myeloid cells (F4/80 staining) | CD3+ T-cell foci | ||
| wt RSV | No | ++/++/++/+/++ | +/++/++/+/++ | ++/++ | ++/++ |
| Yes | −/−/− | −/−/− | −/− | −/− | |
| mG RSV | No | ++/++/+/++/+ | ++/++/+/++/+ | ++/++ | ++/++ |
| Yes | −/−/−/−b | −/−/−/−b | −/− | −/− | |
| Mock | No | −/−/− | −/−/− | ±/− | −/− |
| Yes | −/−/− | −/−/− | −/− | −/− | |
Each of the indicated disease parameters was scored from − to ++, and the scores for individual animals are shown separated by slashes.
An abscess in one lobe; other lobes normal.
Next, we evaluated the effect of this anti-inflammatory treatment on the sG effect in passively immunized animals. Groups of mice were treated with triamcinolone as in the previous experiments and injected with PBS or polyvalent antibodies specific to the F or G protein (Fig. 7A). The day after the antibody administration, the mice were infected with wt RSV or mG RSV. On day 4 after infection, the viral titers in the lungs were determined by plaque titration (Fig. 7B and C). The administration of triamcinolone eliminated the difference in sensitivity of mG RSV and wt RSV to passively transferred antibodies. Indeed, triamcinolone appeared to completely ablate the ability of the passively administered antibodies to restrict RSV replication in vivo. Specifically, in the absence of triamcinolone, the passive transfer of a 1:20 dilution of G-specific serum provided a substantial reduction in the titers of mG RSV (Fig. 4C), whereas no reduction was observed in the presence of the same dose of serum in the presence of triamcinolone (Fig. 7B). Similarly, with the F-specific serum, the administration of a 1:40 or 1:20 dilution in the absence of triamcinolone provided substantial reductions in mG RSV replication (Fig. 4D), whereas the administration of a 1:30 dilution of the same F-specific serum in the presence of triamcinolone had no effect on the virus titer (Fig. 7C). These data indicate that the increased sensitivity of the replication of mG RSV versus wt RSV to passive RSV antibodies was eliminated by anti-inflammatory treatment, an observation consistent with the idea that ADCC- and/or antibody-mediated opsonization was active in restricting the replication of RSV in the lungs of mice.
The greater sensitivity of mG RSV to antibody-dependent neutralization depends on Fc receptors.
A large number of immune cell types bear Fc receptors, and it was of interest to address whether these were involved in the anti-RSV effects indicated above. This was investigated using KO mice lacking the Fc receptor γ chain in the BALB/c background, which is the same background as that used in the experiments presented above. The γ chain is a subunit of the FcγRI, FcγRIII, and FcɛRI receptors in mice; the FcγRI and FcγRIII receptors bind IgG, and FcɛRI is the high-affinity receptor for IgE. Groups of FcR γ chain KO mice were injected with PBS or RSV F-specific antibodies and infected with wt RSV or mG RSV (Fig. 8A). The dose of F serum that was used, 1:20, was high and previously was shown to strongly reduce the replication of mG RSV and wt RSV, with the effect greater for mG RSV (Fig. 4D). On day 4 after infection, the lungs were harvested for virus titration. In the Fc receptor γ KO mice administered PBS, the titers of the two viruses were equal and were similar to that in wt BALB/c mice (Fig. 4), about 104 PFU/g. In mice that received the antibodies, the titers of wt RSV and mG RSV were reduced by similar levels, 5.7-fold (P < 0.05) and 9.3-fold (P < 0.05), respectively. This is in contrast with the normal mice, in which the administration of F-specific serum resulted in a much greater restriction of mG RSV replication than wt RSV replication (Fig. 4). These data suggest that inflammatory cells bearing Fc receptors are partially responsible for mediating the antiviral effects of passively transferred antibodies but are fully responsible for the difference in the sensitivities of wt RSV and mG RSV to these antibodies. Thus, in mice, sG secreted by wt RSV exerts its effect by decreasing the ability of Fc-bearing cells to mediate an antiviral effect. However, Fc receptors involving the γ chain probably do not account for the entire effect of the antibody-dependent restriction of replication, since the passive F antibodies still effected a reduction in virus replication compared to the PBS-treated control. This perhaps is not surprising, since Fc receptors involving the γ chain do not account for all of the receptors for IgG, and the Fc receptors for IgA and IgM do not contain the γ chain.
wt RSV is a more-potent inducer of pulmonary cytokines and chemokines than mG RSV.
The above data indicate that wt RSV and mG are similar in their replication in vivo and in their induction of an inflammatory response in the lung, at least on a gross level, indicating that sG does not appear to affect either of these properties of the viruses in mice. However, sG decreased the ability of FcγR-bearing cells to restrict the replication of RSV in the lungs of mice. Taken together, these results suggest that sG affected the functioning, rather than the influx, of FcγR-bearing inflammatory cells. To further explore the functionality of inflammatory cells in the lungs of wt RSV- and mG RSV-infected mice, the production levels of selected cytokines and chemokines or their mRNAs in whole lung extracts were compared. Since whole lung extracts would include mRNA and protein contributions from both resident cells and infiltrating cells, the levels of cytokines and chemokines or their mRNAs were also compared in mice depleted of the infiltrating cells by triamcinolone. The cytokines and chemokines or their mRNAs affected by this treatment probably originated largely from the inflammatory cell infiltrate, although the steroid probably also affected the cytokine/chemokine output of resident immune cells and pulmonary epithelial cells. Groups of mice were infected with either virus or were mock-infected with or without daily injections of triamcinolone, similarly to the previous experiment. On day 4 postinfection, the animals were euthanized and the lungs were isolated. One lung from each mouse was used for the isolation of total RNA, and the other lung was homogenized and lysed for protein detection. We selected for analysis the Th1-promoting cytokines gamma interferon (IFN-γ) and interleukin-12 (IL-12) (the p40 unit, which is also shared by IL-23); the Th2 cytokines IL-4 and IL-13; the lymphocyte growth factor IL-6; the Th1 suppressor and anti-inflammatory factor IL-10; and the chemokines macrophage inflammatory protein 1α (MIP-1α) and RANTES. All samples were analyzed for the selected cytokines and chemokines at the mRNA level by quantitative RT-PCR (Q-RT-PCR) (Fig. 9A). The levels of cytokines and chemokines present also were assayed at the protein level by ELISA: those cytokines and chemokines that were detected at levels above those in the mock-infected mice are shown in Fig. 9B.
The Q-RT-PCR analysis demonstrated a strong induction of all the selected cytokines and chemokines in response to wt RSV infection (Fig. 9A). In mice infected with mG RSV, the levels of IL-6, IFN-γ, MIP-1α, and RANTES were significantly reduced compared to those of the wt RSV-infected animals, while the levels of IL-4, IL-10, IL-12/23 p40, IL-13, and tumor necrosis factor alpha tended to be lower, but the differences were not statistically significantly different. Treatment of the animals with triamcinolone sharply reduced the induction of cytokines and chemokines by either virus. However, there was one exception, namely, that triamcinolone treatment resulted in only a marginal (∼25%) reduction in the level of RANTES expression in wt RSV-infected mice. This was observed only with wt RSV: in mG RSV-infected mice, RANTES expression was strongly reduced, as with the other cytokines and chemokines. Thus, IL-6, IFN-γ, and MIP-1α mRNAs were differentially expressed (wt RSV > mG RSV) in a triamcinolone-sensitive fashion.
An examination of the cytokine and chemokine protein expression by ELISA demonstrated that only IL-4, IFN-γ, IL-12/23 p40, MIP-1α, and RANTES were induced detectably at the protein level by the viral infection. The level of induction of three of these proteins (IFN-γ, MIP-1α, and RANTES) was significantly less in mG RSV-infected mice than in wt RSV-infected mice; the induction of these same species also was less at the mRNA level, as already noted. Treatment with triamcinolone sharply reduced the induction of cytokines and chemokines at the protein level in both the wt RSV- and mG RSV-infected mice compared to that in the mock-infected mice. Similarly to the Q-RT-PCR data, the one exception was RANTES in the wt RSV-infected animals, for which the reduction associated with triamcinolone was only twofold. This suggests that the sG protein enhances the induction of RANTES even in triamcinolone-treated animals, an effect that was not further analyzed. These data suggest that sG has an immunomodulatory role in RSV infection that augments the expression of inflammatory cytokines and chemokines, including IL-6, IFN-γ, MIP-1α, and, perhaps, RANTES.
DISCUSSION
We evaluated whether the secreted sG protein of RSV might serve as an antigen decoy to bind G-specific antibodies and thereby help the virus evade neutralization. Since sG is secreted substantially more rapidly and in substantially greater abundance than G present in released progeny virus (24, 39), we hypothesized that it might flood the local environment of the infected tissue in vivo to sop up G-specific antibodies. This would not affect neutralization by antibodies specific to F, the other RSV neutralization antigen, but nonetheless would increase the opportunity for progeny RSV to escape neutralization and infect nearby cells. Consistent with this hypothesis, we found that the sG present in the medium supernatants of permissive infected tissue cultures rendered wt RSV less sensitive than mG RSV to neutralization by sera containing G-specific antibodies. However, the decoy activity of sG that we demonstrated in vitro did not appear to play a significant role when mice were given antibodies by passive transfer and infected with wt RSV or mG RSV. This may reflect a deficiency of the mouse model. RSV replication in mice is highly restricted and involves only scattered infected cells, and it is reasonable to suspect that the concentration of sG achieved in the respiratory fluid in mice was too low to be effective. The situation would be very different in humans, fully permissive hosts, where the extent of RSV replication is much higher (51) and would result in a substantially higher level of sG in the respiratory fluids.
The antigen decoy effect of sG probably does not significantly protect the initial RSV inoculum, since whatever sG is present in the inoculum presumably would be diluted in respiratory fluids. However, sG secreted by RSV-infected epithelial cells following the first and subsequent rounds of replication would be at a higher local concentration and would function to decrease the ability of G-specific antibodies present due to maternal transfer or previous infection to neutralize the newly released RSV. It should be noted that serum IgG antibodies reach the mucosal surface by the inefficient mechanism of transudation, such that the concentration at the luminal surface is only a small fraction of the amount present in the serum. Therefore, sG secreted by RSV-infected epithelial cells would have to be effective against relatively low levels of G-specific IgG antibodies. The decoy activity of sG also would be effective against IgA and might also interfere with the antiviral activity of IgA during transcytosis through epithelial cells. The sG secreted by RSV-infected epithelial cells also would be effective against G-specific antibodies elaborated during infection, especially early in the primary response when the concentration of G-specific antibodies would be low. It has been found that during infection with wt virus, the IgA-specific response to RSV G is not affected by the age of the vaccinee, whereas that to the F protein is decreased in young infants (31). If G-specific antibodies indeed constitute a more important arm of the response during primary infection in young infants, the decoy activity of sG might play a correspondingly larger role in young infants. The net effect of this sG antigen decoy effect would be to permit the wt RSV virus to achieve higher titers and an increased duration of shedding, both of which would foster transmission of the virus.
In contrast, in the mouse model, the antibody-mediated restriction of replication by sG was observed not only with G-specific but also with F-specific antibodies. This raised the possibility that the effect in this setting is mediated by the immunomodulatory effects of the protein, effects that remain poorly understood. We hypothesized that sG might interfere with the influx and/or functioning of macrophages, neutrophils, natural killer cells, and other immune cells that bear Fc receptors and contribute to viral clearance by the phagocytosis of antibody-antigen complexes as well as by ADCC. For example, alveolar macrophages and neutrophils are known to contribute to the clearance of influenza virus by binding to virus-antibody complexes through their Fc receptors followed by phagocytosis (15, 25), macrophages have been shown to reduce viral loads during RSV infection (35), and natural killer cells are crucial for RSV clearance (3). Therefore, we evaluated whether the greater sensitivity of mG RSV to passive antibodies could be reversed by interfering with the influx or functioning of immune cells, thus mimicking the proposed effect of sG. In some experiments, we reduced inflammation by the administration of triamcinolone, an anti-inflammatory glucocorticoid. This had the effect of making wt RSV and mG equally sensitive to passive antibodies. A similar effect was observed using Fc KO mice. Thus, both experiments supported the idea that sG spared wt RSV from the antibody-mediated restriction of replication by interfering with Fc-bearing inflammatory cells.
The exact mechanism by which this occurs remains unknown. The available data on the effect of sG on inflammation are controversial. A spontaneous mutant of RSV that does not make sG was shown to increase the production of inflammatory mediators including RANTES (CCL5) and IL-8 (CXCL-8) in infected A549 cells compared to wt RSV (4). Similarly, in infected mice, this same mutant was associated with increased airway inflammation compared to wt RSV (44). One caveat is that partial sequencing of the mutant revealed multiple mutations not related to the expression of sG that might have had untoward effects (4). For example, the replication of this mutant in mice was increased compared to the wt RSV control (44), which might also have contributed to the observed increased inflammation. These two studies suggested that the expression of sG is associated with reduced inflammation. However, an independent study by Maher et al. (29) came to the opposite conclusion. That study employed a recombinantly derived RSV mutant in which the expression of sG was ablated by a single amino acid substitution introduced by reverse genetics, similarly to mG RSV in the present study. However, the viruses used by Maher et al. apparently had not been sequenced to evaluate the possible contributions of adventitious mutations. Maher et al. found that virus lacking sG was associated with reduced pulmonary cellular infiltration and chemokine production in mice compared to those of wt RSV (29). This suggests that sG expressed by wt RSV is a proinflammatory factor. However, the level of replication of the mutant in mice was reduced compared to its wt RSV control, which might also have contributed to the decreased inflammation. The basis for these differing results is not known, although one possibility is that the sG− virus reported by Maher et al. or its respective wt RSV control also contained additional, unidentified, adventitious mutations that influenced replication or the inflammatory response.
In the present study, we similarly compared the host response to wt RSV versus mG RSV using recombinantly derived viruses that, as noted in Results, had minimal adventitious mutations. The two viruses replicated to similar levels, eliminating differences in viral load as a possible confounding factor. We found no apparent difference between wt RSV and mG RSV with regard to the modulation of gross lung histopathology or in the magnitude of pulmonary macrophage and T-cell infiltration in mice. However, the levels of expression of a number of selected cytokines and chemokines were generally less in mice infected with mG RSV than wt RSV. This indicated that sG expressed by wt RSV has a proinflammatory effect, which is consistent with, but less dramatic than, the results of Maher et al. (29). Thus, sG indeed has the potential to affect the antibody-dependent restriction of replication through its immunomodulatory activities. However, since this was not associated with gross differences in lung histopathology and inflammatory cell content, the effect probably is not at the level of inflammatory cell influx. The finding that the ability to express sG was associated with the increased expression of inflammatory cytokines and chemokines seemed contradictory to a role in helping the virus evade the host response, since inflammation typically helps restrict virus replication. However, our results did provide a clue for a possible mechanism, one involving the effects on immune cell function. Specifically, we found that the level of IFN-γ induced by wt RSV was significantly greater than that produced by mG RSV at both the mRNA and the protein levels (Fig. 9). A recently published study demonstrated that interferon γ induced in lungs in response to influenza virus infection causes the functional suppression of pulmonary macrophages (47) and may also account for the observed functional suppression of granulocytes during influenza virus infection (1). In addition, purified sG protein was demonstrated to mediate a dose-dependent suppression of lymphoproliferative responses of peripheral blood mononuclear cells to an unrelated antigen (37). Taken together, these studies suggest that sG may act to suppress the antibody-mediated restriction of replication by interfering with the functioning, rather than the influx, of immune cells. This will be investigated further in future studies.
The observation that sG reduces the antibody-dependent restriction of RSV replication has implications for the development of a live attenuated RSV vaccine. All of the current live attenuated RSV vaccine candidates have an unmodified version of the G gene and thus express both the mG and sG forms of the glycoprotein (54). Candidate live vaccine strains have been shown to infect and induce antibody responses in 1- to 2-month-old infants despite the presence of maternal RSV-specific antibodies (27, 55). The present study suggests that the expression of sG contributes to the ability of RSV to infect young infants that possess maternal antibodies and, on that basis, would be an important element of a live pediatric RSV vaccine. Specifically, the expression of sG by current vaccine strains likely makes the viruses less susceptible to restriction by maternal antibody and likely improves the vaccine “take.” A second factor for consideration is the other immunomodulatory effects of sG, although they are incompletely understood. Of the three independent groups that have examined the effects of sG (including the present study), two (including the present study) provided evidence that sG has proinflammatory effects, as already noted. These effects might contribute to vaccine reactogenicity but also might enhance immunogenicity. The G protein also has additional immunomodulatory effects, although it is not known whether these are specific to mG, sG, or both forms. For example, G contains a CX3C motif and has been shown to function as a mimic of the CX3C chemokine fractalkine, an activity that appears to impede the influx of T lymphocytes and NK cells (21). The G protein also has been shown to inhibit the production of proinflammatory cytokines and chemokines in response to agonists of Toll-like receptors 2, 4, and 9 (34). The G protein is also required for the induction of an efficient RSV-specific cell-mediated response (6). In general, these additional immunomodulatory effects of G remain insufficiently understood for a prediction of how they might influence an RSV vaccine.
The ability of RSV to evade maternal antibodies and infect very young infants, who are particularly vulnerable to respiratory tract disease due to small size, narrow airways, and immunologic immaturity, accounts for much of its pediatric impact. Similarly, the ability of RSV to reinfect RSV-experienced individuals augments its impact later in life, particularly in the elderly. Other respiratory viruses, such as influenza virus and the parainfluenza viruses, are less able to infect and cause significant disease during the first several months of life. This presumably reflects a greater sensitivity of these other viruses to preexisting antibodies compared to RSV, even though all of these viruses share a similar tropism in the respiratory tract. Consistent with a greater sensitivity to neutralizing antibodies, reinfection by influenza virus depends on antigenic shift or drift, rhinoviruses exist in a multitude of serotypes in order to evade host immunity, and parainfluenza viruses have four serotypes for the same reason. In contrast, RSV has a single serotype, with intrasubgroup diversity, yielding at most only a fourfold difference in cross-neutralization between strains. Reinfection occurs readily and frequently in nature and in experimental clinical settings, even by antigenically indistinguishable strains (17, 19, 20, 22). The individual parainfluenza serotypes also can reinfect without the expedient of substantial intraserotype antigenic variability but not to the extent of RSV. One difference between RSV and these other respiratory viruses is the expression of sG. The present study provides the first available explanation for the unusual ability of RSV to evade neutralizing antibodies present from maternal transfer or previous infection. This activity probably also helps shield the virus from neutralization by the burgeoning immune response during infection.
The secreted forms of viral envelope proteins can be produced by diverse strategies in addition to the one described above for RSV. For example, Ebola virus has a single virion glycoprotein GP that is anchored by a transmembrane domain near the C terminus. However, in all known strains of the virus, translation of the unmodified GP mRNA yields a version of the glycoprotein that lacks the transmembrane domain and is secreted; expression of the full-length glycoprotein depends on cotranscriptional stuttering by the viral polymerase on a specific editing motif, which shifts the reading frame and creates the full-length open reading frame (42, 50). Like the full-length glycoprotein, sG contains major virus-neutralizing epitopes (13, 52). The Env glycoprotein of all strains of human immunodeficiency virus is processed by proteolysis into the N-terminal GP120 subunit and the C-terminal GP41 transmembrane subunit (40), with the former being present in the viral particle but also being abundantly shed into the medium (43). The single glycoprotein G of rabies virus, which has a transmembrane domain near the C terminus, is also produced in a secreted form lacking this domain, presumably due to proteolytic cleavage (11). Secreted forms of envelope proteins also have been identified for vesicular stomatitis virus (7, 28), murine leukemia virus (5), herpes simplex virus (45), and Epstein-Barr virus (38). Thus, it is possible that secreted versions of envelope proteins of a variety of viruses help these viruses to evade the antiviral activities of antibodies by acting as antigen decoy molecules, or through their immunomodulatory effects, or both.
Acknowledgments
We thank Ernest Williams and Fatemeh Davoodi for performing the ELISAs.
This project was funded as part of the NIAID intramural program.
Footnotes
Published ahead of print on 8 October 2008.
REFERENCES
- 1.Abramson, J. S., G. S. Giebink, E. L. Mills, and P. G. Quie. 1981. Polymorphonuclear leukocyte dysfunction during influenza virus infection in chinchillas. J. Infect. Dis. 143836-845. [DOI] [PubMed] [Google Scholar]
- 2.Alcami, A. 2003. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 336-50. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, J. J., M. Serin, J. Harrop, S. Amin, G. L. Toms, and R. Scott. 1989. Natural killer cell response to respiratory syncytial virus in the BALB/c mouse model. Adv. Exp. Med. Biol. 257211-220. [DOI] [PubMed] [Google Scholar]
- 4.Arnold, R., B. Konig, H. Werchau, and W. Konig. 2004. Respiratory syncytial virus deficient in soluble G protein induced an increased proinflammatory response in human lung epithelial cells. Virology 330384-397. [DOI] [PubMed] [Google Scholar]
- 5.Bolognesi, D. P., A. J. Langlois, and W. Schafer. 1975. Polypeptides of mammalian oncornaviruses. IV. Structural components of murine leukemia virus released as soluble antigens in cell culture. Virology 68550-555. [DOI] [PubMed] [Google Scholar]
- 6.Bukreyev, A., M. E. Serra, F. R. Laham, G. A. Melendi, S. R. Kleeberger, P. L. Collins, and F. P. Polack. 2006. The cysteine-rich region and secreted form of the attachment G glycoprotein of respiratory syncytial virus enhance the cytotoxic T-lymphocyte response despite lacking major histocompatibility complex class I-restricted epitopes. J. Virol. 805854-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatis, P. A., and T. G. Morrison. 1983. Characterization of the soluble glycoprotein released from vesicular stomatitis virus-infected cells. J. Virol. 4580-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, E. H., and H. J. Lee. 2000. Genetic diversity and molecular epidemiology of the G protein of subgroups A and B of respiratory syncytial viruses isolated over 9 consecutive epidemics in Korea. J. Infect. Dis. 1811547-1556. [DOI] [PubMed] [Google Scholar]
- 9.Coates, H. V., D. W. Alling, and R. M. Chanock. 1966. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am. J. Epidemiol. 83299-313. [DOI] [PubMed] [Google Scholar]
- 10.Collins, P. L., and J. E. J. Crowe. 2007. Respiratory syncytial virus and metapneumovirus, p. 1601-1646. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 11.Dietzschold, B., T. J. Wiktor, W. H. Wunner, and A. Varrichio. 1983. Chemical and immunological analysis of the rabies soluble glycoprotein. Virology 124330-337. [DOI] [PubMed] [Google Scholar]
- 12.Domachowske, J. B., C. A. Bonville, D. Ali-Ahmad, K. D. Dyer, A. J. Easton, and H. F. Rosenberg. 2001. Glucocorticoid administration accelerates mortality of pneumovirus-infected mice. J. Infect. Dis. 1841518-1523. [DOI] [PubMed] [Google Scholar]
- 13.Dowling, W., E. Thompson, C. Badger, J. L. Mellquist, A. R. Garrison, J. M. Smith, J. Paragas, R. J. Hogan, and C. Schmaljohn. 2007. Influences of glycosylation on antigenicity, immunogenicity, and protective efficacy of Ebola virus GP DNA vaccines. J. Virol. 811821-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elango, N., G. A. Prince, B. R. Murphy, S. Venkatesan, R. M. Chanock, and B. Moss. 1986. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc. Natl. Acad. Sci. USA 831906-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujisawa, H. 2008. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J. Virol. 822772-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, O., M. Martin, J. Dopazo, J. Arbiza, S. Frabasile, J. Russi, M. Hortal, P. Perez-Brena, I. Martinez, B. Garcia-Barreno, et al. 1994. Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J. Virol. 685448-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glezen, W. P., A. Paredes, J. E. Allison, L. H. Taber, and A. L. Frank. 1981. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 98708-715. [DOI] [PubMed] [Google Scholar]
- 18.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26153-162. [DOI] [PubMed] [Google Scholar]
- 19.Hall, C. B., A. E. Kopelman, R. G. Douglas, Jr., J. M. Geiman, and M. P. Meagher. 1979. Neonatal respiratory syncytial virus infection. N. Engl. J. Med. 300393-396. [DOI] [PubMed] [Google Scholar]
- 20.Hall, C. B., E. E. Walsh, C. E. Long, and K. C. Schnabel. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163693-698. [DOI] [PubMed] [Google Scholar]
- 21.Harcourt, J., R. Alvarez, L. P. Jones, C. Henderson, L. J. Anderson, and R. A. Tripp. 2006. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J. Immunol. 1761600-1608. [DOI] [PubMed] [Google Scholar]
- 22.Henderson, F. W., A. M. Collier, W. A. Clyde, Jr., and F. W. Denny. 1979. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N. Engl. J. Med. 300530-534. [DOI] [PubMed] [Google Scholar]
- 23.Hendricks, D. A., K. Baradaran, K. McIntosh, and J. L. Patterson. 1987. Appearance of a soluble form of the G protein of respiratory syncytial virus in fluids of infected cells. J. Gen. Virol. 681705-1714. [DOI] [PubMed] [Google Scholar]
- 24.Hendricks, D. A., K. McIntosh, and J. L. Patterson. 1988. Further characterization of the soluble form of the G glycoprotein of respiratory syncytial virus. J. Virol. 622228-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber, V. C., J. M. Lynch, D. J. Bucher, J. Le, and D. W. Metzger. 2001. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 1667381-7388. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, P. R., M. K. Spriggs, R. A. Olmsted, and P. L. Collins. 1987. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. USA 845625-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karron, R. A., P. F. Wright, R. B. Belshe, B. Thumar, R. Casey, F. Newman, F. P. Polack, V. B. Randolph, A. Deatly, J. Hackell, W. Gruber, B. R. Murphy, and P. L. Collins. 2005. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J. Infect. Dis. 1911093-1104. [DOI] [PubMed] [Google Scholar]
- 28.Little, S. P., and A. S. Huang. 1978. Shedding of the glycoprotein from vesicular stomatitis virus-infected cells. J. Virol. 27330-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maher, C. F., T. Hussell, E. Blair, C. J. Ring, and P. J. Openshaw. 2004. Recombinant respiratory syncytial virus lacking secreted glycoprotein G is attenuated, non-pathogenic but induces protective immunity. Microbes Infect. 61049-1055. [DOI] [PubMed] [Google Scholar]
- 30.Mocarski, E. S., Jr. 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10332-339. [DOI] [PubMed] [Google Scholar]
- 31.Murphy, B. R., D. W. Alling, M. H. Snyder, E. E. Walsh, G. A. Prince, R. M. Chanock, V. G. Hemming, W. J. Rodriguez, H. W. Kim, B. S. Graham, et al. 1986. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J. Clin. Microbiol. 24894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy, B. R., A. V. Sotnikov, L. A. Lawrence, S. M. Banks, and G. A. Prince. 1990. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine 8497-502. [DOI] [PubMed] [Google Scholar]
- 33.Olmsted, R. A., N. Elango, G. A. Prince, B. R. Murphy, P. R. Johnson, B. Moss, R. M. Chanock, and P. L. Collins. 1986. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc. Natl. Acad. Sci. USA 837462-7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polack, F. P., P. M. Irusta, S. J. Hoffman, M. P. Schiatti, G. A. Melendi, M. F. Delgado, F. R. Laham, B. Thumar, R. M. Hendry, J. A. Melero, R. A. Karron, P. L. Collins, and S. R. Kleeberger. 2005. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc. Natl. Acad. Sci. USA 1028996-9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pribul, P. K., J. Harker, B. Wang, H. Wang, J. S. Tregoning, J. Schwarze, and P. J. Openshaw. 2008. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 824441-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prince, G. A., A. Mathews, S. J. Curtis, and D. D. Porter. 2000. Treatment of respiratory syncytial virus bronchiolitis and pneumonia in a cotton rat model with systemically administered monoclonal antibody (palivizumab) and glucocorticosteroid. J. Infect. Dis. 1821326-1330. [Google Scholar]
- 37.Ray, R., D. F. Hoft, K. Meyer, R. Brown, L. M. Lagging, and R. B. Belshe. 2001. Immunoregulatory role of secreted glycoprotein G from respiratory syncytial virus. Virus Res. 75147-154. [DOI] [PubMed] [Google Scholar]
- 38.Ressing, M. E., D. van Leeuwen, F. A. Verreck, S. Keating, R. Gomez, K. L. Franken, T. H. Ottenhoff, M. Spriggs, T. N. Schumacher, L. M. Hutt-Fletcher, M. Rowe, and E. J. Wiertz. 2005. Epstein-Barr virus gp42 is posttranslationally modified to produce soluble gp42 that mediates HLA class II immune evasion. J. Virol. 79841-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts, S. R., D. Lichtenstein, L. A. Ball, and G. W. Wertz. 1994. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J. Virol. 684538-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robey, W. G., B. Safai, S. Oroszlan, L. O. Arthur, M. A. Gonda, R. C. Gallo, and P. J. Fischinger. 1985. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science 228593-595. [DOI] [PubMed] [Google Scholar]
- 41.Rooman, R., G. Koster, R. Bloemen, R. Gresnigt, and S. C. van Buul-Offers. 1999. The effect of dexamethasone on body and organ growth of normal and IGF-II-transgenic mice. J. Endocrinol. 163543-552. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez, A., S. G. Trappier, B. W. Mahy, C. J. Peters, and S. T. Nichol. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. USA 933602-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider, J., O. Kaaden, T. D. Copeland, S. Oroszlan, and G. Hunsmann. 1986. Shedding and interspecies type sero-reactivity of the envelope glycopolypeptide gp120 of the human immunodeficiency virus. J. Gen. Virol. 672533-2538. [DOI] [PubMed] [Google Scholar]
- 44.Schwarze, J., and U. Schauer. 2004. Enhanced virulence, airway inflammation and impaired lung function induced by respiratory syncytial virus deficient in secreted G protein. Thorax 59517-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su, H. K., R. Eberle, and R. J. Courtney. 1987. Processing of the herpes simplex virus type 2 glycoprotein gG-2 results in secretion of a 34,000-Mr cleavage product. J. Virol. 611735-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullender, W. M., M. A. Mufson, L. J. Anderson, and G. W. Wertz. 1991. Genetic diversity of the attachment protein of subgroup B respiratory syncytial viruses. J. Virol. 655425-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun, K., and D. W. Metzger. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 14558-564. [DOI] [PubMed] [Google Scholar]
- 48.Teng, M. N., S. S. Whitehead, and P. L. Collins. 2001. Contribution of the respiratory syncytial virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology 289283-296. [DOI] [PubMed] [Google Scholar]
- 49.Trento, A., M. Viegas, M. Galiano, C. Videla, G. Carballal, A. S. Mistchenko, and J. A. Melero. 2006. Natural history of human respiratory syncytial virus inferred from phylogenetic analysis of the attachment (G) glycoprotein with a 60-nucleotide duplication. J. Virol. 80975-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volchkov, V. E., S. Becker, V. A. Volchkova, V. A. Ternovoj, A. N. Kotov, S. V. Netesov, and H. D. Klenk. 1995. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 214421-430. [DOI] [PubMed] [Google Scholar]
- 51.Welliver, T. P., R. P. Garofalo, Y. Hosakote, K. H. Hintz, L. Avendano, K. Sanchez, L. Velozo, H. Jafri, S. Chavez-Bueno, P. L. Ogra, L. McKinney, J. L. Reed, and R. C. Welliver, Sr. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 1951126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson, J. A., M. Hevey, R. Bakken, S. Guest, M. Bray, A. L. Schmaljohn, and M. K. Hart. 2000. Epitopes involved in antibody-mediated protection from Ebola virus. Science 2871664-1666. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. 2008. Initiative for vaccine research (IVR). Respiratory syncytial virus (RSV). Disease burden. World Health Organization, Geneva, Switzerland. http://www.who.int/vaccine_research/diseases/ari/en/index3.html.
- 54.Wright, P. F., R. A. Karron, R. B. Belshe, J. R. Shi, V. B. Randolph, P. L. Collins, A. F. O'Shea, W. C. Gruber, and B. R. Murphy. 2007. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine 257372-7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright, P. F., R. A. Karron, R. B. Belshe, J. Thompson, J. E. Crowe, Jr., T. G. Boyce, L. L. Halburnt, G. W. Reed, S. S. Whitehead, E. L. Anderson, A. E. Wittek, R. Casey, M. Eichelberger, B. Thumar, V. B. Randolph, S. A. Udem, R. M. Chanock, and B. R. Murphy. 2000. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J. Infect. Dis. 1821331-1342. [DOI] [PubMed] [Google Scholar]