Abstract
Human immunodeficiency virus type 1 and simian immunodeficiency virus possess three closely spaced, highly conserved sites for N-linked carbohydrate attachment in the extracellular domain of the transmembrane protein gp41. We infected rhesus monkeys with a variant of cloned SIVmac239 lacking the second and third sites or with a variant strain lacking all three of SIVmac239's glycosylation sites in gp41. For each mutation, asparagine (N) in the canonical N-X-S/T recognition sequence for carbohydrate attachment was changed to the structurally similar glutamine such that two nucleotide changes would be required for a reversion of the mutated codon. By 16 weeks, experimentally infected monkeys made antibodies that neutralized the mutant viruses to high titers. Such antibodies were not observed in monkeys infected with the parental virus. Thus, new specificities were revealed as a result of the carbohydrate attachment mutations, and antibodies of these specificities had neutralizing activity. Unlike monkeys infected with the parental virus, monkeys infected with the mutant viruses made antibodies that reacted with peptides corresponding to the sequences in this region. Furthermore, there was strong selective pressure for the emergence of variant sequences in this region during the course of infection. By analyzing the neutralization profiles of sequence variants, we were able to define three mutations (Q625R, K631N, and Q634H) in the region of the glycosylation site mutations that conferred resistance to neutralization by plasma from the monkeys infected with mutant virus. Based on the reactivity of antibodies to peptides in this region and the colocalization of neutralization escape mutations, we conclude that N-linked carbohydrates in the ectodomain of the transmembrane protein shield underlying epitopes that would otherwise be the direct targets of neutralizing antibodies.
Vaccine-induced protection against a number of pathogens correlates well with neutralizing antibody titers (30). Some have suggested that the most effective vaccine against human immunodeficiency virus (HIV) may be one that is capable of eliciting potent, broadly neutralizing antibodies and broad-spectrum cellular immune responses (37). One major obstacle to the engineering of the antibody component of such a vaccine is the poor immunogenicity of the Env spike that is the target of neutralizing antibodies. Extensive glycosylation of the external surface component of Env, gp120, is now generally believed to contribute importantly to its poor immunogenicity. The gp120 surface glycoproteins of HIV and simian immunodeficiency virus (SIV) each contain approximately 24 sites for N-linked carbohydrate attachment (Asn-X-Ser/Thr). In fact, carbohydrates comprise about 50% of the total mass of gp120. These carbohydrates are required to generate properly folded and processed proteins. However, once fully glycosylated proteins have been produced, these carbohydrate moieties do not appear to be required to maintain native protein structure since enzymatically deglycosylated core envelope proteins retain their ability to bind CD4 and their ability to bind conformation-dependent antibodies (2, 3, 7, 24).
Despite a general requirement of carbohydrate attachment for the generation of functional envelope protein, it is possible to remove some individual carbohydrate attachment sites within gp120 without a loss of the ability to bind CD4 or the ability to yield replication-competent virus. The dispensability of some N-linked glycans for viral replication and the greater sensitivity of some glycan-deficient mutants to antibody-mediated neutralization suggest that these glycans may serve in part as barriers to shield the virus from effective antibody recognition (5, 10, 12, 13, 15, 16, 21, 23, 31, 32, 36). Variations in the number and location of glycosylation sites, particularly within the V1/V2 and V3 loops but also on the “silent face” of gp120, often correlate with altered sensitivity to neutralizing antibodies (1, 6, 11, 21, 22, 34). Patterns of addition and relocation of N-linked glycosylation sites during the course of HIV and SIV infection suggest an evolving “glycan shield” in response to antibody selection (4, 8, 26, 33, 38). Just as the acquisition of particular N-linked sites decreases neutralization sensitivity, the elimination of N-linked sites at the same or nearby locations has been shown to increase neutralization sensitivity for both HIV-1 and SIV (5, 9, 10, 12, 13, 16, 21, 31, 33). Reitter et al. previously demonstrated that a mutation of specific N-linked glycosylation sites in the V1-V2 region of gp120 of SIVmac239 results in replication-competent viruses capable of eliciting increased levels of antibodies with neutralizing activity against the parental wild-type strain SIVmac239 (32, 33). Similarly, Li et al. recently showed that the removal of a single glycan site from HIV-1 gp120 results in an enhanced ability to elicit antibodies with neutralizing activity (19). Thus, an extensive collection of studies have shown that N-linked glycosylation limits both the immunogenicity and antigenicity of gp120.
Effects of glycosylation on the immunogenicity and antigenicity of the gp41 transmembrane subunit have not to our knowledge been previously reported. HIV-1 and SIV contain three closely spaced, highly conserved sites for N-linked carbohydrate attachment in the external domain of the gp41 transmembrane protein. Some strains contain a fourth site in the same general vicinity (18). Although numerous monoclonal antibodies that recognize sequences that flank this stretch in gp41 of HIV-1 have been defined, none recognize amino acid sequences within the region of N-linked carbohydrate attachment itself (17) (Fig. 1A). Thus, there are already data to suggest that the gp41 carbohydrates may be shielding peptide sequences over the region to which they are attached.
FIG. 1.
Antibody reactivity to linear peptides. (A) Locations of peptides in the region of the HIV gp41 protein proximal to the transmembrane domain that interact with antibodies (17). The beginning of the membrane-spanning domain is indicated. The conserved sites of N-linked glycosylation in the ectodomain of gp41 are also indicated by a gray box. (B) Antibody reactivities to overlapping peptides spanning the entire envelope protein by ELISA using a pool of SIV-positive plasmas (AE625) from monkeys infected with SIVmac239. The location of the variable loops, the beginning of the gp41 protein, and the predicted membrane-spanning domain are indicated. The conserved sites of N-linked glycosylation in the ectodomain of gp41 are also indicated by a gray box. (C) Same as B, except that plasma from an individual monkey (monkey 18-01) 16 weeks after infection with SIVmac239 was used as the source of antibody. (D) Same as B, except that plasma from an individual monkey (monkey 414-98) 22 weeks after infection with SIVmac239 was used as the source of antibody. Plasma from monkeys 18-01 and 414-98 was not present in the pool used for B.
For the current studies, we created five mutant strains of SIVmac239 that lack one or more of the three N-linked glycosylation sites in gp41. Two of the multiply mutated strains were used to infect rhesus macaques experimentally so that we could study the effects of these mutations on immunogenicity and antigenicity. Our studies demonstrate that new specificities were revealed as a result of the gp41 carbohydrate mutations, and antibodies of these specificities had neutralizing activity toward mutant viruses. Based on pepscan analysis and the emergence of neutralization escape variants, it seems likely that sequences in the mutated region are the direct targets of neutralizing antibodies.
MATERIALS AND METHODS
Site-specific mutagenesis and subcloning.
Mutations in env were created by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). N-glycan attachment sites in the SIVmac239 gp41 coding sequence were eliminated by changing the asparagine residue in each canonical N-X-S/T motif to a glutamine by site-directed mutagenesis (N-X-S/T to Q-X-S/T) by using a QuikChange mutagenesis kit according to the manufacturer's instructions. Mutations were introduced into plasmid p239SpE3′, which contains the 3′ half of the SIVmac239 genome, and confirmed by direct sequencing.
Primers were purchased from Sigma-Genosys Biotechnologies, Inc. (The Woodlands, TX). All resulting mutants were sequenced using a Beckman Coulter CEQ8000 apparatus. In order to generate full-length versions of all the 3′ mutants, pSIV239SpX5′ (39) was digested with XhoI and SphI, and the corresponding fragment was joined onto each mutant using T4 DNA ligase.
Preparation of virus samples and cell culture.
HEK-293T and C8166-45 SIV-SEAP cells were maintained as previously described (27, 28). Virus was prepared by the transient transfection of HEK-293T cells with plasmids containing full-length SIV proviral genomes. Cells were seeded at 1.5 × 106 cells per flask the day before transfection, and each flask was transfected with 3 μg of each plasmid using the calcium phosphate method (Promega, Madison, WI) according to the manufacturer's instructions. The culture medium was changed on day 2 posttransfection, and supernatants were harvested on day 3. Virus was quantified by determining the concentration of p27 capsid in the supernatant by an antigen capture assay (Advanced BioScience Laboratories, Inc., Kensington, MD).
Monkey infection and sampling.
Indian-origin rhesus macaques (Macaca mulatta) were housed at the New England Primate Research Center in an animal biosafety level 3 containment facility in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care and the Harvard Medical School Animal Care and Use Committee. Research was conducted according to the principles described in the Guide for the Care and Use of Laboratory Animals and was approved by the Harvard Medical School Animal Care and Use Committee (29).
Plasma and peripheral blood mononuclear cells (PBMCs) were isolated from fresh citrate blood of rhesus macaques by density gradient centrifugation (Ficoll 1077; Sigma). For analysis of viral replication in culture, lymphocytes in PBMC samples were activated for 72 h with 1 μg of phytohemagglutinin (Sigma, St. Louis, MO) per ml R10, washed in RPMI 1640 medium, and incubated in R10 supplemented with 10% interleukin-2 overnight before infection.
Viral loads in plasma were determined using a quantitative real-time reverse transcriptase PCR (RT-PCR) assay (20).
Infectivity assays.
Viral infectivity was measured using an immortalized human CD4+ T-cell line (C8166-45 SIV-SEAP). C8166-45 SIV-SEAP cells harbor a Tat-inducible, secreted, engineered alkaline phosphatase (SEAP) reporter construct enabling SIV infection to be measured by SEAP production in the culture supernatant. Aliquots of all virus stocks used in these experiments were subjected to serial twofold dilutions, and SEAP activity from the supernatant was measured at day 3 postinfection according to the manufacturer's recommendations, with modifications as described previously (25).
Neutralization.
The neutralization sensitivity of each virus was tested using an SEAP reporter cell assay as previously described (25). Virus equivalent to 2 ng of p27 capsid protein for SIVmac239 and SIVmac239-gp41/g23 and virus equivalent to 10 ng for SIV239-gp41/g123 were chosen as the lowest levels of viral input that would be sufficient to give a clear SEAP signal within the linear range for each viral strain. SEAP activity was measured when levels were sufficiently over background to give reliable measurements (at least 10-fold). To perform neutralization assays, 96-well plates were set up as follows: 25 μl of medium (RPMI, 10% fetal bovine serum) was added to the first three columns, and 25-μl aliquots of successive twofold dilutions of test plasma in medium were added to each of the other columns. All plasmas were heat inactivated at 56°C for 30 min before use in neutralization assays. Each virus in a total volume of 75 μl was then added to each well in columns 3 through 12. Virus-free medium was added to columns 1 and 2 (mock). The plate was incubated for 1 h at 37°C. After incubation, 5,000 target cells (C8166-45 SIV-SEAP) in a volume of 100 μl were added to each well. The plate was then placed into a humidified chamber within a CO2 incubator at 37°C. SEAP activity was measured using the chemiluminescent Phosphalight SEAP assay system (Applied Biosystems) according to the manufacturer's recommendations, with modifications as described previously (25). Neutralization activity for all antibodies and plasma samples was measured in triplicate using a Victor V multilabel counter (Perkin-Elmer) and reported as a percentage of SEAP activity.
Cloning and sequencing of env from plasma.
SIV RNA was isolated by affinity column purification using a High Pure viral RNA kit (Roche, Indianapolis, IN) according to the manufacturer's protocol. Ten nanograms of viral RNA was amplified by RT-PCR using Titan One tube RT-PCR kit (Roche, Indianapolis, IN) with primers 182EYd (5′-TTTCTCTCTCTTCAGCTGGG-3′) and 183EYu (5′-GAAAGAGAAGAAGAACTCCG-3′) and a nested PCR with primers 184EYu (5′-GCTAAGGCTAATACATCTTCTGC-3′) and 181EYd (5′-CCATGGAGTATTCATATACTGTCCC-3′). The 2.8-kb PCR product was gel purified and used for cloning into the pCR-TOPO cloning vector with the Zero Blunt PCR cloning kit (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. After the transformation of Escherichia coli Stbl2 cells (Invitrogen, Carlsbad, CA), single ampicillin-resistant colonies were grown overnight at 30°C for plasmid preps, which were sequenced as described above.
Generation of mutants with chimeric env genes.
In order to generate NSR, NR, and KLNL, the pCR-TOPO vectors containing the cloned envelope gene amplified from monkey plasmas were digested with PmlI and NheI, and the corresponding fragments were cloned into SIVmac239-FL. In order to generate RNNH, the pCR-TOPO vector containing a cloned envelope from monkey 103-94 was digested with BalI and NheI, and the corresponding fragments were cloned into the SIVmac239 3′ half. The full-length version of this mutant was generated as explained above. Mutant RNH was generated from the corresponding envelope clone including RNNH mutations by site-directed mutagenesis with primers 244EYa (5′-CCATGGCCACGAGCAAGTCTAACACCAAATTGGAACC-3′) and 245EYd (5′-GGTTCCAATTTGGTGTTAGACTTGCTCGTGGCCATGG-3′), amplified, and cloned back in the full-length SIVmac239 clone as explained above.
Env peptide enzyme-linked immunosorbent assay (ELISA).
SIVmac239 Env 15-mer peptides were obtained from the NIH AIDS Research and Reference Reagent Program. Mutant Env 15-mer peptides were synthesized at the Massachusetts General Hospital peptide core facility (Charlestown, MA). Single wells of 96-well half-area, high-binding plates (Costar) were coated with 50 μl of each peptide diluted to 40 μg/ml with phosphate-buffered saline (PBS) and incubated at 4°C overnight. The wells were blocked with 75 μl of 5% nonfat powdered milk in PBS at 37°C for 1 h. Fifty microliters of plasma diluted 1:20 with 5% milk in PBS was added to each well and incubated at 37°C for 2 h. After washing six times with PBS plus 0.05% Tween 20, 50 μl of horseradish peroxidase-conjugated goat anti-human immunoglobulin G antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:2,000 in 5% milk in PBS was added to each well, and the plates were incubated at 37°C for 1 h. The plates were then washed 10 times with PBS-Tween, and 50 μl of tetramethylbenzidine reagent (Calbiochem, Gibbstown, NJ) was added to each well. Thirty minutes later, 50 μl of 250 mM hydrochloric acid was added to each well, and the optical density at 450 nm was measured using the Wallac Victor plate reader (Perkin-Elmer, Waltham, MA).
RESULTS
Lack of antibody recognition in the region spanning the highly conserved sites for N-linked glycosylation in the transmembrane protein.
In order to obtain a baseline characterization of antibody responses to linear epitopes across the SIV envelope protein, we tested pooled plasma from monkeys infected with SIVmac239 for reactivity to overlapping peptides spanning the whole envelope protein. Peptides (15-mer) corresponding to the SIVmac239 envelope sequence and overlapping each adjacent peptide in the sequence by 11 amino acids were used to coat ELISA plates and then tested for reactivity to the pool of plasmas from monkeys infected with SIVmac239. Linear peptide epitopes that clustered in several peaks of strong reactivity across the full amino acid sequence of SIVmac239 were identified (Fig. 1B). The peaks of strong reactivity corresponded to the V1-V2 loops, the C terminus of gp120, one peptide in the ectodomain of the gp41 transmembrane protein, and another peptide located at the beginning of the cytoplasmic domain of gp41. No reactivity was observed in the region spanning the highly conserved sites for N-linked glycosylation in the ectodomain of the transmembrane protein. Very similar patterns of reactivity were observed when the assay was repeated with plasma samples from individual monkeys infected with SIVmac239 (Fig. 1C and D). The pool of plasma samples used for Fig. 1B did not include samples from the monkeys used for Fig. 1C and D. A similar pattern of reactivity with a separate pool of SIV-positive plasma from a different group of SIVmac239-infected monkeys was also observed (data not shown).
The linear antibody epitope map obtained from the peptide scanning with plasmas from monkeys infected with SIVmac239 displayed remarkable concordance with the map of linear epitopes that have been located for the HIV envelope protein (17). The peaks of reactivity observed in the SIV peptide scan corresponded to regions of the HIV envelope protein where a high number of monoclonal antibodies have been mapped. Remarkably, no monoclonal antibody has been mapped to the region encompassing the four N-glycosylation sites in the ectodomain of the HIV transmembrane protein (17) (Fig. 1A).
Effects of removal of conserved N-linked glycosylation sites in the ectodomain of gp41 on infectivity and replication kinetics.
N-linked glycosylation sites in the ectodomain of the transmembrane protein were eliminated in single, double, and triple combinations to create five mutant strains of SIVmac239 (Fig. 2A). For each mutant, an asparagine residue was replaced with a glutamine (N-X-S/T→Q-X-S/T) by site-directed mutagenesis. Extensive sequencing confirmed the absence of off-site mutations for all of the viruses that were used. The infectivity of these mutants was measured under conditions that approximated a single cycle of infection. C8166-45 SIV-SEAP cells were infected with HEK-293T-produced virus normalized to contain known amounts of p27 Gag antigen. C8166-45 SIV-SEAP cells secrete SEAP into the medium in response to infection by SIV. The amount of SEAP secreted correlates directly with the amount of infecting virus and can be sensitively and rapidly quantitated by a chemiluminescent assay (25). SEAP activity was measured at 72 h postinfection; measurements performed at this time reflect primarily the consequences of virus production from the initial round of infection prior to an appreciable spread through the culture from secondary rounds of infection by progeny virions. The results of a representative experiment are shown in Fig. 2. All of the mutants were infectious. Three of the mutants (g2, g3, and g23) showed a moderate decrease in infectivity compared to that of the parental SIVmac239. The only mutants with a dramatic decrease in infectivity were the variants with the first glycosylation site mutated, the SIVmac239-gp41/g12 double mutant and the SIVmac239-gp41/g123 triple mutant (Fig. 2).
FIG. 2.
Comparative infectivities of SIVmac239 and mutants with different N-linked glycosylation sites in the transmembrane protein mutated. (A) Amino acid sequence of the glycosylated region of SIVmac239 gp41. (B and C) Relative infectivities of virus stocks. Virus stocks obtained from the transfection of HEK-293T cells were normalized for the amount of p27 and used to infect C8166-45 SIV-SEAP cells. SEAP activity was measured by use of a Phosphalight kit according to the manufacturer's recommendations at 3 days postinfection.
We also compared the growth curves of the different mutants in rhesus PBMCs. Normalized amounts of mutant and parental virus stocks produced from the transient transfection of HEK-293T cells were used to analyze viral replication in activated rhesus monkey PBMCs from four specific-pathogen-free rhesus monkeys. Cell supernatants were harvested on the indicated days, and the relative levels of p27 were quantitated by antigen capture assays. The replication kinetics observed in PBMCs from the four animals were very similar (Fig. 3). Most of the viruses replicated with kinetics very similar to those of the parental virus; however, two of the mutants (SIVmac239-gp41/g12 and SIVmac239-gp41/g123) replicated with a slight delay (Fig. 3).
FIG. 3.
Replication kinetics of viruses with different N-linked glycosylation sites in the transmembrane mutated in PBMC cultures obtained from four different rhesus macaques. (A) Animal 206-03; (B) animal 330-03; (C) animal 333-03; (D) animal 467-03.
Viral loads following infection of monkeys with SIV mutants.
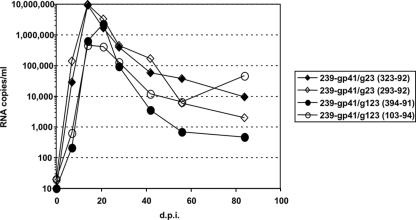
Two Indian-origin rhesus monkeys were infected experimentally with the mutant strain lacking the second and the third glycosylation sites in gp41 (SIVmac239-gp41/g23), and two more monkeys were infected with the mutant strain lacking the first, second, and third sites (SIVmac239-gp41/g123). Viral loads were determined by quantifying viral RNA levels in plasma. Three of the four mutant SIV-infected monkeys displayed peak viral loads in plasma at day 14, the time typically observed with wild-type SIVmac239 (Fig. 4) (12). Mean viral loads at peak in these four mutant SIV-infected monkeys (5 × 106 viral RNA equivalents per ml of plasma) were only modestly decreased from what is typically seen in monkeys infected with the parental SIVmac239 (30 × 106 viral RNA equivalents per ml) (12). Viral loads at set point, estimated from the day 84 time point, were considerably lower than what is typically seen with the parental SIVmac239: means of 1.5 × 104 versus 2 × 106 viral RNA equivalents per ml of plasma, respectively. Thus, these mutant strains appeared to exhibit a slightly attenuated phenotype in rhesus monkeys.
FIG. 4.
Viral RNA loads in plasma after intravenous inoculation of two macaques with an SIVmac239 strain lacking two gp41 N-linked glycosylation sites (animals 323-92 and 293-92) and two macaques lacking three sites (animals 394-91 and 103-94). Each animal was inoculated with virus equivalent to 20 ng of p27. Viral RNA loads in plasma were determined using a quantitative RT-PCR assay. d.p.i., days postinfection.
Removal of conserved N-linked glycosylation sites in the ectodomain of the transmembrane protein exposes epitopes that are targets of neutralizing antibodies.
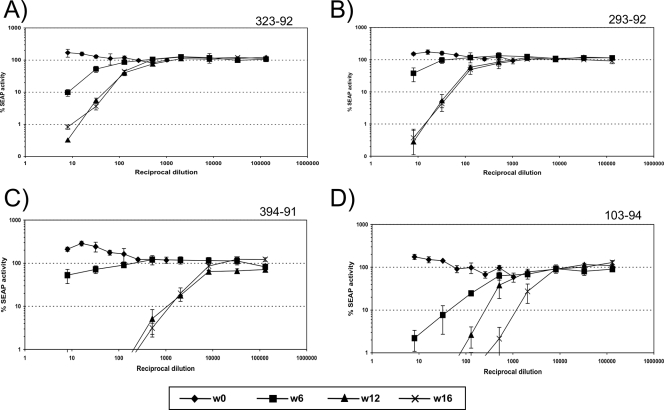
Plasma samples harvested on week 16 postinoculation were tested in vitro for neutralizing activity against the SIVmac239-gp41/g23 and SIVmac239-gp41/g123 mutant viruses. We observed that the presence of carbohydrate attachment site mutations resulted in markedly increased neutralizing responses when measured against the corresponding mutant viruses (Table 1). The neutralizing responses in plasma from monkeys infected with SIVmac239-gp41/g23 and SIVmac239-gp41/g123 were compared to the neutralizing responses observed against the same viruses in plasma from historical control monkeys infected with the parental SIVmac239 in prior experiments (Table 1). At week 16 postinoculation, neutralizing titers to SIVmac239-gp41/g23 were on average 10 times higher in plasma from animals infected with the double mutant than in plasmas from animals infected with SIVmac239. Even more impressive, plasma from animals infected with the triple mutant neutralized the corresponding virus at extremely high titers (5,000 and 4,500). These titers are 1,000 times higher than those obtained from animals infected with SIVmac239 (Fig. 5 and Table 1).
TABLE 1.
Neutralizing activities of plasmas from monkeys infected with SIVmac239-gp41/g23 and SIVmac239-gp41/g123 at week 16 postinoculation
| Virus | Neutralization activity per plasma from animala:
|
||||||
|---|---|---|---|---|---|---|---|
| 323-92b | 293-92b | 394-91c | 103-94c | 414-98d | 189-01d | 18-01d | |
| SIVmac239-gp41/g23 | 150 | 130 | 2000 | 500 | 16 | 20 | 20 |
| SIVmac239-gp41/g123 | 600 | 550 | 5,000 | 4,500 | <8 | <8 | 10 |
| SIV239 | <8 | <8 | <8 | <8 | 8 | <8 | <8 |
| SIV316 | 80,000 | 100,000 | 5,000 | 20,000 | 30,000 | 30,000 | 70,000 |
Titers are expressed as the reciprocal of the dilution of plasma required to reduce infectivity of the indicated virus by 50%.
Plasmas from monkeys infected with SIVmac239gp41/g23.
Plasmas from monkeys infected with SIVmac239gp41/g123.
Plasmas from monkeys infected with SIVmac239.
FIG. 5.
Neutralizing antibody responses. For each monkey, neutralizing activity in plasma was tested against the virus used for the inoculation. (A and B) SIVmac239-gp41/g23 virus against plasmas from animals 323-92 (A) and 293-92 (B). (C and D) SIVmac239-gp41/g123 virus against plasmas from animals 394-91 (C) and 103-94 (D).
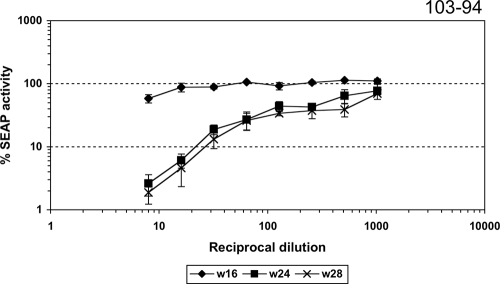
In order to characterize the specificity of this new neutralizing activity, we did some additional testing of the in vitro neutralizing activity in plasmas from monkeys infected with the mutant viruses at week 16 postinoculation against the parental virus and SIVmac316, a macrophage-tropic and neutralization-sensitive variant derived from SIVmac239. This neutralizing activity was compared with the titers obtained against the same viruses from animals infected with the parental SIVmac239 at the same week postinoculation. No significant difference in neutralization titers against SIVmac239 or SIVmac316 was observed between plasmas from monkeys infected with the mutant viruses and plasmas from monkeys infected with the parental strain at this time point. Thus, analysis of samples taken at this relatively early time point revealed that the removal of N-linked glycosylation sites in the ectodomain of the transmembrane resulted in a strong neutralizing response specific for the mutant viruses (Table 1). Beginning at around week 24, monkey 103-94 developed unusually high neutralizing titers against the parental SIVmac239 (titers of approximately 1:350 at weeks 24 and 28) (Fig. 6). These neutralization titers against SIVmac239 persisted at equivalent or higher levels in monkey 103-94 for at least a year (data not shown).
FIG. 6.
Neutralization sensitivity of SIVmac239 to plasma samples from monkey 103-94 at weeks 16, 24, and 28 after infection with SIVmac239-gp41/g123.
Monkeys infected with the mutant viruses made antibodies that reacted with peptides corresponding to the mutant sequences.
Plasma samples obtained from the four mutant SIV-infected monkeys at week 16 postinoculation were tested for reactivity to overlapping peptides spanning the whole envelope gene. Peptides with the parental sequence were used to coat ELISA plates and tested for reactivity to the monkey plasma. The reactivity profiles observed using plasma from the four monkeys infected with the mutant viruses were very similar, with peaks of high reactivity separated by regions with very little reactivity (Fig. 7). This pattern of reactivity is also similar to the one observed with plasma from monkeys infected with the parental SIVmac239 (Fig. 1B, C, and D). Importantly, there was no reactivity in the region encompassing the three N-glycosylation sites that were mutated.
FIG. 7.
Antibody reactivity to overlapping peptides spanning the entire envelope protein by ELISA using plasma from monkeys infected with SIVmac239-gp41/g23 (A and B) and SIVmac239-gp41/g123 (C and D) at week 16 postinoculation. The location of the variable loops, the beginning of the gp41 protein, and the predicted membrane-spanning domain are indicated. The conserved sites of N-linked glycosylation in the ectodomain of gp41 are also indicated by a gray box.
Plasma samples from the infected monkeys at week 16 postinoculation were also tested for reactivity to peptides with the mutant sequence in parallel with peptides that correspond to the parental sequence (Fig. 8A). In this assay, we also included plasmas from historical control monkeys at weeks 16, 17, and 22 after infection with SIVmac239 (Fig. 8A, red). Plasmas from monkeys infected with SIVmac239 showed no reactivity to the peptides spanning the N-linked glycosylation sites, whether the sequence corresponded to the parental or the mutant sequence. In contrast, plasma samples from monkeys infected with the mutant viruses were reactive to the overlapping peptides m6687 (WQEWERKVDFLEEQI) and m6688 (ERKVDFLEEQITALL) with the mutant sequence (Fig. 8B). The appearance of this new reactivity suggests that the presence of carbohydrate on sites 1, 2, and 3 in the ectodomain of the transmembrane protein results in a marked shielding of underlying B-cell epitopes.
FIG. 8.
Antibody reactivity to overlapping peptides of the region spanning the sites for the attachment of N-linked carbohydrates in gp41. Plasma obtained at week 16 postinoculation of monkeys infected with the mutant viruses (monkeys 394-92, 293-92, 323-92, and 103-94) and weeks 16, 17, and 22 postinoculation of monkeys infected with SIVmac239 (monkeys 187-93, 414-98, 189-01, and 18-01) were tested for antibody reactivity to peptides with the parental and mutant sequence. (A) Sequences of the peptides that were used. (B) Pepscan reactivity. O.D., optical density.
Sequence evolution in the env gene during replication in rhesus macaques.
We next examined viral sequences in the envelope gene at week 16 (animals 293-92 and 394-91) and week 22 (animals 323-92 and 103-94) postinoculation. Plasma RNA prepared from monkeys infected with the mutant viruses was used for PCR amplification, cloning, and sequencing of the complete envelope gene. An average of 10.55 (range, 6 to 15) nucleotide substitutions and 8.3 amino acid (aa) (range, 5 to 12) replacements were present in the week 16 and week 22 clones. Mutations that created new N-linked sites were observed in viral RNA from monkeys infected with SIVmac239-gp41/g123. The S627N mutation was common to all the clones from monkeys infected with the triple mutant and created a new N-linked site 2 aa downstream of mutated site g1 in gp41. SIV in one of the monkeys (103-94) infected with the triple mutant had two additional glycosylation sites in gp120 at positions 63 and 254. No additional glycosylation sites were observed in the viral RNA obtained from plasma from monkeys infected with the double mutant (Fig. 9). Additional inspection of the sequences revealed a striking accumulation of mutations in the immediate vicinity of the three mutated N-linked glycosylation sites in the ectodomain of gp41 (aa 621 to aa 660). In this region, a total of 70 nucleotide substitutions (2.9% mutation frequency) that resulted mostly in amino acid substitutions (total nonsynonymous substitutions/total synonymous + nonsynonymous substitutions = 66/70) were present. This accumulation of mutations indicates a strong selective pressure for change within this region.
FIG. 9.
Alignments of SIV envelope sequences from plasma RNA obtained from monkeys infected with the mutant viruses (five clones each) at weeks 16 (animals 293-92 and 394-91) and 22 (animals 323-92 and 103-94) postinfection. The location of the variable loops, the beginning of the gp41 protein, and the predicted membrane-spanning domain (TM) are indicated. Periods indicate conservation with the SIVmac239 sequence. The conserved sites for N-linked glycosylation in the ectodomain of gp41 are highlighted in gray. New sites of N-linked glycosylation (designated by a box) appeared during viral replication in monkeys 394-91 and 103-94.
Mutations in the region proximal to the glycosylation sites in the ectodomain of gp41 confer neutralization resistance.
To better define the specificity of antibodies responsible for the neutralization of the mutant SIVs, four chimeras were constructed. We selected one plasmid per animal containing the envelope viral sequence amplified from plasma RNA at weeks 16 or 22 postinoculation and cut with convenient restriction enzymes. The digested fragments were inserted into digested vectors that contained SIVmac239 sequences, thus creating four SIVs containing envelope proteins chimeric for the region spanning the gp41 N-linked sites (Fig. 10A). The new chimeric viruses NSR, NR, KLNL, and RNNH contain the region spanning the gp41 N-linked sites from plasma viral RNA from animals 323-92, 293-92, 394-91, and 103-94, respectively. Two of the chimeras (KLNL and RNNH) incorporated the same additional N-linked glycosylation site adjacent to g1 in gp41.
FIG. 10.
Characterization of the chimeric viruses used to define mutations that confer neutralization resistance. (A) Sequence alignments in the region spanning the sites for the attachment of N-linked carbohydrates in gp41 of the chimeras and mutants used. The conserved sites of N-linked glycosylation in the ectodomain of gp41 are highlighted in gray. The new sites of N-linked glycosylation are designated by a box. (B and C) Relative infectivity. Virus stocks were obtained from the transfection of HEK-293T cells. Stocks were normalized for the amount of p27 and used to infect C8166-45 SIV-SEAP cells. SEAP activity was measured by use of a Phosphalight kit according to the manufacturer's recommendations at 3 days postinfection.
The infectivities of these mutants were measured under conditions that approximated a single cycle of infection using the protocol described above. All the chimeras except one were able to infect C8166-45 SIV-SEAP cells. The variant with a chimeric KLNL envelope showed no infectivity even after infection with the largest amount of virus (25 ng p27). The other variants displayed infectivities per ng p27 that were similar to those of the parent virus from which they were derived (Fig. 10B and C).
Each of the chimeric viruses was tested for neutralization sensitivity with plasmas from monkeys infected with SIVmac239-gp41/g23 and SIVmac239-gp41/g123 obtained at week 16 postinfection. All replication-competent chimeras were considerably more resistant to neutralization than the mutant viruses from which they were derived (Fig. 11 and Table 2). Chimeras NSR and NR displayed a moderate increase in resistance to neutralization to plasmas from monkeys 323-92, 293-92, and 103-94. Especially significant was the resistance of both chimeras to neutralization by plasma from monkey 394-91, with a 133-fold decrease in the 50% neutralization titer compared with neutralization of the corresponding mutant virus. The most dramatic effect was observed for chimera RNNH. This chimera was resistant to neutralization with plasma from the four monkeys infected with the mutant viruses, changing the 50% neutralization titer from 1:600 to 1:15 with animal 323-92 plasma, from 1:550 to 1:15 with animal 293-92 plasma, from 1:5,000 to less than 1:8 with animal 394-91 plasma, and from 1:4,500 to 1:15 with animal 103-94 plasma compared with the corresponding mutant virus (SIVmac239-gp41/g123).
FIG. 11.
Neutralization sensitivities of SIVmac239-gp41/g123 mutant virus and two chimeric viruses (RNNH and RNH) to plasma samples from monkeys infected with the mutant viruses at week 16 postinoculation. Chimeric virus RNNH has a new site of N-linked glycosylation that has been removed in chimeric virus RNH. Sources of week 16 plasma were animals 323-92 (A), 293-92 (B), 394-91 (C), and 103-94 (D).
TABLE 2.
Neutralization sensitivities of SIVmac239-gp41/g23, SIVmac239-gp41/g123, and the chimeric viruses to plasmas from monkeys inoculated with the mutant virus at week 16 postinoculation
| Virus | Neutralization activity per plasma for animala:
|
|||
|---|---|---|---|---|
| 323-92b | 293-92b | 394-91c | 103-94c | |
| SIVmac239-gp41/g23 | 150 | 130 | 2000 | 500 |
| NSR | 30 | 25 | 15 | 200 |
| NR | 60 | 30 | 15 | 150 |
| SIVmac239-gp41/g123 | 600 | 550 | 5,000 | 4,500 |
| RNNH | 15 | 15 | <8 | 15 |
| RNH | 200 | 250 | 12 | 60 |
Titers are expressed as the reciprocal of the dilution of plasma required to reduce the infectivity of the indicated virus by 50%. Values for chimeric viruses are shown in boldface type.
Plasmas from monkeys inoculated with SIVmac239-gp41/g23.
Plasmas from monkeys inoculated with SIVmac239-gp41/g123.
In order to determine the effect of the newly acquired glycosylation site, we also created a mutant from one of the chimeric viruses (RNNH), in which the S627N mutation was reverted to the parental sequence by site-directed mutagenesis, generating a new chimeric virus with no N-linked sites in the region (RNH) (Fig. 10A). This mutant displayed an intermediate phenotype, being more resistant to neutralization than SIVmac239-gp41/g123 but not to the degree observed for RNNH. The 50% neutralization titers were 1:200 for animal 323-92 plasma, 1:250 for animal 293-92 plasma, 1:12 for animal 394-91 plasma, and 1:60 for animal 103-94 plasma (Fig. 11 and Table 2).
DISCUSSION
Despite the consistent conservation of closely spaced sites for carbohydrate attachment in the ectodomain of gp41 of both HIV-1 and SIV, these sites are quite dispensable for viral replication. The dispensability for SIV gp41 glycosylation sites that we describe here is similar to what was reported previously for HIV-1 gp41 glycosylation sites (14). We have hypothesized in the current study that these carbohydrates may form a barrier to shield amino acids whose recognition by antibodies would result in virus neutralization. The complete absence of antibodies that map to this region of the ectodomain in HIV-1 gp41 is consistent with this hypothesis. Using overlapping peptides that span the full length of the envelope protein of cloned SIVmac239, we have been able to precisely map linear epitopes recognized by antibodies that arise following infection with cloned SIVmac239. This type of analysis is greatly facilitated by the ability to use peptides exactly matched in sequence to the cloned virus used for infection. The pepscan profiles resulting from our analysis of control monkeys infected with wild-type SIVmac239 are remarkably concordant with major sites for antibody recognition of HIV-1 gp41 compiled previously (17). In particular, they confirm the lack of antibody recognition in the stretch that contains the three closely spaced, highly conserved N-linked sites in the SIV transmembrane protein.
By 16 weeks, monkeys infected with the mutant viruses made antibodies that neutralized the mutant viruses to high titer. This neutralizing activity was not observed in plasmas from monkeys infected with the parental virus. Thus, new specificities were revealed as a result of the carbohydrate mutations, and antibodies with these specificities had strong neutralizing activity against the mutant viruses. Pepscan analysis revealed new reactivity to peptides corresponding to the mutant sequences, reactivity that was highly specific for monkeys infected with the mutant viruses. Plasmas from monkeys infected with SIVmac239 at similar weeks postinoculation showed no reactivity to the mutant or parental peptides spanning this region.
Analysis of viral sequences present in monkeys infected with the mutant viruses revealed strong selective pressure for the emergence of variants with sequence changes in this region. Carbohydrate-deficient viruses that were modified to contain these emergent changes were no longer sensitive to neutralization by plasma from the monkeys infected with the carbohydrate-deficient viruses. Especially impressive was the replacement of 4 aa in a 10-aa stretch, which changed SIVmac239-gp41/g123 from extremely sensitive to completely resistant to neutralization; neutralization titers of 1:4,000 and 1:5,000 were changed to 1:15 and 1:8 by this 4-aa replacement. The pepscan profiles and the location of sequence changes that result in escape from neutralization argue strongly that amino acids in the region of the carbohydrate attachment mutations are the direct targets of antibodies with neutralizing activity.
It needs to be noted that while antibodies from mutant-infected monkeys were able to efficiently and specifically bind mutant peptides from aa 637 to 651, escape mutations were mapped at positions 625, 631, and 634. In addition, we were unable to block the neutralizing activity toward mutant virus by linear peptides corresponding to the mutant sequences (data not shown). There are several possible explanations for these observations. It is possible that mutations at positions 625, 631, and 634 alter the ability of antibodies with neutralizing activity to recognize downstream amino acid sequences in the context of the properly folded protein. It is also possible that antibodies with neutralizing activity recognize conformational epitopes in this region that are not represented by the linear peptides. However, we cannot formally exclude the possibility that antibodies with neutralizing activity against the mutant viruses are recognizing epitopes that are located a considerable distance away in the linear sequence.
Ideally, for vaccine purposes, one would like to engineer modified versions of envelope-based immunogens such that they are better capable of eliciting antibodies that can neutralize wild-type virus, as has been described previously by Reitter et al. (33) and by Li et al. (19) using V1/V2 glycan mutations. The gp41 glycan mutants described in this report certainly did not exhibit any increased capacity to elicit antibodies capable of neutralizing SIVmac239 through the first 16 weeks of infection. However, one of the mutant-infected monkeys, animal 103-94, developed neutralizing antibody titers against wild-type SIVmac239 in excess of 1:350 beginning around week 24 following infection that persisted for at least 1 year. Such activity was not observed in the other three mutant-infected monkeys. To what extent the impressive activity in this one animal may have been related to infection by a mutant virus, or just random, is difficult to say. Neutralizing antibody titers against SIVmac239 in animal 103-94 have been exceeded in our experience by only one other monkey that has been recently described (35). It is interesting that SIV in monkey 103-94 developed two new glycosylation sites in gp120 (Fig. 9) that could potentially represent escape mutations for antibodies capable of neutralizing SIVmac239. Monkey 103-94 also developed an unusual reactivity to a peptide just downstream of V1-V2 (Fig. 7). Further work will be needed to precisely define the specificity of monoclonal antibodies from animal 103-94 capable of neutralizing SIVmac239 as well as the precise specificity of antibodies from mutant-infected animals that are responsible for the neutralizing activity against gp41 mutant viruses.
Acknowledgments
We thank William Lauer and Jennifer Morgan for technical assistance, Deborah Letourneau for assistance in preparing the manuscript, the staff of the Division of Primate Resources at NEPRC for monkey acquisition and blood sampling, Michael Piatak, Jr., for viral load measurements, and the NIH AIDS Repository for the peptide collection corresponding to wild-type SIVmac239.
This work was supported by U.S. Public Health Service grants AI025328 and AI150421 to R.D., AI057039 to W.J., and RR00168 to the NEPRC; by an award from the International AIDS Vaccine Initiative; and in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.
Footnotes
Published ahead of print on 1 October 2008.
REFERENCES
- 1.Back, N. K. T., L. Smit, J.-J. De Jong, W. Keulen, M. Schutten, J. Goudsmit, and M. Tersmette. 1994. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 199431-438. [DOI] [PubMed] [Google Scholar]
- 2.Bahraoui, E., A. Benjouad, D. Guetard, H. Kolbe, J. C. Gluckman, and L. Montagnier. 1992. Study of the interaction of HIV-1 and HIV-2 envelope glycoproteins with the CD4 receptor and role of N-glycans. AIDS Res. Hum. Retrovir. 8565-573. [DOI] [PubMed] [Google Scholar]
- 3.Binley, J. M., R. Wyatt, E. Desjardins, P. D. Kwong, W. Hendrickson, J. P. Moore, and J. Sodroski. 1998. Analysis of the interaction of antibodies with a conserved enzymatically deglycosylated core of the HIV type 1 envelope glycoprotein 120. AIDS Res. Hum. Retrovir. 14191-198. [DOI] [PubMed] [Google Scholar]
- 4.Blay, W. M., S. Gnanakaran, B. Foley, N. A. Doria-Rose, B. T. Korber, and N. L. Haigwood. 2006. Consistent patterns of change during the divergence of human immunodeficiency virus type 1 envelope from that of the inoculated virus in simian/human immunodeficiency virus-infected macaques. J. Virol. 80999-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolmstedt, A., S. Sjolander, J. E. Hansen, L. Akerblom, A. Hemming, S. L. Hu, B. Morein, and S. Olofsson. 1996. Influence of N-linked glycans in V4-V5 region of human immunodeficiency virus type 1 glycoprotein gp160 on induction of a virus-neutralizing humoral response. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12213-220. [DOI] [PubMed] [Google Scholar]
- 6.Cheng-Mayer, C., A. Brown, J. Harouse, P. A. Luciw, and A. J. Mayer. 1999. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 735294-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenouillet, E., B. Clerget-Raslain, J. C. Gluckman, D. Guetard, L. Montagnier, and E. Bahraoui. 1989. Role of N-linked glycans in the interaction between the envelope glycoprotein of human immunodeficiency virus and its CD4 cellular receptor. Structural enzymatic analysis. J. Exp. Med. 169807-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frost, S. D., T. Wrin, D. M. Smith, S. L. Kosakovsky Pond, Y. Liu, E. Paxinos, C. Chappey, J. Galovich, J. Beauchaine, C. J. Petropoulos, S. J. Little, and D. D. Richman. 2005. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc. Natl. Acad. Sci. USA 10218514-18519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gowda, S., T. Satyanarayana, M. A. Ayllon, M. R. Albiach-Marti, M. Mawassi, S. Rabindran, S. M. Garnsey, and W. O. Dawson. 2001. Characterization of the cis-acting elements controlling subgenomic mRNAs of citrus tristeza virus: production of positive- and negative-stranded 3′-terminal and positive-stranded 5′-terminal RNAs. Virology 286134-151. [DOI] [PubMed] [Google Scholar]
- 10.Gram, G. J., A. Hemming, A. Bolmstedt, B. Jansson, S. Olofsson, L. Akerblom, J. O. Nielsen, and J. E. Hansen. 1994. Identification of an N-linked glycan in the V1-loop of HIV-1 gp120 influencing neutralization by anti-V3 antibodies and soluble CD4. Arch. Virol. 139253-261. [DOI] [PubMed] [Google Scholar]
- 11.Harouse, J. M., A. Gettie, T. Eshetu, R. C. Tan, R. Bohm, J. Blanchard, G. Baskin, and C. Cheng-Mayer. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIVSF162P3. J. Virol. 751990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, W. E., J. D. Lifson, S. M. Lang, R. P. Johnson, and R. C. Desrosiers. 2003. Importance of B-cell responses for immunological control of variant strains of simian immunodeficiency virus. J. Virol. 77375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, W. E., H. Sanford, L. Schwall, D. R. Burton, P. W. Parren, J. E. Robinson, and R. C. Desrosiers. 2003. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J. Virol. 779993-10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, W. E., J. M. Sauvron, and R. C. Desrosiers. 2001. Conserved, N-linked carbohydrates of human immunodeficiency virus type 1 gp41 are largely dispensable for viral replication. J. Virol. 7511426-11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 753435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolchinsky, P., E. Kiprilov, and J. Sodroski. 2001. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J. Virol. 752041-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korber, B., B. Brander, B. Haynes, R. Koup, J. Moore, B. Walker, and D. Watkins. 2006. HIV Molecular Immunology. LA-UR 07-4752. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, NM.
- 18.Leitner, T., B. Foley, B. Hahn, P. Marx, F. McCutchan, J. Mellors, S. Wolinsky, and B. Korber. 2007. HIV Sequence Compendium 2005. LA-UR 06-0680. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, NM.
- 19.Li, Y., B. Cleveland, I. Klots, B. Travis, B. A. Richardson, D. Anderson, D. Montefiori, P. Polacino, and S. L. Hu. 2008. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J. Virol. 82638-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 7510187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ly, A., and L. Stamatatos. 2000. V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 746769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malenbaum, S. E., D. Yang, L. Cavacini, M. Posner, J. Robinson, and C. Cheng-Mayer. 2000. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. J. Virol. 7411008-11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCaffrey, R. A., C. Saunders, M. Hensel, and L. Stamatatos. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 783279-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Means, R. E., and R. C. Desrosiers. 2000. Resistance of native, oligomeric envelope on simian immunodeficiency virus to digestion by glycosidases. J. Virol. 7411181-11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Means, R. E., T. Greenough, and R. C. Desrosiers. 1997. Neutralization sensitivity of cell culture passaged simian immunodeficiency virus. J. Virol. 717895-7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Means, R. E., J. Reitter, and R. Desrosiers. 1997. N-Glycosylation and avoidance of humoral immunity, p. 137-143. In M. Girard and B. Dodet (ed.), Colloque des cent gardes. Elsevier Paris, Paris, France.
- 27.Mori, K., D. J. Ringler, T. Kodama, and R. C. Desrosiers. 1992. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J. Virol. 662067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison, H. G., F. Kirchhoff, and R. C. Desrosiers. 1993. Evidence for the cooperation of gp120 amino acids 322 and 448 in SIVmac entry. Virology 195167-174. [DOI] [PubMed] [Google Scholar]
- 29.National Research Council. 1996. Guide for the care and use of laboratory animals, p. 86-123. National Academy Press, Washington, DC.
- 30.Parren, P. W., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinones-Kochs, M. I., L. Buonocore, and J. K. Rose. 2002. Role of N-linked glycans in a human immunodeficiency virus envelope glycoprotein: effects on protein function and the neutralizing antibody response. J. Virol. 764199-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reitter, J. N., and R. C. Desrosiers. 1998. Identification of replication-competent strains of simian immunodeficiency virus lacking multiple attachment sites for N-linked carbohydrates in variable regions 1 and 2 of the surface envelope protein. J. Virol. 725399-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4679-684. [DOI] [PubMed] [Google Scholar]
- 34.Rudensey, L. M., J. T. Kimata, E. M. Long, B. Chackerian, and J. Overbaugh. 1998. Changes in the extracellular envelope glycoprotein of variants that evolve during the course of simian immunodeficiency virus SIVMne infection affect neutralizing antibody recognition, syncytium formation, and macrophage tropism but not replication, cytopathicity, or CCR-5 coreceptor recognition. J. Virol. 72209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato, S., E. Yuste, W. A. Lauer, E. H. Chang, J. S. Morgan, J. G. Bixby, J. D. Lifson, R. C. Desrosiers, and W. E. Johnson. 2008. Potent antibody-mediated neutralization of simian immunodeficiency virus strain SIVmac239. J. Virol. 829739-9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schonning, K., B. Jansson, S. Olofsson, J. O. Nielsen, and J.-E. S. Hansen. 1996. Resistance to V3-directed neutralization caused by an N-linked oligosaccharide depends on the quaternary structure of the HIV-1 envelope oligomer. Virology 218134-140. [DOI] [PubMed] [Google Scholar]
- 37.Walker, B. D., and D. R. Burton. 2008. Toward an AIDS vaccine. Science 320760-764. [DOI] [PubMed] [Google Scholar]
- 38.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 39.Yuste, E., H. B. Sanford, J. Carmody, J. Bixby, S. Little, M. B. Zwick, T. Greenough, D. R. Burton, D. D. Richman, R. C. Desrosiers, and W. E. Johnson. 2006. Simian immunodeficiency virus engrafted with human immunodeficiency virus type 1 (HIV-1)-specific epitopes: replication, neutralization, and survey of HIV-1-positive plasma. J. Virol. 803030-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]